Abstract
Our objective was to construct and validate a Multidimensional Prognostic Index (MPI) for 1-year mortality from a Comprehensive Geriatric Assessment (CGA) routinely carried out in elderly patients in a geriatric acute ward. The CGA included clinical, cognitive, functional, nutritional, and social parameters and was carried out using six standardized scales and information on medications and social support network, for a total of 63 items in eight domains. A MPI was developed from CGA data by aggregating the total scores of the eight domains and expressing it as a score from 0 to 1. Three grades of MPI were identified: low risk, 0.0–0.33; moderate risk, 0.34–0.66; and severe risk, 0.67–1.0. Using the proportional hazard models, we studied the predictive value of the MPI for all causes of mortality over a 12-month follow-up period. MPI was then validated in a different cohort of consecutively hospitalized patients. The development cohort included 838 and the validation cohort 857 elderly hospitalized patients. Of the patients in the two cohorts, 53.3 and 54.9% were classified in the low-risk group, respectively (MPI mean value, 0.18 ± 0.09 and 0.18 ± 0.09); 31.2 and 30.6% in the moderate-risk group (0.48 ± 0.09 and 0.49 ± 0.09); 15.4 and 14.2% in the severe-risk group (0.77 ± 0.08 and 0.75 ± 0.07). In both cohorts, higher MPI scores were significantly associated with older age (p = 0.0001), female sex (p = 0.0001), lower educational level (p = 0.0001), and higher mortality (p = 0.0001). In both cohorts, a close agreement was found between the estimated mortality and the observed mortality after both 6 months and 1 year of follow-up. The discrimination of the MPI was also good, with a ROC area of 0.751 (95%CI, 0.70–0.80) at 6 months and 0.751 (95%CI, 0.71–0.80) at 1 year of follow-up. We conclude that this MPI, calculated from information collected in a standardized CGA, accurately stratifies hospitalized elderly patients into groups at varying risk of mortality.
Introduction
A demographic shift has occurred in the last decades in developed countries that has resulted in a considerable rise in the number of elderly patients. Thus, the study and care of elderly subjects have become priority topics for clinicians and researchers.
For patients and caregivers, prognostic information is needed to inform decisions concerning clinical management, discharge plan, and follow-up. This may be particularly important in old age, in which life expectancy may directly influence the care planning of patients. For example, in older patients with limited life expectancy, multiple pathologies and disabilities, and elevated risk of rapid deterioration in health and functional status, the goal of care may be to plan programs of advanced care.1 Conversely, older patients with good life expectancies should receive the standard level of care, including preventive programs and aggressive diagnostic and therapeutic interventions.2,3
Since mortality in older subjects results from a combination of biological, functional, psychological, pathological, and environmental factors, tools that effectively identify patients with low life expectancy should take a multidimensional approach.4 Previous attempts to develop a prognostic index for older patients based on their functional, biological, and environmental characteristics were performed on population-based,5,6 community-dwelling,7,8 or institutionalized subjects.9 Two studies developed a prognostic index for mortality in hospitalized elderly patients from measures of physical and cognitive functions10 or from demographic characteristics, functional disability, co-morbidity, length of hospital stay, and laboratory measures.11 To our knowledge, no prognostic index for mortality in hospitalized elderly patients has been developed that fully utilizes the wide range of information provided by a standardized Comprehensive Geriatric Assessment (CGA), which is the most accurate and sensitive diagnostic tool for evaluating and monitoring elderly patients for clinical decision-making purposes.12,13
The aim of this study was to construct and validate a Multidimensional Prognostic Index (MPI) for 1-year mortality based on information that is available from a standardized CGA commonly used to evaluate hospitalized older patients.
Methods
Subjects
The study was conducted according to the Declaration of Helsinki and the guidelines for Good Clinical Practice and was approved by our Institution Ethics Committee. Written informed consent was obtained from the patients or from relatives of critically ill or demented patients prior to participation in the study.
Two cohorts of elderly hospitalized patients were included in the study. The first cohort (development cohort) was included for developing the MPI; the second cohort (validation cohort) was involved in the study in order to validate the efficacy of MPI as a 1-year mortality risk prognostic index.
For inclusion in the development cohort, we screened all patients aged ≥65 years consecutively admitted from January 1 to December 31, 2004 to the Geriatric Unit of the Casa Sollievo della Sofferenza Hospital (IRCCS, San Giovanni Rotondo, Italy) due to acute disease or relapse of a chronic disease. For inclusion in the validation cohort, we screened all patients ≥aged 65 years consecutively admitted from January 1 to December 31, 2005 to the same geriatric unit.
For both cohorts, inclusion criteria were: (1) age ≥ 65 years; (2) ability to provide an informed consent or availability of a proxy for informed consent and willingness to participate in the study; (3) complete CGA during hospitalization; and (4) availability of mortality/survival information at the dates of study completion.
At baseline, the following parameters were collected by structured interview, clinical evaluation, and review of records from the patients' general practitioners: date of birth, gender, clinical history, current pathologies, and medication history.
