Abstract
Background
Lipid metabolism in mammals is orchestrated by a family of transcription factors called sterol regulatory element-binding proteins (SREBPs) that control the expression of genes required for the uptake and synthesis of cholesterol, fatty acids, and triglycerides. SREBPs are thus essential for insulin-induced lipogenesis and for cellular membrane homeostasis and biogenesis. Although multiple players have been identified that control the expression and activation of SREBPs, gaps remain in our understanding of how SREBPs are coordinated with other physiological pathways.
Methodology
To identify novel regulators of SREBPs, we performed a genome-wide cDNA over-expression screen to identify proteins that might modulate the transcription of a luciferase gene driven from an SREBP–specific promoter. The results were verified through secondary biological assays and expression data were analyzed by a novel application of the Gene Set Enrichment Analysis (GSEA) method.
Conclusions/Significance
We screened 10,000 different cDNAs and identified a number of genes and pathways that have previously not been implicated in SREBP control and cellular cholesterol homeostasis. These findings further our understanding of lipid biology and should lead to new insights into lipid associated disorders.
Introduction
Disruption of intracellular cholesterol metabolism and trafficking is the primary cause of numerous human disorders [1]. It has been shown that the sterol regulatory element binding protein (SREBP) pathway is the master regulator of intracellular lipid homeostasis [2], [3]. SREBPs are generated from two genes, SREBF1 and SREBF2, that are transcribed to form a number of different mRNA and protein species [4]–[8]. The prevalent isoforms are SREBP-1a, SREBP-1c and SREBP-2 [9], [10], but additional splice versions have been described [4], [5], [7], [11], [12]. SREBP-1a and SREBP-1c are both transcribed from the SREBF1 gene and differ in their first and last two exons, while SREBP-2 is the predominant protein produced from the SREBF2 gene [8], [13].
SREBPs are synthesized as inactive precursors that are anchored in the membrane of the ER through two transmembrane domains [14]. The N-terminal domain contain motifs required for dimerization, DNA binding and transactivation [15], [16]. The C-terminal domain of SREBP precursors mediates the formation of complexes with SREBP cleavage-activating protein (SCAP) [17], a membrane protein important for SREBP stability and regulation [18]–[22]. Interaction of SCAP with the COPII machinery leads to the incorporation of the SCAP/SREBP complex into vesicles and transport to the Golgi [20], [23]–[25]. SREBPs are then cleaved by Site-1 and Site-2 proteases (S1P and S2P), leading to the transfer of active transcription factors to the nucleus [26]–[29]. Here, SREBP dimers bind to sterol regulatory elements (SRE) which are present in the promoter regions of genes such as low-density lipoprotein receptor (LDL-R), 3-hydroxy-3-methylglutaryl Coenzyme A reductase (HMGCR), and fatty acid synthase, and multiple other genes involved in the regulation of intracellular lipid metabolism [30], [31]. Thus, regulation of SREBP cleavage and activity is vital for cellular lipid homeostasis and cell survival.
Studies with CHO cells and mice expressing dominant positive versions of SREBPs have shown that the target genes of SREBP-1a and SREBP-2 are largely overlapping. However, SREBP-1a is somewhat more potent at activating genes involved in fatty acid synthesis while SREBP-2 has a preference for genes involved in the biosynthesis of cholesterol. The LDL receptor is controlled equally by both transcription factors [30], [31], [32]. SREBP-1c also controls fatty acid-raising genes and, although significantly weaker than SREBP-1a [30], [32], it is the predominant SREBP isoform in many tissues and in liver regulates the conversion of carbohydrates to triacylglycerol in response to insulin [33].
SREBP-1a and SREBP-2 are subject to negative feedback regulation by cholesterol [34]. Upon binding to cholesterol SCAP undergoes a conformational change that triggers its interaction with one of two ER membrane proteins termed insulin-induced gene(INSIG)-1 and INSIG2 [21], [35]–[40]. Under these circumstances SCAP dissociates from COPII, the SCAP/SREBP complex remains in the ER, and proteolytic activation is blocked [41], [42]. In another feedback loop SREBP-1a and SREBP-1c are suppressed by polyunsaturated fatty acids (PUFA) [43]–[45]. SREBP-1c transcription in the liver is controlled by liver X receptors (LXR), whose activation in turn is blocked by PUFA [43], [46].
In spite of the current research efforts in this field, our knowledge of intracellular cholesterol trafficking and homeostasis is far from complete. To gain a better handle on these events, we performed a genome-wide cDNA over-expression screen to identify modulators of SREBP activity. We used a cell-based luciferase assay that measures expression from an SREBP-specific promoter. We also performed secondary biological assays to further validate these hits. Additionally, employing a novel modification of Gene Set Enrichment Analysis (GSEA) we performed a pathway analysis on the high throughput screening data, as GSEA was originally developed for analyzing microarray experiments [47]. GSEA applies a priori biological knowledge to genome-scale data sets to implicate pathways in the biological system of interest [47]. In addition to known pathways regulating lipid metabolism, such as the SREBP and nuclear hormone receptor pathway, our analysis has led to the identification of a number of pathways previously not associated with the regulation of cellular cholesterol homeostasis. The data suggests that pathways involved in intracellular signal transduction such as tyrosine kinase signaling, G-protein / small GTPase pathways and ephrin signaling positively affect intracellular cholesterol homeostasis, while pathways acting at the extracellular level, such as matrix proteins, cell-matrix and cell-adhesion proteins, and pathways involved in cell structure and organization, negatively regulate cellular cholesterol homeostasis.
We have validated the results of the primary screen through a series of secondary biological assays and find considerable overlap between the genes identified by secondary screening and the pathways identified via GSEA, indicating that pathway-centric analyses of biological screening data is a valid approach that may assist in target identification. Our results implicate multiple novel genes and pathways in intracellular cholesterol homeostasis and open up novel venues for the interrogation of lipid biology and lipid-linked disease.
Results
Optimization of the SREBP signaling assay
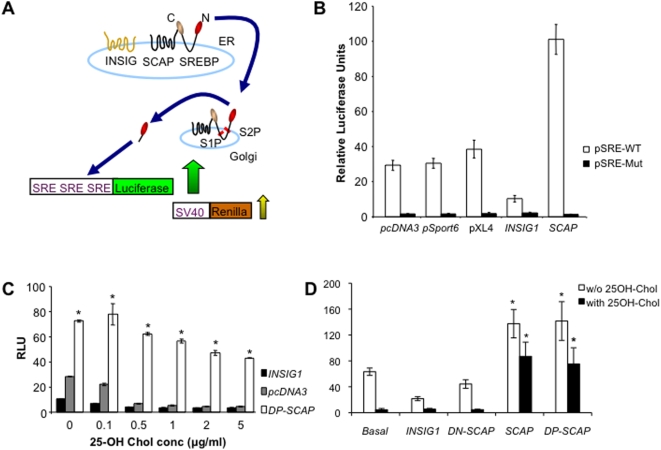
The reporter gene assay used in this study has been previously described [18]. Briefly, this assay is based on endogenous SREBP-mediated activation of a promoter containing three sterol regulatory elements (SREs) driving the expression of a firefly-luciferase gene (reporter construct, Figure 1A). As a transfection control for the luciferase assays, a renilla-luciferase gene, driven by a weak constitutive active SV-40 promoter, was co-transfected along with the firefly-luciferase gene (Figure 1A). The activity of the reporter gene assay was measured as a ratio between the firefly and renilla luciferase levels. Thus, a high luciferase ratio indicates SREBP pathway activation (due to a higher firefly luciferase levels) and vice-versa. For our experiments, this SREBP signaling assay was optimized by a series of steps. First, in order to use an optimal reporter construct the 3×SREs cassette was sub-cloned and tested in a number of luciferase vectors including, pGL3-Basic and pTransLucent. In our hands, the pTransLucent vector displayed higher luciferase ratios and higher signal-to-noise ratio [48] in a 384-well format and was chosen for further experiments (data not shown). Second, two mammalian cell lines HEK-293 and HeLa were tested for cell line of choice. HEK-293 cells displayed higher assay reproducibility, luciferase signals and fold change under different experimental conditions and were thus chosen for this study (data not shown). Third, a mutant SRE promoter [49] driving a luciferase gene was generated and used as a specificity control for our experiments. This mutant SRE-luciferase construct was inactive under all experimental conditions (Figure 1B). Fourth, to optimize the repression of SREBP signaling by cholesterol, a concentration response curve for 25-hydroxy (25-OH) cholesterol with varying times of incubation was performed. The result showed that incubating cells with 1 µg/ml 25-OH cholesterol for 24 hours was sufficient to repress the assay as efficiently as using 5 µg/ml 25-OH cholesterol and hence the lower concentration was used for further studies (Figure 1C).
