Abstract
Targeted expression of foreign genes to the peripheral nervous system is interesting for many applications, including gene therapy of neuromuscular diseases, neuroanatomical studies, and elucidation of mechanisms of axonal flow. Here we describe a microneurosurgical technique for injection of replication-defective viral vectors into dorsal root ganglia (DRG). Adenovirus- and adeno-associated virus-based vectors with transcriptional competence for DRG neurons led to expression of the gene of interest throughout the first neuron of the sensory system, from the distal portions of the respective sensory nerve to the ipsilateral nucleus gracilis and cuneatus, which contains the synapses to the spinothalamic tracts. Use of Rag-1 ablated mice, which lack all B and T lymphocytes, allowed for sustained expression for periods exceeding 100 days. In immunocompetent mice, long-term (52 days) expression was achieved with similar efficiency by using adeno-associated viral vectors. DRG injection was vastly superior to intraneural injection into the sciatic nerve, which mainly transduced Schwann cells in the vicinity of the site of inoculation site but only inefficiently transduced nerve fibers, whereas i.m. injection did not lead to any significant expression of the reporter gene in nerve fibers. The versatile and efficient transduction of genes of interest should enable a wide variety of functional studies of peripheral nervous system pathophysiology.
Keywords: dorsal root ganglia, gene therapy
Successful gene transfer depends on efficient methods of expressing genes of interest into appropriate cell populations. A particularly efficient and safe way of delivering genes to postmitotic cells uses adenoviral vectors and vectors based on recombinant adeno-associated viruses (rAAVs) (1–3).
Gene transfer to the peripheral nervous system (PNS) poses an exceptional challenge because the PNS contains a wide range of cell types, many of which are postmitotic. Delivery of genes selectively to the PNS could have several important applications for gene therapy of neurological diseases. In addition to anatomical studies, direct viral vector-mediated gene transfer allows for exact studies of physiological processes like antero- and retrograde transport and sorting of viruses and/or proteins along axons and dendrites (4–7). Delivery of genes encoding proteins such as neurotrophic factors selectively to the PNS may provide novel therapeutic approaches for spinal muscular atrophy, amyotrophic lateral sclerosis, or hereditary motor sensory neuropathies (8, 9).
The effectiveness of gene therapy using viral vectors depends among other factors on the efficient delivery of the desired genes to the cell population of interest. In past investigations, delivery was mainly accomplished by i.m. injection, which is followed by uptake and retrograde axonal transport of virions (6, 9). In the present study we have reasoned that direct transfer to PNS neurons might be more efficient than methods relying on indirect transduction to neurons. We found direct injection of dorsal root ganglia (DRG) to be the most efficient technique.
Materials and Methods
Adenoviral Vectors.
E1/E3-deleted adenoviral vectors, AdCMVlacZ and AdRSVlacZ, were generated by homologous recombination in E1-transcomplementing 911 cells (10) between a plasmid containing the expression cassette, 5.5 kb of viral DNA, and a ClaI-restricted DNA fragment isolated from AdMLPlacZ. Viral plaques were screened for expression of β-galactosidase (β-gal) by PCR and amplified in 911 cells. Batches were purified by CsCl gradient centrifugation, dialyzed against storage buffer (10% glycerol in PBS at pH 7.2), and kept at −80°C. Viral titers were determined by plaque assay and expressed as plaque-forming units. Transfer plasmids, pAdCMVlacZ and pAdRSVlacZ, contained β-gal linked to the cytomegalovirus (CMV) promoter (11) or the Rous sarcoma virus (RSV) enhancer/promoter (pRcRSV, Invitrogen). To replace the CMV promoter, pAdRSVlacZ was generated in a three-way ligation. The SpeI–SalI 13-kb backbone of pAdCMVlacZ was joined to two fragments generated by PCR with Pfu polymerase (Stratagene), the SpeI–SfiI 450-bp product containing the Ad-inverted terminal repeats was prepared by using pAdCMVlacZ as template and primer P109 (5′-GCTCTAGAACTAGTTGATC) and P110 (5′-GCAGATTCTTCATGCAATGGCCAGCTTGGCCATAATAATAAAACGCCAAC). The SfiI–SalI 500-bp product containing the RSV promoter was prepared by using pRc/RSV as template and primer P111 (5′-CGTTACTAGTGGAGTCGACCTCGGATCCAAGCTTGGAGG) and P112 (5′-ATTATGGCCAAGCTGGCCATTGCATGAAGAATCTGC).
