Abstract
Signals mediated by heterotrimeric G proteins often develop over the course of tens of milliseconds, and could require either conformational rearrangement or complete physical dissociation of Gα βγ heterotrimers. Although it is known that some active heterotrimers are dissociated (into Gα and Gβγ) at steady-state, it is not clear that dissociation occurs quickly enough to participate in rapid signaling. Here we show that fusion proteins containing the c-terminus of GPCR kinase 3 (GRK3ct) and either the fluorescent protein cerulean or Renilla luciferase bind to venus-labeled Gβγ dimers (Gβγ-V), resulting in Förster or bioluminescence resonance energy transfer (FRET or BRET). GRK3ct fusion proteins are freely-diffusible, and do not form preassembled complexes with G proteins. GRK3ct fusion proteins bind to free Gβγ-V dimers but not to rearranged heterotrimers, and thus can report G protein dissociation with high temporal resolution. We find that heterotrimer dissociation can occur in living cells in less than 100 milliseconds. Under the conditions of these experiments diffusion and collision of masGRK3ct fusion proteins and Gβγ-V were not rate-limiting. These results indicate that G protein heterotrimers can dissociate quickly enough to participate in rapid signaling.
Keywords: FRET, BRET, pleckstrin homology domain, collision, diffusion, GPCR
1. Introduction
Signals transduced by G protein-coupled receptors (GPCRs) through heterotrimeric G proteins and their downstream effector molecules can often be characterized as rapid – meaning they can develop and decay in less than a second – and specific – meaning different stimuli can produce different cellular responses even though common signaling molecules are involved in the transduction processes. Recent studies have lent support to the idea that the problems of speed and specificity might be solved by the assembly of multiprotein complexes that contain GPCRs, G proteins and effector molecules and remain intact throughout signal transduction. Signaling within stable complexes would remove the need for active GPCRs, G proteins and effector molecules to encounter each other by random diffusion and collision, which would both eliminate a potential rate-limiting step and might prevent signaling by inappropriate receptor-G protein-effector combinations.
Many of the studies that have provided evidence for signaling by multiprotein complexes have been carried out in living cells using Förster (fluorescence) and bioluminescence resonance energy transfer (FRET and BRET). Some of these studies have suggested that GPCRs and G proteins may be stably associated before and after receptor activation [1–4], and that G protein heterotrimers may rearrange yet remain intact after activation instead of dissociating into their component Gα and Gβγ subunits [5]. Other studies have provided evidence that some active heterotrimers do dissociate in cells, but the methods used in these studies lack the temporal resolution necessary to demonstrate that dissociation occurs over a time course similar to rapid G protein signaling [6–8]. Studies with purified proteins in solution have suggested that heterotrimer dissociation may be a relatively slow process [9], thus it is unclear if dissociation occurs quickly enough to participate in rapid cell signaling.
Studies in living cells have also addressed interactions between G protein subunits and downstream effector molecules. These have demonstrated basal FRET or BRET signals between G protein subunits and adenylyl cyclase (AC), phospholipase Cβ (PLCβ), and inwardly-rectifying potassium (GIRK) channels, again suggesting that these proteins may be part of constitutive signaling complexes [10–12]. Although FRET or BRET studies have measured the time courses of many of the events involved in G protein signaling [13], the time course of effector activation has not been directly measured using these techniques. However, the rate of GIRK channel activation can be measured with high temporal resolution by recording ionic currents, and this rate is comparable to that of G protein activation [5]. It has been argued that the rapid time course of GIRK channel activation is inconsistent with random binding of freely-diffusing Gβγ dimers to these channels, and thus that preexisting G protein-GIRK channel complexes are necessary to explain the lack of a detectable delay between G protein activation and channel activation [12].
In the present study we address both the time course of heterotrimer dissociation and the interaction of G protein subunits with a freely-diffusing effector. We use FRET and BRET to study association of Gβγ dimers with the c-terminus of GPCR kinase 3 (GRK3ct). GRK2 and GRK3 bind to Gβγ dimers via a c-terminal pleckstrin homology domain [14], and GRKct fragments are widely used to inhibit Gβγ signaling by sequestering Gβγ dimers. GRK3ct was of interest as a Gβγ indicator for two reasons, both of which distinguish GRKs from most other Gβγ-binding proteins. First, the structure of a GRK-Gβγ complex has been solved [15]. The surfaces of Gβγ that bind to GRKs overlap extensively with the surfaces that bind to Gα subunits, thus it is likely that GRKs will bind only to free Gβγ dimers and not to rearranged Gα βγ heterotrimers. Second, this fragment is not thought to form preassembled complexes with GPCRs or G proteins, therefore the GRK-Gβγ interaction presumably requires diffusion and collision of these two entities. Changes in FRET or BRET between GRK3ct and Gβγ fusion proteins should then indicate heterotrimer dissociation and collision of freely-diffusing GRK3ct and Gβγ. We use this system to show that G protein heterotrimers dissociate over a time course similar to rapid G protein signaling, and that rapid G protein signaling can occur between freely-diffusing Gβγ dimers and GRK.
2. Materials and Methods
2.1 Plasmid DNA constructs
GRK3 constructs contained amino acids G495-L688 of bovine GRK3 (NP_776925; a.k.a. β-adrenergic receptor kinases 2 or βARK2), preceded either by an initiating methionine, a myristic acid attachment peptide (mas; MGSSKSKTSNS) or a dual palmitoylation peptide derived from the first 20 amino acids of GAP 43 (mem; MLCCLRRTKQVEKNDEDQKI). The stop codon of GRK3 was replaced with a GGG linker, which was followed by either cerulean A206K [16], venus A206K [17], or theRenilla reniformis lucife rase variant Rluc8 [18]. For some experiments the R587Q mutation was introduced into GRK3 by PCR-based mutagenesis. The original masGRK3 construct was a generous gift from Dr. Stephen R. Ikeda (NIAAA, Rockville, MD). Gβγ-V was expressed by cotransfecting plasmids encoding amino acids 1-155 of venus fused to a GGSGGG linker and the n-terminus of human Gγ2 (venus1–155-Gγ2) and amino acids 156–239 of venus fused to a GGSGGG linker and the n-terminus of human Gβ1 (venus155–239-Gβ1) [19]. Gα i1-venus and Gα i1-Rluc8 constructs consisted of either venus A206K or Rluc8 inserted between residues 60 and 61, 91 and 92 or 121 and 122 of human Gα i1 C351G; the venus or Rluc8 polypeptides were flanked by either GGGG (for positions 60 and 91) or GG (for position 121) linkers. All constructs were made using an adaptation of the QuikChange (Stratagene, La Jolla, CA) mutagenesis protocol, were expressed from pcDNA3.1 (Invitrogen, Carlsbad, CA), and were verified by automated sequencing.
