Abstract
Centrosomes control microtubule dynamics in many cell types, and their removal from the cytoplasm leads to a shift from dynamic instability to treadmilling behavior and to a dramatic decrease of microtubule mass (Rodionov et al., 1999; PNAS 96:115). In cadherin-expressing cells, these effects can be reversed: non-centrosomal cytoplasts that form cadherin-mediated adherens junctions display dense arrays of microtubules (Chausovsky et al., 2000; Nature Cell Biol 2:797). In adherens junctions, cadherin’s cytoplasmic domain binds p120 catenin and β-catenin, which in turn binds α-catenin. To elucidate the roles of the cadherin-associated proteins in regulating microtubule dynamics, we prepared GFP-tagged, plasma membrane targeted or untargeted p120 catenin, α-catenin and β-catenin and tested their ability to rescue the loss of microtubule mass caused by centrosomal removal in the poorly adhesive cell line CHO-K1. Only membrane targeting of α-catenin led to a significant increase in microtubule length and density in centrosome-free cytoplasts. Expression of non-membrane-targeted α-catenin produced only a slight effect, while both membrane-targeted and non-targeted p120 and β-catenin were ineffective in this assay. Together, these findings suggest that α-catenin is able to regulate microtubule dynamics in a centrosome-independent manner.
Keywords: alpha-catenin, microtubules, beta-catenin, p120ctn, adherens junction, centrosome, cadherins, cytoplasts
Introduction
The dependence of microtubules (MTs) on the presence of the centrosome is related to the ‘lifestyle’ of the cell. In cells that maintain a centrosomally focused array of MTs, such as most fibroblasts, MT stability depends on the minus end being anchored in the centrosome, more precisely in the pericentriolar material surrounding the mother centriole.1,2 In contrast, epithelial and neuronal cells maintain large populations of MTs that have no apparent connection to the centrosome.2–4 While several mechanisms of the stabilization of non-centrosomal MTs have been proposed,5 the pathways activating these or other mechanisms of this kind specifically in epithelial or neural cells are not known. Recent observations suggest that stabilization of noncentrosomal microtubules is promoted via their interaction with basal cortex of polarized epithelial cells.6
A characteristic feature of epithelial cells is that they form cadherin-based contacts (adherens junctions or AJs) with neighboring cells. Cadherins and associated AJ proteins are serving not only as adhesion molecules but produce a variety of signals determining the major features of epithelial cell phenotype.7–9 Cytoplasmic domains of AJ cadherins bind armadillo family proteins, β-catenin and plakoglobin at the C-terminus,10 and p120-catenin at the juxtamembranal part.11,12 β-catenin and plakoglobin are in turn associated with α-catenin, a protein known to interact with numerous cytoskeletal partners, such as α-actinin, vinculin, actin and formin-1.13 Though α-catenin was thought for many years to form a direct link between actin cytoskeleton and cadherin-based junctional complexes, more recent studies do not confirm this idea, stressing that α-catenin interacts with β-catenin only in monomeric and with actin only in dimeric form.14–16 At the same time α-catenin can potentially affect the actin cytoskeleton both by regulating Arp2/3 complex-dependent actin polymerization,14 and via interaction with an actin-regulatory protein, formin-1.17 In addition, α-catenin is involved in several other signaling pathways.18
In our previous study19 we investigated whether the non-centrosomal pattern of MT organization characteristic of the epithelial ‘lifestyle’ could be induced by driving formation of cadherin cell-cell contacts in a non-epithelial cell. We used well-known Chinese Hamster Ovary cell line (CHO) and its cloned subline CHO-K1 as a model system because these fibroblast-like cells display the radial, centrosomal pattern of MTs characteristic of the ‘fibroblast lifestyle’ and contain no or low levels of E- and N-cadherin. We found that expression of classic cadherins and formation of cell-cell contacts induced a change in the pattern of MT organization to the epithelial-type ‘lifestyle’. Based on this result, we proposed that cadherins initiate a signaling pathway that alters MT organization, promoting formation and/or stabilization of non-centrosomal MTs.19 Here, we begin testing this hypothesis by attempting to identify the immediate downstream steps in the signaling pathway. Our results point to an important role for α-catenin.