Figure 1. The SREBP cleavage assay.
(A) Schematic representation of the SREBP cleavage assay. (B) Activity of wild-type (WT) versus mutant (Mut) SRE promoter. HEK-293 cells were set up in a 96-well plate (in triplicate). After 24 hours, cells were transfected with either WT (open bars) or mutant (black bars) luciferase reporter constructs, along with renilla luciferase construct and the indicated plasmid /cDNA. Cells were grown for an additional 24 hours before performing the assay. (C) Effects of 25-hydroxy cholesterol (25-OH chol.) on SREBP signaling. The assay was carried out under varying 25-OH cholesterol concentrations (0.1–5 µg/ml) and for different incubation periods (6, 12, and 24 hours). 25-OH chol. was added to cells 1 day after transfecting with the reporter plasmids, SRE-luciferase and renilla luciferase. Maximum suppression of SRE-luciferase signals was observed after 24 hours of incubation with 25-OH chol (shown here). The effects of DP-SCAP under high 25-OH cholesterol levels are significantly higher at all concentrations (Student's t-Test, p<0.005). (D) Effects of known repressors, activators and high cholesterol (25OH chol., 1 µg/ml) on the SREBP signaling pathway. The assay was carried out as in B. Basal refers to pcDNA3 overexpression. SCAP and DP-SCAP significantly activate the assay in the absence (white bars) or presence (black bars) of 25-OH cholesterol (Student's t-Test, p<0.05). (B–D) Error bars indicate standard deviations (n = 3). Where not visible, error bars are smaller than symbols. The graphs are representative of at least 2 independent experiments.
We tested the robustness and sensitivity of the assay by evaluating the effects of SCAP and INSIG1 overexpression on SREBP signaling under normal cell culture conditions (cells grown in medium containing 10% serum and antibiotics). Full-length plasmids encoding hamster SCAP [18] and human INSIG1 [40] were co-transfected along with the wild-type SRE-luciferase reporter and changes in luciferase ratios were measured. We noted an approximate three and a half fold activation or repression of basal (empty vector over-expression) SREBP activity in the presence of SCAP or INSIG1, respectively (Figure 1B). A dominant-positive form of SCAP (DP-SCAP) which no longer binds INSIG1 as it contains a point mutation in its INSIG1 interacting domain [24], was equally active in enhancing SREBP signaling as wild-type SCAP. In addition, a dominant negative form of SCAP (DN-SCAP) which lacks the INSIG-binding domain [50], repressed SREBP cleavage as efficiently as over-expression of INSIG1 (Figure 1D). Next, we examined the effects of SCAP and INSIG1 over-expression in the presence of high cholesterol (1 µg/ml 25-OH cholesterol). The repressed luciferase levels found under high cholesterol conditions were rescued by the over-expression of positive components of the SREBP pathway such as wild-type SCAP or DP-SCAP as expected (Figure 1D). Under these conditions of repressed luciferase activity, we found no further measurable inhibitory effects of INSIG1 (Figure 1D).
Genome-wide screen for regulators of cellular cholesterol homeostasis
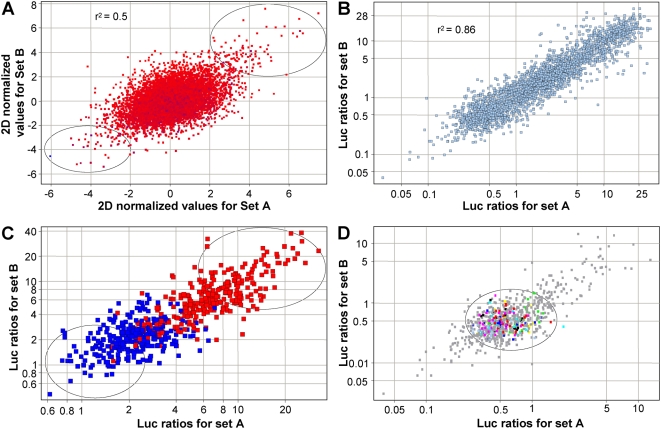
Having determined the optimal conditions for the SREBP signaling assay, we made use of the sufficient fold difference under normal cell culture conditions to identify novel activators and repressors of the SREBP pathway. To this end, a collection of 10,000 random full-length human cDNAs was screened using a ‘gene-by-gene’ unbiased assay. The screen was carried out in duplicate so that the data could be subjected to 2-dimensional (2D) normalization i.e. normalization to remove both well-to-well and plate-to-plate variation (see Materials and Methods for details). A scatter plot for the primary screen was obtained by plotting the 2D normalized luciferase ratios for a clone in the first experiment against that obtained in the second experiment (Figure 2A). The clones lying at the extremities displayed highest activity and were selected for further validation (circles). Clones which modulated luciferase ratios by at least 2-fold were re-tested for their effects in the SREBP signaling assay. With this cut-off, a total of 176 activators and repressors were selected for re-confirmation assays. Each clone was assayed in triplicate for all the subsequent follow-up experiments. The scatter plot of the total re-screening data showed that the clones lie along the diagonal, indicating internal consistency of the experimental conditions (Figure 2B). The first of these experiments confirmed the behavior of each clone under identical conditions to that used for the original gene-by-gene unbiased screen. Furthermore, we found that genes identified in the primary screen as activators (red) clustered separately from the repressors (blue), confirming the reproducibility of the results (Figure 2C). Clones showing no clear discrimination as either activators or repressors were removed from final analysis (Figure 2C, central overlapping red and blue points and Figure S1). Genes that activated or suppressed SREBP cleavage to the greatest extent were found at the extremities of the scatter plot.
Figure 2. Primary and secondary screen results.
(A) Scatter plot showing the result from the primary screening of 10,000 putative full-length human cDNA's in the SREBP cleavage assay. The 2D-normalized z-scores for a clone in the first experiment (x-axis) are plotted against that obtained in the second experiment (y-axis). Genes at the top right corner represent potential activators of SREBP signaling, while those at the bottom left corner represent potential repressors of SREBP signaling (circles). (B) Scatter plot representing the combined data from all secondary screens. Each clone was re-tested in triplicate in two separate experiments. Firefly to renilla luciferase ratios for a clone in the first experiment (x-axis) were plotted against the ratios for the same clone in the second experiment (y-axis). (C) Analysis of the selected 176 clones under conditions identical to those used in the primary screen. Scatter plot shows luciferase ratios obtained for a clone in the first experiment against ratios obtained for the same clone in the second experiment. Activators are represented in red and repressors in blue. Circles represent clones displaying the highest activation or repression of luciferase ratios. (D) Effect of the 176 selected activators and suppressors (grey points) on mutant SRE promoter. The scatter plot shows the luciferase ratios obtained for a clone in the first experiment against luciferase ratios obtained in the second experiment. Central data points (circle) represent genes that did not have an effect on the mutant SRE-luciferase. Grey points falling at the extremities represent clones that activated the mutant SRE-luciferase or had higher levels of renilla luciferase. Control genes are color coded as: red, DN-SCAP; dark blue, DP-SCAP; yellow, INSIG1; black, SCAP; green, pSport6; sky blue, pXL4; pink, pcDNA3.