2.2 Cell culture, transfection and avidin-mediated crosslinking
HEK 293 cells (ATCC, Manassas, VA) were propagated in plastic flasks and on polylysine-coated glass coverslips according to the supplier’s protocol. Cells were transfected using polyethyleneimine or Fugene 6 (Roche Diagnostics GmbH, Mannheim, Germany) and were used for experiments 12–24 hours later. For most experiments human A1 adenosine receptors (A1Rs), Gα , Gβ, Gγ and GRK plasmids were transfected at a 1:2:1:1:1 ratio, and PTX (100 ng ml−1; List Biologicals, Campbell, CA) was added to the culture medium immediately after transfection. For avidin-mediated crosslinking cells were rinsed 3 times in buffer containing 150 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 12 mM glucose, 0.5 mM CaCl2, and 0.5 mM MgCl2 (pH 8.0), and incubated at room temperature for 15 minutes in 0.5 mg ml−1 NHS-sulfo-LC-LC-biotin (Pierce, Rockford, IL). Cells were washed an additional 3 times and incubated for 15 minutes in 0.1 mg ml−1 avidin. Avidin-crosslinked cells were used for experiments within 1 hour of crosslinking.
2.3 Confocal microscopy and FRAP
Confocal images were acquired using a Leica (Wetzlar, Germany) SP2 scanning confocal microscope and a 63X, 1.4 NA objective. For documenting FRET (Figure 1) cells were illuminated with the 458 nm line of a krypton-argon laser, and emission was monitored at 465–500 nm and 510–600 nm. For FRAP experiments (Figure 4) low intensity illumination (514 nm) was used during a control (prebleach) period, after which a 4 μm segment of the plasma membrane edge was irreversibly photobleached by increasing the laser intensity to 100%. Recovery of fluorescence into the bleached segment of plasma membrane was monitored for up to 3 minutes using low intensity illumination. Average pixel intensity in the bleached region was corrected for photobleaching during low intensity illumination, normalized and plotted versus time.
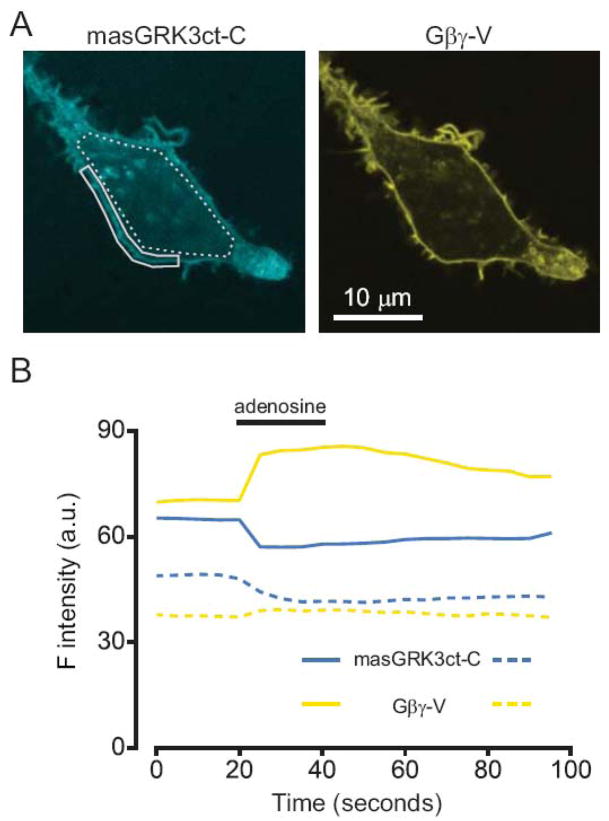
Figure 1.
masGRK3ct-cerulean is localized at the plasma membrane, and transfers energy to Gβγ-venus at the plasma membrane. (A) Confocal images of an HEK 293 cell expressing masGRK3ct-C and Gβγ-V; cerulean (C) was excited at 458 nm, and venus (V) was excited at 514 nm. Both proteins are expressed primarily at the plasma membrane. Regions of interest used for measurements in panel B are outlined in solid (for the plasma membrane) and broken (for the cell interior) lines. (B) Activation of A1 adenosine receptors (A1Rs) with adenosine (30 μM) reversibly increased V emission intensity (510–600 nm; yellow lines) and decreased C emission (465–500 nm; blue lines) after excitation at 458 nm, indicating an increase in FRET between masGRK3ct-C and Gβγ-V. The FRET increase occurred largely at that plasma membrane.
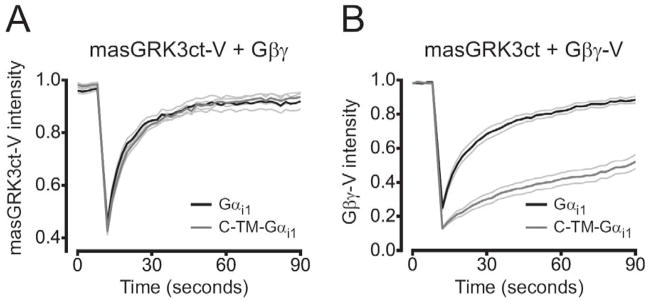
Figure 4.
masGRK3ct-V diffuses freely in the plasma membrane. (A) Recovery of masGRK3ct-V fluorescence into photobleached regions of the plasma membrane in cells expressing unlabeled Gβγ and either mobile Gα i1 (n=9) or immobile C-TM-Gα i1 (n=9). Immobile heterotrimers did not decrease the mobility of masGRK3ct-V. (B) Recovery of Gβγ-V fluorescence in cells expressing unlabeled masGRK3ct and either Gα i1 (n=11) or immobile C-TM-Gα i1 (n=10). Immobile heterotrimers significantly decreased the mobility of Gβγ-V. Lines represent the mean ± s.e.m.; photobleaching occurred at time=10 seconds.
