Abstract
Abuse of prescription opioids has risen precipitously in the United States. Few controlled comparisons of the abuse liability of the most commonly abused opioids have been conducted. This outpatient study employed a double-blind, randomized, within-subject, placebo-controlled design to examine the relative abuse potential and potency of oral oxycodone (10, 20 & 40 mg), hydrocodone (15, 30 & 45 mg), hydromorphone (10, 17.5 & 25 mg) and placebo. Healthy adult volunteers (n=9) with sporadic prescription opioid abuse participated in 11 experimental sessions (6.5 hr in duration) conducted in a hospital setting. All three opioids produced a typical mu opioid agonist profile of subjective (increased ratings of liking, good effects, high and opiate symptoms), observer-rated, and physiological effects (miosis, modest respiratory depression, exophoria and decrements in visual threshold discrimination) that were generally dose-related. Valid relative potency assays revealed that oxycodone was roughly equipotent to or slightly more potent than hydrocodone. Hydromorphone was only modestly more potent (less than two-fold) than either hydrocodone or oxycodone, which is inconsistent with prior estimates arising from analgesic studies. These data suggest that the abuse liability profile and relative potency of these three commonly used opioids do not differ substantially from one another and suggest that analgesic potencies may not accurately reflect relative differences in abuse liability of prescription opioids.
Keywords: hydrocodone, oxycodone, hydromorphone, human, opioid, abuse liability, analgesia, prescription opioids
1. Introduction
Rates of prescription opioid abuse have been on the rise in the United States for the past several years (Compton and Volkow, 2006; Zacny et al., 2003) and may be increasing elsewhere in the world, including, for example, in Australia (Mouzos et al., 2007). Data from the 2006 National Survey on Drug Use and Health (NSDUH) indicate that 4.7 million Americans used a prescription opioid illicitly in the month preceding the survey (SAMHSA, 2007b), and approximately one million people in 2006 were estimated to be prescription opioid-dependent (based upon DSM-IV criteria), adding to the nearly one million people estimated by the Office of National Drug Control Policy to be chronic users of heroin (ONDCP, 2001). The number of new illicit users of prescription opioids has grown to over 2 million, up from 1.5 million in 1999, representing over a 5-fold increase from the mid 1980’s when there were approximately 400,000 new initiates per year. One review suggests that the relative prevalence of opioid prescription abuse may be comparable to the prevalence of heroin and cocaine abuse (Zacny et al., 2003). For those individuals initiating illicit drug use for the first time, the most recent epidemiological data indicate that prescription opioids have now surpassed marijuana as the most common drug(s) of initiation (SAMHSA, 2007b). The 2006 Monitoring the Future survey, a national survey of drug use among 8th, 10th and 12th graders, reported stable or declining rates of use for most illicit drugs; however, rates of Oxycontin® and Vicodin® use increased in nearly all grade levels, with the highest rate estimates indicating that nearly 10% of all 12th graders have used Vicodin® (Johnston et al., 2007). Moreover, survey data indicate that about half of teens (grades 7–12) do not believe that there is a great risk in abusing prescription medicine and 30% believe that prescription pain relievers are non-addictive (PATS, 2005).
Hydrocodone (also known as dihydrocodeinone) is a semi-synthetic full mu opioid agonist related to codeine that is metabolized by cytochrome P450 2D6 and transformed into another mu agonist, hydromorphone (Otton et al., 1993); however, this conversion does not appear to be critical to the abuse potential of hydrocodone (Kaplan et al., 1997). Hydrocodone is marketed in the United States for use as an antitussive and analgesic and, while hydrocodone itself is listed under Schedule II, it is only sold in combination formulations (e.g., with acetaminophen - Lortab® and Vicodin®), which are regulated under the less restrictive Schedule III category. Hydrocodone is the most frequently prescribed opioid in the United States with an estimated 130 million prescriptions in 2006, up from approximately 88 million in 2000 (IMS National Prescription Audit Plus™). Given its broad availability, it is no surprise that hydrocodone accounts for the greatest proportion of emergency room drug use mentions for the prescribed opioids (and has shown successive increases since 1996) according to the Drug Abuse Early Warning Network (DAWN), a national data collection system that systematically monitors drug abuse through hospital emergency departments in the contiguous United States (SAMHSA, 2007a).
Oxycodone (dihydrodydroxycodienone) is a semi-synthetic opioid that has been in clinical use for more than 90 years. Oxycodone is manufactured and marketed in the United States in numerous oral formulations, although oxycodone is widely used in other countries by the intravenous, intramuscular and rectal routes. Oxycodone is a full mu opioid agonist, metabolized by the P450 2D6 enzyme to at least one active metabolite, oxymorphone (Beaver et al., 1978), which is itself marketed as an analgesic. Historically, oxycodone was considered to be associated with a lower abuse liability, similar to that of codeine, because it was initially introduced to the United States in 1981 in combination with over-the-counter non-opioid analgesics (Poyhia et al., 1993). Oxycontin®, a sustained release formulation of oxycodone, has been identified in the lay press as an especially problematic and dangerous drug of abuse because it is available in higher dosages than other oxycodone formulations, and the sustained release feature can be readily bypassed by crushing the tablets. However, Oxycontin®, immediate release oxycodone (e.g., Roxicodone®) and oxycodone combination products with either acetaminophen (e.g., Percocet®) or aspirin (e.g., Percodan®), all regulated under Schedule II, have historically accounted for a smaller portion of prescription opioid sales (estimated to be about 15% of the market in 2001 based upon the IMS National Prescription Audit Plus™) than those for hydrocodone, propoxyphene or codeine products. However, recent data from the Drug Enforcement Agency (http://www.deadiversion.usdoj.gov/quotas/quota_history.htm) indicate that, while licit aggregate production for hydrocodone has increased approximately 2.8-fold between 1998 and 2008 (from 16,314,000 kg to 45,200,000 kg), production of oxycodone has increased nearly 6-fold (from 12,118,000 kg to nearly 75,000,000 kg) over the same 10-year period, reflecting the increasing availability of, demand for, and exposure to both prescription opioids. Moreover, data from recent DAWN reports suggest that oxycodone mentions are increasing in number, approaching the number of hydrocodone mentions (SAMHSA, 2007a).
Hydromorphone, a semi-synthetic opioid derived from morphine, is a full mu agonist that has been used clinically for more than 75 years and is typically reserved for the treatment of severe pain. It is presently marketed in formulations for oral, rectal and parenteral use. While production of hydromorphone has also increased over the past decade, its clinical use is less widespread as evidenced, in part, by substantially lower aggregate production (e.g., only 3.3. million kg in 2008) compared to oxycodone and hydrocodone. In early 2005, a novel sustained release formulation (Palladone®) of hydromorphone was introduced onto the U.S. market; however, sale of the product was suspended within months subsequent to pronounced synergistic effects when taken in combination with alcohol, which were reported to represent a substantively greater risk than the typical interaction of other mu opioid agonists when taken in combination with alcohol. The purpose of this study was to examine the relative abuse liability of hydrocodone and oxycodone in comparison to hydromorphone by examining a range of doses, including supratherapeutic doses, in individuals who reported sporadic recreational use of prescription opioids. Despite the widespread clinical use of oxycodone and hydrocodone, comparatively few studies have directly evaluated the abuse liability and clinical pharmacology of these commonly abused opioids. Most available data assessing the relative potency of these compounds to produce their direct effects has emanated from the pain literature where the drugs are compared on their relative efficacy as analgesics rather than measures related to abuse liability.
2. Methods
2.1 Participants
Participants were adult volunteers who used prescription opioids illicitly for their psychoactive effects but were not physically dependent on opioids at the time of the study (for details see Section 3.1). Volunteers were recruited through local advertisements and were paid for their participation. Individuals who were seeking treatment for their substance abuse or successfully sustaining abstinence in the community were excluded. Study enrollment was continuous, and a maximum of four volunteers could participate simultaneously.