Adeno-Associated Viral Vectors.
PsubCMV-β was obtained by inserting the EcoRI/HindIII CMV-lacZ-poly(A) expression cassette from pCMV-β (CLONTECH) in XbaI sites of psub201 (12). To produce psubCMV-WPRE, lacZ was replaced by a multiple cloning site into which the 0.59-kb ClaI/ClaIWPRE fragment from p138WPRE was cloned (13). The CMV promoter was replaced by a 1.48-kb Xbal/SalI fragment from the human platelet-derived growth factor (PDGF) β-chain promoter to generate psub-PDGF-WPRE. The rAAV vector plasmid psubPDGF-WPRE-EGFP was obtained by cloning the NheI–NotI enhanced green fluorescent protein (EGFP) fragment from pEGFP-N1 (CLONTECH) between the PDGF promoter and the WPRE element of psub-PDGF-WPRE. For generation of the adeno helper plasmid pBS-E2A-VA-E4, the 1.38-kb SalI/ApaI VA fragment, the 5.77-kb BamHI/EcoRI E2A fragment and the 4.0-kb Apal 3′ terminal E4 fragment from adenovirus type 5 genomic DNA were cloned in pBluescript SK+ (Stratagene). The rAAV packaging plasmid pAAV/Ad-rep(ACG) was generated by PCR mutagenesis of the Rep78 start codon from ATG to ACG in the pAAV/Ad plasmid (12).
rAAV-PDGF-EGFP virus particles were produced in an adenovirus-free system (14) by cotransfection of 293T cells with psubPDGF-WPRE-EGFP, the rAAV packaging plasmid pAAV/Ad-rep(ACG), which contains the rep and cap genes and the adenovirus helper plasmid pBS-E2A-VA-E4, by using the calcium phosphate method. Cells were collected and lysed by three freeze-thaw cycles, and debris was eliminated by centrifugation. Some contaminant proteins were removed by precipitation with 25% ammonium sulfate. rAAV-PDGF-EGFP was further purified over two consecutive CsCl gradients, dialyzed against PBS containing 1 mM MgCl2, and stored at −80°C in the presence of 10% glycerol. Viral particle numbers, as determined by slot blot hybridization, were 1.5 × 1010/ml.
Procedures for Viral Administration to Animals.
For all experiments with rAAV vectors we used C57BL/6 mice, whereas all experiments with replication-deficient adenovirus were performed with Rag-1−/− mice (15). Three microliters of PBS or storage buffer containing 2.5 × 107 plaque-forming units of AdCMVlacZ was injected into the musculus tibialis cranialis over 5 min. One mouse of the adenovirally transduced group received additionally 3 μl PBS into the contralateral musculus tibialis cranialis. For intranerval injection, mice were anaesthetized with xylazin/ketamin, and the right sciatic nerve was surgically exposed by dislodging the musculus gluteus superficialis and the musculus biceps femoris. The nerve was gently placed onto a metal plate (20 × 5 × 0.5 mm), and 2.5 × 107 plaque-forming units of AdCMVlacZ or of AdRSVlacZ (3 μl) were injected with a 34-gauge Hamilton syringe over 5 min (16). The nerve was anatomically repositioned, and the skin was closed with USP 4.0 nylon sutures. Two mice of the adenovirally transduced group received additionally 3 μl of PBS in the contralateral sciatic nerve. One additional control mouse received 3 μl of PBS in the right sciatic nerve.
For DRG injection, mice were anaesthetized with xylazin/ketamin. DRG (levels L4/L5) were surgically exposed by dissecting the musculus multifidus and the musculus longissimus lumborum and by removing the processus accessorius and parts of the processus transversus. AdCMVlacZ or AdRSVlacZ (2.5 × 107 plaque-forming units) or 6 × 107 particles of rAAV-PDGF-EGFP suspended in 4 μl (2 μl per ganglion) were injected. Two mice of the adenovirally transduced group received additionally 4 μl of PBS in the contralateral L4/L5 DRG. One (adenoviral group) or three (rAAV group) control mice received 4 μl of PBS in the right L4/L5 DRG. All injections were done by using a stereotaxic frame and a Hamilton syringe with a 34-gauge needle over 5 min. Muscles then were adapted by using USP 5.0 catgut. Skin closure was achieved with 4.0 nylon suture.