2.4 FRET microfluorimetry
Single-cell photometry was carried out using an inverted Olympus IX-70 epifluorescence microscope equipped with a 100W Hg lamp, a heated stage (Warner Instruments, Hamden, CT) and a 40X 1.15NA water-immersion objective or a 60X 1.4 NA oil-immersion objective equipped with an objective heater (Bioptechs, Butler, PA). Excitation was controlled by a mechanical shutter (Uniblitz, Vincent Associates, Rochester, NY), and dual emission was measured using a pair of photodiodes (Till Photonics GmbH, Munich, Germany). For donor excitation the microscope was equipped with a donor excitation filter (420–450 nm; 440AF21) and dichroic mirror (455DCLP). For direct acceptor excitation the microscope was equipped with an acceptor excitation filter (485–510 nm; 500AF25) and dichroic mirror (525DCLP; Omega Optical, Brattleboro, VT). Fluorescence emission was directed through a dichroic mirror (505DCLP) to either a donor channel (460–500 nm; D480/40) or an acceptor channel (>515 nm; 515LP). Throughout each experiment fluorescence intensity was measured after donor excitation in both the donor (IDD) and acceptor (IDA) channels. At the end of each experiment acceptor intensity was measured after direct acceptor excitation (IAA). The FRET index NFRET [20] was calculated as:
where a is a correction factor for direct acceptor excitation (IDA/IAA measured in cells expressing acceptor only; a=0.40 for this setup), and d is a correction factor for donor bleedthrough into the acceptor channel (IDA/IDD measured in cells expressing donor only; d=0.56).
Cells were continuously perfused with or bathed in a solution containing 150 mM NaCl, 5mM KCl, 10 mM HEPES, 10 mM glucose, 1.5 mM CaCl2, and 2.5 mM MgCl2 (pH 7.2, ~320 mOsm kg−1 H2O). Solution exchanges were made using a multiport attachment and perfusion capillary positioned directly in front of the cell under study. In some experiments (Figure 6) adenosine was applied to cells in a heated static bath using a Picospritzer (General Valve Corp., Fairfield, NJ) and a patch pipette containing the above solution and 200 μM adenosine.
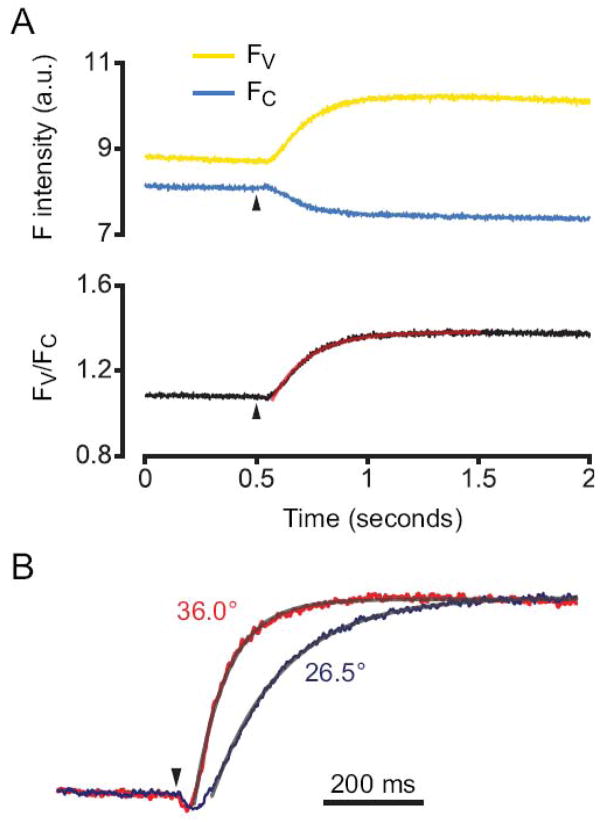
Figure 6.
Rapid, temperature-sensitive increases in FRET between masGRK3ct-C and Gβγ-V. FRET recordings are shown from cells expressing A1Rs, Gα oA, Gβγ-V and masGRKct-C. (A) Fluorescence intensity for Gβγ-V (FV; yellow line) and masGRK3ct-C (FC; blue line) and the ratio of the two (FV/FC; black line) recorded from an exemplary cell are plotted as a function of time before an after pressure application of adenosine from a pipette containing 200 μM adenosine at time=0.5 seconds (arrowhead). (B) The time course of FRET between masGRK3ct-C and Gβγ-V is temperature-sensitive with a Q10 of ~3. Normalized average FV/FC from cells recorded at 36.0°C (n=13; red line) and 26.5°C (n=10; blue line). In both panels the smooth lines were generated by fitting the rise in FV/FC to an exponential function.
2.5 BRET
Cells were harvested in PBS and transferred to opaque 96-well microplates (Nunc, Thermo Scientific, Rochester, NY). Benzyl-coelenterazine (coelenterazine h; 5 μM; Nanolight Technologies, Pinetop, AZ) was added immediately prior to making measurements, which were made using a photon-counting mulitmode plate reader (Mithras LB940; Berthold Technologies GmbH, Bad Wildbad, Germany). The raw BRET signal (em535/480) was calculated as the emission intensity at 520–545 nm divided by emission intensity at 475–495 nm. Net BRET was this ratio minus the same ratio measured from cells expressing only the BRET donor (Rluc8). Steady-state BRET measurements (e.g. Figures 2C and 5) were made ~5 minutes after the addition of agonist.