All participants were determined to be in good health by medical history and physical examination, an electrocardiogram and laboratory tests. Subjects were carefully screened to eliminate those presenting with seizure disorders, history of asthma or other respiratory disorders, head injury, hypertension, cardiovascular disease or abnormal ECG. All participants reported illicit use of opioids, and this use was confirmed by objective urinalysis results during the screening period. This study was approved by the University of Kentucky Institutional Review Board, and subjects gave their written informed consent prior to participation. A Certificate of Confidentiality was obtained from the National Institute on Drug Abuse for the project. This study was conducted in accordance with the Helsinki guidelines for ethical human research.
Subjects participated as outpatients in this study and were instructed that they needed to abstain from illicit drug use during their participation. Each study day urine specimens and breathalyzer tests were obtained and tested for illicit drugs, including methadone, cocaine, THC, benzodiazepines, morphine-derived opioids, amphetamine, barbiturates, methamphetamine, phencyclidine, tricyclic antidepressants (Multi-Drug Screen Test Dip Card 10 panel; American Screen Corp., Louisiana), oxycodone (Single Oxy Dip Card; American Screen Corp., Louisiana), and alcohol (Alcosensor, Alcoscan AL5000, Sentech, South Korea), to ensure the absence of recent use. Females were tested for pregnancy during each screening visit and on the morning of each session. Subjects were instructed to eat breakfast 2 hr prior to arrival to the laboratory; they were not allowed to eat or smoke throughout the duration of the experimental test sessions.
2.2. Drugs
An Investigational New Drug Application was obtained from the Food and Drug Administration to support the conduct of this study (#69,214). All study medications were prepared in the Investigational Pharmacy at the University of Kentucky. Commercial suppliers were used to obtain hydrocodone bitartrate (Cardinal Health, Knoxville, TN), oxycodone hydrochloride (Spectrum Laboratory, Gardena, CA), and hydromorphone hydrochloride (Cardinal Health, Knoxville, TN). Powder was weighed and packed into identically-appearing size 0 capsules (Health Care Logistics). Doses were administered as the salt weight. Commercially available cornstarch was used for the placebo condition and for filler in the active dose capsules. The experimental doses were placebo (cornstarch), oxycodone (10, 20 & 40 mg), hydrocodone (15, 30 & 45 mg) and hydromorphone (10, 17.5 & 25 mg).
2.3 Dose Selection Rationale
There was little information on the direct acute effects of oxycodone and hydrocodone in this population, and therefore, a pilot study was completed to help guide the choice of safe, tolerable, and equipotent doses of oxycodone, hydrocodone and hydromorphone for use in the current study. In all respects, the pilot data collected were identical in nature and scope to that collected in the present study except: 1) doses were administered in ascending order for safety purposes, and 2) there were a greater number of test conditions (17 total, five doses each of oxycodone, hydrocodone and hydromorphone, placebo and an active practice condition) and a wider range of test doses (oxycodone [5 mg – 50 mg], hydrocodone [7.5 mg – 60 mg], and hydromorphone [2.5 mg – 35 mg]. Results showed that oxycodone (5 mg) and hydromorphone (2 and 5 mg) were inactive in sporadic opioid users. Based upon these data, highest test doses selected for the randomized study were those that produced maximum scores on subjective visual analog scales (i.e., liking, good effects and high) while being safely tolerated by the pilot subjects, and lowest test doses were selected with the expectation that they would exhibit some pharmacological activity. The range of test doses overlapped with the range of illicit doses of oxycodone and hydrocodone reported by the participants in this study.
2.4 Study Design
A double-blind, within-subject, randomized, placebo-controlled design was used. There were eleven outpatient sessions conducted at the University of Kentucky General Clinical Research Center (UK GCRC). The first session served as a practice and safety session during which subjects received the intermediate dose of hydromorphone (17.5 mg, p.o.) under single-blind conditions (data not reported from this session). This allowed the volunteer to acclimate to the experimental environment, screen for idiosyncratic reactions to hydromorphone, and confirm that the volunteers were able to provide subjective reports of opioid drug effects (no subjects were excluded based upon this test session). Subjects then participated in ten double-blind, randomized, experimental test sessions separated by at least 72 hr in which they received a single test drug. Baseline data were collected for 30 min prior to drug administration at 9:00 AM, and data collection proceeded for 6 hr thereafter. Subjects were retained for an additional 2 hr post-session for continued observation and were offered a full meal, if desired, during that time. Subjects could complete the study within 6 weeks; however, it could take longer due to subject and staff scheduling.
2.5 Experimental Sessions
Subjects arrived at the UK GCRC at approximately 8:00 am on session days accompanied by study staff after providing a urine drug test and alcohol breathalyzer test negative for illicit drugs and alcohol, respectively. An intravenous catheter was placed into an antecubital vein prior to the start of session as a precautionary measure to ensure immediate venous access in the event of an emergency. Subjects were seated in a cushioned chair in an experimental test room directly in front of a Macintosh Mini, OSX (Apple Computer, Cupertino, CA) that was used to collect the data. The computer was programmed to record physiological measures (except pupil diameter and respiratory rate) and to present questionnaires in the appropriate order; volunteers entered their responses by using a computer mouse and/or keypad (for analytic details see Section 2.6). A research assistant was seated behind the computer with a keyboard to initiate tasks and to enter observer-rated measures. Data printouts were collected after the completion of each session, and data were transferred to Macintosh Excel spreadsheets (Microsoft Corp., Redmond, WA) for analyses.
Physiological Measures
Oxygen saturation, skin temperature, blood pressure and heart rate were collected every minute using an automatic physiologic monitoring device (Scholar III model 507ELC2, Criticare Systems INC, Waukesha, WI). Data collection began 30 min before the drug administration, and continued for 6 hr after drug administration. The research assistant recorded the respiration rate by counting the number of breaths within 30-sec. Pupil diameter was determined from Polaroid camera photographs (Polaroid Corp., Cambridge, MA) using a two-fold magnification taken in constant room lighting provided by halogen lamps. Both respiratory rate and pupil diameter were collected at regular intervals throughout the session according to the time course described below.
Subject and Observer-rated Measures
Subject-rated measurements included visual analog scales, the Addiction Research Center Inventory (ARCI) short form (Martin et al., 1971), a pharmacological class questionnaire (from Jasinski et al., 1977) and a 17-item adjective checklist that encompassed both an Agonist Scale and the Fraser Scale (see Walsh et al., 1995 for scale descriptions). An observer-rated opioid adjective rating scale was also used. The subject- and observer-rated adjectives and the visual analog scales were collected 30 min before drug administration, every 15-min for 2 hr after drug administration, and then at 0.5 hr intervals for the remainder of the session (along with pupil photographs and manual respiratory rate measurements). The ARCI and pharmacological class questionnaire were collected at baseline and at 0.5 hr intervals throughout the session.