Results
Assessment of microtubule total length in cytoplasts containing and lacking centrosomes
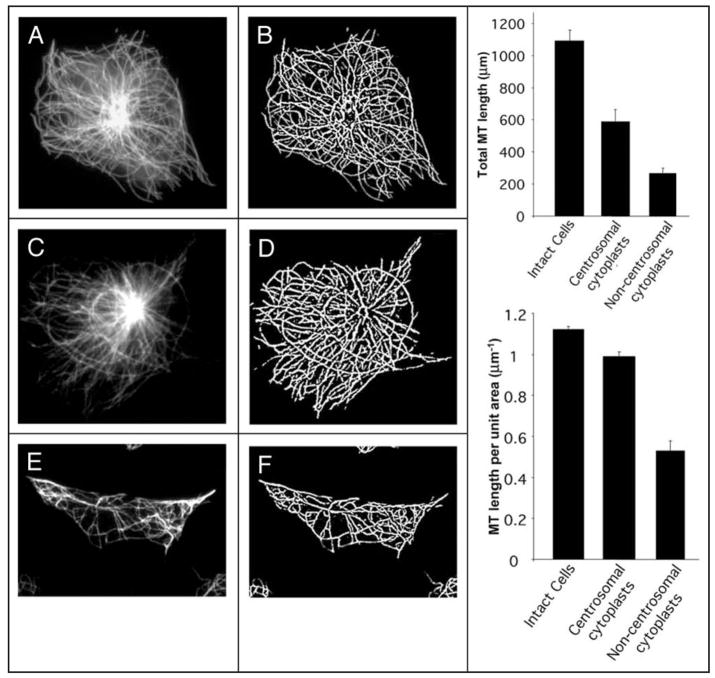
In cytoplasts containing a centrosome, the MT array is radial and the density is high, similar to that of intact cells (Fig. 1A–D). In cytoplasts lacking the centrosome, if prepared from control CHO-K1 cells, the MT array is random and sparse (Fig. 1E and F). We used total length and density of the MTs in centrosome-free cytoplasts as quantitative parameters for the assessment of the effects of experimental manipulations on the non-centrosomal MTs.
Figure 1.
Quantification of the MT length and density in CHO-K1 cells and cytoplasts. (A, C and E) α-tubulin antibody staining of MTs in intact cell (A), centrosome-containing (C), and centrosome-lacking (E) cytoplasts. (B, D and F) Processed images of MT networks produced from A, C and E images, respectively, using the “FiberScore” program described by Lichtenstein et al. (2003). Graphs on the right represent (top) average total MT length per cell or per cytoplast with or without centrosome; (bottom) average MT densities (total MT length per cell or cytoplast area) in cells and in cytoplasts. Error bars correspond to standard errors of mean (SEM).
In our previous study, we estimated the density of MTs in a cell or cytoplast by total tubulin immunofluorescence after triton X-100 extraction. This procedure gave a value for MT density in terms of arbitrary fluorescence units per unit area.19,20 It provided reliable relative measurements for examination of variables within a single experiment, but did not provide data that could be directly compared between experiments. In this study, we improved our measurements of tubulin polymer by means of custom software21 that recognized and segmented fibrillar elements in the immunofluorescence image satisfying the criteria of MT staining (Fig. 1A–F). The derived images (Fig. 1B, D and F) allowed measurement of total MT length (Fig. 1, upper right graph) and MT density as the integrated length per unit of projected cell area (lower right graph). Thus, the procedure provides parameters characterizing the MT network that can be compared across experiments and even in different cell systems.
Average value of MT length for cytoplasts containing centrosomes is 585 ± 77(SEM) μm per cytoplast. This value is apparently lower than the amount of MT polymer in intact cells (1092 ± 66 μm), which simply reflects the fact that the cytoplasts are on average smaller than cells. Indeed, the MT polymer amount normalized per unit of projected area was essentially the same for intact cells and centrosome-containing cytoplasts (about 0.99 ± 0.02 μm/μm2). Cytoplasts lacking centrosomes, however, gave significantly lower values of MT length, 262 ± 38 μm per cytoplast, or 0.52 ± 0.05 μm/μm2, which are approximately 50% of the values for centrosome-containing cytoplasts. Thus, centrosome-free cytoplasts contain significantly lower amounts of microtubule polymer than intact cells or centrosome-containing cytoplasts.