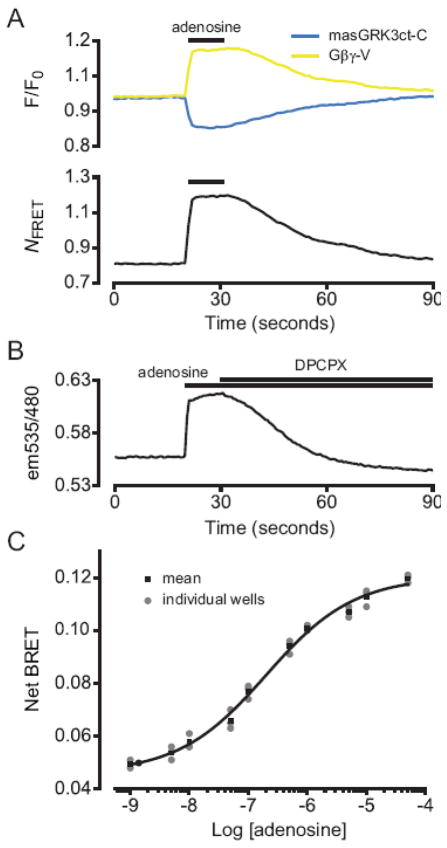
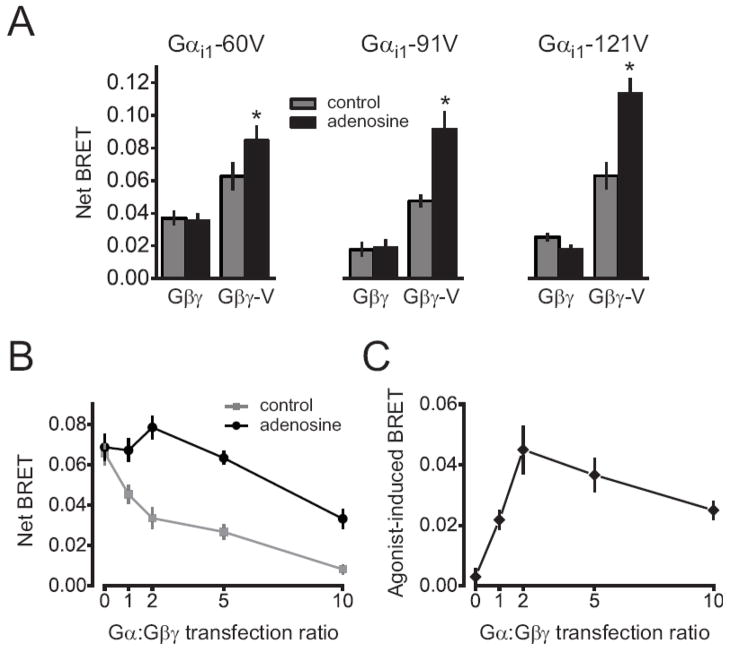
Figure 2.
Agonist-induced increases in FRET and BRET between masGRK3ct fusion proteins and Gβγ-V. (A) Activation of A1Rs with 30 μM adenosine reversibly increases normalized V emission intensity (F/F0; yellow line) and decreases C emission (blue line), resulting in an increase in the FRET index NFRET (see Materials and Methods). V and C emission were monitored simultaneously using microfluorimetry from the entirety of single cells expressing A1Rs, Gα i1, masGRK3ct-C and Gβγ-V; traces represent the average of 10 cells. (B) Activation of A1Rs with 30 μM adenosine increases BRET, as indicated by an increase in the ratio of acceptor (Gβγ-V) to donor (masGRK3ct-Rluc8) emission (em535/480). The agonist-induced BRET increase was reversed by subsequent addition of the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 2 μM). Cells expressed A1Rs, Gα i1, masGRK3ct-Rluc8 and Gβγ-V. Acceptor and donor emission were monitored sequentially for each time point using a plate reader; the average of signals recorded from 8 wells of a 96-well plate is shown. (C) Net BRET (see Materials and Methods) is plotted as a function of adenosine concentration; the smooth line was generated by fitting the mean data to a logistic function (EC50 = 186 nM).
Figure 5.
masGRK3ct-Rluc8 binds to free Gβγ dimers instead of rearranged Gα βγ heterotrimers. (A) A1R activation increases BRET between masGRK3ct-Rluc8 and Gβγ-V, but not between Gα-V and masGRK3ct-Rluc8. Net BRET is plotted in the absence (gray bars) and presence (black bars) of 10 μM adenosine. Cells expressed Gα i1 with venus fused after amino acid 60, 91 or 121, masGRK3ct-Rluc8, A1Rs and either unlabeled Gβγ or Gβγ-V. Basal BRET was observed between all Gα i1-V variants and masGRK3ct-Rluc8. Each bar represents the mean ± s.e.m. of 6 independent experiments performed in triplicate; *, P<0.005, paired t-test. (B and C) Increasing Gα expression decreases basal and agonist-induced BRET between masGRK3ct-Rluc8 and Gβγ-V. Cells expressed C-TM-Gα i1, masGRK3ct-Rluc8, A1Rs and Gβγ-V. In panel B net BRET is plotted as a function of the ratio of C-TM-Gα i1 to Gβ (and Gγ) plasmid DNA used for transfection, in the absence (gray points) and presence (black points) of 10 μM adenosine. In panel C the agonist-induced BRET change is plotted versus transfection ratio. Each data point represents the mean ± s.e.m. of 4 independent experiments performed in triplicate.
3. Results
3.1 RET between GRK3ct fusion proteins and Gβγ-V
The crystal structure of the GRK2-Gβγ complex indicates that the c-terminus of GRK2 extends in the general direction of the n-termini of Gβ and Gγ [15], suggesting that labels attached to these points would be close enough to each other to transfer energy. Therefore, we fused the n-termini of Gβ1 to and Gγ2 to complementary fragments of the yellow fluorescent protein venus (V) [17]. Coexpression of these proteins produces functional Gβγ dimers labeled with venus (Gβγ-V) [19] to serve as FRET or BRET acceptors. The c-terminal pleckstrin homology domain of GRK3 (the final 141 amino acids) was fused to the blue fluorescent protein cerulean (C) to serve as a FRET donor [16] or to a Renilla luciferase mutant (Rluc8) [18] to serve as a BRET donor. These GRK constructs were targeted to the plasma membrane by adding n-terminal peptides directing either myristoylation (mas; e.g. masGRK3ct-C) or dual palmitoylation (mem; e.g memGRK3ct-C; see Materials and Methods).
Gβγ-V and masGRK3ct-C were expressed in HEK 293 cells together with unlabeled Gα subunits and A1 adenosine receptors (A1Rs). Confocal microscopy indicated that both of the labeled proteins were located at the plasma membrane (Figure 1A). Activation of A1Rs with 30 μM adenosine increased Gβγ-V emission and decreased masGRK3ct-C emission after excitation of masGRK3ct-C (at 458 nm), consistent with an increase in FRET between the two proteins (Figure 1B). Agonist-induced changes were greatest in regions of interest centered over the plasma membrane, indicating that FRET signals generated by masGRK3ct-C and Gβγ-V originate primarily at the plasma membrane. Smaller changes in V and C emission occurred over intracellular regions, most likely due to stray emission from the plasma membrane and translocation of some masGRK3ct-C from the intracellular compartments to the plasma membrane. In either case signals reflect the interaction between masGRK3ct-C and Gβγ dimers.