The visual analog questions were “Do you feel any DRUG EFFECT?” “ Do you LIKE the drug?” “How HIGH are you?” “Does the drug have any GOOD EFFECTS?” “Does the drug have any BAD EFFECTS?” and “How much do you DESIRE opiates?” The subjects responded by positioning an arrow along a 100-point line labeled with “not at all” at one end and “extremely” at the other. The ARCI consisted of 49 true/false questions that are subdivided into scales that are sensitive to euphoria (morphine-benzedrine group: MBG), sedation (pentobarbital-chlorpromazine-alcohol group: PCAG), dysphoria (lysergic acid diethylamide: LSD), and amphetamine-like effects (benzedrine group: BG and amphetamine: A). On the pharmacological class questionnaire, subjects were asked to categorize their drug effect as being most similar to one of the following ten classes of psychoactive drugs: placebo, opiates, phenothiazines, barbiturates and sleeping medications, opiate antagonists, antidepressants, hallucinogens, benzodiazepines, stimulants, phencyclidine and other. Descriptive titles and examples were given for each drug class. The subject and observer-rated adjective checklists used a 5-point Likert scale (0 not at all – 4 extremely) to rate the following items: [subject and observer] sleepy, skin itchy, relaxed, coasting, drunken, nervous, nodding, sluggish feeling, friendly, good mood, energetic, [subject only] stomach turning, talkative/soapboxing, pleasant sick, drive, dry mouth, and carefree.
Ocular and Performance Tasks
Three additional measures were collected at baseline and at 1 hr intervals throughout the session to assess motor impairment, including two ocular tasks sensitive to disruptions of perception and ocular motor control and a gross motor task. Critical flicker fusion threshold, a measure of temporal discrimination of a visual stimulus, was measured using an automated flicker fusion device (Model 12021, Lafayette Instrument, Lafayette, IN). The flicker and the fusion tasks were each run for two trials at every data collection time point. The flicker task requires the subject to gaze at a steady light stimulus and to respond when the changing presentation interval makes the light appear to flicker- that is, to be a noncontinuous light source. In contrast, the fusion task requires the subject to gaze at a flickering light stimulus and respond when the changing presentation interval makes the light appear to be a continuous light source- that is to be fused. Outcome measures include the individual fusion and flicker thresholds and the Critical Flicker/Fusion (CFF; average value of the fusion and flicker value); all variables are reported in frequency (Hz; range from 1.0 – 100).
The second ocular task was the Maddox-Wing test (Model CE0120, Clement Clarke Ltd., London, UK). For this task, subjects are instructed to place their eyes against the eye pieces and focus on an arrow at the end of the device. The apparent position of the arrow after focusing is reported as a numeric position along a metric on the device (measured in prism diopters) and is dependent on the gaze of the individual. This is a measure of exophoria or under convergence, which is the tendency for gaze to shift outward from the midline as a function of changes in ocular motor control.
A simple balance task was included for which subjects were instructed to hold their arms directly out to their sides and, with eyes closed, to stand first on one foot for as long as possible (maximum trial length was 30 sec). The time that their foot touched down (or 30 sec if the trial was successfully completed) was recorded, and then the same instruction was repeated while standing on the opposite foot.
2.6 Statistical Analyses
All measures collected during the experimental sessions were analyzed initially as raw time course data using a two-factor analysis of variance (ANOVA with Proc Mixed to account for any missing data: drug condition × time). The on-line physiological measures, collected on a minute-by-minute basis during the experimental sessions, were averaged across time to yield intervals (ranging from 15 min to 30 min), which corresponded to the subjective reporting intervals. In addition to the raw score analyses, peak scores (either minimum or maximum depending upon the direction of effects) for individuals were obtained for repeated measures collected during the experimental sessions; these were analyzed using 1-factor ANOVA. Tukey’s post-hoc tests for repeated measures were used to analyze further the time course and provide information on the time of onset and offset of effects across drugs and doses. Time-to-peak effect (minimum or maximum depending upon the outcome measure) was calculated first for individual subjects and dose conditions (placebo was excluded), and Proc Mixed ANOVA was used to compare the time-to-peak values among the drugs.
Peak effect data from measures where significant main effects of dose were observed were analyzed further using the Finney (Finney, 1964) method for parallel line bioassays. The analysis of parallel line bioassays is used to determine the relative potency of two compounds. This analysis was used to determine that the dose-response functions (excluding placebo) of oxycodone, hydrocodone and hydromorphone did not differ with respect to linearity and parallelism (p > .05) and showed slopes significantly different from 0 (p < .01) without differences in effect magnitude across drugs (p > .05). Six-point bioassays were used for all measures (three doses per drug), log doses were employed, and hydromorphone served as the reference compound in the estimates. Data from all measures meeting these criteria were used to calculate relative potency estimates and 95% confidence intervals for those estimates.
3. Results
3.1 Participants
Fifty volunteers signed consent to be screened for the study. Twelve were lost to follow-up, seven did not meet the drug use criteria, thirteen were excluded for medical reasons, and five decided not to join the study. Thirteen subjects were enrolled into the study; four had at least one experimental session but left before study completion, and nine volunteers completed. Of those completing, there were 8 males/1 female, all Caucasian who had an average age of 28.6 (±2.8) years. Current use (i.e., in the 30 days prior to screening) of other drugs was common; eight were cigarette smokers, seven reported marijuana use (average 15 of past 30 days), two reported sporadic benzodiazepine use and five reported current alcohol use (average 4 of past 30 days). They all reported current use of illicit opioids with an average history of 9.1 (±2.4) years of use and an average reported current use of 1–2 days weekly. The most common opioids mentioned for current illicit use were hydrocodone, oxycodone and methadone. Only one participant had used heroin; none reported intravenous use nor a past episode of physical dependence. All reported using opioids by the oral route, and three reported using intranasally.
3.2 Time Course and Peak Effect Outcomes
Physiological Outcomes
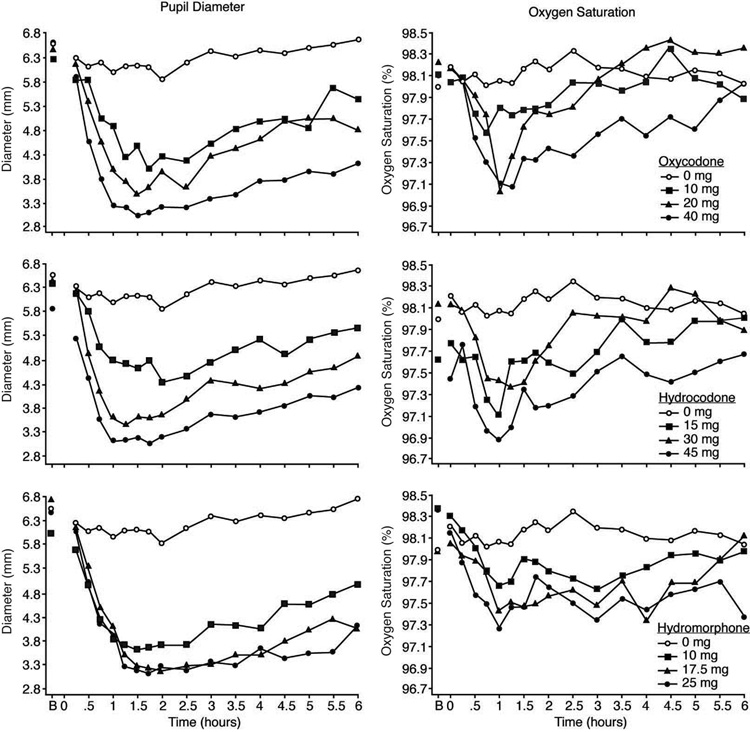
Data illustrating the time-action profile of pupil diameter for oxycodone, hydrocodone and hydromorphone are depicted in the left column of Figure 1 (see Table 1 for peak minimum score outcomes). The onset of effects, as assessed by the first time point to differ significantly from baseline, were generally comparable across the three drugs with most active doses producing evident effects within 30 – 45 minutes after administration. The effects of oxycodone hydrocodone and hydromorphone were dose-related; however, the two highest doses of hydromorphone produced nearly comparable constriction. The miotic effects persisted until the end of the 6-hr session for the highest doses of hydromorphone and oxycodone (Tukey tests; p<.05), but not for hydrocodone. There were no significant differences among the three drugs on the time-to-reach nadir on this measure or any of the other physiological measures.
Figure 1.