We next utilized the mutant SRE-luciferase reporter to identify non-specific regulators of SRE-luciferase. When compared to the internal controls (colored central points), a set of genes that significantly altered renilla levels (data not shown) and/or changed mutant-SRE promoter activity (Figure 2D, extremities of the scatter plot) were discarded as being false positives. Genes in the activator set that did not affect the mutant SRE promoter were deemed as candidates that regulate SREBP signaling (Figure 2D, circled central grey points). Thus, starting from 176 clones this analysis resulted in 27 activators and 40 repressors (Tables S1, S2) that showed specific effects in regulating the SREBP assay (Figure S1), while not affecting the mutant SRE promoter.
Gene set enrichment analysis (GSEA) of high throughput screening data
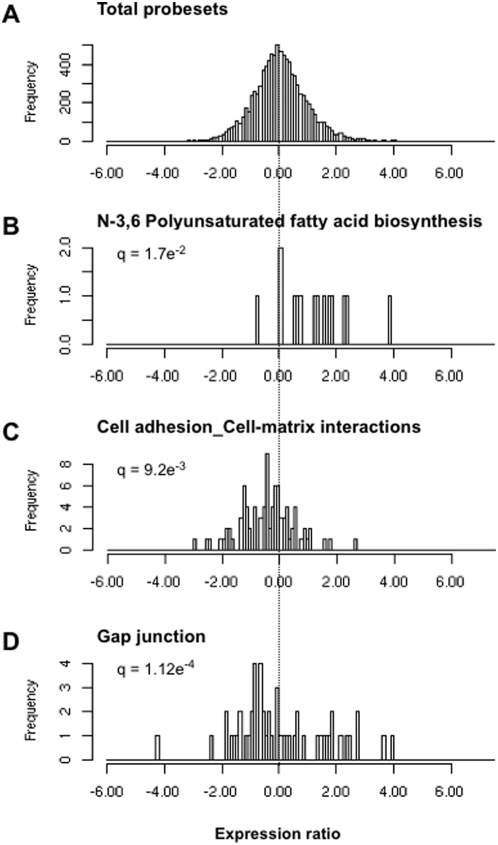
Results from the primary gene-by gene screen were analyzed by a novel application of the Gene Set Enrichment Analysis (GSEA) technique modified for high throughput screening data (for details see Materials and Methods). We identified a number of pathways whose members coordinately modulate SREBP activity as measured in this screen. The GSEA results included pathways which are known to positively regulate intracellular cholesterol homeostasis, such as polyunsaturated and unsaturated fatty acid biosynthesis (Figure 3B) and sphingolipid metabolism pathways, as well as the nuclear hormone receptor pathway (Table 1). Additionally, signaling pathways relating to heterotrimeric G-proteins, small GTPases (including the Rab family of GTPases), RAS- and RAS-related GTPases and angiotensin signaling via PYK2, all of which have been implicated in the regulation of intracellular cholesterol metabolism (see discussion), were identified as activators of SREBP signaling. We also identified pathways previously not associated with the regulation of lipid homeostasis including ephrin signaling and epidermal growth factor receptor (EGFR) signaling pathways. In contrast to the identified activators, a majority of which impacted intracellular signaling events, the repressors from our screen were enriched for pathways associated with the extracellular matrix, cell adhesion & cell matrix interactions (Figure 3C) and matrix glycoproteins (Table 2). Proteins regulating the cytoskeleton and cell architecture and serine proteases were also found to repress the cholesterol pathway.
Figure 3. Gene set enrichment results.
Distribution of 2D normalized z-scores (NZ2D) for (A) all cDNA clones used in this screen and clones assigned to the (B) N-3,6 Polyunsaturated fatty acid biosynthesis, (C) Cell Adhesion / Cell Matrix Interaction and (D) Gap Junction pathways. The rightward shift of NZ2D scores among fatty acid synthesis genes (B) relative to background (A) indicates that overexpression of genes in this pathway on balance tend to activate SREBP transcriptional activity, whereas the leftward shift in (C) indicates that the cell adhesion/cell matrix tend to inhibit SREBP transcriptional activity in this screen. Gap junction genes were spread on either side of the median (D) indicating that a sub-set of gap junction genes activated the SREBP pathway, while others repressed it.
Table 1. List of pathways designated as activators of SREBP signaling by Wilcoxon test.
| Pathway name | Pathway source | Probestes | Wilcoxon p-value | Wilcoxon FDR q-value |
| Hyperplasia | MetaCore | 14 | 2.80E-05 | 0.012 |
| Tyrosine protein kinase | PANTHER | 52 | 3.04E-05 | 0.012 |
| n-3,6 Polyunsaturated fatty acid biosynthesis | MetaCore | 14 | 1.11E-04 | 0.018 |
| Angiotensin signaling via PYK2 | MetaCore | 38 | 1.74E-04 | 0.023 |
| G-protein | PANTHER | 118 | 2.16E-04 | 0.023 |
| Epidermal cell differentiation | MetaCore | 64 | 2.21E-04 | 0.023 |
| Ephrins signaling | MetaCore | 46 | 2.44E-04 | 0.023 |
| Sphingolipid metabolism | MetaCore | 17 | 2.50E-04 | 0.023 |
| Unsaturated fatty acid biosynthesis | MetaCore | 11 | 3.30E-04 | 0.027 |
| T-cell activation | PANTHER | 53 | 3.43E-04 | 0.027 |
| Small GTPase | PANTHER | 97 | 3.96E-04 | 0.028 |
| Pancreatic neoplasms | MetaCore | 111 | 4.28E-04 | 0.029 |
| Lupus erythematosus, systemic | MetaCore | 38 | 4.37E-04 | 0.029 |
| Pituitary diseases | MetaCore | 17 | 4.45E-04 | 0.029 |
| Immunoglobulin receptor family member | PANTHER | 37 | 5.07E-04 | 0.032 |
| RAS-related GTPase | PANTHER | 55 | 5.47E-04 | 0.033 |
| Neoplasms, complex and mixed | MetaCore | 38 | 5.49E-04 | 0.033 |
| Nuclear hormone receptor | PANTHER | 20 | 6.27E-04 | 0.035 |
| Axon guidance | KEGG | 87 | 6.75E-04 | 0.036 |
| EGFR signaling via small GTPases | MetaCore | 24 | 7.03E-04 | 0.036 |
| Ras-GDP/GTP | PANTHER | 6 | 9.56E-04 | 0.040 |
| Non-receptor tyrosine protein kinase | PANTHER | 21 | 9.84E-04 | 0.040 |
| EGF signaling pathway | MetaCore | 39 | 1.03E-03 | 0.040 |
| RAC1 in cellular process | MetaCore | 18 | 1.16E-03 | 0.044 |
| Transcription factor Tubby signaling pathways | MetaCore | 22 | 1.28E-03 | 0.048 |
Table 2. List of pathways designated as repressors of SREBP signaling by Wilcoxon test.
