Abstract
Objectives
To investigate muscle fatigue of the knee flexors and extensors in people with cerebral palsy (CP) compared with those without motor disability during performance of a voluntary fatigue protocol and to investigate the relationship with functional mobility.
Design
A case-control study.
Setting
A biomechanics laboratory.
Participants
Ambulatory subjects with CP (n=18; mean age, 17.5y) in Gross Motor Function Classification System (GMFCS) levels I, II, and III and a comparison group of age-matched subjects (n=16) without motor disability (mean age, 16.6y).
Interventions
Not applicable.
Main Outcome Measures
The voluntary muscle fatigue protocol consisted of concentric knee flexion and extension at 60° a second for 35 repetitions on an isokinetic dynamometer. Peak torque for each repetition was normalized by the maximum peak torque value. Muscle fatigue was calculated as the rate of decline in normalized peak torque across all repetitions, represented by the slope of the linear regression. Self-selected and fast gait velocities were measured as well as the Pediatric Outcomes Data Collection Instrument (PODCI).
Results
Greater fatigability (slope) was observed in the comparison group for both knee flexors and extensors than in the group with CP. Within CP, lower knee extensor fatigue (slope) was associated with lower functioning GMFCS levels and lower levels of activity and participation as measured by the PODCI transfers and basic mobility.
Conclusions
Even after adjusting for maximum peak torque, the knee flexors and extensors of participants with CP were observed to be less fatigable than age-matched peers without motor disability. The lower rate of muscle fatigue was also associated with lower functional mobility in CP. These results may be related to strength or activation differences and/or muscle property alterations. Future investigations are warranted.
Keywords: Muscle fatigue, Muscle spasticity, Muscle strength, Quadriceps muscle, Rehabilitation
Measures of physiologic capacity, such as lower-extremity muscle strength, have been correlated with functional measures in people with CP and other disabilities. 1–3 However, neither muscle strength nor measures of physical function have been shown to be related to psychosocial aspects of QOL, such as comfort and happiness.4 Self-reported physical fatigue, on the other hand, has been significantly associated with QOL measures of psychosocial well being, such as bodily pain, limitations in physical and emotion role function, and low life satisfaction in adults with CP.5 Furthermore, adults with CP report fatigue as a main cause of the deterioration or cessation of their walking ability.6–8 However, these studies assessed fatigue using questionnaires and interviews and did not attempt to differentiate among the different types of fatigue.
Previous objective clinical measures of fatigue in people with CP were focused primarily on the cardiorespiratory system. Although it has been well documented that children and adolescents with CP have lower V̇O2max than their typically developing peers, most authors concluded that local muscle factors, such as muscle fatigue, were responsible for the lower V̇O2 max and limitations in activity.9–11 Following this same argument, Lundberg10 and Hoofwijk et al9 suggested that spastic muscles may have decreased venous return and inhibited muscle lactate clearance during exercise, thereby increasing muscle fatigue and leading to a decrease in V̇O2 max values.
There are different definitions of muscle fatigue in the literature. Muscle fatigue is defined here as a reduction in force output that occurs during sustained voluntary activity.12 Fatigue resistance, or the ability to withstand fatigue, is also referred to as muscle endurance in the literature. Muscle fatigue can occur at any point along the activation process, from the central nervous system to the level of the motor neuron. Techniques that use electric stimulation (ie, twitch interpolation, electrically elicited contractions) are capable of isolating peripheral aspects of muscle fatigue from central aspects. For example, Stackhouse et al13 investigated peripheral muscle fatigue of the quadriceps and triceps surae through the use of electrically elicited contractions in children with CP. In that study, the quadriceps, but not the triceps surae, were observed to be less fatigable compared with a control group. However, in their study, the influence of the central nervous system was removed. Because muscle activity depends on the integration of the entire chain of events, muscle fatigue as assessed through voluntary performance may occur at both central and peripheral sites simultaneously.14
Because muscles adapt to the amount and type of neural stimulation being imposed on them, secondary effects of spasticity on muscle tissue can also have a profound impact on the ability to generate and maintain muscle force. Muscle abnormalities such as alterations in muscle fiber size and fiber type distribution, excessive collagen accumulation, and increased muscle stiffness (elastic modulus) of spastic muscle cells have also been reported in CP.15–20 These alterations of muscle properties can have significant implications for essential aspects of muscle performance, such as the ability to generate force and to sustain force output. In CP and other motor disorders, different muscle groups can be affected to varying degrees; therefore, these changes may be muscle-specific, rather than generalizable across all muscles.