We then measured adenosine-induced FRET changes with microfluorometry (Figure 2A), collecting fluorescence emission from the entirety of single cells. Adenosine rapidly increased Gβγ-V emission and decreased masGRK3ct-C emission after excitation of masGRK3ct-C (at 420–450 nm). Similar results were obtained when either Gα i1 or Gα oA were coexpressed with Gβγ-V, masGRKct-C and A1Rs. Adenosine reversibly increased the FRET index NFRET [20] by 52 ± 10% in cells expressing Gα i1 (n=10), and by 59 ± 9% in cells expressing Gα oA (n=10). Myristoylated (masGRK3ct-C; Figure 2A) and dually-palmitoylated (memGRK3ct-C; data not shown) GRK3 FRET donors were functionally indistinguishable, and most experiments were carried out with masGRK3ct-C.
FRET microfluorimetry is ideal for measuring the kinetics of protein interaction (see below). However, this technique is less convenient for measuring parameters such as agonist sensitivity, or for comparing multiple cell populations. For this type of experiment we instead employed masGRK3ct-Rluc8 as a BRET donor. Adenosine reversibly increased BRET between masGRK3ct-Rluc8 and Gβγ-V in cell populations (Figure 2B). Steady-state agonist sensitivity was measured using adenosine concentrations ranging from 10−9 M to 10−4 M (e.g. Figure 2C). In three independent experiments EC50 values derived by fitting the concentration-response data to the Hill equation were 201 nM, 362 nM and 186 nM. As a means of demonstrating the specificity of these signals, we performed parallel experiments with masGRK3ct-Rluc8 bearing a point mutation (R587Q, numbered relative to full-length GRK3) of a contact residue that has previously been shown to abolish Gβγ sensitivity in an in vitro assay [21]. Adenosine (50 μM) failed to increase BRET between masGRK3ct-Rluc8 R587Q and Gβγ-V (change in net BRET −0.003 ± 0.001, n=3 independent experiments).
3.2 GRK3ct fusion proteins are diffusible Gβγ sensors
One of our goals was to produce a Gβγ indicator that could be shown to be freely-diffusible, i.e. one that is not part of a preassembled signaling complex. Intact GRK2 and GRK3 are located in the cytosol of unstimulated cells, and are recruited to the plasma membrane only after G protein activation [14]. We confirmed this general arrangement by fusing venus to the c-terminus of GRK3ct (without a membrane-targeting peptide), and acquiring confocal images of cells expressing GRK3ct-V, A1 adenosine receptors (A1Rs), Gα oA and unlabeled Gβ1 and Gγ2 subunits. As shown previously for full-length GRK2 [22], GRK3ct-V was located primarily in the cytosol, and receptor activation induced a rapid translocation of GRKct-V from the cytosol to the plasma membrane (Figure 3). GRK3ct-V translocation occurred with a single-exponential time constant of 431 ± 35 ms (n=9) at room temperature. This result suggests that GRK3ct fusion proteins do not form high-affinity complexes with membrane proteins prior to G protein activation.
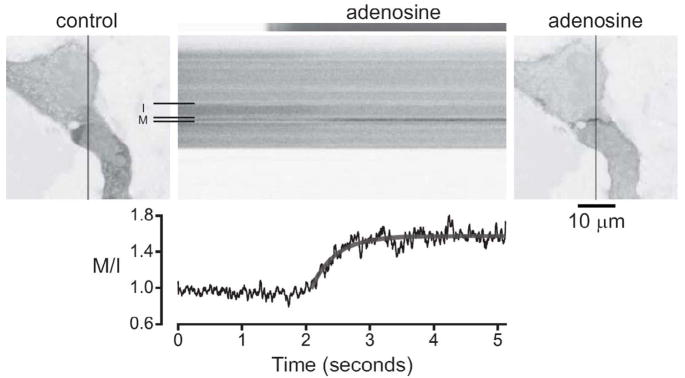
Figure 3.
GRK3ct-V is rapidly translocated to the plasma membrane. Confocal images of HEK 293 cells expressing GRK3ct-V, A1Rs, Gagr;oA and Gβγ are shown before (control) and after (adenosine) application of 30 μM adenosine. Line scanning was performed during the experiment (shown in the center panel) to capture translocation of GRK3ct-V from intracellular (I) to membrane (M) compartments (indicated by the horizontal lines) with high temporal resolution. The adenosine solution exchange time was measured by performing an identical series of line scans during perfusion with a fluorescent dye (shown at the top of the center panel). The graph shows the M/I intensity ratio as a function of time, scaled to correspond to the scan series. The smooth line was generated by fitting the rising phase of the translocation to an exponential function. Vertical lines in the images indicate the position of the line scan; an inverted grayscale lookup table is used to enhance contrast and clarity.