All patients admitted in our unit received a standard CGA for clinical purposes. Vital status up to December 31, 2005 (development cohort), and up to December 31, 2006 (validation cohort), was assessed by directly contacting the participants or consulting the registry offices of the cities where the patients were residents at the time of hospital admission. Dates of death were identified from death certificates.
Comprehensive Geriatric Assessment
Comprehensive Geriatric Assessment (CGA) was carried out using assessment instruments widely employed in geriatric practice. Functional status was evaluated by the Activities of Daily Living (ADL) index,14 which defines the level of dependence/independence of six daily personal care activities, including bathing, toileting, feeding, dressing, urine and bowel continence, and transferring (in and out of bed or chair), and by the Instrumental Activities of Daily Living (IADL) scale,15 which assesses independence in eight activities that are more cognitively and physically demanding than those in the ADL index, including managing finances, taking medications, using the telephone, shopping, using transportation, preparing meals, doing housework, and washing.
Cognitive status was assessed by the Short Portable Mental Status Questionnaire (SPMSQ), a 10-item questionnaire that assesses orientation, memory, attention, calculation, and language.16
Co-morbidity was examined using the Cumulative Illness Rating Scale (CIRS).17 The CIRS uses 5-point ordinal scales (score 1–5) to estimate the severity of pathology in each of 13 systems, including cardiac, vascular, respiratory, eye-ear-nose-throat, upper and lower gastroenteric diseases, hepatic, renal, genitourinal, musculoskeletal, skin disorders, nervous system, endocrine-metabolic, and psychiatric behavioral problems. Based on the ratings, the two following scores are derived: (1) the Co-morbidity Index (CIRS-CI), which reflects the number of concomitant diseases and is derived from the total number of categories in which moderate or severe levels (grades 3–5) of disease are quoted (ranging 0–13); and (2) the Severity Index (CIRS-SI), which reflects the overall severity of diseases and the average rating of 13 disease categories, excluding psychiatric behavioral problems (ranging 1–5).
Nutritional status was explored with the Mini Nutritional Assessment (MNA),18 which includes information on (1) anthropometric measures (body mass index [BMI]: body weight/height2, mid-arm circumference in cm [MAC], calf circumference in cm [CC], and weight loss); (2) lifestyle, medication, and mobility; (3) number of meals, food, and fluid intake and autonomy of feeding; and (4) self-perception of health and nutrition.
The Exton-Smith Scale (ESS) was used to evaluate the risk of developing pressure sores. This five-item questionnaire determines physical condition, mental condition, activity, mobility, and incontinence. For each item, a score from 1 to 4 is assigned.19
Medication use was defined according to the Anatomical Therapeutics Chemical Classification code system (ATC classification),20 and the number of drugs used by patients at admission was recorded. Patients were defined as drug users if they took a medication of any drug included in the ATC classification code system at the time of admission.
Social aspects included household composition, home services, and institutionalization.
Multidimensional Prognostic Index
In order to develop a Multidimensional Prognostic Index (MPI) that correctly reflects the multidimensional impairment of the hospitalized geriatric patient, a cluster analysis on CGA data of the development cohort population was initially made for evaluating the independence of variables and identifying the most relevant domains of the CGA in predicting mortality outcome. The cluster analysis showed a correlation among ADL, IADL, SPMSQ, ESS, and MNA and evident independence among the previous variables and co-morbidity (CIRS) and medication use, which were correlated with each other and social aspects. Thus we started to develop a MPI considering only three variables: ADL, medication use, and social aspects. This “three-domain” MPI, while in a Cox regression analysis produced an acceptable separation among the survival curves of the three groups of patients (low, moderate, and severe risk of death), resulted in an unsatisfactory prognostic index for 1-year mortality (OR, 0.635; 95%CI, 0.141–2.871). Following a step-wise method, other domains of the CGA, one at a time, were progressively included in the model, and relative Cox and logistic regression analyses were performed. Thus the eight-domain MPI (i.e., a total of 63 items in eight domains of the CGA) resulted in the best index in predicting 1-year mortality in this population.
For each domain, a tripartite hierarchy was used (0, no problems; 0.5, minor problems; and 1, major problems) based on conventional cut-off points derived from the literature for the SPMSQ,16 MNA,18 EES,19 and ADL/IADL22 or by observing the frequency distribution of the patients at various levels to identify points of separation for co-morbidities and number of drugs. The specific threshold used to define the three categories are shown in Table 1. The sum of the calculated scores from the eight domains was divided by 8 to obtain a final MPI score from 0 to 1. For analytical purposes, absolute values of MPI were not considered; we preferred to express the MPI as low (MPI value ≤0.33), moderate (MPI between 0.34 and 0.66), and severe risk (MPI >0.66), according to previous rule-based indices used for exploring multidimensional impairment in elderly subjects.21 In order to determine the best MPI cut-off points, MPI values were classified into three categories: low, moderate and severe risk as follows:
Table 1. Multidimensional Prognostic Index Score Assigned to Each Domain Based on the Severity of the Problem.
