Point of care (POC) sensors for the determination of immune status are an important part of the preparation for possible pandemics such as that created by avian influenza.[1,2] NMR based magnetic relaxation switches (MRSw's) are attractive for this application because they are indifferent to light and involve no immobilization of materials on vessel walls.[3] MR relaxometers are practical as POC sensors since their requirements for magnetic field strength, volume and homogeneity are minimal.[4-6]
To date, MRSw's have used magnetic nanoparticles (NPs) that react with molecular targets to aggregate and decrease T2. Although MRSw's detect highly multivalent viruses or bacteria with high sensitivity,[7,8] their sensitivity for proteins is far lower.[9,10] Our goal was to improve the sensitivity of MRSw sensors for divalent antibodies, so the immune status of birds or humans might eventually be determined using a POC MR relaxometer. A monoclonal antibody recognizing the Tag peptide from a hemagglutinin of a human influenza virus was used as a target to assess strategies for improving MRSw sensitivity.
An obvious strategy for improving MRSw sensor sensitivity was to employ the equivalence principle of antibody-antigen reactions involved in precipitin formation,[11-13] reducing the concentration of Tag peptide-magnetic particles (a synthetic multivalent antigen), so that lower concentrations of anti-Tag antibodies might achieve aggregation. The Tag peptide from the influenza hemagglutinin was therefore conjugated to nanoparticles (NPs) and far larger micron-sized magnetic particles (MPs), to yield Tag-NPs and Tag-MPs. As indicated in Table 1, a Tag-MP contained 350,000 times more iron than a Tag-NP and had a correspondingly higher magnetic moment per particle. With a typical initial T2 for MRSw assays (100 msec), the concentration of particles decreased from 2.8 × 10−9 M with Tag-NPs to 5.1 × 10−15 M using Tag-MPs.
Table 1.
Properties of magnetic particles (MPs) and nanoparticles (NPs).
| Particle | MP | NP |
|---|---|---|
| Size (nm) | 1000 | 30 |
| Settling | <5% | None |
| Peptides per particle | 3.0 × 105 | 20-30 |
| R2 (sec−1/mM Fe) | 43 | 50 |
| M (emu/g Fe) | 105 | 86.6 |
| Fe atoms per particle | 2.8 × 109 | 8000[a] |