To further test the possibility that GRK3ct fragments (including membrane-associated fragments) preassociate with G proteins, we made use of a fluorescence recovery after photobleaching (FRAP) assay that detects stable protein-protein interactions [7]. In these experiments the lateral mobility of masGRK3ct-V was measured in the presence of mobile or immobile heterotrimers, with the expectation that immobilizing heterotrimers would decrease the mobility of masGRKct-V if the two formed a stable complex. In addition to masGRKct-V cells expressed unlabeled Gβ1 and Gγ2 subunits, and either Gα i1 or C-TM-Gα i1, which consists of an extracellular ECFP moiety, a transmembrane domain and Gα i1. We have shown previously that C-TM-Gα i1 forms functional heterotrimers [7], and we verified the ability of heterotrimers containing C-TM-Gα i1 to signal to GRK3ct fusion proteins (see below). C-TM-Gα i1-containing heterotrimers were immobilized with avidin-mediated crosslinking (see Materials and Methods), whereas Gα i1-containing heterotrimers were protected from crosslinking and remained mobile. The mobility of masGRK3ct-V was identical when this protein was expressed with heterotrimers containing immobile C-TM-Gα i1 or mobile Gα i1 (Figure 4). The time to half recovery (T½) of masGRK3ct-V fluorescence after photobleaching was 11 ± 1 s with C-TM-Gα i1, and 10 ± 1 s with Gα i1 (n=9; P=0.33). The extent of fluorescence recovery at 90 seconds was 88 ± 4% with C-TM-Gα i1, versus 83 ± 5% with Gα i1 (n=9; P=0.46). As a positive control, immobilizing C-TM-Gα i1 (in the presence of unlabeled masGRK3ct) dramatically decreased the mobility of Gβγ-V [7]. In seven of ten cells Gβγ-V fluorescence did not recover to half of the prebleach intensity within 90 seconds, and T½ was >40 seconds in the three remaining cells, whereas T½ was 15 ± 2 s with Gα i1 (n=11). The extent of Gβγ-V fluorescence recovery at 90 seconds was 45 ± 4% with C-TM-Gα i1 (n=10), and 86 ± 3% with Gα i1 (n=11; P<0.001). One possible reason why immobile C-TM-Gα i1 failed to decrease masGRK3ct-V mobility is that there was an excess of the latter protein. However, the intensity of masGRK3ct-V in the bleached regions of interest (131 ± 15 arbitrary units; a.u.; n=9) was not significantly different from the intensity of Gβγ-V (113 ± 10 a.u.; n=10; P=0.34), suggesting there was not a relative overabundance of masGRK3ct-V. Expression of C-TM-Gα i1 was also similar in these two conditions (76 ± 15 a.u. vs. 64 ± 10 a.u.; P=0.49). These results suggest that GRK3ct fusion proteins are not stably preassociated with heterotrimeric G proteins.
3.3 GRK3ct fusion proteins bind to free Gβγ
The primary goal of our experiments was to produce an indicator that detected free Gβγ dimers as opposed to Gβγ dimers that are still associated with Gα subunits. It has been suggested that heterotrimers are sufficiently flexible to allow the binding of effectors while Gα and Gβγ remain associated via the n-terminus of Gα [23]. However, GRK2 binds to regions Gβγ that interact with both the switch regions and the n-terminus of Gα [15], suggesting it is unlikely that Gβγ can bind to a GRK and Gα simultaneously. Nevertheless we designed experiments to determine if GRK3ct fusion proteins could bind to rearranged Gα βγ-V.
We first attempted to detect agonist-induced increases in BRET between masGRK3ct-Rluc8 and venus-labeled Gα subunits. In order to increase the likelihood of producing a permissive orientation of the V and Rluc8 moieties we inserted V in three different locations (after amino acids 60, 91 and 121; Gα i1-60V, Gα i1-91V and Gα i1-121V) in the all-helical domain of Gα i1. All three locations have been previously shown to support agonist-induced changes in energy transfer between Gα and Gβγ [1, 5]. However, adenosine failed to change BRET between these Gα i1-V contstructs and masGRK3ct-Rluc8 when they were coexpressed with A1Rs and unlabeled Gβ1 and Gγ2 subunits (Figure 5A). Modest basal BRET between Gα i1-V and masGRK3ct-Rluc8 was observed. However, this signal was not decreased by overexpression of unlabeled masGRK3ct, and was therefore considered to be non-specific. All three Gα i1-V fusion proteins were functional, as each could support adenosine-induced increases in BRET if Gβγ was also labeled with venus (Figure 5A). These results suggest that if masGRK3ct-Rluc8 binds to rearranged heterotrimers, it does so in a manner that does not alter net energy transfer to Gα i1-60V, Gα i1-91V or Gα i1-121V.
The failure to detect BRET between masGRK3ct-Rluc8 and Gα-V is consistent with binding of the former to free Gβγ dimers, but the possibility remains that masGRK3ct-Rluc8 binds to rearranged heterotrimers in a configuration that does not change energy transfer to Gα-V subunits. Therefore, we designed an additional experiment to differentiate binding of masGRK3ct-Rluc8 to rearranged heterotrimers from binding to free Gβγ dimers. This experiment is based on the premise that excess free Gα subunits can bind to (and sequester) free Gβγ dimers, but can not bind to Gβγ dimers that are still associated with another Gα subunit. The second possibility would require Gβγ dimers to bind to two Gα subunits at the same time (see below). If free Gα subunits can interact only with free Gβγ dimers, then free Gα subunits will have no effect on binding of masGRK3ct-Rluc8 to Gβγ-V dimers that are part of rearranged heterotrimers. Therefore, overexpression of Gα would be expected to have no effect on the agonist-induced increase in BRET between Gα βγ-V and masGRK3ct-Rluc8. In contrast, free Gα subunits will compete with masGRK3ct-Rluc8 for binding to free Gβγ-V, and thus overexpression of Gα would decrease the agonist-induced increase in BRET between Gβγ-V and masGRK3ct-Rluc8.
To test this idea we progressively increased the amount of plasmid DNA used to express Gα subunits, while keeping the amount of DNA used to express Gβγ dimers constant. For this experiment we again used C-TM-Gα i1, as we have shown previously that expression of C-TM-Gα subunits at the plasma membrane can be increased by increasing the amount of plasmid DNA used for transfection [8]. Basal BRET (prior to receptor activation) between Gβγ and masGRK3ct-Rluc8 was greatest without C-TM-Gα i1 expression, and gradually decreased as C-TM-Gα i1 expression increased (Figure 5B). The adenosine-induced BRET increase also decreased from 0.050 ± 0.007 to 0.025 ± 0.003 (n=6 independent experiments; P<0.05, paired t-test) as the relative expression of C-TM-Gα i1 increased. Thus overexpression of C-TM-Gα subunits interfered with the interaction between masGRK3ct-Rluc8 and Gβγ-V. Similar results were obtained with graded transfection of unlabeled Gα subunits (data not shown). To test the possibility that Gβγ-V bound to two Gα subunits simultaneously we measured BRET between Gα i1-V and Gα i1-Rluc8 before and after activation of A1Rs. If receptor activation in the presence of excess Gα subunits leads to the formation of Gα-Gβγ-Gα complexes, then BRET between Gα i1-V and Gα i1-Rluc8 subunits that become part of individual quaternary complexes might be detectable. Basal BRET was detected between Gα i1-91V and Gα i1-121Rluc8 or Gα i1-121V and Gα i1-91Rluc8 (expressed with unlabeled Gβγ and A1Rs), but adenosine did not increase this BRET signal. The change in the BRET ratio for these two combinations was −0.003 ± 0.001 and −0.002 ± 0.001 respectively (n=3 independent experiments; P>0.05). Therefore, if Gβγ dimers interacted with two Gα subunits simultaneously, they did so in a manner that did not detectably increase or decrease BRET between these subunits. Taken together these results support the conclusion that masGRK3ct binds to free Gβγ dimers, and thus serves as an indicator of heterotrimer dissociation.