| Problem | |||
|---|---|---|---|
| Assessment | No (value = 0) |
Minor (value = 0.5) |
Severe (value = 1) |
| Activities of Daily Living (ADL)a | 6–5 | 4–3 | 2–0 |
| Instrumental ADL (IADL)a | 8–6 | 5–4 | 3–0 |
| Short Portable Mental Status Questionnaire (SPMSQ)b | 0–3 | 4–7 | 8–10 |
| Comorbidity Index (CIRS-CI)c | 0 | 1–2 | ≥3 |
| Mini Nutritional Assessment (MNA)d | ≥24 | 17–23.5 | <17 |
| Exton Smith Scale (ESS)e | 16–20 | 10–15 | 5–9 |
| Number of medications | 0–3 | 4–6 | ≥7 |
| Social support network | Living with family | Institutionalized | Living alone |
Number of active functional activities.
Number of errors.
Number of diseases (see text).
MNA score: ≥24, satisfactory nutritional status; 17–23.5, at risk of malnutrition; <17, malnutrition.
ESS score: 16–20, minimum risk; 10–15, moderate risk; 5–9, high risk of developing scores.
Then we fixed d = (B – A) value to 0.1, obtaining the following cut-off point sequence:
Setting the d values to 0.2, 0.3, …, 0.8, for each cut-off point sequence, the degree of separation between the MPI curves for patients with low, moderate, and severe risk was computed: thus we found that the point values that produced the best separation among the curves corresponding to the different MPI grades were A = 0.33 and B = 0.66. In this model the term “separation among the curves” refers to the maximal value of R, that is, R = Δr1 + Δr2 where
and
In the integral, rL, rM, and rS refer to the survival curves for a low, moderate, and severe patient risk.
Statistical analysis
All analyses were performed using the SPSS v13 software for Windows (SPSS Inc., Chicago, IL).23 Continuous variables were shown as mean ± standard deviation and comparisons between men and women used the Mann-Whitney U test. The Kruskall-Wallis analysis of variance was used to compare age, gender, educational level, and mortality across the MPI groups.
The relationship between MPI score group and time to death was analyzed by the age- and sex-adjusted Cox proportional hazard regression model. Time to death was calculated as the time between admission and time of death or end of the follow-up, whichever came first. The proportionally of the hazard assumption was graphically checked by plotting log (-log (survival function)].
Martingale residuals were used to explore the functional form of the relationship between the MPI and mortality and to verify whether the thresholds for the definition of the MPI group were appropriate.
The proportionality assumption was verified by plotting Ln[-ln(survival function)] with time.
To test the hypothesis that the prognostic value of the aggregated MPI was superior to the prognostic value of its single components considered individually, a logistic model was carried out on the individual parameters. Age, ADL, IADL, SPMSQ, CIRS, MNA, EES, and number of drugs were evaluated as continuous variables, while social support network and MPI were evaluated as ordinal variables, based on the assumption of an equidistance between single unit values. Sex was analyzed as a dichotomous variable.
We assessed the predictive accuracy of the final model by looking at the two components of accuracy: calibration and discrimination. Calibration of the model was assessed by comparing the predicted mortality with the actual mortality in the development and validation cohorts. The discrimination of the model was assessed by calculating the receiver operating characteristic (ROC) curves for the development and validation cohorts.
A p value of <0.05 was considered for statistical significance.
Results
Development of the Multidimensional Prognostic Index
During the 1-year inclusion period of the development cohort, 1549 patients were consecutively admitted to our Geriatric Unit. Seventy-five subjects were excluded because they were younger than 65 years; 281 patients were excluded because information on their vital status after 1 year of follow-up was not available; 152 patients were excluded because the CGA was not completed; and 203 patients were excluded because they refused to participate in the study. Thus, the final study population included 838 elderly patients—373 men, 465 women with a mean age of 79.2 ± 7.3 years, a range of 65–101 years.
Table 2 reports the characteristics of the patients included in the development cohort, divided according to gender. The MPI value in the development cohort was 0.37 ± 0.23 (range 0–1). According to their MPI value, 447 patients (53.3%) were included in MPI grade 1 (low risk, MPI mean value 0.18 ± 0.09); 262 patients (31.2%) in MPI grade 2 (moderate risk, MPI 0.48 ± 0.09); 129 patients (15.4%) in MPI grade 3 (severe risk, MPI 0.77 ± 0.08). Higher MPI grades were significantly associated with older age (mean age grade 1, 77 ± 7.0 years; grade 2, 81.1 ± 7.2 years; grade 3, 83.0 ± 7.3 years; p = 0.0001), female sex (low risk, 48.3%; moderate risk, 64.1%; severe risk, 62.7%; p = 0.0001), lower educational level (low risk, 4.5 ± 3.1; moderate risk, 3.7 ± 3.0; severe risk, 3.8 ± 3.5 years; p = 0.0001), and progressively higher mortality after 6 months (grade 1, 5.6%; grade 2, 16.7%; grade 3, 34.1%) and 1 year of follow-up (grade 1, 8.1%; grade 2, 21.3%; grade 3, 43.4%; p = 0.0001) (Table 3).