Morphological and DNA Analysis.
All mice were anaesthetized with xylazin/ketamin and perfused with PBS containing 2% paraformaldehyde and 0.5% glutaraldehyde at defined time points. For β-gal analysis, brains, spinal cords, and both sciatic nerves were removed and postfixed in the same solution (4 h). Tissue was rinsed with PBS, reacted in 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0,01% (wt/vol) sodium deoxycholate, 0,02% NP-40, and 1 mg/ml Bluo-gal (Sigma) in PBS for 4–6 h. For EGFP detection, mice were perfused with the same fixative as above, and sciatic nerves and spinal cord were processed for whole mounts and cryosectioning. Samples were embedded in paraffin, cut in 4-μm sections, and counterstained with nuclear fast red or Sudan black. Selected slides were double-stained for neurofilaments (mAb against neurofilament protein 70 kDa and 200 kDa, 1:20, Bio-Science, Emmenbrücke, Switzerland). Visualization was achieved with biotinylated secondary antibody (Dako, 1:20), biotin/avidin-peroxidase (Dako), and diaminobenzidine.
After postfixation with 0.5% glutaraldehyde, selected samples were fixed with osmic tetroxide and embedded in epoxy resin. Sections (3 μm) were stained with toluidine blue. Expression of EGFP was detected by fluorescence and confocal laser scanning microscopy on whole-mount preparations of the sciatic nerve.
Whole-mount sections were double or separately stained for neurofilament and EGFP. For detection of EGFP, a mAb (Chemicon, 1:500) was used, and neurofilaments were detected with a polyclonal rabbit anti-mouse antibody (Chemicon, 1:200). Visualization was achieved by using alexa 546-labeled goat anti-mouse (Molecular Probes, 1:2,000) or FITC-labeled goat anti-rabbit antibody (Sigma, 1:750).
For PCR, DNA was extracted from DRG and midsciatic nerves of mice upon microdissection of frozen sections. Two primers (5′-CAGTGGCCAATGGTGAGCAAGGGCGAGGAGC-3′ and 5′-TGTAGGTACCCTCTGCCATGCCGAGAGTGATCC-3′) were used to amplify the entire ORF of EGFP.
Results
No β-Gal Activity in the PNS After i.m. Injection of Adenoviral Vectors Containing the lacZ Gene into the Musculus Tibialis Cranialis.
AdCMVlacZ was injected i.m. in three mice. Another mouse received PBS. For detection of β-gal activity, sciatic nerves and spinal cords were examined as whole mounts under a dissection microscope. In addition, histological sections from the sciatic nerve or the spinal cord were examined at various magnifications. However, in contrast to previous reports, no β-gal activity was detectable in any of the examined samples at any time point (9) (7, 14, or 21 days) (Table 1).
Table 1.
Summary of the results from the i.m., intranerval, and DRG injections
| Injection site | Construct | n | Expression of reporter gene | Time points of analysis, days | Cell types transduced | |
|---|---|---|---|---|---|---|
| Muscle | AdCMVlacZ | 3 | − | 7–21 | – | − |
| Sciatic nerve | AdRSVlacZ | 4 | ++ | 2–21 | Epineurium | ++ |
| Schwann cells | +++ | |||||
| Neurons | + | |||||
| AdCMVlacZ | 6 | ++ | 2–102 | Epineurium | ++ | |
| Schwann cells | +++ | |||||
| Neurons | + | |||||
| DRG | AdRSVlacZ | 4 | +++ | 2–24 | Epineurium | + |
| Schwann cells | + | |||||
| Neurons | +++ | |||||
| AdCMVlacZ | 6 | +++ | 2–50 | Epineurium | + | |
| Schwann cells | + | |||||
| Neurons | +++ | |||||
| rPDGF-AAV-EGFP | 6 | +++ | 16–52 | Epineurium | − | |
| Schwann cells | − | |||||
| Neurons | +++ | |||||
Localized β-Gal Activity After Intranerval Injection in the Sciatic Nerve of Adenoviral Vectors Containing the lacZ Gene.