| Pathway name | Pathway source | Probestes | Wilcoxon p-value | Wilcoxon FDR q-value |
| Extracellular matrix | PANTHER | 136 | 2.97E-06 | 0.006 |
| Cell adhesion_Cell-matrix interactions | MetaCore | 89 | 8.85E-06 | 0.009 |
| Extracellular matrix glycoprotein | PANTHER | 41 | 1.84E-05 | 0.012 |
| Skeletal development | PANTHER | 54 | 6.14E-05 | 0.017 |
| Serine protease | PANTHER | 61 | 8.10E-05 | 0.017 |
| Cell structure | PANTHER | 238 | 8.60E-05 | 0.017 |
| Cytoskeletal regulation by Rho GTPase | PANTHER | 66 | 9.04E-05 | 0.017 |
| Actin binding cytoskeletal protein | PANTHER | 191 | 2.38E-04 | 0.023 |
| Proteolysis_Connective tissue degradation | MetaCore | 52 | 2.40E-04 | 0.023 |
| Protocadherin alpha | PANTHER | 9 | 2.48E-04 | 0.023 |
| Myosin | PANTHER | 31 | 3.52E-04 | 0.027 |
| NF-kappaB cascade | PANTHER | 17 | 3.52E-04 | 0.027 |
| Serine protease related | PANTHER | 25 | 6.23E-04 | 0.035 |
| Microtubule family cytoskeletal protein | PANTHER | 97 | 7.15E-04 | 0.036 |
| Cytoskeleton_Cytoplasmic microtubules | MetaCore | 71 | 7.42E-04 | 0.037 |
| Urea cycle and metabolism of amino groups | KEGG | 10 | 1.02E-03 | 0.040 |
| Synthetase | PANTHER | 41 | 1.03E-03 | 0.040 |
| Proteolysis_ECM remodeling | MetaCore | 38 | 1.08E-03 | 0.042 |
Application of a GSEA variant [51], the Levene test for homogeneity of variance as modified by Brown and Forsythe (LBF), [52] identified several pathways that included both positive and negative regulators of cholesterol homeostasis. The significant pathways identified once again included known regulators of cellular cholesterol homeostasis such as lipid metabolism, regulation of metabolism and Ras pathways as well as novel pathways such as Gap junction (Figure 3D), B-cell receptor and the Slit-Robo signaling pathways (Table 3). Notably, our screening results suggest a reciprocal relationship between gap junction formation and cholesterol homeostasis (see discussion and Table S3).
Table 3. List of SREBP pathway modulators by LBF test.
| Pathway name | Pathway source | Probestes | LBF p-value | LBF FDR q-value |
| Gap junction | KEGG | 52 | 3.00E-07 | 1.12E-04 |
| Ras | PANTHER | 8 | 9.81E-07 | 2.75E-04 |
| Long-term depression | KEGG | 32 | 1.38E-06 | 3.09E-04 |
| Lipid metabolism | PANTHER | 78 | 2.02E-06 | 4.11E-04 |
| Zinc finger protein | PANTHER | 19 | 3.77E-06 | 6.49E-04 |
| Neoplasms, fibrous tissue | MetaCore | 26 | 6.79E-06 | 1.01E-03 |
| Neoplasms, connective and soft tissue | MetaCore | 144 | 3.39E-05 | 4.22E-03 |
| Cell adhesion_Platelet-endothelium-leucocyte interactions | MetaCore | 76 | 5.22E-05 | 6.15E-03 |
| Autoimmune diseases | MetaCore | 189 | 5.58E-05 | 6.25E-03 |
| Mucinoses | MetaCore | 12 | 7.72E-05 | 8.23E-03 |
| Plasmacytoma | MetaCore | 60 | 8.87E-05 | 9.03E-03 |
| Blood protein disorders | MetaCore | 63 | 9.47E-05 | 9.22E-03 |
| B-cell- and antibody-mediated immunity | PANTHER | 33 | 1.21E-04 | 1.13E-02 |
| Multiple myeloma/paraproteinemias | MetaCore | 58 | 1.57E-04 | 1.15E-02 |
| Hemorrhagic disorders | MetaCore | 104 | 1.44E-04 | 1.15E-02 |
| Anterior/posterior patterning | PANTHER | 22 | 1.59E-04 | 1.15E-02 |
| Mesoderm development | PANTHER | 221 | 1.66E-04 | 1.16E-02 |
| Neuroendocrine tumors | MetaCore | 207 | 2.00E-04 | 1.32E-02 |
| B cell receptor signaling pathway | KEGG | 34 | 2.18E-04 | 1.36E-02 |
| Macrophage-mediated immunity | PANTHER | 46 | 2.65E-04 | 1.60E-02 |
| Regulation of metabolism | MetaCore | 135 | 3.64E-04 | 2.09E-02 |
| Cell adhesion_Attractive and repulsive receptors | MetaCore | 125 | 4.89E-04 | 2.70E-02 |
| Slit-Robo signaling | MetaCore | 29 | 5.06E-04 | 2.70E-02 |
| Interleukin signaling pathway | PANTHER | 66 | 4.96E-04 | 2.70E-02 |
| Arachidonic acid production | MetaCore | 7 | 7.20E-04 | 3.75E-02 |
| Segment specification | PANTHER | 31 | 8.43E-04 | 4.20E-02 |
| Sarcoma | MetaCore | 116 | 8.91E-04 | 4.29E-02 |
| Vascular hemostatic disorders | MetaCore | 73 | 9.01E-04 | 4.29E-02 |
| Carcinoma, neuroendocrine | MetaCore | 27 | 1.11E-03 | 4.87E-02 |
Novel modifiers of SRE-luciferase act by stimulating or repressing SREBP activity
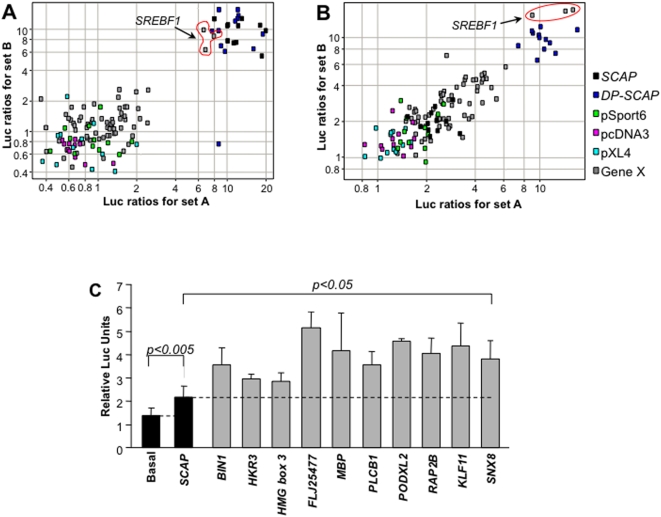
To further understand the influence of the candidate genes on regulating SREBP signaling, we tested the activators in the presence of excess sterol (1 µg/ml 25-OH cholesterol). As shown in Figure 4A, under high 25OH-cholesterol conditions, a majority of the genes identified as activators in our screen were clustered in the bottom left corner of the scatter plot indicating that the activity of these genes was attenuated in the presence of excess cholesterol. In contrast, we found only one gene (shown in triplicate) that was able to overcome high cholesterol levels (Figure 4A). This clone corresponded to sterol regulatory element binding protein 1c (SREBP1c).
Figure 4. Novel activators of the SREBP pathway.
(A) Effects of activator genes in presence of 25-OH cholesterol. The cDNA's were assayed in the presence of 1 µg/ml 25-hydroxy cholesterol. Scatter plot shows the luciferase ratios obtained for a clone in the first experiment against that obtained in the second experiment. Data points lying at the bottom left corner of the scatter plot represent genes that do not activate SRE-luciferase in the presence of high cholesterol. SCAP (black points), DP-SCAP (dark blue points) and SREBF1 overcome cholesterol repression (top right corner). Empty vectors color coded as: green, pSport6; sky blue, pXL4; pink, pcDNA3. (B) Effects of INSIG1 overexpression (15 ng/well) on novel activator genes. SCAP (central black points), DP-SCAP (dark blue points) and SREBF1 (top right corner) escape repression by INSIG1. In addition, a number of candidate genes (grey points) activated SRE-luciferase to a greater extent than SCAP. Colored points represent empty vectors: green, pSport6; sky blue, pXL4; pink, pcDNA3. (C) Graphical representation of the ten novel activator genes that activate SREBP signaling under high INSIG1 levels. These genes activate the SREBP signaling assay to a significantly greater extent than SCAP (Student's t-Test, p<0.005 or p<0.05).