Data are shown for mean values (n=9) for pupil diameter (left column) and oxygen saturation (right column) after administration of oxycodone (upper panels), hydrocodone (middle panels) and hydromorphone (lower panels) as a function of time (ordinate) since drug administration during the 6-hr session. Time course analysis revealed significant effects of dose for all three drugs for pupil diameter (F[9,72] = 13.4; p<.001) and oxygen saturation (F[9,72] = 2.3; p = .024). B indicates the baseline time point.
Table 1.
Only measures for which a significant effect of drug condition are shown below. Measures were analyzed as peak maximum score unless minimum is indicated (*). Values are mean scores (n=9) for placebo (PCB), oxycodone (OXY), hydrocodone (HYC) and hydromorphone (HYM) in mg.
| PCB | OXY | OXY | OXY | HYC | HYC | HYC | HYM | HYM | HYM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Measure | p | 0 | 10 | 20 | 40 | 15 | 30 | 45 | 10 | 17.5 | 25 |
| Physiological | |||||||||||
| Respiratory Rate* | .002 | 13.3 | 12.0 | 10.7 | 10.4 | 13.1 | 11.6 | 11.1 | 11.3 | 11.8 | 10.4 |
| Pupil Diameter * | <.001 | 5.4 | 3.6 | 3.2 | 2.8 | 3.8 | 3.1 | 2.8 | 3.4 | 3.0 | 2.9 |
| Subject-Rated | |||||||||||
| Visual Analogs | |||||||||||
| High | .002 | 1.1 | 23.3 | 31.3 | 43.8 | 24.3 | 31.2 | 50.7 | 39.3 | 42.9 | 48.7 |
| Drug Effect | <.0001 | 1.0 | 22.9 | 34.3 | 50.2 | 30.1 | 32.2 | 53.2 | 46.1 | 44 | 49.8 |
| Good Effect | <.0001 | 1.0 | 27.8 | 37 | 59.4 | 34.3 | 38.7 | 57.3 | 47.2 | 41.1 | 54.8 |
| Like | <.0001 | 1.1 | 29.2 | 37.8 | 53 | 35.7 | 36.6 | 61.3 | 51.2 | 42.6 | 57.9 |
| Desire Opiates | .025 | 50.3 | 34.4 | 37.7 | 48.8 | 33.3 | 52.8 | 34.6 | 55.4 | 24.7 | 50.1 |
| ARCI | |||||||||||
| PCAG | .036 | 3.7 | 5.2 | 5.9 | 6.2 | 4.2 | 6.6 | 6.2 | 4.6 | 6.0 | 5.7 |
| AMPH | .024 | 2.1 | 2.4 | 3.7 | 4.6 | 4.0 | 3.8 | 4.4 | 3.9 | 4.2 | 4.4 |
| MBG | .041 | 3.0 | 3.6 | 4.9 | 7.6 | 6.4 | 5.2 | 7.1 | 5.7 | 5.6 | 7.7 |
| Adjectives | |||||||||||
| Sleepy | .009 | 0.8 | 1.2 | 1.1 | 1.4 | 1 | 2 | 1.4 | 1.3 | 2.3 | 1.7 |
| Stomach Turning | .024 | 0.1 | 0.3 | 0.3 | 0.4 | 0 | 0.4 | 0.7 | 0.7 | 1.4 | 0.6 |
| Skin Itchy | <.0001 | 0.2 | 0.7 | 1.6 | 1.9 | 0.8 | 1.6 | 2 | 1.1 | 1.9 | 2 |
| Coasting | .006 | 0.3 | 0.9 | 0.8 | 1.2 | 1 | 1.2 | 1.4 | 1.2 | 1.6 | 2 |
| Pleasant Sick | .012 | 0 | 0.2 | 0.4 | 0.7 | 0.2 | 0.1 | 0.6 | 0.4 | 1.4 | 0.8 |
| Drunken | .034 | 0 | 0.1 | 0.1 | 0.4 | 0.3 | 0.1 | 1 | 0.1 | 0.7 | 0.3 |
| Nodding | <.0001 | 0.2 | 0.6 | 1.2 | 1.8 | 0.7 | 1.6 | 1.7 | .8 | 2.3 | 1.4 |
| Sluggish | .01 | 0.3 | 0.7 | 0.6 | 1.2 | 0.4 | 1.2 | 1 | 0.4 | 1.3 | 1.3 |
| Dry Mouth | .005 | 0.7 | 0.6 | 0.9 | 0.8 | 0.4 | 0.9 | 1.3 | 0.8 | 1.3 | 1.3 |
| Carefree | .007 | 1 | 1.7 | 1.7 | 2.1 | 1.6 | 1.7 | 2.2 | 2 | 2.1 | 1.9 |
| Agonist Scale | <.0001 | 10.9 | 13.7 | 16.4 | 18.1 | 14.9 | 16 | 21.3 | 16 | 20 | 20.9 |
| Fraser Scale | <.0001 | 4.8 | 6.6 | 8.1 | 9 | 6.9 | 8.8 | 9 | 7.6 | 9.4 | 9.2 |
| Observer-Rated | |||||||||||
| Skin Itchy | <.0001 | 0 | 0.8 | 1.4 | 2.3 | 0.9 | 1.6 | 2.2 | 1.2 | 1.7 | 1.6 |
| Relaxed | .007 | 1.6 | 2.4 | 1.9 | 2.4 | 2.2 | 2.2 | 2.4 | 1.7 | 2 | 2.4 |
| Coasting | .001 | 0.1 | 1.1 | 1.3 | 2.4 | 1.3 | 1.6 | 2.3 | 1.3 | 1.6 | 2.1 |
| Talkative | <.0001 | 0.9 | 1.9 | 2.4 | 2.7 | 1.6 | 1.9 | 2.2 | 2.1 | 2.4 | 2.2 |
| Drunken | .002 | 0 | 0 | 0.3 | 0.9 | 0.1 | 0.1 | 0.6 | 0.2 | 0.4 | 0.8 |
| Nodding | .008 | 0.2 | 1 | 0.8 | 2.1 | 0.7 | 1.7 | 1.6 | 0.9 | 1.7 | 1.8 |
| Sluggish | .005 | 0.2 | 1.2 | 1.3 | 1.9 | 0.8 | 1.4 | 1.2 | 0.8 | 1 | 1.7 |
| Agonist Scale | <.0001 | 7.9 | 10.9 | 13 | 16.8 | 10.8 | 11.9 | 15.1 | 12 | 13.7 | 15.7 |
| Fraser Scale | <.0001 | 3.2 | 5.6 | 6.2 | 7.6 | 5.2 | 6.6 | 8.2 | 5.4 | 7.2 | 7.7 |
| Ocular Tasks | |||||||||||
| Maddox Wing | .005 | 4.2 | 6.3 | 7.7 | 10.2 | 7.2 | 7.5 | 10 | 8 | 9.2 | 9.7 |
| Flicker/Fusion* (#1) | <.0001 | 39.4 | 36.2 | 36.4 | 34.6 | 37.3 | 35.9 | 34.8 | 35.9 | 34 | 33.9 |
| Flicker/Fusion* (#2) | <.0001 | 38.9 | 35.8 | 35.4 | 34.2 | 36 | 36.4 | 33.9 | 35.9 | 34.2 | 33.8 |
Oxygen saturation was significantly reduced according to the time course analysis (Figure 1, right column; see legend for statistical outcome). For all three drugs, the peak reduction in oxygen saturation occurred within about 1 hr of drug administration. Analyses revealed no differences among active doses of the three drugs for the absolute peak reductions in oxygen saturation; however, higher doses produced more sustained reductions. Similarly, respiratory rate was significantly reduced after administration of all three drugs according to the time course (F[9,72] = 8.67; p<.001) and peak nadir analysis (Table 1); maximal reductions were only on the order of about 3 breaths/min in comparison to the placebo condition (data not shown). None of the three opioids significantly altered heart rate, systolic or diastolic blood pressure, and there were no adverse medical consequences as a result of drug administration.