Table 2. Characteristics of Patients in the Development and Validation Cohorts Divided According to Gender.
| Development cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 373) |
Females (n = 465) |
p value | All (n = 838) |
Males (n = 402) |
Females (n = 454) |
p value | All (n = 856) |
|
| Patients (%) | 44.5 | 55.4 | — | 100 | 46.9 | 53.0 | — | 100 |
| Age (years)a | 78.6 ± 7.3 | 79.7 ± 7.2 | 0.03 | 79.2 ± 7.3 | 77.6 ± 6.9 | 78.9 ± 7.3 | 0.017 | 78.3 ± 7.1 |
| Age range (years)a | 65–98 | 65–101 | — | 65–101 | 65–98 | 65–100 | — | 65–100 |
| Educational level (years)a | 4.6 ± 3.4 | 3.7 ± 2.8 | 0.0001 | 4.2 ± 3.1 | 4.9 ± 3.7 | 3.6 ± 2.9 | 0.0001 | 4.2 ± 3.3 |
| ADL (score)a | 4.6 ± 2.2 | 4.1 ± 2.3 | 0.001 | 4.3 ± 2.3 | 4.6 ± 2.2 | 4.3 ± 2.3 | 0.006 | 4.4 ± 2.2 |
| IADL (score)a | 4.4 ± 3.0 | 4.3 ± 3.3 | 0.52 | 4.3 ± 3.2 | 4.3 ± 2.9 | 4.6 ± 3.3 | 0.208 | 4.4 ± 3.1 |
| SPMSQ (score)a | 2.3 ± 2.8 | 3.0 ± 3.0 | 0.0001 | 2.7 ± 2.9 | 2.2 ± 2.7 | 2.9 ± 2.9 | 0.0001 | 2.6 ± 2.8 |
| Exton Smith (score)a | 16.7 ± 3.6 | 15.8 ± 3.8 | 0.0001 | 16.2 ± 3.7 | 16.8 ± 3.4 | 15.9 ± 3.6 | 0.0001 | 16.3 ± 3.5 |
| CIRS-CI (score)a | 2.8 ± 1.7 | 2.8 ± 1.6 | 0.86 | 2.8 ± 1.7 | 2.8 ± 1.7 | 2.6 ± 1.6 | 0.108 | 2.7 ± 1.7 |
| CIRS-SI (score)a | 1.6 ± 0.3 | 1.6 0.3 | 0.27 | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.5 ± 1.7 | 0.032 | 1.57 ± 0.4 |
| MNA (score)a | 22.6 ± 5.3 | 20.9 ± 6.0 | 0.0001 | 21.6 ± 5.8 | 22.6 ± 5.3 | 21.6 ± 5.5 | 0.001 | 22.1 ± 5.4 |
| Number of drugsa | 3.5 ± 2.4 | 3.8 ± 2.3 | 0.026 | 3.6 ± 2.4 | 3.9 ± 2.8 | 4.2 ± 2.5 | 0.066 | 4.0 ± 2.6 |
| Prognostic index (score)a | 0.33 ± 0.22 | 0.40 ± 0.24 | 0.0001 | 0.37 ± 0.23 | 0.33 ± 0.21 | 0.38 ± 0.23 | 0.007 | 0.36 ± 0.22 |
| Mortality (%) at 6 months | 16.9 | 10.8 | 0.011 | 15.7 | 15.9 | 10.1 | 0.008 | 12.9 |
| Mortality (%) at 12 months | 20.1 | 15.7 | 0.101 | 17.7 | 19.4 | 14.3 | 0.029 | 16.7 |
Values are mean ± SD.
Table 3. Characteristics of Patients in the Development and Validation Cohorts Divided According to the Multidimensional Prognostic Index.