Of 10 mice injected into the sciatic nerve, eight showed expression of β-gal predominantly confined to the immediate neighborhood of the site of injection. Histologically, expression was seen mainly in Schwann cells and perineural tissue. Axonal expression also was seen, but to a much lesser extent (Figs. 1 and 2). The amount and distribution of β-gal activity was identical in animals that had received the adenoviral vector encoding CMV-lacZ and those injected with the RSV-lacZ vector. In the contralateral noninjected side and in mice receiving PBS there was no detectable β-gal activity.
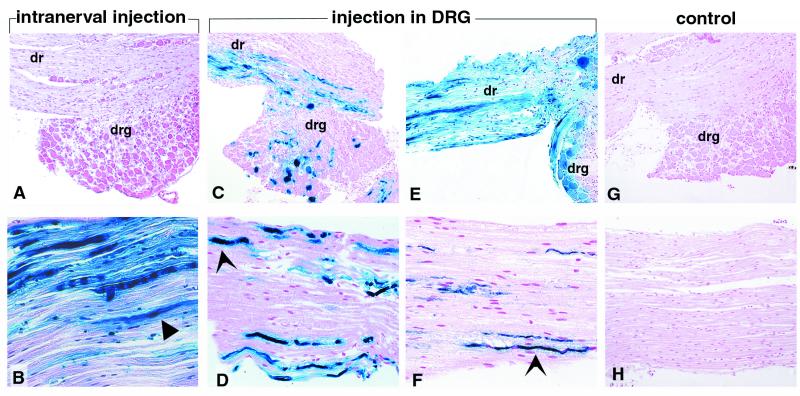
Figure 1.
Expression of β-gal activity in DRG (A, C, E, and G) and sciatic nerves (B, D, F, and H) after injection of AdCMVlacZ in the sciatic nerve (A and B), AdCMVlacZ in the DRG (C and D), AdRSVlacZ in the DRG (E and F), and PBS in the DRG (G and H). Sections were stained with bluo-gal and counterstained with nuclear fast red. The blue color visualizes cells expressing β-gal. (A and B) 102 days after injection (A, ×65; B, ×100). (C and D) 14 days after injection (C, ×65; D, ×100). (E and F) 24 days after injection (E, ×65; F, ×100). (G and H) Control animal, 14 days after injection (G, ×65; H, ×100). (A and B) expression of β-gal in Schwann cells (block arrow), cells of the epineurium, and axons. There is no significant expression of β-gal in the DRG. (C–F) DRG neurons and axons (arrow) express β-gal. Expression of β-gal is detectable to a lesser extent in Schwann cells and cells of the epineurium. dr, dorsal root; drg, dorsal root ganglia.
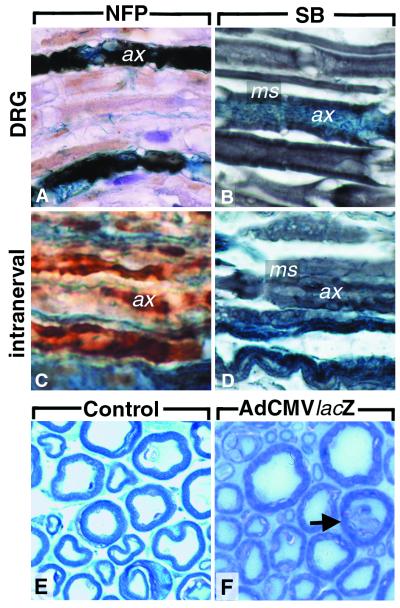
Figure 2.
Immunohistochemistry for neurofilament protein (A and C) and Sudan black stain (B and D) of sciatic nerves 50 days after injection of AdCMVlacZ in the DRG L4/L5 (A and B) or the sciatic nerve (C and D). β-gal expression and immunopositivity for neurofilament proteins colocalize in A. (E and F) Semithin sections of the plexus lumbalis: no significant demyelination is visible. Occasional axonal degeneration is marked with an arrow. (F) After AdCMVlacZ injection in the DRG. (E) Healthy nonmanipulated mouse. ax, axon; ms, myelin sheath; NFP, neurofilament immunohistochemistry; SB, sudan black stain. Magnifications: ×260.
At 2 days and 1 week, β-gal activity was prominent mainly in Schwann cells in the area of the injection site. At later time points (2 and 3 weeks) activity was detected in the distal sciatic nerve and the plexus lumbalis including the DRG up to 30 mm from the injection site (Figs. 3 and 4). Two of four mice displayed expression of β-gal in the fasciculus gracilis at later time points (2 and 3 weeks). Expression was stable and strong in all mice taken at later time points. One mouse taken at 102 days still showed a strong expression of β-gal (Fig. 1B).