Subject-Rated Measures
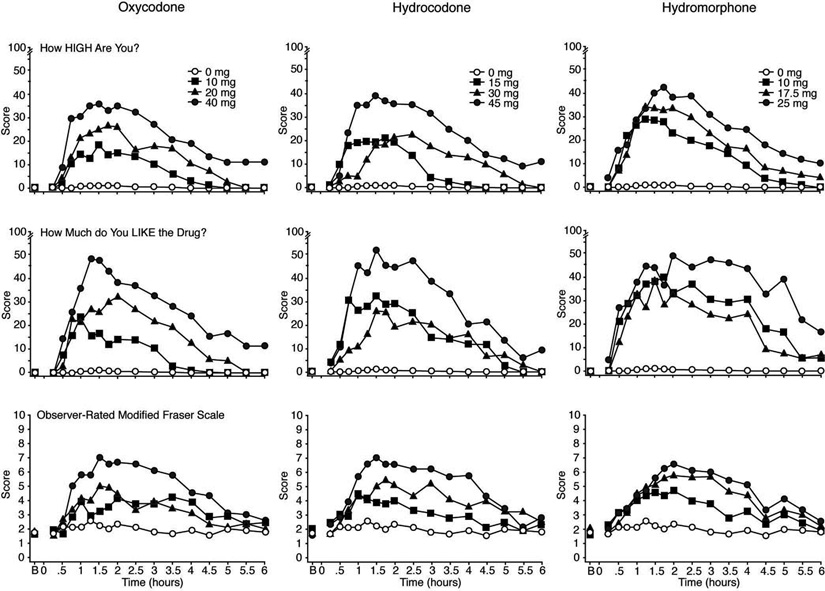
Time course data for the visual analog ratings of “How High Are You?” are shown in Figure 2 (top row). As shown, increased ratings of “high” were dose-dependent after dosing with oxycodone, hydrocodone and hydromorphone, and all active doses differed significantly from placebo (F[9,72] = 2.03; p = .048; Tukey tests p<.05). A similar profile of effects was observed for the visual analog measures of “liking” (also shown in Figure 2, middle row), “drug effect,” and “good effects,” although ratings were not uniformly dose-dependent for the two lower doses hydromorphone as shown (see also peak score data in Table 1). While the time course analysis did not reveal significant dose effects for ratings of “desire for opiates,” the peak score analysis did (Table 1). There were no significant increases on ratings of “bad effects” or “sick.” The analysis of the time-to-reach peak revealed few statistically significant differences among the three drugs. For example, comparisons for the main effect of drug failed to yield statistically significant differences, but there was a significant drug × dose interaction for ratings of “liking” (F[4, 32]= 2.9; p=.035).
Figure 2.
Data are shown for mean values (n=9) for the subject-rated visual analog questionnaires “How High Are You?” (top row), “How Much Do You Like the Drug?” (middle row) and the observer-rated Modified Fraser Scale of opioid agonist signs (bottom row; maximum score 10) after administration of oxycodone (left column), hydrocodone (middle column) and hydromorphone (right column) as a function of time (ordinate) since drug administration during the 6-hr session. Time course analyses revealed significant effects of dose for all three drugs for ratings of “high” (F [9, 72]=2.03; p = .048), “like the drug” (F [9, 72]=3.24; p=.002) and the Fraser scale (F [9,72] = 10.7; p<.0001). B indicates the baseline time point.
Results for the subject-rated adjective scales revealed significant (p <.05) endorsement of typical mu agonist effects (see Table 1), including sleepy, stomach turning, skin itchy, coasting, pleasant sick, drunken, nodding (see Figure 3), sluggish, dry mouth and carefree. The increased subject ratings for the constellation of mu opiate agonist-like effects are reflected in the findings for the composite Fraser and Agonist Scales (p<.001; see Table 1 and Figure 3; middle panels). There were significant increases on scores for the PCAG, AMPH and MBG scales of the ARCI but not for the LSD scale. The responses to the pharmacological class questionnaire generally revealed increases in percent of subjects identifying the test drug as an opioid as a function of dose (oxycodone 10, 20 & 40 mg yielding 56%, 67% & 100%, hydrocodone 15, 30 & 45 mg yielding 89%, 89%, & 100%, and hydromorphone 10, 17.5 & 25 mg yielding 78%, 89% and 100%, respectively).
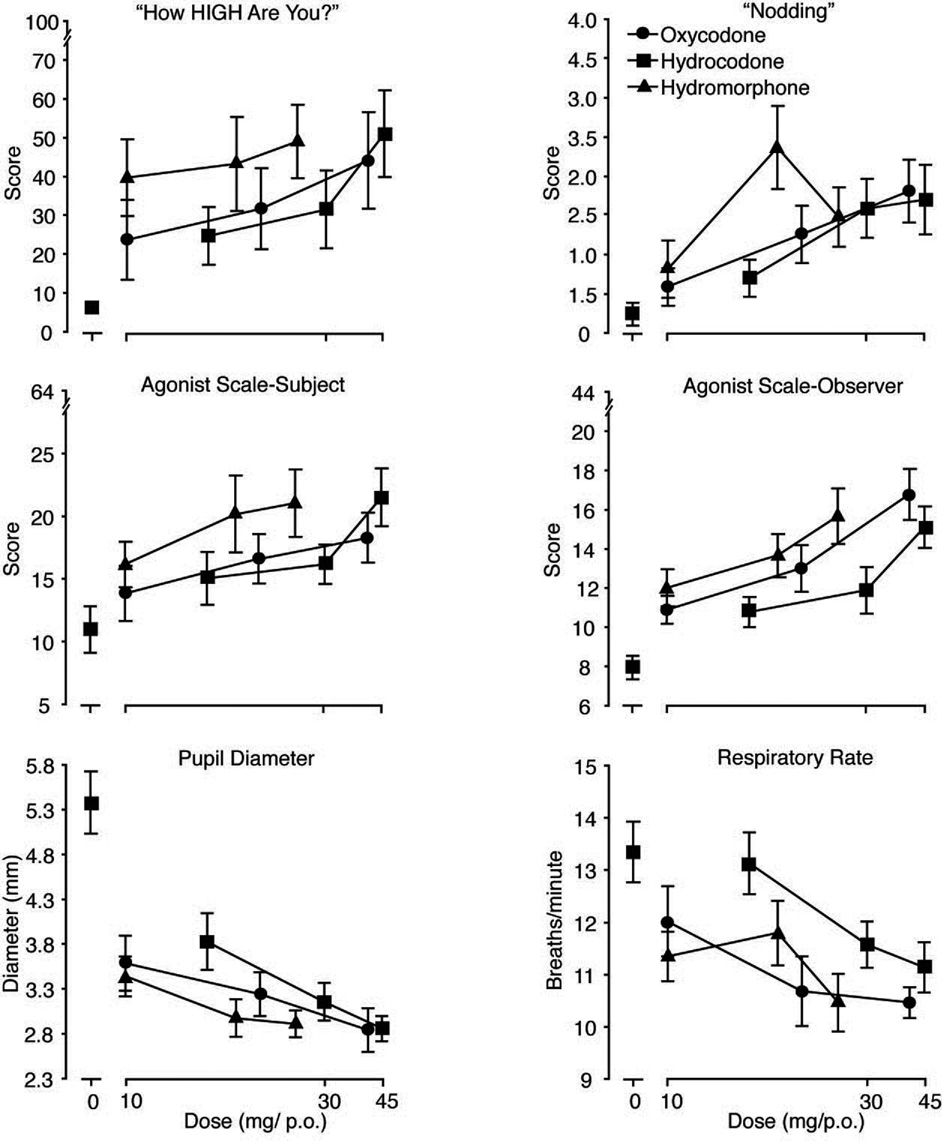
Figure 3.