| Development cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|
| Characteristics | Low risk (0.0–0.33) |
Moderate risk (0.34–0.66) |
Severe risk (0.67–1.0) |
Low risk (0.0–0.33) |
Moderate risk (0.34–0.66) |
Severe risk (0.67–1.0 |
| Patients: no. (%) | 447 (53.3) | 262 (31.2) | 129 (15.4) | 471 (55.0) | 263 (30.7) | 122 (14.2) |
| Women (%)* | 48.3 | 64.1 | 62.7 | 48.8 | 57.8 | 59.0 |
| Prognostic index score | ||||||
| Range | 0.0–0.33 | 0.34–0.66 | 0.67–1.00 | 0.00–0.33 | 0.36–0.64 | 0.67–1.00 |
| Mean ± SD | 0.18 ± 0.09 | 0.48 ± 0.09 | 0.77 ± 0.08 | 0.18 ± 0.09 | 0.49 ± 0.09 | 0.75 ± 0.07 |
| Age (years) | ||||||
| Range | 65–97 | 66–101 | 66–100 | 65–95 | 66–100 | 66–100 |
| Mean ± SD* | 77.0 ± 7.0 | 81.1 ± 7.2 | 83.0 ± 7.3 | 76.2 ± 6.4 | 80.5 ± 7.2 | 81.5 ± 7.2 |
| Educational level (years) | ||||||
| Mean ± SD* | 4.5 ± 3.1 | 3.7 ± 3.0 | 3.8 ± 3.5 | 4.4 ± 3.3 | 4.2 ± 4.0 | 3.8 ± 3.1 |
| Mortality | ||||||
| 6 months (%)* | 5.6 | 16.7 | 34.1 | 4.2 | 17.1 | 36.9 |
| 1 year (%)* | 8.1 | 21.3 | 43.4 | 5.7 | 23.2 | 45.1 |
p = 0.0001.
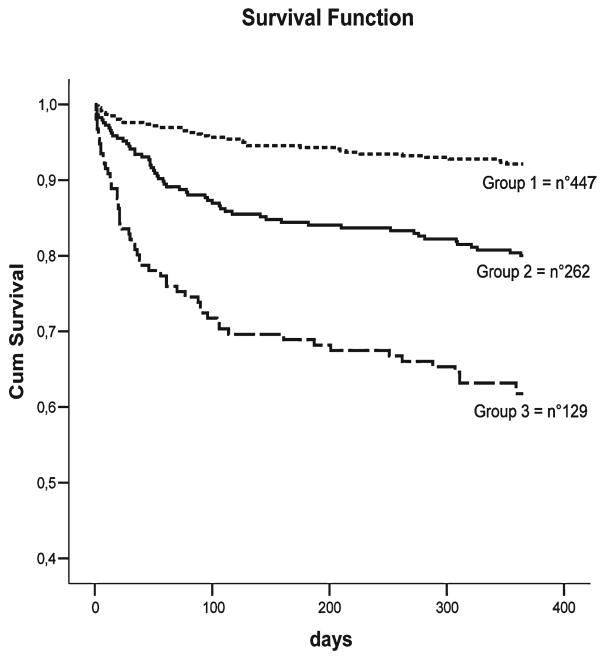
Figure 1 shows the age- and sex-adjusted survival curves for different grades of MPI: patients with higher MPI demonstrated higher mortality rates (p = 0.001). Table 4 reports the corresponding HR and 95% confidence intervals by different grades of MPI and the observed mortality after 6 months and 1 year of follow-up. A very close agreement was found between the estimated mortality by the three MPI grades and the observed mortality after both 6 months and 1 year of follow-up.
FIG. 1.
Survival curves, adjusted for age and gender, for different grades of Multidimensional Prognostic Index as obtained in the development cohort of patients.
Table 4. Cumulative HR in the Development and Validation Cohorts According to Different Grades of Severity of MPI after 180 Days and 1 Year of Follow-Up.
| Development cohort (no. 838) |
Validation cohort (no. 857) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CI (95%) | CI (95%) | ||||||||
| MPI grades | Time (months) |
HR | Lower | Upper | Observed mortality | HR | Lower | Upper | Observed mortality |
| Low risk, | 6 | 0.053 | 0.033 | 0.073 | 0.056 | 0.041 | 0.023 | 0.059 | 0.042 |
| MPI 1 | 12 | 0.082 | 0.057 | 0.107 | 0.081 | 0.057 | 0.035 | 0.079 | 0.057 |
| Moderate risk, | 6 | 0.168 | 0.125 | 0.211 | 0.167 | 0.177 | 0.132 | 0.222 | 0.171 |
| MPI 2 | 12 | 0.223 | 0.174 | 0.272 | 0.213 | 0.246 | 0.195 | 0.297 | 0.232 |
| Severe risk, | 6 | 0.350 | 0.270 | 0.430 | 0.341 | 0.438 | 0.350 | 0.526 | 0.369 |
| MPI 3 | 12 | 0.482 | 0.396 | 0.568 | 0.434 | 0.559 | 0.469 | 0.649 | 0.451 |
To test the hypothesis that the prognostic value of the aggregated MPI was superior to the prognostic value of its single components considered individually, a logistic model was applied on individual parameters. The analyses demonstrated that the MPI was significantly associated with the mortality rates after 1 year of follow-up; standardized beta coefficients showed that the prognostic value of the MPI was higher compared to those of the individual parameters (Table 5).