| Particle Conc. @ T2 = 100 msec, (M) |
5.1 × 10−15 | 2.8 × 10−9 |
From[22].
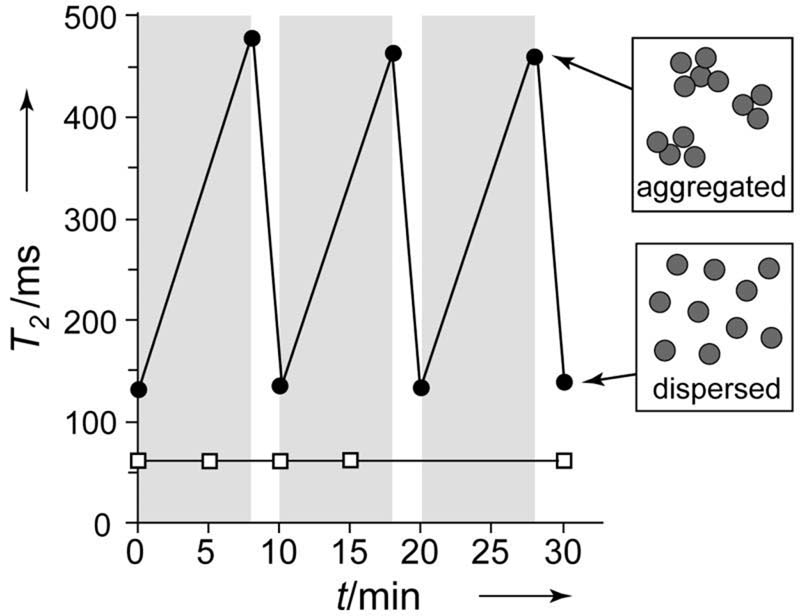
Attempts to use Tag-MPs for MRSw assays indicated Tag-MPs differed from earlier NPs in two key respects. First, as shown in Figure 1, Tag-MP's underwent a reversible increase in T2 in the 0.47 T relaxometer magnet. Micrographs indicated the T2 increase was associated with MP aggregation resulting from magnetic attractions between Tag-MP's(see Figure 2 of reference [14]), with the randomizing effects of thermal energy causing dispersion when the field was removed. Second, Tag-MP aggregation resulted in a T2 increase while Tag-NP aggregation resulted in a T2 decrease,[3,15] observations consistent with the outer sphere diffusion theory used to describe the effects of magnetic particles on T2.[16-19] This theory employs two parameters, τd, the diffusion time for water, and Δω, the difference in angular frequency between the magnetic field experienced by a proton at the particle (or aggregate) surface versus that in the bulk. Outer sphere diffusion theory predicts T2 will decrease as Tag-NPs aggregate, since the motional averaging condition is fulfilled with both dispersed and aggregated materials (Δω*τd <1). With Tag-MP aggregation, the resulting magnetic field inhomogeneities become so few and infrequent water molecules must traverse long distances to encounter them. Here the effects on T2 become diffusion limited (Δω*τd >1). The effects of NP and MP aggregation on T2 have been discussed.[14]
Figure 1. Magnetic particles respond to a homogeneous magnetic field.
T2 of a MP solution (●), but not a NP solution (□), increases when in a 0.47 T field (gray). White, no field.
Figure 2. Methods of increasing MRSw sensitivity.
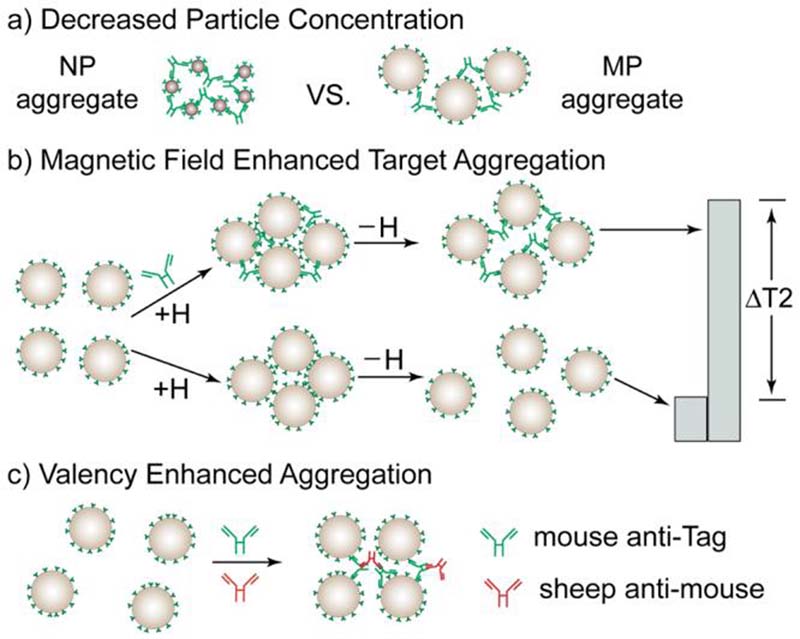
a) Decrease in particle concentration. Anti-Tag forms bridges between larger MPs. b) Magnetic field enhanced target aggregation. A magnetic field (+H) produces magnetic attractions between MPs that result in aggregation. In the presence of anti-Tag (top) the aggregate is maintained when the field is removed (−H) and a high T2 results. c) Valency enhanced aggregation. The addition of an anti-Fc antibody permits anti-Tag to bind more than two MPs simultaneously.