In addition to excess cholesterol, we also tested the activators in the presence of high INSIG1 levels to probe whether any of the genes in the activator set could overcome INSIG mediated pathway inhibition. As expected, co-overexpression of SCAP was found to overcome INSIG1-mediated SREBP stabilization. Of the 27 novel genes (Table S1) that promoted SREBP signaling, ten new genes were identified that were able to overcome the inhibitory effects of INSIG1 in a manner similar to that of SCAP, under conditions of excess INSIG1 (Figure 4B and 4C). These genes include bridging integrator-1 (BIN1), GLI-Kruppel family member, HKR3 (HKR3), high-mobility group box 3 (HMG3), the hypothetical protein FLJ25477, myelin basic protein (MBP), phospholipase C, beta 1 (PLCB1), podocalyxin-like 2 (PODXL2), RAP2B member of RAS oncogene family (RAP2B), kruppel-like factor 11 (KLF11) and sorting nexin 8 (SNX8).
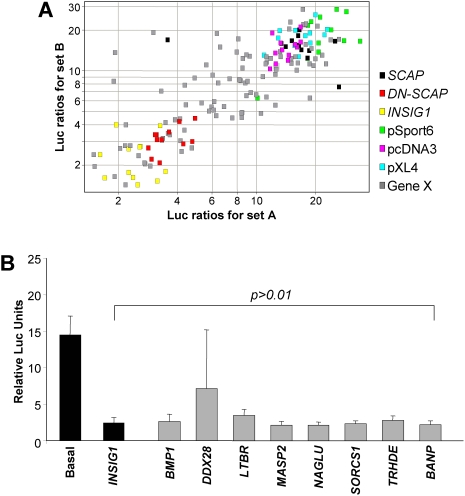
Activation of SREBP cleavage by over-expression of SCAP can be repressed by co-overexpression of INSIG1 [40]. To examine if any of the 40 novel repressors could exert a similar effect as INSIG1, candidate repressors were tested for their ability to down-regulate elevated luciferase ratios resulting from SCAP overexpression. INSIG1 and DN-SCAP could down-regulate SCAP induced SREBP signaling (Figure 5A, yellow and red points respectively) and served as controls. Interestingly, a number of candidate genes (grey points) localizing with INSIG1 (yellow points) in the bottom left corner of the scatter plot were identified (Figure 5A), indicating these genes effectively repressed SREBP signaling despite SCAP over-expression. Eight genes repressed SCAP mediated activation of SREBP signaling as efficiently as INSIG1 (Figure 5B). These included bone morphogenetic protein 1 (BMP1), DEAD box polypeptide 28 (DDX28), lymphotoxin beta receptor (LTBR), mannan-binding lectin serine peptidase 2 (MASP2), N-acetyl-glucosaminidase, alpha (NAGLU), sortilin-related VPS10 domain containing receptor 1 (SORCS1), thyrotropin-releasing hormone degrading enzyme (TRHDE) and BTG3 associated nuclear protein (BANP).
Figure 5. Novel repressors of the SREBP pathway.
(A) Effects of SCAP (15 ng/well) over-expression on novel repressor genes. Scatter plot depicting the luciferase ratios obtained for a clone in the first experiment against ratios obtained in the second experiment. Internal control genes are color coded as: yellow, INSIG1; red, DN-SCAP; green, pSport6; sky blue, pXL4; pink, pcDNA3. (B) Graphical representation of eight repressors that can inhibit SCAP-mediated SREBP activation as efficiently as INSIG1 (Student's t-Test, p>0.01).
To rule out the possibility that the effects of the novel genes identified in this screen are due to variations in transfection-control renilla luciferase levels, we have analyzed these values separately. We observe about a 2-fold variation in renilla luciferase values across the samples (Tables S1, S2). We believe that this variation is to be expected for a transient transfection experiment and does not influence the outcome of the luciferase assays significantly. The only cases where we have noticed the renilla luciferase values to be low are for the hypothetical protein FLJ25477 and RAP2B (Table S1).
Discussion
Starting with a gene-by-gene approach to screen for modifiers of SRE-luciferase activity, we have identified several known and novel modulators of SREBP transcriptional activity.
With the aim of identifying novel activators of SREBP activity, we tested the primary hit list in the presence of high cholesterol and INSIG1 co-overexpression. Only one gene (SREBF1) was able to overcome these repressive conditions (Figure 4A and 4B). The activation of the SRE-luciferase reporter by SREBF1 even in the presence of sterols is most likely due to the production of the cleaved N-terminal transcriptional activator [14] . However, in the presence of INSIG1 co-overexpression, we identified ten novel genes that could overcome the inhibitory effects of INSIG1 (Figure 4C). Our finding that KLF11 and HMG3 act as SREBP modulators is in keeping with previous studies implicating these two classes of transcription factors in SREBP modulation [53]–[55]. Intriguingly, MBP, an integral component of myelin also activates SREBP signaling. A recent study implicates SREBP-1c and SREBP2 in the regulation of lipid metabolism and modulation of gene expression in Schwann cells, the myelinating cell of the peripheral nervous system [56]. Sorting nexin 8 (SNX8), another novel activator of SREBP signaling is a member of a diverse family of proteins that are grouped together based on the presence of a phospholipid binding Phox-homology (PX domain) that impact various intracellular trafficking and sorting events. Whether SNX8 regulates SREBP transcriptional activity by regulating intracellular trafficking events remains to be evaluated.
In contrast, by co-overexpression of SCAP, we have identified eight novel repressors of SREBP signaling (Figure 5B). Notably, recent results suggest that the immune system, through LTBR signaling, directly influences the enzymatic regulation of lipid homeostasis [57], underscoring the potential value of the novel modifiers identified in our screen. In addition, NAGLU is a lysosomal enzyme involved in the degradation of heparin sulfate [58]. Loss of NAGLU results in the lysosomal storage disease, Sanfilippo syndrome type B [58], [59]. SorCS1 is a type 1 transmembrane protein implicated in intracellular protein trafficking and sorting and predominantly localized to neurons [60], [61] and it is tempting to speculate that SorCS1 might play a role in lipid storage disorders of the brain. These novel repressors may mediate their effect via direct / indirect regulation of SCAP, via repressing SREBP transport or by modulating INSIG1 levels. Further characterization and validation studies are needed to distinguish between these possibilities and determine the precise mechanism of action of the identified repressors and activators.
With an aim to elucidate pathways involved in the coordinate control of SREBP signaling and cholesterol homeostasis we analyzed primary results from the gene by gene screen using a Gene Set Enrichment Analysis approach. In addition to known regulators, this analysis unraveled novel roles for several pathways including the ephrin receptor (EphR) and EGF receptor (EGFR) signaling pathways, as putative activators or inhibitors of SREBP signaling. Both EGFR and EphR have been shown to associate with caveolin-1 positive microdomains [62], [63] and signal via cholesterol-rich lipid rafts, implying that these pathways might be positively regulated by cellular cholesterol levels. Also, receptor tyrosine kinases such as EGFR and EphRs activate MAPK signaling which has been shown to stimulate SREBP transcriptional activity [64]. Signaling via the transcription factor Tubby represents another novel positive regulator of cholesterol homeostasis identified in our screen (Table 1). Tubby has been shown to bind to plasma membrane phophoinositides and participate in heterotrimeric G-protein coupled receptor signaling (GPCR) and there is ample evidence in support of a crucial role for cholesterol in modulating GPCR function (reviewed in [65]). It is worth noting that recent studies have also unraveled a novel role for tubby in regulating a Rab-dependent endocytic trafficking pathway [66]. Enrichment for serine proteases as gathered from our GSEA results indicates that in addition to the critical S1P/S2P mediated cleavage of SREBP, and PCSK9 catalyzed LDLR trafficking [67], [68], additional important nodes in the SREBP signaling pathway might be regulated by proteases. Also, the notion that efficient and directional intracellular trafficking of vesicles is closely dependent on microtubule and cytoskeleton dynamics is supported by our analysis where we have identified associated pathways as modifiers.