The six panels illustrate mean (n=9; ± 1 SEM) values for peak maximum scores (from left to right and top to bottom) on 1) the subject-rated visual analog scale “How High Are You?,” 2) subject ratings of nodding, 3) the subject-rated Agonist Scale, 4) the observer-rated Agonist Scale and for the peak minimum values for 5) pupil diameter (mm) and 6) respiratory rate (breaths/min).
Observer-Rated Measures
Scores on a number of adjective rating scales and the two composite scale scores (Agonist and Fraser) were significantly elevated by the active drug conditions compared to placebo (Table 1). Illustrative time course data are shown in Figure 2 (bottom row) for the Fraser Scale (see figure legend for statistical outcomes). As shown, observable signs of opioid intoxication increased in a generally dose-dependent fashion for oxycodone, hydrocodone and hydromorphone. Specific signs of opioid intoxication that were significantly elevated include skin itchy (i.e., scratching), relaxed, coasting, talkative, drunken, nodding, sluggish, friendly and energetic (p <.05). There were no differences among the three drugs with regard to the calculated time-to-peak for any observer-rated measure and occurred generally within 1.5 to 2 hr post-dosing.
Ocular and Performance Measures
Time course data revealed that there was a significant effect of dose (data not shown graphically) on the Maddox-Wing test (F[9,72] = 2.8; p = .008), whereby scores increased after active doses compared to placebo, indicative of significant exophoria, with the highest dose of each drug producing the greatest increase (see peak maximum scores in Table 1).
Analysis of the peak minimum scores for all outcome measures of the flicker-fusion task, including individual ascending and descending trials and the critical flicker-fusion threshold for both trials, revealed statistically significant differences (p < .05) as a function of dose (see Table 1 for peak minimum scores of threshold values) indicative of poorer detection after active drug administration compared to placebo. There were no significant impairments on the gross motor balance task. Observed scores were consistently 30 seconds, the maximum possible time for each trial, under all test conditions.
3.3 Relative Potency Assays
Relative potency estimates were made with hydromorphone as the reference compound (and excluded placebo). The results of these analyses are shown in Table 2; only measures for which valid assays were obtained are listed. Figure 3 illustrates six measures for which valid assays were obtained across the various domains of collected data: subject-rated measures (“high,” “nodding,” composite Agonist-Scale), observer-rated (composite Agonist Scale), and physiological (pupil diameter and respiratory rate). Relative potency estimates for hydrocodone ranged from 0.69 to 0.87 and from 0.70 to 0.99 for oxycodone. Overall, relative potency estimates were higher for observer-rated measures compared to subject-rated measures. When averaged across all estimates, the mean relative potency of hydrocodone:hydromorphone (based upon a total of 17 dependent measures) was 0.772:1.0 mg. The overall mean relative potency for oxycodone to hydromorphone was slightly higher at 0.85:1.0 mg (based upon a total of 19 dependent measures).
Table 2.
Listed below are the relative potency estimates and confidence intervals based upon Finney’s (1964) bioassay results using the logarithmic doses. All outcome measures, for which significant effects of dose were found in the peak maximum score analyses unless minimum is denoted (*), were assayed. Only those yielding a valid bioassay are reported here based upon the following criteria: no significant differences in preparation, linearity or parallelism and a significant regression. Hydromorphone is the reference drug. Relative potency is expressed as mg of hydromorphone necessary to produce the same effect of 1 mg of the test agent.
| Hydromorphone Relative to Hydrocodone |
Confidence Intervals |
Hydromorphone Relative to Oxycodone |
Confidence Intervals |
|
|---|---|---|---|---|
| Subject-Rated | ||||
| Visual Analog | ||||
| High | .71 | 0.23 – 0.92 | .70 | 0.16 – 0.93 |
| Drug Effect | - | - | .72 | 0.07 – 0.97 |
| Good Effects | - | - | .82 | 0.38 – 1.12 |
| Adjective | ||||
| Skin Itchy | .79 | 0.61 – 0.94 | .84 | 0.64 – 1.01 |
| Nodding | .78 | 0.43 – 1.0 | .83 | 0.53 – 1.05 |
| Dry Mouth | .75 | 0.57 – 0.88 | - | - |
| Sluggish | .79 | 0.27 – 1.2 | - | - |
| Agonist Scale | .77 | 0.62 – 0.88 | .73 | 0.55 – 0.86 |
| Fraser Scale | .78 | 0.65 – 0.89 | .80 | 0.61 – 0.95 |
| ARCI Scales | ||||
| AMPH | - | - | .79 | 0.45 – 1.0 |
| MBG | - | - | .82 | 0.51 – 1.05 |
| Observer-Rated | ||||
| Skin Itchy (scratching) | .87 | 0.73 – 1.1 | .96 | 0.81 – 1.14 |
| Nodding | .80 | 0.55 – 1.0 | .89 | 0.31 – 1.46 |
| Coasting | .87 | 0.65 – 1.3 | .93 | 0.67 – 1.25 |
| Drunken | .72 | 0.25 – 0.92 | .77 | 0.77 – 1.04 |
| Talkative | .62 | .99 | 0.75 – 1.49 | |
| Agonist Scale | .76 | 0.57 – 0.90 | .92 | 0.75 – 1.13 |
| Fraser Scale | .83 | 0.73 – 0.95 | .88 | 0.71 – 1.05 |
| Physiological | ||||
| Pupil Diameter* | .78 | 0.65 – 0.90 | .88 | 0.75 – 1.0 |
| Respiratory Rate* | .71 | 0.37 – 0.87 | .99 | 0.58 – 2.7 |
| Ocular | ||||
| Maddox Wing | - | - | .83 | 0.65 – 0.98 |
| Flicker-Fusion* (#1) | .69 | 0.43 – 0.83 | - | |
| Flicker-Fusion* (#2) | .73 | 0.37 – 0.93 | - | |
| Mean Relative Potency | .764 | .847 | ||
4. Discussion
This study examined the direct pharmacodynamic effects and relative potency of oxycodone and hydrocodone in comparison to hydromorphone over a range of oral doses in individuals with recreational prescription opioid abuse who were without physical dependence. All three opioid drugs were well tolerated and produced the prototypic constellation of opioid agonist effects as assessed by the participants, the observers and physiological outcomes. This profile included dose-related ratings of euphoric-like subjective effects, sedation, miosis, respiratory depression, and exophoria. The relative potency estimates suggest that oral oxycodone and oral hydrocodone are nearly equipotent on most measures, while oral hydromorphone is only modestly more potent than oxycodone and hydrocodone.