Three techniques were then explored separately and in combination to increase the sensitivity of MRSw assays, see Figure 2. First, a decrease in particle concentration was achieved by replacing the magnetic nanoparticle (NP) with a micron-sized particle (MP), see Figure 2a. The second technique, magnetic field enhanced, molecular target aggregation, exploited the reversible magnetic field induced MP aggregation in the absence of a target ligand shown in Figure 1. We hypothesized that the magnetic field aggregation of Tag-MPs might enhance anti-Tag mediated aggregation (Figure 2b). Therefore solutions of Tag-MPs were exposed to anti-Tag in the relaxometer magnet, resulting in both magnetic field induced and anti-Tag antibody mediated Tag-MP aggregation. Samples were then removed from the magnetic field, to allow Tag-MPs to disaggregate which occurred only in the absence of particle crosslinking by anti-Tag. T2 was determined in less than 30 seconds by placing samples in the relaxometer so that magnetic field induced aggregation during T2 measurement was minimal. Finally, we employed valency enhancement, where the valency of the monoclonal anti-Tag was increased above the normal two antigen-combining sites per IgG by adding a sheep F(ab′)2 antibody to the Fc fragment of the mouse anti-Tag monoclonal antibody (Figure 2c).
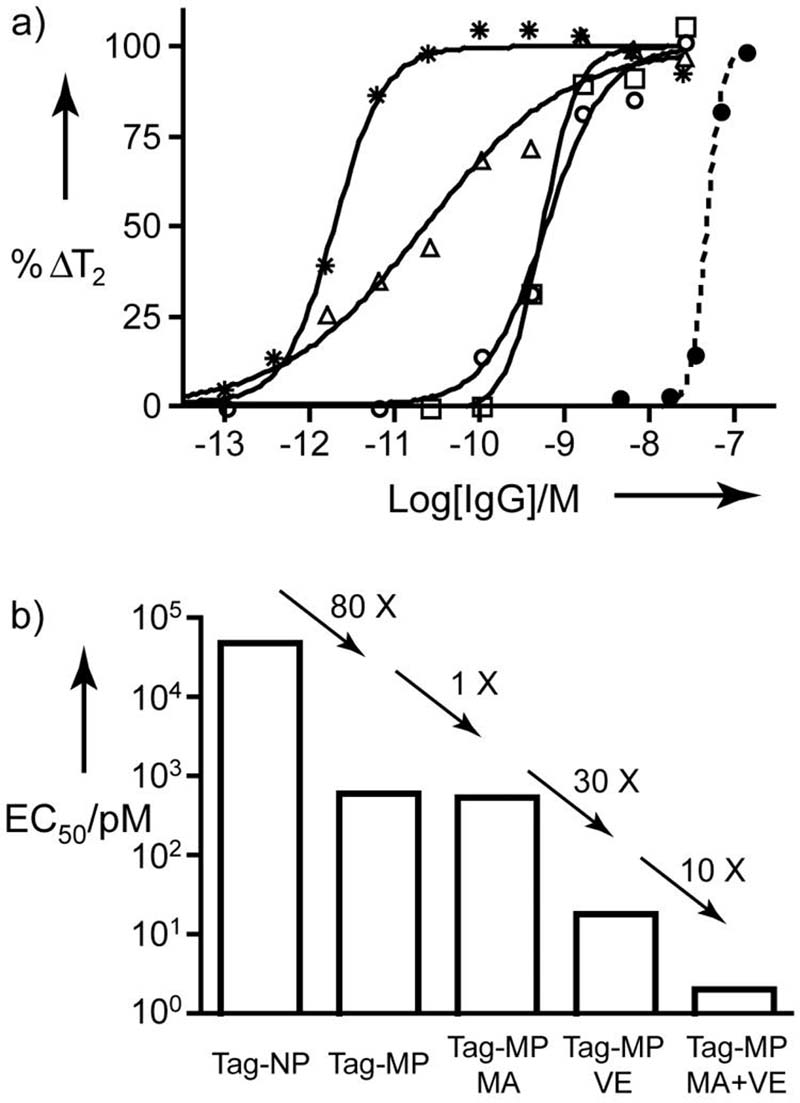
We first measured T2 as a function of anti-Tag concentration using Tag-NPs and Tag-MPs (decreasing particle concentration but without magnetic field or valency enhancement) as shown in Figure 3a. Data were fit to a 4-parameter equation, to obtain the EC50 (midpoint), the Hill coefficient (curve slope), and computer generated maximum and minimum values of T2. Table 2 provides EC50's, Hill coefficients, and ΔT2's for the curves shown in Figure 3a. ΔT2, the T2 for the fully dispersed state minus the T2 for the fully aggregated state, is positive for anti-Tag induced Tag-NP aggregation but negative for anti-Tag induced Tag-MP aggregation. %ΔT2 is the difference in T2 in the presence and absence of anti-Tag divided by ΔT2 and expressed in percent.