Finally, using a series of biological validation assays we successfully matched number of GSEA significant pathways to the genes identified in our screen (Table 4). It is worth noting that the genes validated in this study, namely the activators RAB20, RAB8A and BIN1 [69], [70] and the repressors, SNX8 [71], [72] and SorCS1 [60] have all been implicated in membrane and vesicle trafficking events. Interestingly, a recent gene expression profiling comparison of normal and Niemann Pick disease type C (NPC) patient fibroblasts revealed changes in several genes important for membrane traffic including RAB20 and SorCS1 [73]. The LBF GSEA analysis additionally points to a potential link between gap junction formation and cholesterol homeostasis (Table S3). Our screening results indicate that growth factors [74], protein kinase A, and protein kinase C [75]–[77] signaling cascades that dampen the formation and/or function of gap junctions also induce SREBP activity. Conversely, microtubule related tubulins involved in hemichannel transport [78], and casein kinases which promote the formation of gap junctions [75], [77], [79] , inhibit SREBP activity in our screen. Taken together, these results suggest a reciprocal relationship between gap junction formation and cholesterol biosynthesis that may warrant further investigation. The novel genes and pathways identified in the screen and subsequent pathway analysis detailed in this study, when validated in disease relevant contexts could represent novel therapeutic entry points or pathway nodes that enhance our understanding of lipid biology.
Table 4. Overlap between GSEA and biological validation.
| Pathway name from GSEA | Gene from biological analysis |
| Regulation of metabolism | SREBF1, INSIG2, ACSL4 |
| Cell adhesion_Platelet-endothelium-leucocyte interactions | SCARF1 |
| Serine protease | LACTB, ABHD4, MASP2 |
| n-3,6 Polyunsaturated & unsaturated fatty acid biosynthesis | ACSL4 |
| Arachidonic acid production | ACSL4 |
| G-protein, small GTPase & RAS-related GTPase | RAB20, RAB8A, RAP2B |
| Neuroendocrine tumors | BIN1, RAB8A |
| Lipid & sphingolipid metabolism | SPTLC1, PLCB1 |
| Autoimmune diseases | MBP, SPTLC1 |
| Cell structure & Gap junction | CSNK1E, DGCR14, MBP |
Novel genes regulating SREBP signaling that were identified in the biological re-screens were mapped onto the pathway analysis data. Shown here are the novel genes that showed up in both biological as well as pathway analyses.
Materials and Methods
Reporter constructs
Three copies of the sterol regulatory element (SRE, AAAATCACCCCACTGCAAACTCCTCCCCCTGC) from the low-density lipoprotein receptor gene promoter [18] were subcloned upstream of a pTransLucent (Panomics) and pGL3-Basic (Promega) luciferase vector to create a SRE-luciferase plasmid. As a control, a mutated version of this promoter was synthesized (Medigenomics) to contain four point mutations in each SRE element as previously reported [49] (AAAAGAACCCCTATGCAAACTCCTCCCCCTGC, mutations underlined). As an internal transfection normalization control, a humanized renilla luciferase gene driven by a weak ubiquitous SV-40 promoter (phRL-SV40, Promega) was used.
Plasmids and human cDNA clone collection
The cDNA used as controls for the SREBP cleavage assay, namely full length hamster SREBP-cleavage activating protein (SCAP), human INSIG1, hamster dominant positive and dominant negative SCAP (DP-SCAP/DN-SCAP) have been previously described [18], [40], [50], [80]. For the genome-wide study, approximately 10,000 full-length cDNA clones were purchased from OriGene Technologies (Rockville, MD) and prepared for screening as previously described [81].
Cell lines and growth conditions
CHO wild-type and HEK-293 cells were obtained from ATCC. The CHO wild-type and mutant cell lines were grown in F-12 (HAM) media (Invitrogen), supplemented with 5% new-born calf serum (NCS), 10 mM HEPES buffer and 1× Penicillin-Streptomycin (Invitrogen) antibiotic. HEK-293 cells were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and containing 1× Penicillin-Streptomycin (Invitrogen). All cell lines were grown in a humidified incubator at 37°C and with 5% CO2. 25-hydroxy (25-OH) cholesterol (Sigma) was dissolved added to media as indicated in figure legends.
Genome-wide cDNA study and luciferase assays
A reverse transfection protocol was followed for testing the 10,000 genes for their effect in the SREBP signaling assay. Trypsinized HEK-293 cells were added to 384-well white opaque bottom plates (Nunc), containing the cDNA clone and transfection mix, at density of 2500 cells/well at 25 µl per well using a Multidrop 384 (Thermo Labsystems) and incubated at 37°C in 5% CO2. The transfection mix consisted of 17.5 ng reporter plasmid/well, 0.7 ng Renilla/well and Fugene 6 (Roche) at a ratio of 3 µl Fugene to 1 µg of DNA in 5 µl of Optimem (Invitrogen). The transfection mixture was added to the 384-well plates containing the cDNA clone using a FlexDrop (Perkin Elmer). The cDNA's were screened at a concentration of 120 ng/well. For cholesterol stimulation, 25-hydroxycholesterol (Sigma) in 0.01% ethanol was added to cells at a concentration of 1 µg/ml, and incubated for 24 hours. Firefly and Renilla luciferase activity was measured 40 hours post transfection using the Dual Glow assay system (Promega). Plates were allowed to cool for 10 minutes before 30 µl of each assay reagent was added. The plates were shaken for 10 minutes on a multi-plate shaker. Luminescence was determined using an EnVision plate reader (Perkin Elmer) with a 100 msec integration time. For luciferase assays carried out in 96-well plates, all reagents were proportionally increased 4-fold.
Data analysis and 2D-normalization of genome-wide study
Results were analyzed using Spotfire Decisionsite software and Microsoft Excel. For the genome-wide study, luciferase ratios were normalized as follows. The firefly-luciferase readout values were first normalized using the corresponding renilla-luciferase readout values. The firefly-renilla ratio was further normalized as follows. The one-dimensional (1D) values were obtained by scaling the ratios with the plate median in order to remove plate-to-plate variation. The two-dimensional (2D) values were obtained by further removing the well-to-well variations through an iterative procedure. Finally, the normalized values were standardized to obtain the NZ score, which is a more robust equivalent of the more commonly used Z score. If the distribution is perfectly normal, NZ score will be the same as the Z score.
GSEA methodology
Screening results were analyzed with a modified version of the Gene Set Enrichment Analysis (GSEA) technique previously described elsewhere [47], [51], [82]. As input, 2D normalized z-scores (NZ2D) were first computed to estimate the effect of each cDNA on the SREBP assay readout. NZ2D scores were averaged per cDNA across replicates. These averaged NZ2D values were used to rank the cDNAs for input to the GSEA method. Two variants of the GSEA method were applied to these ranked scores. The first method represented the standard GSEA approach [47], [82] and used the Wilcoxon ranked sum test [83] to identify pathways whose members tended to activated or inhibited the assay. The second GSEA variant applied a robust test for homogeneity of variance [51], the Levene test as modified by Brown and Forsythe (LBF) [52]. Application of the LBF test was used to identify pathways that contain similar numbers of activators and repressors of the assay. Such cases may elude detection by the Wilcoxon test, as the contributions of activators and inhibitors tend to cancel each other out. The presence of activators and inhibitors within a pathway will yield a larger variance of NZ2D scores than is generally present in the assay and is thus detectable by the LBF test. Finally, a false discovery rate (FDR) [84] correction was applied to the computed p-values to account for multiple hypothesis testing. This process transforms the original p-values into FDR q-values that were used for significance testing. The GSEA results were then filtered to identify interesting pathways by 1) removing pathways with <5 clones; 2) removing pathways with >250 clones; 3) removing pathways with FDR q-values>0.05 for the Wilcoxon and LBF tests. This resulted in 103 moderately-sized pathways that had hits at q-values<0.05 in at least one test.
