Abstract
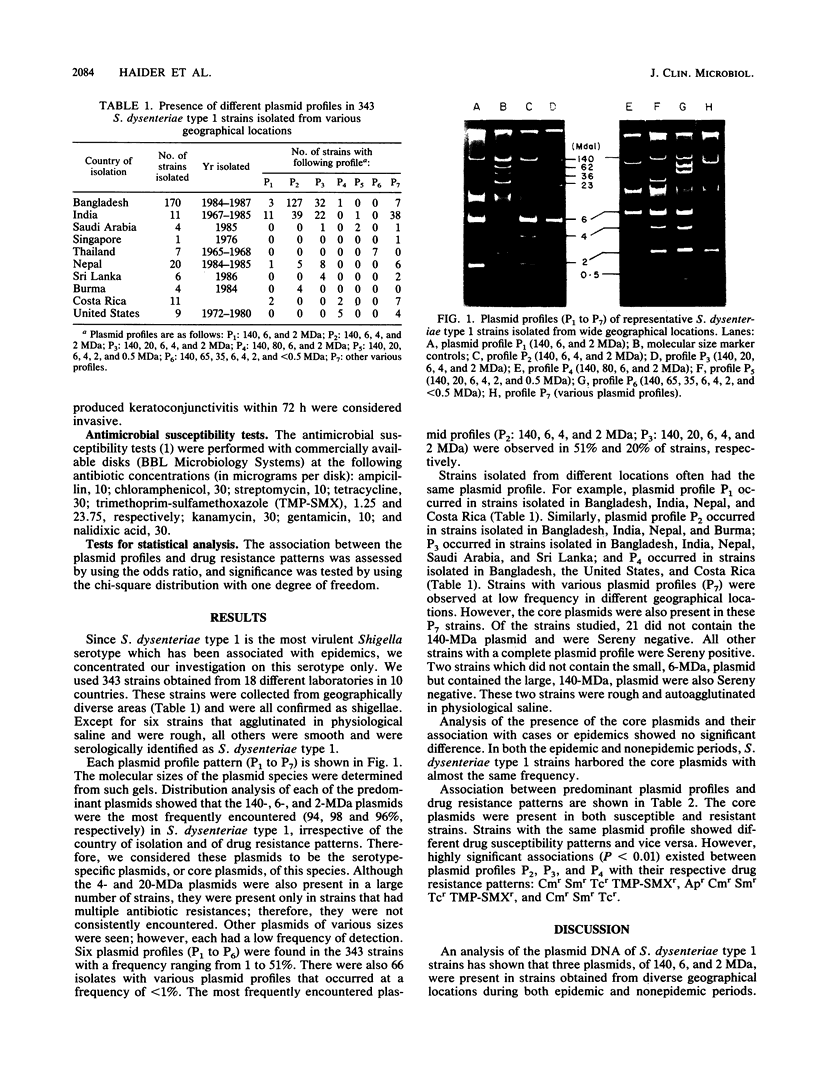
Plasmid profiles and antimicrobial susceptibility patterns of 343 strains of Shigella dysenteriae type 1, obtained from 18 different geographical locations, were analyzed. Three plasmids, with molecular sizes of 140, 6, and 2 megadaltons (MDa), were present in 94, 98, and 96%, respectively, of the 343 strains isolated during either epidemic or nonepidemic periods from 1965 to 1987. In addition to these plasmids, 83% of the strains harbored a 4-MDa plasmid and 25% harbored a 20-MDa plasmid. Various plasmid profiles were observed in which the 140-, 6-, and 2-MDa plasmids occurred commonly, irrespective of the place of isolation and drug resistance pattern of the strains. Certain profiles showed significant association with drug resistance patterns. These findings suggest that three plasmids, of molecular sizes 140, 6, and 2 MDa, are unique to S. dysenteriae type 1 strains and may indicate the global spread of a pathogenic bacterial clone. Additionally, these core plasmids, plus plasmids of various other sizes, could be used to identify emerging subclones which are causing both epidemic and sporadic disease. Thus, plasmid profiles of S. dysenteriae type 1 strains can be used to monitor possible pandemic strains as well as individual epidemic strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner F., Margadant A., Peduzzi R., Piffaretti J. C. The plasmid pattern as an epidemiologic tool for Salmonella typhimurium epidemics: comparison with the lysotype. J Infect Dis. 1983 Jul;148(1):7–11. doi: 10.1093/infdis/148.1.7. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr Molecular analysis of plasmids in epidemiologic investigation. J Infect Dis. 1983 Jul;148(1):1–6. doi: 10.1093/infdis/148.1.1. [DOI] [PubMed] [Google Scholar]
- Haider K., Huq M. I., Samadi A. R., Ahmad K. Plasmid characterization of Shigella spp. isolated from children with shigellosis and asymptomatic excretors. J Antimicrob Chemother. 1985 Dec;16(6):691–698. doi: 10.1093/jac/16.6.691. [DOI] [PubMed] [Google Scholar]
- Jamieson A. F., Bremner D. A., Bergquist P. L., Lane H. E. Characterization of plasmids from antibiotic-resistant Shigella isolates by agarose gell electrophoresis. J Gen Microbiol. 1979 Jul;113(1):73–81. doi: 10.1099/00221287-113-1-73. [DOI] [PubMed] [Google Scholar]
- Mata L. J., Gangarosa E. J., Cáceres A., Perera D. R., Mejicanos M. L. Epidemic Shiga bacillus dysentery in Central America. I. Etiologic investigations in Guatemala, 1969. J Infect Dis. 1970 Sep;122(3):170–180. doi: 10.1093/infdis/122.3.170. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi M. H., Sack D. A., Haider K., Ahmed Z. U., Rahaman M. M., Morshed M. G. Plasmid-mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet. 1987 Aug 22;2(8556):419–421. doi: 10.1016/s0140-6736(87)90957-3. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Hopkins J. D., Gilleece E. S., Medeiros A. A., Kent R. L., Blackburn B. O., Holmes M. B., Reardon J. P., Vergeront J. M., Schell W. L. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982 Jul 1;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- Prado D., Murray B. E., Cleary T. G., Pickering L. K. Limitations of using the plasmid pattern as an epidemiological tool for clinical isolates of Shigella sonnei. J Infect Dis. 1987 Feb;155(2):314–316. doi: 10.1093/infdis/155.2.314. [DOI] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Tompkins L. S., Falkow S. Use of agarose gel electrophoresis of plasmid deoxyribonucleic acid to fingerprint gram-negative bacilli. J Clin Microbiol. 1981 Jun;13(6):1105–1108. doi: 10.1128/jcm.13.6.1105-1108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid N. S., Rahaman M. M., Haider K., Banu H., Rahman N. Changing pattern of resistant Shiga bacillus (Shigella dysenteriae type 1) and Shigella flexneri in Bangladesh. J Infect Dis. 1985 Dec;152(6):1114–1119. doi: 10.1093/infdis/152.6.1114. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Shahid N., Huq M. I., Alim A. R., Cohen M. L. Usefulness of plasmid profiles for differentiation of Shigella isolates in Bangladesh. J Clin Microbiol. 1984 Aug;20(2):300–301. doi: 10.1128/jcm.20.2.300-301.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth K. Molecular epidemiology of bacterial infections: examples of methodology and of investigations of outbreaks. Rev Infect Dis. 1986 Sep-Oct;8(5):682–692. doi: 10.1093/clinids/8.5.682. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]