While the acute effects of oxycodone, hydrocodone and hydromorphone were very robust for many measures (e.g., mioisis and subjective responses), there were limited respiratory depressant effects even at these supratherapeutic doses. The highest doses tested in the present study (40 mg oxycodone, 45 mg hydrocodone, 25 mg hydromorphone) were approximately 8-, 9- and 12-fold greater than the lowest recommended starting analgesic doses (PDR, 2007) for oxycodone (5 mg), hydrocodone (5–10 mg) and hydromorphone (2 mg), respectively. The profile of subjective responses was consistent with that typically reported by opioid abusing individuals after administration of mu opioid agonists, including increased ratings of measures related to euphoria (e.g., liking for the drug, good effects, high, MBG scores) and sedation (PCAG scores, sleepy, nodding); these reports were also concordant with the observers’ ratings. Few studies have examined the acute effects of these common opioids when administered to non-dependent, opioid-abusing individuals; however, the observed subjective effects profile is consistent with available earlier reports from post-addicts and/or active opioid abusers for parenteral or oral oxycodone (original data unpublished but described in Epstein et al., 2006), hydrocodone (Fraser and Isbell, 1950; Jasinski and Martin, 1967) and hydromorphone ( e.g., Jasinski et al., 1977; Preston et al., 1989; Preston et al., 2004; Walsh et al., 1996). Recent studies have examined the abuse liability profile of these opioids when given to individuals with limited recreational drug abuse histories. Acute oral doses of oxycodone (Zacny and Gutierrez, 2003; Zacny and Lichtor, 2008) and two different hydrocodone products (Zacny, 2003; Zacny et al., 2005) given to individuals without opioid abuse produced similar physiological effects and positive mood effects, but also produced some unpleasant or aversive subjective effects (e.g., increased LSD scores, unpleasant bodily sensations). The absence of aversive effects here (i.e., no increase in LSD scores) may reflect differences between the two study populations in regard to their respective preference for using opioids and/or due to differential tolerance.
Two ocular tests, the Maddox-Wing [a test of divergence] and the flicker fusion [a test of visual discrimination threshold], were included as potentially sensitive indices to assess opioid-induced motor impairment, as performance on gross motor tasks is not reliably impaired by opioids (e.g., Preston et al., 1989). Both measures proved to be sensitive yielding dose-dependent impairment with all three opioids. Findings of increased exophoria (Saarialho-Kere et al., 1989; Zacny and Gutierrez, 2003) and impaired critical flicker discrimination (Saarialho-Kere et al., 1989) are consistent with earlier findings for oxycodone in normal, healthy volunteers. In the study by Saarialho-Kere and colleagues, volunteers received oxycodone (0.13 mg/kg or 9.1/70 kg, i.m.), estimated to be equivalent to ≤ 5 mg of oral oxycodone [assuming 40 – 60% oral bioavailability (Leow et al., 1992; Poyhia et al., 1993)]. While flicker fusion was reported to be the most sensitive index of oxycodone effects in that study, impairments in standing balance were also observed, which were absent in the present study. Exophoria (as measured by the Maddox-Wing) has also been reported to occur after administration of hydromorphone (Walker et al., 2001) and hydrocodone (Zacny, 2003; Zacny et al., 2005); however, this may be the first report of flicker fusion detection disruption with these two agents.
There was no evidence to suggest that the profile of subjective effects produced by these three opioid agonists, particularly as they relate to abuse liability, differed in any substantive way. An important factor believed to contribute to differential abuse potential between drugs and between routes of administration is the rate of drug onset (e.g., Abreu et al., 2001; de Wit et al., 1992; de Wit et al., 1993); whereby, more rapid onset of positive subjective effects is associated with greater abuse liability. Examination of the time-to-reach peak effect following oral administration of these three drugs revealed very few statistically significant differences, suggesting no substantive difference in onset. The observed Tmax values in the present study are consistent with those found in earlier studies of oral oxycodone (Zacny and Gutierrez, 2003), hydrocodone (Zacny, 2003) and hydromorphone (Preston et al., 2004).
The primary findings of interest from the present study arise from the relative potency assays comparing hydrocodone and oxycodone to hydromorphone. There were numerous dependent measures for which the requisite criteria were met for a valid relative potency assay; thus, these estimates stem from numerous outcomes. The data suggest that the relative potency of these three commonly abused opioids do not differ greatly from one another, which is somewhat surprising based upon previous studies on their relative potencies as analgesics, particularly for hydromorphone. However, regarding the comparison of relative potency estimates across studies, there are a number of factors warranting consideration. First, relative potency estimates arising from analgesia studies typically employ morphine as the comparator, as it is the standard drug of choice whenever possible (Pereira et al., 2001); thus comparisons may need to be made to (or converted to) morphine-equivalents. Second, while some potency estimates are derived from single dose crossover studies as in the present design, many studies have used repeated (chronic) dosing designs, and these may yield different findings. For example, some data suggest that initial estimates of differences between drugs may decline with repeated dosing (Dunbar et al., 1996), while other data suggest that the order in which drugs are tested may influence potency estimates in crossover designs (Lawlor et al., 1997). Third, potency estimates with parenteral administration assume 100% delivery and, thus, do not account for potential between-drug differences in oral bioavailability, which is important in cases where differences may exist (for example, oxycodone is recognized as being one of the opioids with highest oral bioavailability). Fourth, potency estimates from analgesia studies use pain outcome measures, while the outcome measures in this study were related to abuse liability and non-analgesic physiological outcomes. Thus, relative potencies may differ depending upon the outcome of interest (although here estimates were comparable for physiologic versus subjective/observer measures).
Using a standard equianalgesic estimates table for parenteral opioid equivalents of 10 mg morphine (Jaffe and Martin, 1991), one would predict that hydrocodone would be ½ - 1 times, oxycodone would be 1 – 2 times (Poyhia et al., 1993), and hydromorphone would be 6 –7 times as potent as morphine. These parenteral estimates are consistent with the present findings for oral oxycodone and hydrocodone producing fairly comparable effects. In regard to comparative potency studies of abuse liability, rather than analgesia, an early study comparing identical doses of hydrocodone and morphine (15 and 30 mg/70 kg, i.m.) in non-dependent post-addicts concluded that hydrocodone was approximately equipotent to morphine at producing opiate-like symptoms (Jasinski and Martin, 1967), consistent with the analgesic potency estimates. With oxycodone, a recent study examined the relative potency of oral oxycodone compared to morphine for abuse liability outcomes in non-drug abusers and concluded that oxycodone was three times as potent as morphine (Zacny and Lichtor, 2008), which may, in part, be accounted for by the superior oral bioavailability of oxycodone. Comer and colleagues (2007) examined intravenous oxycodone in heroin dependent individuals who were maintained on morphine during participation and concluded that oxycodone was equipotent to morphine when given intravenously (Comer et al., 2007). Thus, through extrapolation across routes, the present findings of oxycodone and hydrocodone having approximately equipotent abuse liability when given orally are reasonably consistent with previous studies of these agents tested by other routes.
Based upon published analgesic tables, parenteral hydromorphone may have been predicted to be up to 7-times as potent than either hydrocodone or oxycodone (Jaffe and Martin, 1991); however, the present findings suggest that it was far less potent in this abuse liability assessment (less than two-fold greater than either oxycodone or hydrocodone). Available estimates for equianalgesic oral dosing suggest that hydromorphone at 7.5 mg would be equivalent to oxycodone at 20 mg, suggesting approximately a 3-fold difference in potency by the oral route (American Pain Society, 1999), while review of analgesia studies involving repeated dosing would predict an approximate 4-fold difference (see Pereira et al., 2001). The finding that hydromorphone was less potent than expected was also supported by the initial pilot testing, in which the lowest doses (2 and 5 mg) were not endorsed by subjects as active and failed to produce miosis. Perhaps the only study to compare hydromorphone to morphine on abuse liability outcomes was done at the Lexington Narcotics Prison Farm; however, that study was interrupted prior to completion due to a change in regulations that prohibited the use of Federal prisoners as research subjects (Jasinski et al., 1977). Using a cross-over design with acute oral dosing, it was reported (with an incomplete data set as some subjects withdrew from the study, and the study was halted) that relative potency estimates ranged from 6.1 – 13 (i.e., mg of morphine needed to produce the effects equivalent to 1 mg of hydromorphone) across a range of measures (pupil diameter, subject and observer ratings of “drug liking”) with an average estimate of 9:1 morphine:hydromorphone (although the inverse relationship was found when the drugs were given subcutaneously). Interestingly, in that study, the range of oral hydromorphone doses was comparable to that used in the present study, but the acute morphine doses were quite substantial (130 and 260 mg) for non-dependent participants. As that study did not include the other comparator agents tested in the present study, it is impossible to reconcile these findings.