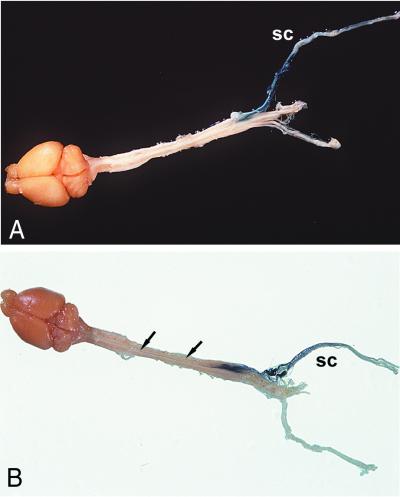
Figure 3.
Expression of β-gal in sciatic nerve and spinal cord of mice after stereotaxic administration of AdRSVlacZ into DRG (segments L4 and L5). (A and B) Five days after injection of AdRSVlacZ: Expression of β-gal extends to the middle part of the sciatic nerve and is visible in the dorsal root of the spinal cord. (C) Twenty-four days after injection of AdRSVlacZ, β-gal expression is prominent throughout the sciatic nerve. (D and E) Cross-section of the thoracic spinal cord (D) and dorsal view of the spinal cord and the medulla oblongata (E) 34 days after injection of AdRSVlacZ. Strong β-gal expression is now seen in the thoracic fasciculus gracilis (fg) and to a lesser extent in the medulla oblongata. Arrows in E mark the fasciulus gracilis: left arrow, caudal part of the fg; right arrow, cranial part of the fg. sc, sciatic nerve; drg, dorsal root ganglia.
Figure 4.
Expression of β-gal activity in the brain, spinal cord, and sciatic nerves 7 days after administration of AdCMVlacZ (A), 34 days after injection of AdRSVlacZ (B) into DRG of lumbar segments L4 and L5. Strong expression of β-gal along the sciatic nerve (sc), in DRG (A and B), and in the fasciculus gracilis (B).
Stable and Widespread β-Gal Activity in the Sensory Pathway After Injection of Adenoviral Vectors Containing the lacZ Gene in the DRG L4/L5.
Of 10 mice injected in the DRG, all showed strong expression of β-gal activity. On histological sections, expression was seen predominantly in neurons, whereas satellite cells and perineural tissue showed lower levels of β-gal activity (Figs. 1 and 2). Ganglion cells in the injected DRG showed strong expression of β-gal in their cytosol (Fig. 1). The use of a CMV promoter provided no significant advantages over the RSV promoter in the DRG injections. In two mice receiving PBS in contralateral DRG and in two mice receiving PBS only in the right DRG no β-gal activity could be detected.
Even at early time points (5 days and 1 week) β-gal activity was strong both in the vicinity of the injection sites and in areas situated up to 12 mm from the injection site (Figs. 1 and 3). At later time points (2 weeks and 24 days) β-gal activity could be seen in all mice in areas very distant from the injection site, such as the distal parts of the sciatic nerve and the cranial third of the fasciculus gracilis in the spinal cord (Figs. 3 and 4). In AdCMVlacZ-injected mice, 2 weeks after injection β-gal activity was detected in the posterior thoracic portion of the spinal cord. Three weeks after injection β-gal activity was detected in the cervical portion of the spinal cord and in the medulla oblongata. In AdRSVlacZ-injected mice, β-gal activity was detected in the thoracic portion of the spinal cord at 24 days and in the cervical spinal cord and medulla oblongata at 34 days (Figs. 3 and 4). Expression was stable and strong in all mice up to 50 days.
Stable and Widespread Expression of EGFP in the Sensory Pathway After Injection of rAAV Containing the EGFP Gene in the DRG L4/L5.