Figure 3. Techniques for increasing the response to anti-Tag antibody.
a) The initial reference assay with Tag-NPs is shown with a dotted line and (●). To obtain a decrease in particle concentration, Tag-MPs (○) were used. The Tag-MP was then used with magnetic field aggregation (□), with valency enhancement (△), and with both magnetic aggregation and valency enhancement (✩). b) EC50's obtained by decreasing particle concentration, magnetic field aggregation (MA), and valency enhancement (VE). Additional curve parameters are provided in Table 2.
Table 2.
Sensitivity of NP and MP Based MRSw Sensors.
| Magnetic Aggre- gation |
Valency Enhance- ment |
EC50 (nM) |
Hill slope |
ΔT2 (msec) |
PSC (nM) |
|
|---|---|---|---|---|---|---|
| Tag- NP |
N | N | 49 | 4.7 | +110 | 26 |
| Tag- MP |
N | N | 0.60 | 1.4 | −20 | 0.41 |
| Y | N | 0.55 | 2.1 | −140 | 0.060 | |
| N | Y | 0.017 | 0.55 | −64 | 0.00020 | |
| Y | Y | 0.0020 | 1.5 | −250 | 0.00014 |
Using Tag-NPs, increasing concentrations of anti-Tag gave an EC50 of 49 nM, versus an EC50 of 0.60 nM for Tag-MPs. Further reductions in the EC50's of the MP based MRSw assays were obtained by the use of magnetic field and valency enhancement as shown in Figure 3b. Through a combination of all three techniques, the EC50's for anti-Tag were progressively decreased from a starting value of 49 nM with Tag-NPs to 0.0020 nM (2.0 pM), obtained with decreased particle concentration, magnetic field aggregation, and valency enhancement (Figure 3c).
To obtain a detection limit from these results, we determined a “projected sensitivity concentration” or PSC. Five msec was added to (or subtracted from) the value of T2 in the absence of anti-Tag and the concentration at this T2 determined from curve parameters (Table 2). The PSC for anti-Tag with Tag-NP sensor, the starting point of our investigation, was 26 nM. By using our three techniques for enhancing sensitivity, the PSC was reduced to 0.00014 nM or 186,000 fold. The PSC is discussed further in Supporting Information. This detection sensitivity is comparable with those of most advanced nanostructure-based antibody assays.[20]
We obtained a high sensitivity MRSw sensor for influenza antibody by using MPs and exploiting a previously unrecognized feature of their response to a magnetic field. Application of a homogeneous magnetic field enhanced antibody-based crosslinking between particles, providing a novel method of increasing the sensitivity of a homogeneous, particle aggregation based immunoassay. Together with high throughput methods of peptide and peptide-MP conjugate synthesis,[21] MRSw sensors might be used, both to analyze the immune response to mutating viruses in laboratory settings, and for an MRSw based POC antibody sensor.
Acknowledgments
This work was supported by R01EB0004626 and U54CA119349.
Footnotes
Justification
New sensors for the detection of antibodies to the structural features of viral proteins are an important part of the preparation for possible viral pandemics. Here we show that micron-sized particles that are 350,000 times more magnetic (per particle) than earlier magnetic nanoparticles provide a high sensitivity, NMR based sensor for detecting antibodies to influenza virus. A homogeneous magnetic field was employed to enhance antibody-based crosslinking between particles, providing a novel method of increasing the sensitivity of a homogeneous (non-separation based) immunoassay.