Targeting of cadherin-catenin complex components to plasma membrane
Previously, we suggested that cadherins regulation of MT dynamics was mediated by the cadherin cytoplasmic tail.19 Here, we intend to investigate, which cytoplasmic partner(s) of cadherin are responsible for the cadherin-dependent survival of free MTs in the centrosome-lacking cytoplasts. Since formation of cadherin-mediated cell-cell junctions recruits to the membrane cadherin partners, β-catenin and p120 catenin, as well as the β-catenin partner α-catenin, we decided to deliver these proteins one by one to the membrane in a cadherin-independent manner and to check whether such targeting would affect microtubule length and density in the centrosome-free cytoplasts. A requirement of this experimental approach was to prepare constructs of the cadherin-catenin complex components containing a membrane targeting domain. For this purpose we chose a membrane targeting domain consisting of the extracellular and transmembrane portions of the α subunit of the interleukin 2 receptor (IL2R) described previously22 and used in several studies.23,24 The enhanced GFP fused to the C-terminus of each fusion protein made it possible to identify transfected cells. Figure 2 shows the fusion chimeras that were constructed.
Figure 2.
Expression of chimeric fusion constructs in CHO-K1 cells. Right panel represents the scheme of chimeric fusion constructs of α-catenin (A), β-catenin (B) or p120 catenin (C) with either enhanced GFP (EGFP) only, or the extracellular-transmembrane domain of the interleukin-2 receptor α-subunit and EGFP. Left panel presents Western blots showing expression of endogenous α-catenin (A), β-catenin (B) and p120ctn (C) and exogenous transfected EGFP-derivatives of corresponding proteins and their membrane-targeted IL2R fusion constructs.
Membrane targeting of transfected constructs was confirmed by immunostaining cells with an antibody to the extracellular domain of IL2R (not shown). The staining shows that the IL2R-fusions of α- and β-catenin and p120 were detected on the surface of transiently transfected CHO-K1 cells. Western blotting of extracts prepared from the transiently transfected cells (Fig. 2) showed that these fusion constructs were expressed as a single major species of the correct molecular weight.
CHO-K1 cells expressing IL2R-fused β- and α-catenins, as well as α- or β-catenin lacking the membrane targeting IL2R domain, did not demonstrate any apparent phenotypic abnormalities. Some of p120-catenin-overexpressing cells had typical arborized (“branching”) phenotype in accordance with published results.26,28,42 Surprisingly, cells expressing IL2R-p120 did not demonstrate any branching.
Effects of membrane targeting of catenins on the MTs in non-centrosomal cytoplasts
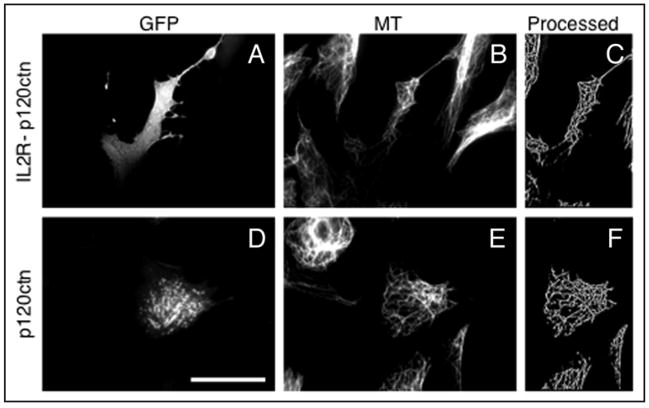
We first tested p120 catenin, which according to some studies can interact with MTs, and bind a MT-based motor, kinesin.25–28 Nevertheless, expression of neither GFP-p120, nor IL2R-p120-GFP increased the total MT length in centrosome-free cytoplasts to the level that would be significantly higher than the MT length in the cytoplasts prepared from cells transfected with EGFP vector alone (Figs. 3 and 6). Thus, membrane targeting of p120 is not sufficient for the stabilization of non-centrosomal microtubules.
Figure 3.