Table 5. Individual Risk Factors for 1-Year Mortality in Patients in the Development and Validation Cohorts.
| Development cohort (no. 838) | Validation cohort (no. 856) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||
| Risk factors | Standardized β coefficient |
OR | Lower | Upper |
p value |
Standardized β coefficient |
OR | Lower | Upper |
p value |
| Multidimensional Prognostic Index | 8.775 | 2.961 | 2.323 | 3.773 | 0.0001 | 9.954 | 3.647 | 2.827 | 4.705 | 0.0001 |
| Age | 6.147 | 1.082 | 1.055 | 1.110 | 0.0001 | 5.073 | 1.068 | 1.041 | 1.096 | 0.0001 |
| Sex (male) | 1.662 | 1.351 | 0.947 | 1.929 | 0.0970 | 1.983 | 1.441 | 1.005 | 2.066 | 0.0470 |
| ADL | 8.373 | 1.347 | 1.276 | 1.480 | 0.0001 | 8.533 | 1.386 | 1.286 | 1.494 | 0.0001 |
| IADL | 6.694 | 1.234 | 1.160 | 1.313 | 0.0001 | 8.100 | 1.320 | 1.234 | 1.411 | 0.0001 |
| SPMSQ | 5.901 | 1.188 | 1.122 | 1.258 | 0.0001 | 5.948 | 1.191 | 1.125 | 1.262 | 0.0001 |
| CIRS co-morbidity | 4.813 | 1.303 | 1.170 | 1.452 | 0.0001 | 6.864 | 1.461 | 1.311 | 1.629 | 0.0001 |
| MNA | 7.581 | 1.134 | 1.098 | 1.171 | 0.0001 | 9.337 | 1.179 | 1.139 | 1.221 | 0.0001 |
| Exton Smith | 8.770 | 1.250 | 1.189 | 1.314 | 0.0001 | 9.575 | 1.300 | 1.232 | 1.372 | 0.0001 |
| No. of drugs | 1.307 | 1.051 | 0.976 | 1.131 | 0.1910 | 3.697 | 1.135 | 1.061 | 1.213 | 0.0001 |
| Social support network | 1.810 | 1.442 | 0.970 | 2.145 | 0.0700 | 0.834 | 0.835 | 0.547 | 1.275 | 0.4040 |
Validation of the Multidimensional Prognostic Index
During the 1-year inclusion period of the validation cohort, 1544 patients were consecutively admitted to our Geriatric Unit. Fifty-two subjects were excluded because they were younger than 65 years; 231 patients were excluded because information on their vital status was not available after 1 year of follow-up; 184 patients were excluded because the CGA was not completed; and 221 patients were excluded because they refused to participate in the study. Thus the validation cohort included 857 elderly patients, 402 men and 454 women, with a mean age of 78.3 ± 7.1 years, and a range of 65 to 100 years. Table 2 reports the characteristics of patients included in the validation cohort divided according to gender.
According to their MPI value, 471 patients (55%) resulted in MPI grade 1 (low risk, MPI mean value 0.18 ± 0.09); 263 patients (30.7%) in MPI grade 2 (moderate risk, MPI 0.49 ± 0.09); and 122 patients (14.2%) in MPI grade 3 (severe risk, MPI 0.75 ± 0.07). As for the development cohort, higher MPI grades were significantly associated with older age (mean age grade 1, 76.2 ± 6.4 years; grade 2, 80.5 ± 7.2; grade 3, 81. ± 7.2 years; p = 0.0001), female sex (grade 1, 48.8%; grade 2, 57.8%; grade 3, 59.0%, p = 0.0001), lower educational level (grade 1, 4.4 ± 3.3; grade 2, 4.2 ± 4.0; grade 3, 3.8 ± 3.1 years; p = 0.0001), and progressively higher mortality after both 6 months (grade 1, 4.2%; grade 2, 17.1%; grade 3, 36.9%; p = 0.0001) and 1 year (grade 1, 5.7%; grade 2, 23.2%; grade 3, 45.1%; p = 0.0001) (Table 3).
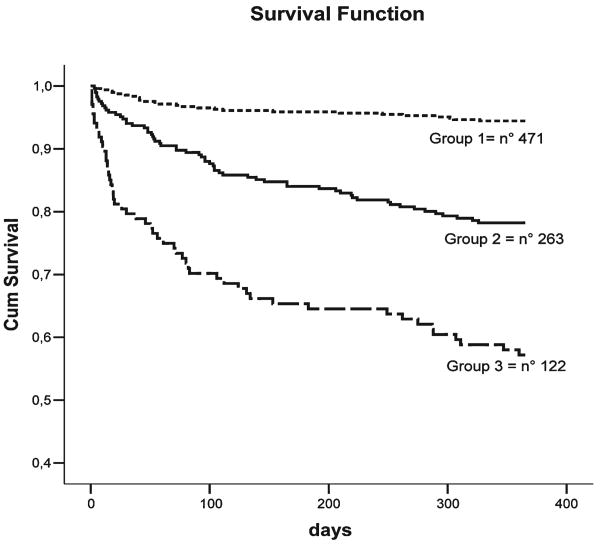
Figure 2 shows the age- and sex-adjusted survival curves for different grades of MPI: patients with higher MPI demonstrated higher mortality rates (p = 0.001). Table 4 reports the corresponding HR and 95% confidence intervals by different grades of MPI and the observed mortality after 6 months and 1 year of follow-up. As for development cohort, a close agreement was found between the estimated mortality by the three MPI grades and the observed mortality after both 6 months and 1 year of follow-up. The discrimination of the MPI was also good, with a ROC area of 0.751 (95%CI, 0.70–0.80) at 6 months and 0.751 (95%CI, 0.71–0.80) at 1 year of follow-up.