Isokinetic fatigue protocols for the knee flexors and extensors have been extensively developed in the healthy adult population21,22 and have been modified for use with children23 and with other neurologic populations such as multiple sclerosis.24 An advantage of isokinetic dynamometry is that it provides a safe, controlled environment in which a muscle or group of muscles can be isolated with stabilization of other joints.25 An isokinetic fatigue protocol similar to the ones mentioned was developed recently by the authors26 for use in children and young adults with mild-to-moderate CP and was shown to be feasible for testing the knee flexors and extensors.
The primary purpose of this study was to determine whether muscle fatigue in the knee flexors and extensors in people with CP differs from those without a motor disability during the performance of a voluntary fatigue test. A secondary purpose of the study was to determine the relationship of muscle fatigue to functional level, walking velocity, and activity/participation as measured by the PODCI. The quadriceps and hamstrings muscle groups were chosen because of the importance of these muscles in gait and function.1–3 We hypothesized that people with CP would have greater levels of muscle fatigue than nondisabled peers and that muscle fatigue would be inversely related to functional level, walking velocity, and activity and participation.
METHODS
Participants
A group of 18 participants with CP and a comparison group of 16 participants without motor disability between the ages of 10 and 25 years were recruited for the study. An attempt was made to ensure that sex and age distributions were similar across groups. Participant characteristics are listed in table 1. Participants with CP were ambulatory with or without assistive devices. They were excluded if they had orthopedic surgery within 12 months prior to the testing, received botulinum toxin injections to the quadriceps or hamstrings within 6 months prior to the testing, or complained of existing knee pain.
Table 1.
Participant Characteristics
| Group | Sex | Age Range (y) | Age ± SD (y) | Height ± SD (m) | Weight ± SD (kg) |
|---|---|---|---|---|---|
| CP | 13F/5M | 10–25 | 17.49± 5.03 | 1.52± 0.08 | 47.57± 9.92 |
| Comparison | 13F/3M | 10–23 | 16.61± 4.45 | 1.59± 0.09* | 53.97± 9.72 |
Abbreviations: F, female; M, male.
The comparison group was significantly taller than the CP group (P=.02).
The study was approved by the human studies committee at our institution. Written informed consent from each participant over 18 years of age was obtained. Parental consent forms for participants younger than 18 years of age were obtained from their parents or legal guardians.
Fatigue Testing
An isokinetic dynamometera was used to record torque of the knee flexors and extensors during maximum voluntary exertions throughout the available passive ROM. Participants performed 8 to 12 submaximal concentric, reciprocal knee flexion and extension repetitions to familiarize themselves with the task. The fatigue protocol consisted of reciprocal, maximal concentric knee extension and flexion at 60° a second for 35 repetitions and has been published elsewhere.26 During pilot testing, peak torque during the fatigue test for both knee extension and flexion showed a consistent pattern of decline during the first 30 to 40 repetitions and then began to level off in subjects with and without CP who were asked to complete 100 repetitions. This pattern has been demonstrated for the knee extensors during similar protocols in other studies.27,28 Therefore, 35 repetitions were chosen for this protocol. Strong verbal encouragement on every repetition as well as visual biofeedback of torque production was provided to encourage maximal effort on all repetitions. Data were gravity-corrected, and only the constant velocity portion was used for calculation. The following calculations were made separately for knee flexion and extension repetitions. For each subject, peak torque was measured as the highest value achieved during each repetition. Maximum peak torque for each subject was measured as the single highest peak torque value across all repetitions. Peak torque for each repetition was then normalized by the maximum peak torque value. Normalized peak torque data were averaged for each group, and muscle fatigue was calculated as the rate of decline in normalized peak torque across all repetitions, represented by the slope of the linear regression.21 The first repetition was excluded from analysis because it is usually unreliable.
Functional Measures
Each subject was assigned a GMFCS level as a measure of functional mobility, restricted to I, II, or III because of the ambulatory requirements of the study. Although the GMFCS was not originally intended for those over 12 years of age, it has been shown to be reliable29 and stable over time in adults with CP.30 Activity and participation were assessed with the PODCI.31 The PODCI questionnaire was completed by the parents of participants under 18 or by the participants themselves who were 18 or older (American Academy of Orthopaedic Surgeons/Pediatric Orthopaedic Society of North America, Version 2.0). The PODCI was designed to assess self-reported physical function and psychosocial aspects of health status deemed relevant to musculoskeletal disability. Five scales were computed with a percent score from 0 (worst) to 100 (best).