Effect of expression of membrane-targeted and cytoplasmic p120 catenin on the microtubule density in the centrosome-lacking cytoplasts. (A–C): cells transfected with membrane targeted IL2R- GFP-p120. (D–F): cells transfected with the p120-GFP. From left to right: GFP fluorescence in cytoplasts produced from transfected cells (left), microtubule staining of the same field of view (middle), processed microtubule image of the transfected cytoplasts (right). Scale bar represents 10 μm.
Figure 6.
Quantification of the microtubule amount in the centrosome-lacking cytoplasts expressing cytoplasmic and membrane-targeted adherens junction proteins. (A) Bars represent mean total microtubule length per unit area (μm−1) ± SEM. (B) Bars represent mean total microtubule length per cytoplast ± SEM.
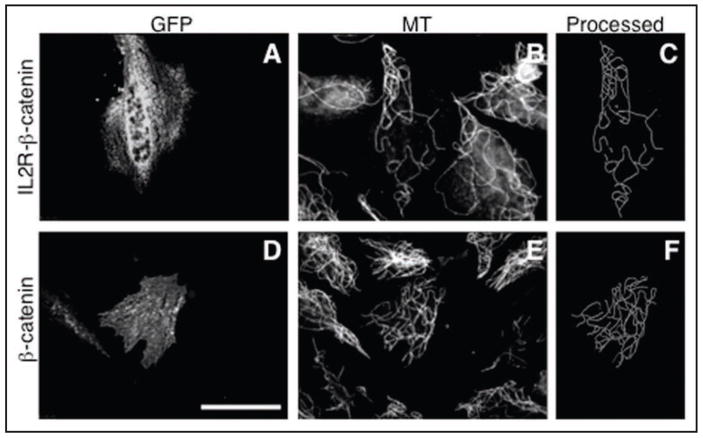
To test the role of β-catenin and its membrane-targeted derivative, it was necessary to protect these proteins from rapid proteasomal degradation.29 To this end, we used its mutated form, β-catenin-S33Y, known to be insensitive to proteasomal degradation, but fully functional.24,30 β-catenin-S33Y was used also for the production of the membrane targeted fusion with the IL2 receptor. Cytoplasmic expression of the β-catenin-S33Y (data not shown), or β-catenin-S33Y-GFP (Figs. 4 and 6) did not lead to any increase of MT density in non-centrosomal cytoplasts as compared to transfection with GFP vector alone. Moreover, membrane targeting of β-catenin-S33Y by IL2R fusion also failed to produce a greater MT polymer level from that of the GFP control. Thus, membrane targeting of β-catenin alone is also not sufficient to protect non-centrosomal microtubules from depolymerization.
Figure 4.
Effect of expression of membrane-targeted and cytoplasmic β-catenin on the microtubule density in the centrosome-lacking cytoplasts. Upper: cells transfected with membrane targeted IL2R-β-catenin-GFP chimera (A–C), lower: cells transfected with the β-catenin-GFP (D–F). From left to right: GFP fluorescence in cytoplasts produced from transfected cells (left), microtubule staining of the same field of view (middle), processed microtubule image of the transfected cytoplasts (right). Scale bar represents 10 μm.
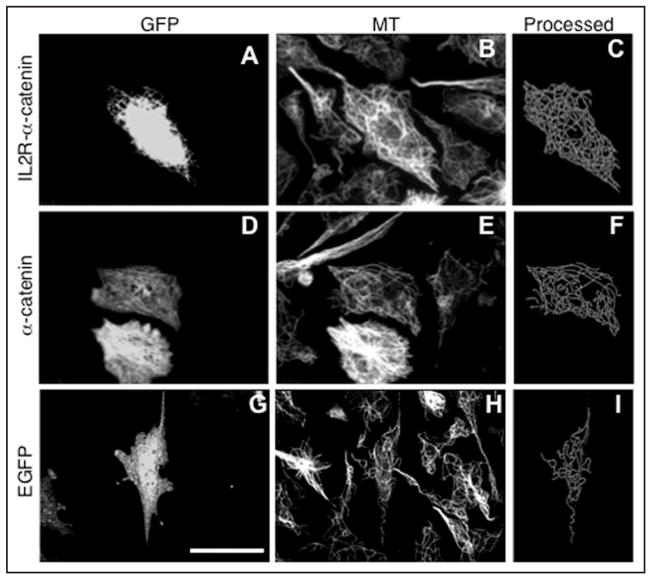
As previously shown,19 CHO-K1 cells express low or undetectable levels of N- or E-cadherin and β-catenin. They also express low levels of α-catenin, as shown here in Figure 2A. Consequently, it seemed possible that the negative effects of expressing membrane-targeted β-catenin were a consequence of the low levels of endogenous α-catenin. We therefore tested the effects on MTs of expression of α-catenin (Fig. 5). Cytoplasts expressing non-tagged or GFP-tagged α-catenin demonstrated levels of MT polymer slightly greater than in the cytoplasts expressing GFP alone (Figs. 5 and 6). Remarkably, expression of membrane-targeted IL2R-α-catenin (both with or without GFP-tag) produced a dramatic effect. MT levels were significantly higher than in the GFP control and were comparable to levels seen in centrosome-containing cytoplasts (Figs. 1, 5 and 6).