FIG. 2.
Survival curves, adjusted for age and gender, for different grades of Multidimensional Prognostic Index as obtained in the validation cohort of patients.
Discussion
We developed an MPI based on information available from a standard CGA that included clinical, functional, cognitive, nutritional, and social parameters. The MPI can be used to stratify hospitalized older patients into categories that experienced a highly differential 1-year mortality. Adjusting for age and sex, the prognostic effect of MPI on mortality was highly significant; moreover, logistic analysis confirmed that the aggregate MPI was significantly associated with mortality, and its prognostic value was higher than those provided by the individual parameters utilized for constructing the MPI (i.e., ADL, IADL, SPMSQ, MNA, ESS, CIRS, medications, and co-habitation status).
Thereafter, we tested and validated this MPI on a second cohort of hospitalized elderly patients. The results confirmed that MPI has a good prognostic effect on 1-year mortality with a close agreement between the estimated mortality and the observed mortality after both 6 months and 1 year of follow-up.
These findings support the importance of considering multidimensional aggregate information for predicting post-hospitalization mortality in older patients. While a previous prognostic “frailty” index based on a CGA was developed in elderly subjects who were living at their homes,24 reporting a good prognostic value of this index for adverse outcomes (i.e., institutionalization or mortality25), to our knowledge this was the first description of a prognostic index based on data available from a standard CGA for older patients hospitalized in an acute ward.
Previously, two studies described a prognostic index for mortality in hospitalized older patients. The first demonstrated the importance of functional measures (i.e., IADL, Mini-Mental State Examination (MMSE), and shortened Geriatric Depression Scale) in improving the predictive ability of five standard burden of illness indices (Charlson, Acute Physiology and Chronic Health Evaluation [APACE II], Disease Staging, All Patient Refined Diagnosis Related Groups, and the clinician's subjective rating) in predicting 90-day and 2-year mortality in a population of 207 hospitalized patients of 70 years or older.10 The second study described a prognostic index for 1-year mortality that used six risk factors (male sex, the number of dependent ADLs, the diagnosis of congestive heart failure and/or cancer, high creatinine, and/or low albumin levels) in older patients discharged from general medical or teaching hospitals.11
Unlike the prognostic indices calculated in community dwelling or institutionalized elderly people,5–9 for constructing the present MPI we used parameters that particularly characterize the hospitalized elderly patient. Indeed, the MPI included the co-morbidity CIRS score, the evaluation of nutritional status according to MNA, while functional, cognitive, and social parameters were derived from the basal ADL and IADL, the SPMSQ, and co-habitation status. These sensitive instruments have been validated in several settings of geriatric populations.14–18,26,27
This MPI includes parameters obtained from a standardized CGA and was excellent in identifying three groups of 1-year mortality risk as demonstrated by the three distinct and uncrossed survival curves in both the development and validation cohorts. Moreover, the finding that the prognostic value of the aggregated MPI was superior to the prognostic value of its individual components considered singularly confirmed that the multidimensional approach can be successfully used in hospitalized elderly patients both for clinical28 and for administrative purposes.29 Indeed, a significant correlation was found between multidimensional impairments, as assessed by the CGA criteria and the all-patients-refined-(APR)-DRG system that is an administrative tool useful in identifying elderly inpatients at high risk of high health resource consumption.29
Interestingly, in constructing the MPI by using a step-wise method, we found that the eight-domain MPI, which included a total of 63 items in eight domains of the CGA, resulted in a better index in predicting 1-year mortality than an index including a lower number of domains. This finding is in agreement with recent studies performed in older individuals that demonstrated that a cumulative index of health deficiencies can significantly predict health outcomes and death, suggesting that development of prognostic indices on the basis of a limited number of health problems have to be performed with great caution.30,31
In this study, the main outcome was defined by mortality status during the 1-year follow-up period. Mortality was selected as an outcome because it is dichotomous, easily distinguishable, and unequivocal.
The MPI that we have constructed has allowed us to define three grades of risk. We found that higher MPI grades were significantly associated with increasing age and lower educational level. Moreover, the MPI was significantly higher in females than in males (0.40 ± 0.02 vs. 0.33 ± 0.02; p = 0.0001 in the development cohort; 0.38 ± 0.23 vs. 0.33 ± 0.21; p = 0.007 in the validation cohort) while mortality rates were lower in women than in men. These findings are in agreement with recent studies from Canadian,32 American,33 and Chinese elderly populations,34 which reported that the gender difference in life expectancy was not entirely due to differences in impairment, because men with the same chronological age and biological impairments (e.g., frailty index) had a higher risk of death compared with that of women.