3.4 The time course of masGRK3ct-C binding to Gβγ-V
Finally, we were interested in measuring the time course of Gβγ binding to masGRK3ct. For these experiments we returned to FRET microfluorimetry, as this technique allows measurements with high temporal resolution. The most rapid signals known to be mediated by Gβγ dimers are activation of inwardly-rectifying potassium (GIRK) currents and inhibition of voltage-gated calcium channels in CNS neurons [24–28], both of which develop over the course of tens to hundreds of milliseconds. Since the most abundant Gα isoform is CNS neurons is Gα o [29, 30], we focused on the onset kinetics of FRET signals mediated by Gα oA and A1Rs. Adenosine was pressure-applied to individual cells from a micropipette positioned within one cell diameter of the cell under study. FRET between masGRK3ct-C and Gβγ-V increased after adenosine application monotonically with a time constant of 213 ± 32 ms (n=10) at 26.5 °C (Figure 6A). Raising the temperature to 37 °C decreased this time constant to 67 ± 13 ms (n=13; Figure 6B). The onset kinetics and temperature-sensitivity of FRET between masGRK3ct-C and Gβγ-V are thus very similar to the onset kinetics and temperature-sensitivity of Gβγ-mediated ion channel modulation [26].
4. Discussion
The main objective of this study was to produce an indicator of free Gβγ dimers that could be used to study the time course of heterotrimer dissociation in living cells. Previous studies of FRET between Gα and Gβγ have shown that G proteins are activated rapidly [5], but this approach does not provide unambiguous evidence of physical dissociation. Dissociation is revealed by methods that measure macroscopic translocation of G protein subunits [6, 7], but these methods lack the temporal resolution necessary to measure the time course of dissociation. In the present study we used GRK3ct fusion proteins as indicators of Gβγ subunits. We conclude that GRK3ct fusion proteins bind to free Gβγ dimers and not to Gβγ dimers that are part of rearranged heterotrimers, and thus can report heterotrimer dissociation with high temporal resolution.
The conclusion that GRK3ct binds only to free Gβγ dimers is based on several arguments and observations. First, the GRK-Gβγ interface includes Gβγ residues that contact both the switch regions and the α N helix of Gα [15]. Therefore, binding of GRK3ct to Gβγ is likely to require complete dissociation of Gα . Previous biochemical studies have shown that GRK and Gα binding to Gβγ are mutually exclusive, and that this competition is only partially relieved by activation of Gα with GTPγS [31]. This finding does not rule out the possibility that GRK binds to rearranged heterotrimers that contain Gα-GTPγS and Gβγ, but it does suggest that even active (GTPγS-bound) Gα interferes with the interaction between GRK and Gβγ.
Second, we observed non-specific basal BRET between three different Gα i1-V subunits and masGRK3ct-Rluc8, but activation did not change this signal. This suggests that the two proteins were randomly arranged in a manner that permitted BRET to occur, and that activation did not significantly change this arrangement. Therefore, if masGRK3ct-Rluc8 bound to Gβγ dimers that were still associated with Gα i1-V subunits, then it must have done so in a manner that did not change BRET with Gα i1-V. This seems like a remote possibility, particularly in light of the fact that no agonist-induced change was observed when the BRET acceptor (venus) was located at any of three different positions in the Gα all-helical domain. When both Gα i1 and Gβγ were labeled with venus the agonist-induced BRET signal was comparable to that observed with unlabeled Gα i1, suggesting the addition of the venus moiety did not interfere with the masGRK3ct-Rluc8-Gβγ interaction.
Third, overexpression of unlabeled Gα decreased agonist-induced BRET between masGRK3ct-Rluc8 and Gβγ-V. The most likely explanation for this is that free Gα subunits served as a sink for Gβγ-V dimers. If masGRK3ct-Rluc8 bound to Gβγ-V dimers that were part of rearranged heterotrimers, then free Gα subunits could only interfere with this process by also binding to rearranged heterotrimers, meaning a single Gβγ dimer would have to interact with two Gα subunits at once. Again, we think that this is an unlikely possibility, particularly since we did not observe agonist-induced BRET between Gα i1-Rluc8 and Gα i1-V subunits under these conditions. Taken together these observations strongly suggest that GRK3ct binds to free Gβγ dimers and not to rearranged heterotrimers.
The conclusion that GRK3ct binds exclusively to free Gβγ dimers has several important implications. FRET signals between masGRK3ct-C and Gβγ-V developed over the course of ~100 milliseconds, which is similar to previously-reported rates of G protein activation measured using FRET and BRET between labeled Gα subunits and Gβγ dimers [1, 5]. This suggests that physical dissociation follows G protein activation in living cells without a significant delay. This time course is also comparable to the time course of regulation of several Gβγ effectors, including GIRK channels [26, 28] and voltage-gated calcium channels [27], suggesting that these effectors may be engaged by free Gβγ dimers. Indeed, agonist-induced regulation of several Gβγ effectors (including GIRK channels and voltage-gated calcium channels) is inhibited by GRKct peptides [32–34]. If these peptides bind only to free Gβγ dimers, this suggests that physical dissociation of heterotrimers is a common prerequisite for Gβγ signaling.