Figure 5.
Effect of membrane targeted α-catenin expression on microtubule density in the centrosome-lacking cytoplasts. Upper: cells transfected with membrane targeted IL2R-α-catenin-GFP chimera (A–C), middle: cells transfected with the α-catenin-GFP (D–F), lower: cells transfected with the enhanced GFP alone (G–I). From left to right: GFP fluorescence in cytoplasts produced from transfected cells (left), microtubule staining in the same field (middle), processed microtubule image of the transfected cytoplasts (right). Scale bar represents 10 μm.
We conclude that expression specifically of membrane-targeted α-catenin is sufficient to stabilize MTs, and thus represents a likely downstream effector of cell-cell contact regulation of MT dynamics.
Discussion
In this study, we targeted major adherens junction components to the plasma membrane in a cadherin-independent manner, and checked whether forced membrane localization of some of these proteins could reproduce one effect of cadherin signaling, an increase of MT mass in the centrosome-free cytoplasts.19 We employed a well-established membrane-targeting method based on the fusion of proteins of interest with extracellular and transmembrane domains of the α subunit of the interleukin-2 receptor.22
Comparison of the effects produced by plasma membrane-delivered β-catenin, p120-catenin, and α-catenin revealed straightforward results. Overexpression of membrane-targeted α-catenin was sufficient to increase the total length and density of microtubules in non-centrosomal cytoplasts more than two fold, while neither β-catenin, nor p120-catenin produced any effects. Overexpression of regular α-catenin produced minor but noticeable effect in the same direction as membrane-targeted α-catenin. Thus, membrane targeting of α-catenin seems to play a primary role in cadherin-dependent microtubule regulation. This finding is in line with other recent studies addressing the function of α-catenin, which is regarded now as a signaling molecule and a regulator of actin dynamics, rather than just a physical link connecting cadherin complex with the actin cytoskeleton.13–18,31
How could α-catenin membrane targeting affect stability of non-centrosomal MTs? α-Catenin was shown to function as an element of several signaling cascades in different cellular systems.31 In particular, its conditional knockout in the skin led to sustained activation of the Ras-Erk/MAPK pathway and augmentation of proliferation within several days of α-catenin ablation.32 Direct binding of Rho to α-catenin was detected in Drosophila33 suggesting that α-catenin may participate in the regulation of Rho-activity. Finally, α-catenin can modulate the actin cytoskeleton via its effect on Arp2/3 dependent actin polymerization and filament bundling.14
α-Catenin interacts directly with a coiled-coil (cc) domain of formin-1.17 Formin-1, similarly to other formins, demonstrates an ability to nucleate actin filament assembly via its FH2 and FH1 domains.17 Moreover, some formins are thought to interact with MTs and, directly or indirectly, stabilize them.3,34–36 Notably, our preliminary studies show that expression of a constitutively active FH1-FH2 containing portion of mDia1 formin, as well as formin-1, significantly augmented the amount of MT polymer in the centrosome-free cytoplasts.37,38 Thus, a possible scenario for the cadherin-dependent stabilization of non-centrosomal MTs includes the α-catenin-dependent recruitment of formin-1 to the plasma membrane. The submembrane localization of formin-1 might by itself be sufficient to stabilize MTs. Another possibility is that delivery of formin-1 to the membrane leads to its activation. The mechanism of formin-1 activation is not known, but one can imagine that phosphorylation by some membrane associated kinases, or other type of modification occurring only at the membrane, may play a critical role in it. Then activated formin-1 may dissociate from α-catenin, return into cytoplasm and stabilize the non-centrosomal MTs there.