Of six mice that received rAAV-PDGF-EGFP in the DRG, four showed expression of EGFP in the PNS. Confocal fluorescent analysis of rAAV-PDGF-EGFP-injected mice showed selective expression of the gene in axons and cell bodies of DRG (Fig. 5). Simultaneous immunofluorescent staining for neurofilaments, (which are expressed in axons) and EGFP showed colocalization of the EGFP and neurofilament expression (Fig. 5). Three control mice receiving PBS did not show any EGFP-like fluorescence. In the rAAV genomic context, the neuron-specific PDGF promoter led to highly selective expression of EGFP in DRG and their dendrites. In two mice taken at 16 days we could not observe any expression of EGFP. In contrast, expression of EGFP in mice taken at 45 days was strong, and no reduction of expression was detectable at the latest time point of 52 days (Fig. 5). PCR analysis confirmed persistence of rAAV in DRG at 45 days; viral DNA was detectable in DRG but not in sciatic nerves (Fig. 5F).
Figure 5.
Expression of EGFP in the sciatic nerves and the DRG 45 days (A and B) and 52 days after injection of rAAV-PDGF-EGFP in the DRG L4/L5 (C–E). (A) Strong expression of EGFP in some DRG neurons (L4). (B) In the middle region of the sciatic nerve, axons express EGFP. (C) Alexa 546-labeled EGFP expression in the middle sciatic nerve. (D and E) Colocalization of Alexa 546-labeled EGFP (D) and FITC-labeled neurofilament (E) expression. (F) PCR analysis of DRG and mid-sciatic nerves (scn) in rAAV-PDGF-EGFP-injected (gfp) and PBS-injected (pbs) animals 45 days after injection. A 700-bp GFP fragment can be amplified from DRG, but not from scn, of AAV-injected animals. +, 25 pg of PDGF-EGFP plasmid. (A and B) Confocal laser scanning microscopy, ×25. (B–E) Conventional fluorescence microscopy, ×100. Arrows, axons; arrowhead, DRG.
Neurotoxic Effects.
The occurrence of possible neurotoxic effects secondary to expression of transduced proteins, or to direct neurotoxicity of the adenoviral structural proteins, was studied by observing mice for signs of paresis and by scoring histologically sciatic nerves for the presence of demyelination, digestion chambers, and axonal loss. One mouse was eliminated because it showed paresis persisting at day 5 after injection. This mouse is not included in Table 1. Of 23 mice injected with adenoviral vectors, six showed transient paresis lasting 3–4 days. In the group of mice that received i.m. injections no transient or permanent pareses were observed. There was no difference in incidence or severity of paresis between mice injected in the sciatic nerve or DRG (data not shown). Histologically, there was no apparent demyelination in mice injected in the sciatic nerve nor in the DRG (Fig. 2). However in one DRG-injected mouse minimal Wallerian degeneration was visible in semithin sections 50 days after viral administration (Fig. 2F).
All of the rAAV-injected mice fully recovered within 2 days of the operation. With the exception of transient paresis observed in two of six mice there were no clinical signs of neurotoxic effects. Selected mice were histologically scored for the presence of demyelination or digestion chambers: We could not observe neurotoxic effects in any of these mice.
Discussion
Adenoviral and rAAV-based vectors are routinely used for transferring genes to postmitotic cells (17–20). Transfer of genes to the PNS was described by injection of the adenovirus into the sciatic nerve or muscles (2, 9). However administration of adenoviral vectors by intranerval injection leads to predominant expression in the injected area, which typically exceeds the expression levels achieved along the actual neural pathway. According to published studies, this problem may be obviated by i.m. injection of the virus (8, 9), yet in our study i.m. injection did not lead to any detectable expression of the reporter gene in either the PNS or the spinal cord. In our hands, injection into the sciatic nerve led to β-gal activity in the vicinity of the injection site and to a much lesser extent in regions distant from the injection site. Moreover the cell populations successfully transduced consisted predominantly of Schwann cells and mesenchymal cells in the perineurium (21).
Here we introduce a strategy for delivering genes to the PNS, which leads to specific and strong expression along sensory neural pathways. By injecting viral vectors directly into the DRG we achieved strong expression of the gene in remote segments of the corresponding neuronal processes, such as the distal sciatic nerve and the entire length of the fasciculus gracilis. Therefore, this strategy allows for expression of the gene of interest along the entire length of a sensory dorsal root neuron, the longest neural pathway in vertebrates. Neurons were predominantly transduced, whereas Schwann and perineurial cells were transduced to a lesser extent.