In summary, the present data reveal that oral hydrocodone, oxycodone and hydromorphone all produced a profile of pharmacodynamic effects typical of mu opioid agonists, including robust increases on subjective and observer-rated measures reflecting euphoric-like responses and other opioid symptoms, miosis and impaired visual motor function. The subjective effects profile related to abuse liability did not differ substantively among the three drugs when evaluated in a population of prescription opioid abusers. Supratherapeutic doses of all three agents were well tolerated in this experienced group of participants, and only modest changes in respiratory function were observed. The relative potency assays revealed that oral hydrocodone and oxycodone were roughly equipotent, while hydromorphone, surprisingly, was only modestly more potent (less than two-fold) than either hydrocodone or oxycodone; these estimates are substantially lower than would have been predicted by relative potency estimates based upon analgesic outcomes. These data suggest that the abuse liability of these three commonly used opioids does not differ substantially from one another with respect to profile of action and suggest that there may be a dissociation between relative potency estimates for measures of abuse liability and analgesia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sharon L. Walsh, Department of Behavioral Sciences, Center on Drug and Alcohol Research, University of Kentucky, 515 Oldham Court Lexington, KY 40502
Paul A. Nuzzo, Department of Behavioral Sciences, Center on Drug and Alcohol Research, University of Kentucky, 515 Oldham Court Lexington, KY 40502 USA
Michelle R. Lofwall, Department of Psychiatry, University of Kentucky, 3470 Blazer Parkway Lexington, Kentucky 40509 USA
Joseph R. Holtman, Jr., Department of Anesthesiology, University of Kentucky, Suite 290 101 Prosperous Place Lexington, Kentucky 40509 USA
REFERENCES
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacol. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. Fourth Edition. Glenview, IL: American Pain Society; 1999. [Google Scholar]
- Angst MS, Drover DR, Lotsch J, Ramaswamy B, Naidu S, Wada R, Stanski DR. Pharmacodynamics of orally administered sustained-release hydromorphone in humans. Anesthesiology. 2001;94:63–73. doi: 10.1097/00000542-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer; II: Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. J Pharmacol Exp Ther. 1978;207:101–108. [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosberg SK, Kowalczyk WJ. Abuse liabliity of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301479. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology. 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- de Wit H, Dudish S, Ambre J. Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology. 1993;112:324–330. doi: 10.1007/BF02244928. [DOI] [PubMed] [Google Scholar]
- Dunbar PJ, Chapman CR, Buckley FP, Gavrin JR. Clinical analgesic equivalence for morphine and hydromorphone with prolonged PCA. Pain. 1996;68:265–270. doi: 10.1016/s0304-3959(96)03213-7. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychiatry. 2006;73:90–99. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Statistical Method in Biological Assay. Second edition. New York: Hafner; 1964. [Google Scholar]
- Fraser HF, Isbell H. Addiction liabilities of morphinan, 6-methyldihydromorphine and dihydrocodeinone. J Pharmacol Exper Ther. 1950;100:128–135. [PubMed] [Google Scholar]
- Jaffe JH, Martin WR. Opioid analgesics and antagonists. In: Gilman AG, Rall RW, Nies AS, Taylor P, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. Eighth Edition. Volume 1. Elmsford, New York: Maxwell Macmillan International Editions; 1991. pp. 485–521. [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick J, Gorodetzky C, Cone E, Kay D. Progress report from the clinical pharmacology section of the NIDA Addiction Research Center. 39th Annual Meeting, The Committee on Problems of Drug Dependence; Washington, D.C. National Research Council, National Academy of Sciences; 1977. pp. 133–168. [Google Scholar]
- Jasinski DR, Martin WR. Assessment of the dependence-producing properties of dihydrocodeinone and codoxime. Clin Pharmacol Ther. 1967;8:266–270. doi: 10.1002/cpt196782266. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Bethesda, MD: National Institute on Drug Abuse; Monitoring the Future: National Results on Adolescent Drug Use, Overview of Key Findings 2006. 2007
- Kaplan HL, Busto UE, Baylon J, Cheung SW, Otton SV, Somer G, Sellers EM. Inhibition of cytochrome P450 2D6 metabolism of hydrocodone to hydromorphone does not importantly affect abuse liability. J Pharmacol Exp Ther. 1997;281:103–108. [PubMed] [Google Scholar]
- Lawlor P, Turner K, Hanson J, Bruerra E. Dose ratio between morphine and hydromorphone in patients with cancer pain: a retrospective study. Pain. 1997;72:79–85. doi: 10.1016/s0304-3959(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Leow KP, Smith MT, Watt JA, Williams BE, Cramond T. Comparative oxycodone pharmacokinetics in humans after intravenous, oral, and rectal administration. Ther Drug Monit. 1992;14:479–484. doi: 10.1097/00007691-199212000-00008. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan BS, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mouzos J, Hind N, Smith L, Adams K. Research and Public Policy Series, 75. Canberra, Australia: Australian Institute of Criminology; 2007. Drug use monitoring in Australia: 2006 annual report on drug use among police detainees. [Google Scholar]
- Office of National Drug Control Policy. What America's Users Spend on Illegal Drugs, 1988–2000 (December, 2001) 2001 http://www.whitehousedrugpolicy.gov/publications/drugfact/american%5Fusers%5Fspend2002/execsummary.pdf.
- Otton SV, Schadel M, Cheung SW, Kaplan HL, Busto UE, Sellers EM. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clin Pharmacol Ther. 1993;54:463–472. doi: 10.1038/clpt.1993.177. [DOI] [PubMed] [Google Scholar]
- PATS- Partnership Attitude Tracking Study. Partnership for a Drug-Free America (Ed.) New York, New York: 2005. [Google Scholar]
- PDR. 2008 Physician's Desk Reference. 62nd Edition. Montvale, NJ: Thomson Healthcare, Inc.; 2007. [Google Scholar]
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Poyhia R, Vainio A, Kalso E. A review of oxycodone's clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manage. 1993;8:63–67. doi: 10.1016/0885-3924(93)90101-z. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human postaddicts: Agonist-antagonist opioids. J Pharmacol Exper Ther. 1989;250:184–196. [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Schroeder JR, Abreu ME, Epstein DH, Pickworth WB. Cyclazocine: comparison to hydromorphone and interaction with cocaine. Behav Pharmacol. 2004;15:91–102. doi: 10.1097/00008877-200403000-00001. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Drug Abuse Warning Network, 2005: National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; DAWN Series D-29. 2007a
- SAMHSA. Rockville, MD: Substance Abuse and Mental Health Services Administration; Results from the 2006 National Survey on Drug Use and Health: National Findings. 2007b
- Walker DJ, Zacny JP, Galva KE, Lichtor JL. Subjective, psychomotor, and physiological effects of cumulative doses of mixed-action opioids in healthy volunteers. Psychopharmacology. 2001;155:362–371. doi: 10.1007/s002130100723. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exper Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exper Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Zacny JP. Characterizing the subjective, psychomotor, and physiological effects of a hydrocodone combination product (Hydodan) in non-drug abusing volunteers. Psychopharmacology. 2003;165:146–156. doi: 10.1007/s00213-002-1245-5. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Bigelow GE, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence Task Force on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug -abusing volunteers. Psychopharmacology. 2003;170:242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, Bolbolan SA. Profiling the subjective, psychomotor, and physiological effects of a hydrocodone/acetaminophen product in recreational drug users. Drug Alcohol Depend. 2005;78:243–252. doi: 10.1016/j.drugalcdep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology. 2008;196:105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]