References
- 1.Lu PS. Science. 2006;312:337. doi: 10.1126/science.1128199. [DOI] [PubMed] [Google Scholar]
- 2.Butler D. Nature. 2006;439:248. doi: 10.1038/439248a. [DOI] [PubMed] [Google Scholar]
- 3.Perez JM, Josephson L, O'Loughlin T, Hogemann D, Weissleder R. Nat. Biotechnol. 2002;20:816. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 4.Manz B, Coy A, Dykstra R, Eccles CD, Hunter MW, Parkinson BJ, Callaghan PT. J. Magn. Reson. 2006;183:25. doi: 10.1016/j.jmr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Blumich B, Blumler P, Eidmann G, Guthausen A, Haken R, Schmitz U, Saito K, Zimmer G. Magn. Reson. Imaging. 1998;16:479. doi: 10.1016/s0730-725x(98)00069-1. [DOI] [PubMed] [Google Scholar]
- 6.Sillerud LO, McDowell AF, Adolphi NL, Serda RE, Adams DP, Vasile MJ, Alam TM. J. Magn. Reson. 2006;181:181. doi: 10.1016/j.jmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R. J. Am. Chem. Soc. 2003;125:10192. doi: 10.1021/ja036409g. [DOI] [PubMed] [Google Scholar]
- 8.Kaittanis C, Naser SA, Perez JM. Nano Lett. 2007;7:380. doi: 10.1021/nl062553z. [DOI] [PubMed] [Google Scholar]
- 9.Kim GY, Josephson L, Langer R, Cima MJ. Bioconjugate Chem. 2007;18:2024. doi: 10.1021/bc070110w. [DOI] [PubMed] [Google Scholar]
- 10.Perez JM, O'Loughin T, Simeone FJ, Weissleder R, Josephson L. J. Am. Chem. Soc. 2002;124:2856. doi: 10.1021/ja017773n. [DOI] [PubMed] [Google Scholar]
- 11.DeLisi C. J. Theor. Biol. 1974;45:555. doi: 10.1016/0022-5193(74)90130-1. [DOI] [PubMed] [Google Scholar]
- 12.Chak KC, Hart H. Bull. Math. Biol. 1980;42:37. doi: 10.1007/BF02462365. [DOI] [PubMed] [Google Scholar]
- 13.van Oss CJ. J. Immunoassay. 2000;21:143. doi: 10.1080/01971520009349532. [DOI] [PubMed] [Google Scholar]
- 14.Hong R, Cima MJ, Weissleder R, Josephson L. Mag. Reson. Med. 2008 doi: 10.1002/mrm.21526. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephson L, Perez JM, Weissleder R. Angew. Chem. Int. Ed. 2001;40:3204. doi: 10.1002/1521-3773(20010903)40:17<3204::AID-ANIE3204>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Gillis P, Koenig SH. Magn. Reson. Med. 1987;5:323. doi: 10.1002/mrm.1910050404. [DOI] [PubMed] [Google Scholar]
- 17.Brooks RA. Magn. Reson. Med. 2002;47:388. doi: 10.1002/mrm.10064. [DOI] [PubMed] [Google Scholar]
- 18.Yung KT. Magn. Reson. Imaging. 2003;21:451. doi: 10.1016/s0730-725x(02)00640-9. [DOI] [PubMed] [Google Scholar]
- 19.Roch A, Gossuin Y, Muller RN, Gillis P. J. Magn. Magn. Mater. 2005;293:532. [Google Scholar]
- 20.Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat. Biotechnol. 2005;23:1418. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds F, O'Loughlin T, Weissleder R, Josephson L. Anal. Chem. 2005;77:814. doi: 10.1021/ac049307x. [DOI] [PubMed] [Google Scholar]