To assess the impact of different transcriptional regulatory elements on the efficiency of transduction and gene expression, we evaluated adenoviral vectors in which the gene of interest was driven by the CMV immediate early promoter or the RSV enhancer/promoter: both promoters have been shown to transduce cells of the nervous system (17, 18). The use of the CMV promoter was particularly attractive because CMV has been implicated as a causative agent of dorsal root ganglionitis (22), suggesting a strong tropism for DRG neurons. However, we did not find any differences in efficiency of cell transduction or duration of gene expression between the CMV and RSV promoters. The neuron-specific PDGF promoter within an rAAV vector led to highly selective and long-lasting transduction of ganglion cells. For transduction of central nervous system neurons the PDGF promoter within the context of the rAAV vector had been shown to be superior to other tested promoters (23).
We were concerned about possible neurotoxic effects induced by viral or transgenic expression (24). However, there was no significant difference in the incidence of neurological side effects between the groups of mice administered intranervally and those injected into the DRG despite the much higher efficiency of the latter procedure. Fibers with very high expression of β-gal showed signs of axonal degeneration with occasional digestion chambers resembling Wallerian degeneration. In contrast, neurotoxic effects were absent from mice injected with rAAV apart from transient paresis, in accordance with the lack of central nervous system neurotoxicity of rAAV (3).
Although adenoviral vectors represent a promising tool for transferring foreign genes to many tissue compartments, expression of transduced genes in immunocompetent animals is typically transient. We therefore used Rag-1−/− mice that lack all differentiated B and T cells. In these mice we achieved stable, strong expression for up to 102 days. Because there was no decline in expression we conclude that expression is likely to last for periods of time well exceeding our last time point. The above findings confirm and add to the large body of evidence pointing to the rate-limiting role of the immune response to genes expressed by adenoviral vectors on the duration of expression (15, 19, 24–27). We have not attempted to systematically investigate duration of expression in immunocompetent mice; however, in many experimental models the use of immunodeficient animals may be acceptable.
To overcome the problem of immune response we used an rAAV vector in immunocompetent mice. Histological analysis did not reveal any inflammatory response to the viral vector or EGFP. The delayed expression of the gene of interest is most likely caused by the conversion of the AAV single-stranded DNA to double-stranded DNA, which was shown to be the rate-limiting step for efficient expression (28).
The methods detailed in the present study will allow investigations of neuroregeneration and studies on the neurotropism of infectious agents. For instance, we are using the system described here to express the prion protein in the peripheral nervous system of prion protein knockout mice (29) to study the role of normal cellular prion protein in the spread of infectious prions from peripheral sites to the central nervous system (30). In addition, the strong expression in neural processes renders this system particularly attractive for neuroanatomical tracing studies (31).
As an ultimate goal, delivery of genes specifically to the PNS of humans could be useful in treating diseases affecting the PNS, such as toxic or metabolic axonal neuropathies. With this goal in mind, the delivery of genes via injection into DRG seems to be an efficient addition to the collection of tools for gene therapy.
Acknowledgments
We thank Dr. P. Gambetti for suggestions, Dr. M. Perricaudet for providing the parental adenoviral vector ADMLPlacZ, Dr. T. Bächi for assistance with confocal microscopy, and M. König and M. Peltola for technical help. This study was supported by grants of the Swiss Nationalfonds (NFP38/38+), the European Union (98.6040, 98.6015), and the National Institutes of Health (5P01 AG-1435902) to A.A., and a long-term EMBO fellowship to B.N.