Thus, α-catenin might be involved in a variety of regulatory pathways affecting both global signaling cascades and local cytoskeletal rearrangements. Involvement of α-catenin in the microtubule regulation demonstrated in the present study reveals a novel important facet in its signaling function.
Materials and Methods
Cell culture and transfection
CHO-K1 cells were obtained from American Type Tissue Culture (ATCC, Rockville, MD, USA) and cultured in Ham’s F12 medium supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and antibiotics (complete medium). Transient transfections were performed with Lipofectamine PLUS reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
DNA constructs
pIL2R-α-catenin and pIL2R-N-cadherin expression vectors were obtained from Dr. Ben-Zion Katz.39,40 pHA-β-catenin-S33Y plasmid (non-degradable β-catenin) was described in.41 GFP-p120 construct was described in our paper.42
For preparation of pIL2R-α-catenin-GFP construct, containing extracellular and transmembrane domain of alpha subunit of IL2 receptor fused with mouse α-catenin and C-terminal enhanced GFP (EGFP), 3′ part of α-catenin was amplified by PCR using sense primer A6 CAGACTCGGCATGCAAGCAG, containing Sph I site, and antisense primer A7 TGCTCACCATGTCGACGATGCTGTCCATGGCTTTGA. EGFP was amplified by PCR from pEGFP plasmid (Clontech, Mountain View, CA) using sense primer A8 GGACAGCATCGTCGACATGGTGAGCAAGGGCGAGGAG and antisense primer A9 ACCTTCTA GATCACTTGTAC AGCTCGTCCATGC, containing Xba I site. Both PCR products were mixed, and fusion DNA containing 3′ part of α-catenin fused to EGFP was amplified using A6 and A9 primers. Obtained PCR product was digested using Sph I and Xba I enzymes and introduced into pIL2R-α-catenin vector by Sph I/Xba I sites.
For construction of pHA-β-catenin-S33Y-GFP, containing GFP cDNA fused to C-terminal end of β-catenin, 3′ part of β-catenin was amplified using sense primer B1 GACTGCAGATCTTGGACTGG and anisense primer B2 TGCTCACCATG TCGACCAGGTCAGTATCAAA CCAGGC from pHA-β-catenin-S33Y plasmid, cDNA encoding EGFP was amplified using sense primer G1 TACTGACCTGGTCGACAT GGTGAGCAAGGGCGAGGAG and antisense primer G2 ACCTGGATCCTCACT TGTACAGCTCGTCCATGC from pEGFP plasmid DNA (Clontech, Mountain View, CA). Then both PCR products were mixed and fusion product, containing 3′ part of β-catenin fused to EGFP sequence was obtained by amplification primers. Fusion PCR product was inroduced into with B1 and G2 pHA-β-catenin-S33Y plasmid41 by EcoR V and Bam H1 sites.
For construction of pIL2R-β-catenin-S33Y-GFP expression vector, containing IL-2 receptor fused to β-catenin-S33Y-GFP, 5′-end of β-catenin-S33Y was amplified by PCR from pHA-β-catenin-S33Y DNA using sense primer B3 ACCTAAGCTTATGGCTACT CAAGCTGACCTG, containing Hind III site, and antisense primer B4 ATGAGCAGCGTCAAACTGCG. Obtained PCR product was digested using Hind III and Sph I and 3′ part of β-catenin was prepared by digestion of pHA-β-catenin-S33Y-GFP using Sph I and Sal I. Both inserts was introduced into pIL2R-α-catenin-GFP using Hind III and Sal I sites instead of α-catenin sequence.
For construction of pHA-α-catenin-GFP, 5′ part ofα-catenin cDNA was amplified by PCR from pIL-2R-α-catenin-GFP using sense primer A1 ACCTTCTAGAATGACTGCCGTCCACGCAGGC, containing Xba I site, and antisense primer A2 GTAACCTGTGTAACAAGAGG. Obtained PCR product was digested using Xba I and Bst XI enzymes, 3′ part of α-catenin was obtained from pIL2R-α-catenin-GFP via digestion by Bst XI and Sal I sites. Both inserts were introduced into pHA-β-catenin-S33Y-GFP by Xba I and Sal I sites instead of β-catenin sequence.