This application of GSEA is a natural extension of a methodology that has enjoyed great success when applied to microarray data [47], [51], [82]. Nevertheless, there are fundamental differences between these types of experiments that impact the interpretation of results. Whereas a simple microarray experiment consists of a single perturbation and readouts for tens of thousands of genes, this screen includes thousands of cDNA overexpression perturbations and a single readout. When applied to microarray data, GSEA identifies pathways that are modulated in response to a specific perturbation. In this application, GSEA should identify pathways that modulate SREBP activity. The recovery of several pathways known to modulate cholesterol homeostasis validates the application of pathway-centric methodologies for analyzing cDNA overexpression screens.
Supporting Information
Scatter plot of the novel activators (red) and repressors (blue) of SREBP signaling after removal of the false positives and clones with high renilla luciferase levels.
(1.31 MB TIF)
Complete list of validated activators of SREBP signaling.
(0.04 MB XLS)
Complete list of validated repressors of SREBP signaling.
(0.04 MB XLS)
Genes that affect the formation and function of Gap Junctions also modulate SREBP activity: Several branches of the KEGG Gap Junction (HSA04540) pathway were notable for their coordinate regulation of the SREBP assay. Notably, positive regulators of gap junctions tended to inhibit SREBP activity (Casein Kinases, Microtubules), whereas negative regulators tended to activate SREBP (Growth Factors, Protein Kinase C, Protein Kinase G). The most potent inhibitor and activator among these were validated in secondary assays (PLCB1, CSNK1E). Some genes showed isoform selectivity for SREBP activity, e.g. the epsilon isoform of casein kinase 1 was a more potent activator than the delta and gamma isoforms. Nevertheless, the consistency of results across several independent branches of gap junction formation signaling pathways suggests an inverse causal relationship between the function of gap junctions and cholesterol homeostasis.
(0.03 MB XLS)
Acknowledgments
We thank Justin Warner, John Alford, and Charles Tao (Developmental and Molecular Pathways, NIBR, Cambridge) for technical assistance with the genome-wide screen.
Footnotes
Competing Interests: We have a patent filed relating to some of the material that is presented in the manuscript: (WO/2007/141346) GENES REGULATING INTRACELLULAR CHOLESTEROL TRAFFICKING AS TARGETS FOR TREATMENT OF CHOLESTEROL-RELATED DISEASES. SC, JDS, NRM, CM, MAL and RS are employed by Novartis.
Funding: This work was funded by an Ara Parseghian Medical Research Foundation grant (which went solely towards funding the post doctoral researcher and first author, Sandipan Chatterjee) and by the Novartis Institutes for Biomedical Research. Novartis Institutes for Biomedical Research played an active role in study design, data collection and analysis, decision to publish as well in preparing the manuscript.
References
- 1.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 3.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Felder TK, Klein K, Patsch W, Oberkofler H. A novel SREBP-1 splice variant: tissue abundance and transactivation potency. Biochim Biophys Acta. 2005;1731:41–47. doi: 10.1016/j.bbaexp.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Harada N, Yonemoto H, Yoshida M, Yamamoto H, Yin Y, et al. Alternative splicing produces a constitutively active form of human SREBP-1. Biochem Biophys Res Commun. 2008;368:820–826. doi: 10.1016/j.bbrc.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Liu F, Millette CF, Kilpatrick DL. Expression of a novel, sterol-insensitive form of sterol regulatory element binding protein 2 (SREBP2) in male germ cells suggests important cell- and stage-specific functions for SREBP targets during spermatogenesis. Mol Cell Biol. 2002;22:8478–8490. doi: 10.1128/MCB.22.24.8478-8490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 9.Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001;276:4365–4372. doi: 10.1074/jbc.M007273200. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue J, Sato R. A novel splicing isoform of mouse sterol regulatory element-binding protein-1 (SREBP-1). Biosci Biotechnol Biochem. 1999;63:243–245. doi: 10.1271/bbb.63.243. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Sartini BL, Millette CF, Kilpatrick DL. A developmental switch in transcription factor isoforms during spermatogenesis controlled by alternative messenger RNA 3′-end formation. Biol Reprod. 2006;75:318–323. doi: 10.1095/biolreprod.106.052209. [DOI] [PubMed] [Google Scholar]
- 13.Hua X, Wu J, Goldstein JL, Brown MS, Hobbs HH. Structure of the human gene encoding sterol regulatory element binding protein-1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11.2 and 22q13. Genomics. 1995;25:667–673. doi: 10.1016/0888-7543(95)80009-b. [DOI] [PubMed] [Google Scholar]
- 14.Hua X, Sakai J, Brown MS, Goldstein JL. Regulated cleavage of sterol regulatory element binding proteins requires sequences on both sides of the endoplasmic reticulum membrane. J Biol Chem. 1996;271:10379–10384. doi: 10.1074/jbc.271.17.10379. [DOI] [PubMed] [Google Scholar]
- 15.Parraga A, Bellsolell L, Ferre-D'Amare AR, Burley SK. Co-crystal structure of sterol regulatory element binding protein 1a at 2.3 A resolution. Structure. 1998;6:661–672. doi: 10.1016/s0969-2126(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 16.Sato R, Yang J, Wang X, Evans MJ, Ho YK, et al. Assignment of the membrane attachment, DNA binding, and transcriptional activation domains of sterol regulatory element-binding protein-1 (SREBP-1). J Biol Chem. 1994;269:17267–17273. [PubMed] [Google Scholar]
- 17.Sakai J, Nohturfft A, Cheng D, Ho YK, Brown MS, et al. Identification of complexes between the COOH-terminal domains of sterol regulatory element-binding proteins (SREBPs) and SREBP cleavage-activating protein. J Biol Chem. 1997;272:20213–20221. doi: 10.1074/jbc.272.32.20213. [DOI] [PubMed] [Google Scholar]
- 18.Hua X, Nohturfft A, Goldstein JL, Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 1996;87:415–426. doi: 10.1016/s0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, et al. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15:1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nohturfft A, Yabe D, Goldstein JL, Brown MS, Espenshade PJ. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102:315–323. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Rawson RB, DeBose-Boyd R, Goldstein JL, Brown MS. Failure to cleave sterol regulatory element-binding proteins (SREBPs) causes cholesterol auxotrophy in Chinese hamster ovary cells with genetic absence of SREBP cleavage-activating protein. J Biol Chem. 1999;274:28549–28556. doi: 10.1074/jbc.274.40.28549. [DOI] [PubMed] [Google Scholar]
- 23.Espenshade PJ, Li WP, Yabe D. Sterols block binding of COPII proteins to SCAP, thereby controlling SCAP sorting in ER. Proc Natl Acad Sci U S A. 2002;99:11694–11699. doi: 10.1073/pnas.182412799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nohturfft A, Brown MS, Goldstein JL. Sterols regulate processing of carbohydrate chains of wild-type SREBP cleavage-activating protein (SCAP), but not sterol-resistant mutants Y298C or D443N. Proc Natl Acad Sci U S A. 1998;95:12848–12853. doi: 10.1073/pnas.95.22.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nohturfft A, DeBose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc Natl Acad Sci U S A. 1999;96:11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawson RB, Zelenski NG, Nijhawan D, Ye J, Sakai J, et al. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol Cell. 1997;1:47–57. doi: 10.1016/s1097-2765(00)80006-4. [DOI] [PubMed] [Google Scholar]
- 27.Sakai J, Duncan EA, Rawson RB, Hua X, Brown MS, et al. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell. 1996;85:1037–1046. doi: 10.1016/s0092-8674(00)81304-5. [DOI] [PubMed] [Google Scholar]
- 28.Sakai J, Rawson RB, Espenshade PJ, Cheng D, Seegmiller AC, et al. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol Cell. 1998;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 30.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai JT, Guryev O, Brown MS, Goldstein JL. Differential stimulation of cholesterol and unsaturated fatty acid biosynthesis in cells expressing individual nuclear sterol regulatory element-binding proteins. J Biol Chem. 1998;273:26138–26148. doi: 10.1074/jbc.273.40.26138. [DOI] [PubMed] [Google Scholar]