Abbreviations
- PNS
peripheral nervous system
- DRG
dorsal root ganglia
- rAAV
recombinant adeno-associated virus
- β-gal
β-galactosidase
- CMV
cytomegalovirus
- RSV
Rous sarcoma virus
- PDGF
platelet-derived growth factor
- EGFP
enhanced green fluorescent protein
References
- 1.Federoff H J, Brooks A, Muhkerjee B, Corden T. J Neurosci Methods. 1997;71:133–142. doi: 10.1016/s0165-0270(96)00133-1. [DOI] [PubMed] [Google Scholar]
- 2.Hermens W T, Giger R J, Holtmaat A J, Dijkhuizen P A, Houweling D A, Verhaagen J. J Neurosci Methods. 1997;71:85–98. doi: 10.1016/s0165-0270(96)00129-x. [DOI] [PubMed] [Google Scholar]
- 3.Xiao X, Li J, McCown T J, Samulski R J. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner B J, Shine H D. Exp Neurol. 1998;153:102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- 5.Ghadge G D, Roos R P, Kang U J, Wollmann R, Fishman P S, Kalynych A M, Barr E, Leiden J M. Gene Ther. 1995;2:132–137. [PubMed] [Google Scholar]
- 6.Kuo H, Ingram D K, Crystal R G, Mastrangeli A, Ghadge G D, Roos R P, Kang U J, Wollmann R, Fishman P S, Kalynych A M, et al. Brain Res. 1995;705:31–38. doi: 10.1016/0006-8993(95)01065-3. [DOI] [PubMed] [Google Scholar]
- 7.Terada S, Nakata T, Peterson A C, Hirokawa N. Science. 1996;273:784–788. doi: 10.1126/science.273.5276.784. [DOI] [PubMed] [Google Scholar]
- 8.Haase G, Kennel P, Pettmann B, Vigne E, Akli S, Revah F, Schmalbruch H, Kahn A. Nat Med. 1997;3:429–436. doi: 10.1038/nm0497-429. [DOI] [PubMed] [Google Scholar]
- 9.Haase G, Pettmann B, Vigne E, Castelnau-Ptakhine L, Schmalbruch H, Kahn A. J Neurol Sci. 1998;160, Suppl. 1:97–105. doi: 10.1016/s0022-510x(98)00207-x. [DOI] [PubMed] [Google Scholar]
- 10.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, Van Ormondt H, Hoeben R C, Van Der Eb A J. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 11.Schneider S D, Rusconi S, Seger R A, Hossle J P. Gene Ther. 1997;4:524–532. doi: 10.1038/sj.gt.3300432. [DOI] [PubMed] [Google Scholar]
- 12.Samulski R J, Chang L S, Shenk T. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donello J E, Loeb J E, Hope T J. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X, Li J, Samulski R J. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Bassant M H, Baron H, Gumpel M, Cathala F, Court L. Brain Res. 1986;383:397–401. doi: 10.1016/0006-8993(86)90048-x. [DOI] [PubMed] [Google Scholar]
- 17.Akli S, Caillaud C, Vigne E, Stratford Perricaudet L D, Poenaru L, Perricaudet M, Kahn A, Peschanski M R. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- 18.Bajocchi G, Feldman S H, Crystal R G, Mastrangeli A. Nat Genet. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- 19.Benihoud K, Saggio I, Opolon P, Salone B, Amiot F, Connault E, Chianale C, Dautry F, Yeh P, Perricaudet M. J Virol. 1998;72:9514–9525. doi: 10.1128/jvi.72.12.9514-9525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 21.Shy M E, Tani M, Shi Y J, Whyatt S A, Chbihi T, Scherer S S, Kamholz J. Ann Neurol. 1995;38:429–436. doi: 10.1002/ana.410380313. [DOI] [PubMed] [Google Scholar]
- 22.Fuller G N, Jacobs J M, Guiloff R J. Lancet. 1989;2:937–941. doi: 10.1016/s0140-6736(89)90952-5. [DOI] [PubMed] [Google Scholar]
- 23.Peel A L, Zolotukhin S, Schrimsher G W, Muzyczka N, Reier P J. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- 24.Kajiwara K, Byrnes A P, Charlton H M, Wood M J, Wood K J. Hum Gene Ther. 1997;8:253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- 25.Smith T A, White B D, Gardner J M, Kaleko M, McClelland A. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 26.Parr M J, Wen P Y, Schaub M, Khoury S J, Sayegh M H, Fine H A. J Neurovirol. 1998;4:194–203. doi: 10.3109/13550289809114519. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H G, Zhou T, Yang P, Edwards C K, Curiel D T, Mountz J D. Hum Gene Ther. 1998;9:1875–1884. doi: 10.1089/hum.1998.9.13-1875. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari F K, Samulski T, Shenk T, Samulski R J. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Büeler H R, Fischer M, Lang Y, Bluethmann H, Lipp H P, DeArmond S J, Prusiner S B, Aguet M, Weissmann C. Nature (London) 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 30.Blättler T, Brandner S, Raeber A J, Klein M A, Voigtländer T, Weissmann C, Aguzzi A. Nature (London) 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 31.Kuo H, Ingram D K, Crystal R G, Mastrangeli A. Brain Res. 1995;705:31–38. doi: 10.1016/0006-8993(95)01065-3. [DOI] [PubMed] [Google Scholar]