If physical dissociation of heterotrimers is required for signaling by Gβγ dimers, then lateral diffusion of free Gβγ and collision with effector molecules will also be required. Diffusion could impose a limit on the rate of signaling if active G proteins and effectors are, on average, separated by sufficiently large distances. Signaling by preformed complexes that contain GPCRs, G proteins and acceptors would not require diffusion, thus the assembly of such complexes could be important for rapid signaling. The rapid activation of GIRK channels is one signaling event that is thought to involve preassembled complexes [11, 12]. In contrast, GRKs are not known to form preassembled complexes with GPCRs or G proteins, and our results suggest that GRKct fusion proteins and G proteins diffuse freely and independently. Our results do not directly address GIRK channel activation. However, we do show that signaling that involves diffusion and collision of Gβγ dimers and GRK3ct fusion proteins can be as rapid as activation of GIRK channels. The rates of agonist-induced FRET between Gβγ-V and masGRK3ct-C and GIRK channel activation are both temperature-sensitive, with similar Q10 values of ~3 [26]. This suggests that neither process is rate-limited by diffusion, and thus that both are likely to be limited by intramolecular (or intracomplex) processes such as guanine nucleotide exchange. We emphasize that our present results were obtained in a model preparation where the involved signaling molecules were almost certainly overexpressed compared to their abundance in native tissues. The rate limit imposed by diffusion and collision of GPCRs, G proteins and effectors in native cells could be significantly slower. Additional experiments will be required to determine the relative importance of signaling within complexes and signaling by diffusion and collision.
5. Conclusions
GRK3ct fusion proteins (in combination with labeled Gβγ dimers) can be used as time-resolved indicators of heterotrimeric G protein activation and dissociation. G protein heterotrimers dissociate over a time course similar to rapid G protein signaling. Rapid G protein signaling can occur between freely-diffusing Gβγ dimers and GRK3ct fusion proteins.
Acknowledgments
We thank Dr. Steve Ikeda for the gift of masGRK3ct and Dr. Sanjiv Sam Gambhir for Rluc8. This work was supported by grants from the American Heart Association (0715111 to G.J.D.), the National Science Foundation (MCB 0620024 to N.A.L.) and the National Institutes of Health (GM078319 to N.A.L.).
Abbreviations
- FRET
Förster resonance energy transfer
- BRET
bioluminescence resonance energy transfer
- GRK
G protein receptor kinase
- HEK
human embryonic kidney
- Rluc8
Renilla luciferase 8
- C
cerulean
- V
venus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 2.Gales C, Rebois RV, Hogue M, Trieu P, Breit A, Hebert TE, Bouvier M. Nat Methods. 2005;2(3):177–184. doi: 10.1038/nmeth743. [DOI] [PubMed] [Google Scholar]
- 3.Nobles M, Benians A, Tinker A. Proc Natl Acad Sci U S A. 2005;102(51):18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hein P, Frank M, Hoffmann C, Lohse MJ, Bunemann M. Embo J. 2005;24(23):4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunemann M, Frank M, Lohse MJ. Proc Natl Acad Sci U S A. 2003;100(26):16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akgoz M, Kalyanaraman V, Gautam N. J Biol Chem. 2004;279(49):51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 7.Digby GJ, Lober RM, Sethi PR, Lambert NA. Proc Natl Acad Sci U S A. 2006;103(47):17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Digby GJ, Sethi PR, Lambert NA. J Physiol. 2008;586(14):3325–3335. doi: 10.1113/jphysiol.2008.153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Buranda T, Simons PC, Lopez GP, McIntire WE, Garrison JC, Prossnitz ER, Sklar LA. Anal Biochem. 2007;371(1):10–20. doi: 10.1016/j.ab.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowal L, Provitera P, Scarlata S. J Biol Chem. 2006;281(33):23999–24014. doi: 10.1074/jbc.M512330200. [DOI] [PubMed] [Google Scholar]
- 11.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. J Cell Sci. 2006;119(Pt 13):2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 12.Riven I, Iwanir S, Reuveny E. Neuron. 2006;51(5):561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Lohse MJ, Nikolaev VO, Hein P, Hoffmann C, Vilardaga JP, Bunemann M. Trends Pharmacol Sci. 2008;29(3):159–165. doi: 10.1016/j.tips.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Science. 1992;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 15.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Science. 2003;300(5623):1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo MA, Springer GH, Granada B, Piston DW. Nat Biotechnol. 2004;22(4):445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 17.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. Nat Biotechnol. 2002;20(1):87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 18.Loening AM, Fenn TD, Wu AM, Gambhir SS. Protein Eng Des Sel. 2006;19(9):391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- 19.Hynes TR, Tang L, Mervine SM, Sabo JL, Yost EA, Devreotes PN, Berlot CH. J Biol Chem. 2004;279(29):30279–30286. doi: 10.1074/jbc.M401432200. [DOI] [PubMed] [Google Scholar]
- 20.Xia Z, Liu Y. Biophys J. 2001;81(4):2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carman CV, Barak LS, Chen C, Liu-Chen LY, Onorato JJ, Kennedy SP, Caron MG, Benovic JL. J Biol Chem. 2000;275(14):10443–10452. doi: 10.1074/jbc.275.14.10443. [DOI] [PubMed] [Google Scholar]
- 22.Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM. J Biol Chem. 1999;274(11):7565–7569. doi: 10.1074/jbc.274.11.7565. [DOI] [PubMed] [Google Scholar]
- 23.Rebois RV, Warner DR, Basi NS. Cell Signal. 1997;9(2):141–151. doi: 10.1016/s0898-6568(96)00133-7. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda SR. Nature. 1996;380(6571):255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 25.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. Nature. 1987;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 26.Otis TS, De Koninck Y, Mody I. J Physiol. 1993;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfrieger FW, Gottmann K, Lux HD. Neuron. 1994;12(1):97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 28.Sodickson DL, Bean BP. J Neurosci. 1996;16(20):6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huff RM, Axton JM, Neer EJ. J Biol Chem. 1985;260(19):10864–10871. [PubMed] [Google Scholar]
- 30.Sternweis PC, Robishaw JD. J Biol Chem. 1984;259(22):13806–13813. [PubMed] [Google Scholar]
- 31.Touhara K, Inglese J, Pitcher JA, Shaw G, Lefkowitz RJ. J Biol Chem. 1994;269(14):10217–10220. [PubMed] [Google Scholar]
- 32.Delmas P, Brown DA, Dayrell M, Abogadie FC, Caulfield MP, Buckley NJ. J Physiol. 1998;506(Pt 2):319–329. doi: 10.1111/j.1469-7793.1998.319bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. J Biol Chem. 1994;269(8):6193–6197. [PubMed] [Google Scholar]
- 34.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Nature. 1994;370(6485):143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]