Antibodies and reagents
Antibodies for α-tubulin (DM-1A) and γ-tubulin (GTU-88) were from Sigma (Sigma, St. Louis, MO, USA), the polyclonal anti γ-tubulin antibodies were previously described.20 Antibodies to α-catenin, β-catenin and p120ctn were obtained from BD Bioscience (BD, Franklin Lakes, NJ, USA). Anti GFP antibodies were obtained from Roche (Roche, Basel, Switzerland).
Secondary antibodies coupled to Cy-3, Cy-5 were purchased from Jackson Laboratories (West Grove, PA, USA), Alexa-350 was from Molecular Probes (Molecular Probes Inc., Eugene, OR, USA). HSP-conjugated anti-mouse antibodies were from GE Healthcare (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA).
Nocodazole, cytochalasin D, rhodamine-phalloidin and fibronectin were all purchased from Sigma (Sigma, St. Louis, MO, USA). Tissue culture media, antibiotics (penicillin, streptomycin) and glutamine were obtained from Invitrogen (Carlsbad, CA, USA), and the FCS was from Biological Industries (Kibbutz Beit Haemek, Israel).
Preparation of cytoplasts
The key assay in our experiments is the preparation of cytoplasts either containing or lacking the centrosome. The assay had been introduced by43 and then extensively used in our previous studies.19,20 CHO-K1 cells were first transiently transfected with control vector or experimental constructs. About 24 h after transfection, cells were plated on fibronectin coated glass coverslips and the cytoplasts were then prepared by a drug treatment and centrifugation procedure. The cytoskeleton was weakened by simultaneous nocodazole (1 μg/ml, Sigma) and cytochalasin D (1.25 μg/ml, Sigma) treatment for 90 min to depolymerize MTs and disrupt actin filaments, respectively. Centrifugation at 10,000 g for 25 min of cells adherent to a coverslip in a direction perpendicular to the cover-slip caused the nucleus to be removed, leaving cytoplasts behind. The proportion of cytoplasts that contain or lack the centrosome depended on the details of the drug concentration and centrifugation conditions. After centrifugation, coverslips were placed in fresh medium for 1–2 h to allow the recovery and reassembly of MTs.
Fluorescence microscopy
For immunofluorescence, cells were fixed with glutaraldehyde. After fixation cells were rinsed with PBS and permeabilized with 0.5% Triton X-100 for 5 minutes. For antibody staining, cells were incubated with primary antibodies diluted in PBS. Then cells were washed in PBS three times and stained with secondary antibodies. After three final washes fluorescent labeling was analyzed using an Axiovert 100 TV inverted microscope (Zeiss, Oberkochen, Germany).
Image analysis
For image acquisition and analysis the Priism software written within the Image Visualization Environment (University of California San Francisco, www.msg.ucsf.edu/IVE/) was used. Total lengths of microtubules in cells and cytoplasts were calculated using the fiber score algorithm.21 The algorithm was implemented in Priism environment, m and enabled the evaluation of fiber length in μm, density and co-alignment.
Acknowledgments
We are grateful to Dr. Ben-Zion Katz (The Hematology Institute, Tel Aviv Sourasky Medical Center) for providing plasmids and Prof. Alexey Khodjakov (Wadsworth Center, Albany, NY) for critically reading the manuscript. This study was funded in part by grants from the Israel Sciences Foundation, Minerva Foundation, Pasteur-Weizmann Council and Maurice Janin fund to A.D.B. A.D.B. holds the Joseph Moss Professorial Chair in Biomedical Research.