Gait velocity was assessed using a stop watch during level walking over a 10-m distance. Subsequent marks were placed 2m from the starting point and 2m from the ending point, thus allowing a 6-m timed middle section for the test. Each subject was tested at a comfortable walking speed and a fast walking speed. Velocity was calculated as meters a second divided by the participant’s height. Two trials at each speed were averaged.32
Statistical Analysis
Comparisons between the 2 groups (with and without CP) and the 2 muscle groups (knee flexors and extensors) were made using independent and paired t tests, respectively. Within the group with CP, Pearson r correlation procedures were used to determine the relationship of gait velocity and PODCI scores with the slope for the knee flexors and extensors. Spearman ρ correlations were used to determine the relationship between GMFCS level and the slope (α = .05).
RESULTS
Mean age and weight did not differ across groups, but the comparison group without CP was slightly taller than the group with CP (t=2.66; P=.02) (see table 1). The average maximum peak torque values of the knee extensors obtained during the fatigue protocol for the group with CP and the comparison group were 54.2±20.8 and 108.4±37.9Nm, respectively (t=5.24; P<.001). For the knee flexors, the average maximum peak torque values were 24.2±11.3 and 57.7±21.3 Nm for the group with CP and the comparison group, respectively (t=5.83; P<.001).
Fatigue
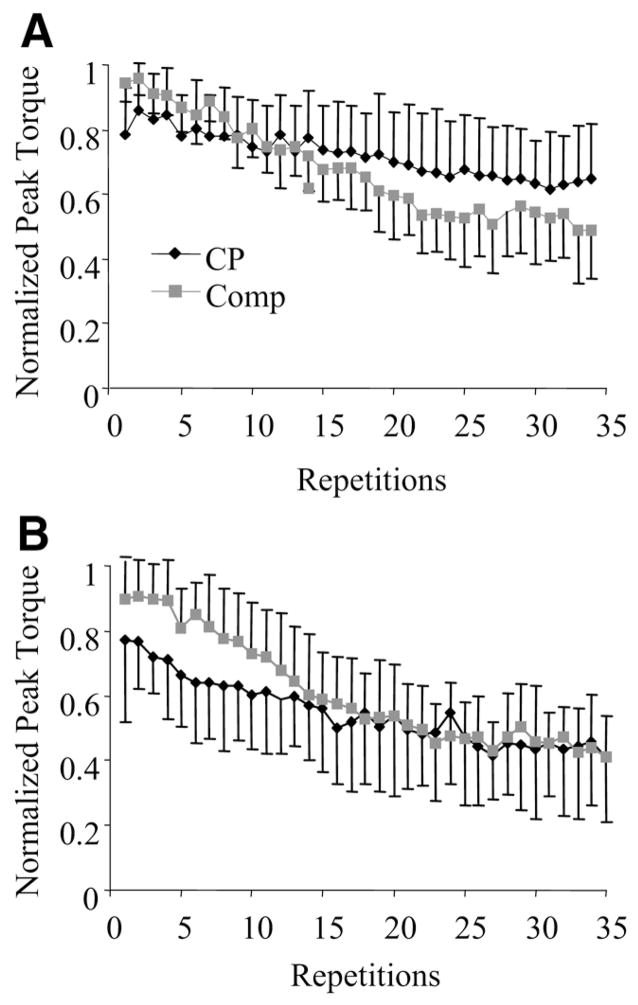
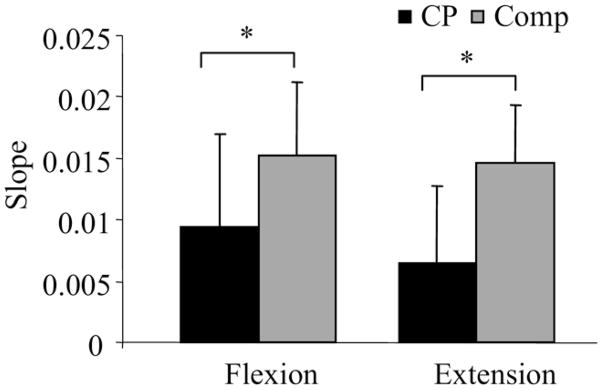
Figure 1 illustrates the group means and SDs of the normalized peak torques over the 35 repetitions for the knee flexors and extensors. The normalized peak torque of the comparison group declined more (greater slope) than the group with CP for both the knee flexors (t=−2.44; P=.02) and extensors (t=−4.22; P=.001), indicating greater muscle fatigue of the comparison group. Means and SDs of the slope values for each group are illustrated in figure 2. The magnitude of the slope values are presented as positive values for ease of interpretation. The slope values did not differ between the knee flexors and extensors for either the comparison (t=−.80; P=.434) or the group with CP (t=−2.01; P=.061).