- 33.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Adams CM, Goldstein JL, Brown MS. Cholesterol-induced conformational change in SCAP enhanced by Insig proteins and mimicked by cationic amphiphiles. Proc Natl Acad Sci U S A. 2003;100:10647–10652. doi: 10.1073/pnas.1534833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, et al. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 37.Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- 38.Janowski BA. The hypocholesterolemic agent LY295427 up-regulates INSIG-1, identifying the INSIG-1 protein as a mediator of cholesterol homeostasis through SREBP. Proc Natl Acad Sci U S A. 2002;99:12675–12680. doi: 10.1073/pnas.202471599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci U S A. 2002;99:12753–12758. doi: 10.1073/pnas.162488899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 41.Sun LP, Li L, Goldstein JL, Brown MS. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J Biol Chem. 2005;280:26483–26490. doi: 10.1074/jbc.M504041200. [DOI] [PubMed] [Google Scholar]
- 42.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci U S A. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, et al. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci U S A. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thewke DP, Panini SR, Sinensky M. Oleate potentiates oxysterol inhibition of transcription from sterol regulatory element-1-regulated promoters and maturation of sterol regulatory element-binding proteins. J Biol Chem. 1998;273:21402–21407. doi: 10.1074/jbc.273.33.21402. [DOI] [PubMed] [Google Scholar]
- 45.Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J Biol Chem. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
- 47.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 49.Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem. 1993;268:14490–14496. [PubMed] [Google Scholar]
- 50.Sakai J, Nohturfft A, Goldstein JL, Brown MS. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J Biol Chem. 1998;273:5785–5793. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- 51.Kemp DM, Nirmala NR, Szustakowski JD. Extending the pathway analysis framework with a test for transcriptional variance implicates novel pathway modulation during myogenic differentiation. Bioinformatics. 2007;23:1356–1362. doi: 10.1093/bioinformatics/btm116. [DOI] [PubMed] [Google Scholar]
- 52.Conover WJ, Johnson ME, Johnson MM. A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics. 1981;21:351–361. [Google Scholar]
- 53.Cao S, Fernandez-Zapico ME, Jin D, Puri V, Cook TA, et al. KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J Biol Chem. 2005;280:1901–1910. doi: 10.1074/jbc.M407941200. [DOI] [PubMed] [Google Scholar]
- 54.Najima Y, Yahagi N, Takeuchi Y, Matsuzaka T, Sekiya M, et al. High mobility group protein-B1 interacts with sterol regulatory element-binding proteins to enhance their DNA binding. J Biol Chem. 2005;280:27523–27532. doi: 10.1074/jbc.m414549200. [DOI] [PubMed] [Google Scholar]
- 55.Natesampillai S, Fernandez-Zapico ME, Urrutia R, Veldhuis JD. A novel functional interaction between the Sp1-like protein KLF13 and SREBP-Sp1 activation complex underlies regulation of low density lipoprotein receptor promoter function. J Biol Chem. 2006;281:3040–3047. doi: 10.1074/jbc.M509417200. [DOI] [PubMed] [Google Scholar]
- 56.de Preux AS, Goosen K, Zhang W, Sima AA, Shimano H, et al. SREBP-1c expression in Schwann cells is affected by diabetes and nutritional status. Mol Cell Neurosci. 2007;35:525–534. doi: 10.1016/j.mcn.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, et al. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 58.Weber B, Blanch L, Clements PR, Scott HS, Hopwood JJ. Cloning and expression of the gene involved in Sanfilippo B syndrome (mucopolysaccharidosis III B). Hum Mol Genet. 1996;5:771–777. doi: 10.1093/hmg/5.6.771. [DOI] [PubMed] [Google Scholar]
- 59.Zhao HG, Li HH, Bach G, Schmidtchen A, Neufeld EF. The molecular basis of Sanfilippo syndrome type B. Proc Natl Acad Sci U S A. 1996;93:6101–6105. doi: 10.1073/pnas.93.12.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermey G, Keat SJ, Madsen P, Jacobsen C, Petersen CM, et al. Characterization of sorCS1, an alternatively spliced receptor with completely different cytoplasmic domains that mediate different trafficking in cells. J Biol Chem. 2003;278:7390–7396. doi: 10.1074/jbc.M210851200. [DOI] [PubMed] [Google Scholar]
- 61.Hermey G, Riedel IB, Rezgaoui M, Westergaard UB, Schaller C, et al. SorCS1, a member of the novel sorting receptor family, is localized in somata and dendrites of neurons throughout the murine brain. Neurosci Lett. 2001;313:83–87. doi: 10.1016/s0304-3940(01)02252-2. [DOI] [PubMed] [Google Scholar]
- 62.Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, et al. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–356. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U. Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- 64.Kotzka J, Muller-Wieland D, Roth G, Kremer L, Munck M, et al. Sterol regulatory element binding proteins (SREBP)-1a and SREBP-2 are linked to the MAP-kinase cascade. J Lipid Res. 2000;41:99–108. [PubMed] [Google Scholar]
- 65.Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Mukhopadhyay A, Pan X, Lambright DG, Tissenbaum HA. An endocytic pathway as a target of tubby for regulation of fat storage. EMBO Rep. 2007;8:931–938. doi: 10.1038/sj.embor.7401055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 68.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpaa K, Laakkonen P, et al. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- 70.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, et al. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. 1997;137:1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins–unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 72.Verges M. Retromer and sorting nexins in development. Front Biosci. 2007;12:3825–3851. doi: 10.2741/2355. [DOI] [PubMed] [Google Scholar]
- 73.Reddy JV, Ganley IG, Pfeffer SR. Clues to neuro-degeneration in Niemann-Pick Type C disease from global gene expression profiling. PLoS ONE. 2006;1:e19. doi: 10.1371/journal.pone.0000019. doi:10.1371/journal.pone.0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leithe E, Rivedal E. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci. 2004;117:1211–1220. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- 75.Cruciani V, Mikalsen SO. Connexins, gap junctional intercellular communication and kinases. Biol Cell. 2002;94:433–443. doi: 10.1016/s0248-4900(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 76.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 77.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 79.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 80.Nohturfft A, Hua X, Brown MS, Goldstein JL. Recurrent G-to-A substitution in a single codon of SREBP cleavage-activating protein causes sterol resistance in three mutant Chinese hamster ovary cell lines. Proc Natl Acad Sci U S A. 1996;93:13709–13714. doi: 10.1073/pnas.93.24.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szustakowski JD, Lee JH, Marrese CA, Kosinski PA, Nirmala NR, et al. Identification of novel pathway regulation during myogenic differentiation. Genomics. 2006;87:129–138. doi: 10.1016/j.ygeno.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 83.Siegal S. Nonparametric statistics for the behavioral sciences. New York: McGraw Hill; 1956. [Google Scholar]
- 84.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plot of the novel activators (red) and repressors (blue) of SREBP signaling after removal of the false positives and clones with high renilla luciferase levels.
(1.31 MB TIF)
Complete list of validated activators of SREBP signaling.
(0.04 MB XLS)
Complete list of validated repressors of SREBP signaling.
(0.04 MB XLS)
Genes that affect the formation and function of Gap Junctions also modulate SREBP activity: Several branches of the KEGG Gap Junction (HSA04540) pathway were notable for their coordinate regulation of the SREBP assay. Notably, positive regulators of gap junctions tended to inhibit SREBP activity (Casein Kinases, Microtubules), whereas negative regulators tended to activate SREBP (Growth Factors, Protein Kinase C, Protein Kinase G). The most potent inhibitor and activator among these were validated in secondary assays (PLCB1, CSNK1E). Some genes showed isoform selectivity for SREBP activity, e.g. the epsilon isoform of casein kinase 1 was a more potent activator than the delta and gamma isoforms. Nevertheless, the consistency of results across several independent branches of gap junction formation signaling pathways suggests an inverse causal relationship between the function of gap junctions and cholesterol homeostasis.
(0.03 MB XLS)