Abbreviations
- APC
adenomatous polyposis coli protein
- AJ
adherens junction
- CHO
chinese hamster ovary cells
- DMEM
Dulbecco’s Modified Eagle’s Medium
- EGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- IL2R
interleukin-2 receptor
- MAPK
mitogen-activated protein kinase
- mDia1
mouse diaphanous related formin 1
- MT
microtubule
- PBS
phosphate buffered saline
- SD
standard deviation
- SEM
standard error of mean
References
- 1.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 2.Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–9. [PubMed] [Google Scholar]
- 3.Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–63. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen MM. Microtubule release and capture in epithelial cells. Biol Cell. 1999;91:331–41. [PubMed] [Google Scholar]
- 5.Dammermann A, Desai A, Oegema K. The minus end in sight. Curr Biol. 2003;13:614–24. doi: 10.1016/s0960-9822(03)00530-x. [DOI] [PubMed] [Google Scholar]
- 6.Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J Cell Biol. 2005;171:845–55. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erez N, Bershadsky A, Geiger B. Signaling from adherens-type junctions. Eur J Cell Biol. 2005;84:235–44. doi: 10.1016/j.ejcb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 9.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–96. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–89. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobielak A, Fuchs E. Alpha-catenin: At the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–25. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin and adherens junctions. Cell. 2005;123:769–72. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin JM, Nelson WJ. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- 20.Rodionov V, Nadezhdina E, Borisy G. Centrosomal control of microtubule dynamics. Proc Natl Acad Sci USA. 1999;96:115–20. doi: 10.1073/pnas.96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenstein N, Geiger B, Kam Z. Quantitative analysis of cytoskeletal organization by digital fluorescent microscopy. Cytometry A. 2003;54:8–18. doi: 10.1002/cyto.a.10053. [DOI] [PubMed] [Google Scholar]
- 22.LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–47. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz BZ, Romer L, Miyamoto S, Volberg T, Matsumoto K, Cukierman E, Geiger B, Yamada KM. Targeting membrane-localized focal adhesion kinase to focal adhesions: roles of tyrosine phosphorylation and SRC family kinases. J Biol Chem. 2003;278:29115–20. doi: 10.1074/jbc.M212396200. [DOI] [PubMed] [Google Scholar]
- 24.Sadot E, Simcha I, Shtutman M, Ben Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA. 1998;95:15339–44. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–94. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 27.Roczniak-Ferguson A, Reynolds AB. Regulation of p120-catenin nucleocytoplasmic shuttling activity. J Cell Sci. 2003;116:4201–12. doi: 10.1242/jcs.00724. [DOI] [PubMed] [Google Scholar]
- 28.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512–21. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 29.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 31.Scott JA, Yap AS. Cinderella no longer: alpha-catenin steps out of cadherin’s shadow. J Cell Sci. 2006;119:4599–605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- 32.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–17. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 33.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–82. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 34.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 35.Zhou F, Leder P, Martin SS. Formin-1 protein associates with microtubules through a peptide domain encoded by exon-2. Exp Cell Res. 2006;312:1119–26. doi: 10.1016/j.yexcr.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–36. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballestrem C, Magid N, Zonis J, Shtutman M, Ishizaki T, Narumiya S, Bershadsky A. Regulation of microtubule dynamics by the formin homology protein, mDia1. Molecular biology of the cell. 2002;13:422. [Google Scholar]
- 38.Ballestrem C, Magid N, Zonis J, Shtutman M, Bershadsky A. Interplay between the actin cytoskeleton, focal adhesions, and microtubules. In: Ridley A, Peckham M, Clark P, editors. Cell Motility: From Molecules to Organisms. New York, NY: John Wiley & Sons; 2004. pp. 75–99. [Google Scholar]
- 39.Katz BZ, Levenberg S, Yamada KM, Geiger B. Modulation of cell-cell adherens junctions by surface clustering of the N-cadherin cytoplasmic tail. Exp Cell Res. 1998;243:415–24. doi: 10.1006/excr.1998.4194. [DOI] [PubMed] [Google Scholar]
- 40.Sadot E, Conacci-Sorrell M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben Ze’ev A, Geiger B. Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. J Cell Sci. 2002;115:2771–80. doi: 10.1242/jcs.115.13.2771. [DOI] [PubMed] [Google Scholar]
- 41.Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, Ben Ze’ev A. Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin. J Cell Biol. 1998;141:1433–48. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 43.Karsenti E, Kobayashi S, Mitchison T, Kirschner M. Role of the centrosome in organizing the interphase microtubule array: properties of cytoplasts containing or lacking centrosomes. J Cell Biol. 1984;98:1763–76. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]