This MPI has several potential uses, both in clinical and research settings. In clinical settings, this index may be useful in identifying both high- and low-risk patients so that specific interventions can be targeted to each group. This could be important particularly for identifying those low-risk elderly patients who can benefit from cancer screening and/or chronic disease prevention programs and who are excluded merely due to old age.35 Conversely, the MPI is useful in identifying those high-risk patients for whom appropriate advance care assistance programs could be helpful and cost-effective.
In research settings, the MPI may be useful in epidemiological studies that examine the impact of exposure and treatments on mortality, as well as in basic science studies that attempt to evaluate and identify biological and/or genetic markers of frailty in the elderly.
This MPI has some limitations. Since the index focused on hospitalized older patients, it is likely that it is not applicable to institutionalized and community dwelling subjects. Indeed, in healthier groups of elderly subjects, the MPI would probably include other predictor variables that take into account the different setting populations.
In conclusion, we have described a Multidimensional Prognostic Index derived from a standardized CGA in hospitalized elderly patients. This index is a reliable and sensitive measurement of risk assessment that might be useful for clinical and research purposes.
Acknowledgments
This work was fully supported by the Ministero della Salute, IRCCS Research Program, Ricerca Corrente 2006-2008, Linea no. 2, Malattie di rilevanza sociale.
References
- 1.Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389–2395. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 3.American Geriatrics Society Clinical Practice Committee. Breast cancer screening in older women. J Am Geriatr Soc. 2000;48:842–844. [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 5.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Elliott M, Rubenstein LZ, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 7.Carey EC, Walter LC, Lindquist K, et al. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 9.Fillenbaum GG, Pieper CF, Cohen HJ, et al. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol. 2000;55:M84–89. doi: 10.1093/gerona/55.2.m84. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 11.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 12.Consensus Development Panel. Solomon David. National Institutes of Health Consensus Development Conference Statement: Geriatric assessment methods for clinical decision-making. J Am Geriatr Soc. 2003;51:1490–1494. doi: 10.1046/j.1532-5415.2003.51471.x. Chairman. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein LZ. Comprehensive Geriatric Assessment: from miracle to reality. J Gerontol Med Sci. 2004;59A:473–477. doi: 10.1093/gerona/59.5.m473. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Downs TD, Cash HR, et al. Progress in the development of an index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 15.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 16.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 17.Linn B, Linn M, Gurel L. The Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 18.Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Progr. 1999;1:3–11. doi: 10.1159/000062967. [DOI] [PubMed] [Google Scholar]
- 19.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minist Health Public Health Lab Serv. 1966;25:238–268. [PubMed] [Google Scholar]
- 20.L'informatore farmaceutico 2004. OEMF International, Milano 2002. Guidelines for ATC Classification, NLN publication no.16. Uppsala, Sweden: Nordic Council on Medicines; 1985. [Google Scholar]
- 21.Rockwood K, Stadnyk K, MacKnight C, et al. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 22.Thomas VS, Rockwood K, McDowell I. Multidimensionality in instrumental and basic activities of daily living. J Clin Epidemiol. 1998;51:315–321. doi: 10.1016/s0895-4356(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 23.SPSS, version 13. Instruction Manual. SPSS; Chicago: 2002. [Google Scholar]
- 24.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized Comprehensive Geriatric Assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones D, Song X, Mitniski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. doi: 10.1007/BF03327413. [DOI] [PubMed] [Google Scholar]
- 26.Parmelee PA, Thuras PD, Katz IR, et al. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 27.De Groot LC, Beck AM, Schroll M, et al. Evaluating your Nutritional Health Checklist and the Mini Nutritional Assessment as tools to identify nutritional problems in elderly Europeans. Eur J Clin Nutr. 1998;52:877–883. doi: 10.1038/sj.ejcn.1600658. [DOI] [PubMed] [Google Scholar]
- 28.Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull. 2004;71:45–49. doi: 10.1093/bmb/ldh033. [DOI] [PubMed] [Google Scholar]
- 29.Pilotto A, Scarcelli C, D'Ambrosio LP, et al. All Patient Refined Diagnosis Related Groups: A new administrative tool for identifying elderly patients at risk of high resource consumption. J Am Geriatr Soc. 2005;53:1–2. doi: 10.1111/j.1532-5415.2005.53031_3.x. [DOI] [PubMed] [Google Scholar]
- 30.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Med Sci. 2007;62A:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 31.Kulminski A, Ukraintseva SV, Akushevich IV, et al. Cumulative index of health deficiencies as a characteristics of long life. J Am Geriatr Soc. 2007;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 33.Kulminski A, Yashin A, Arbeev K, et al. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goggins WB, Woo J, Sham A, et al. Frailty index as a measure of biological age in a Chinese population. J Gerontol. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 35.Gross CP, McAvay GJ, Krumholz HM, et al. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145:646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]