Fig 1.
Mean and SD of normalized peak torque data across all repetitions in the CP and the comparison (Comp) groups for (A) knee extensors and (B) knee flexors.
Fig 2.
Mean and SD of the slope of the decline in normalized peak torque across repetitions in the CP and comparison (Comp) groups for knee extension and flexion. *Significant difference (P<.05).
Functional Measures
Participants with CP who had greater mobility (eg, lower GMFCS levels) had a greater rate of decline in normalized peak force (greater slope) of the knee extensors (r=−.50; P=.035). The transfers and basic mobility scale of the PODCI was directly correlated with the slope of the knee extensors (r=.61; P=.008). A positive correlation indicates that participants with greater levels of functioning (eg, greater scores on the PODCI) had greater rates of decline. None of the scales related to psychosocial well being (pain and comfort, happiness) were correlated with the slope. Self-selected and fast walking velocities approached significance with the knee extensors only (P=.08, P=.06, respectively). Correlation coefficients are listed in table 2.
Table 2.
Correlations of the Slope With Functional Measures
| Functional Measures | Slope KE | Slope KF |
|---|---|---|
| PODCI sports and physical function | .35 | .28 |
| PODCI transfers and basic mobility | .61* | .36 |
| PODCI global functioning | .41 | .34 |
| PODCI happiness scale | −.36 | −.09 |
| PODCI pain and comfort | .15 | −.04 |
| Self-selected velocity | .43 | .40 |
| Fast velocity | .45 | .38 |
| GMFCS† | −.50* | −.24 |
Abbreviations: KE, knee extension; KF, knee flexion.
P<.05.
Spearman ρ correlation.
DISCUSSION
Contrary to our hypotheses, the knee flexors and extensors in our sample of participants with CP were observed to be less fatigable than the age-matched comparison group as indicated by the lower rate of decline in peak torque. Furthermore, greater knee extensor fatigue was associated with higher functioning GMFCS levels and greater levels of activity and participation as measured by the PODCI transfers and basic mobility. Counterintuitively, it appears as though lower levels of muscle fatigue of the knee flexors and extensors, as measured by this test, are characteristic of greater disability.
Although knee extensor fatigue was significantly associated with the GMFCS and transfers and basic mobility, it was not associated with any of the other functional measures; however, the relationship with self-selected and fast velocity approached significance (see table 2). Perhaps this is because the transfers and basic mobility scale contain items related to overall physical performance, which would be most closely associated with lower-extremity muscle function. The sports and physical function scale, on the other hand, consists of more challenging and specific performance items, whereas the global functioning scale is very generalized and includes items from both of the aforementioned scales as well as upper-extremity activities and items related to pain and comfort. It should be noted that although we tested participants with CP up to 25 years of age, the PODCI has not been validated in people over 18 years.
The fatigue protocol employed here was designed as a measure of volitional activity, which would be more representative of everyday function versus an electrically elicited fatigue test or an isometric test. Nevertheless, our results are similar to a study that used an electrically elicited fatigue test in which the quadriceps in children with CP were found to be less fatigable than a control group.13 Because the muscles were electrically stimulated in that study, only peripheral aspects of muscle fatigue distal to the peripheral motor nerve were assessed. Our study, on the other hand, encompassed both central and peripheral aspects of muscle fatigue, from the central nervous system command to the contractile apparatus of the muscle itself. Therefore, peripheral versus central contributions to the measured fatigue can not be determined from these results. Similarities between the 2 studies should be interpreted within this context.
Several mechanisms can be suggested to explain the lower rate of fatigue in the group with CP, the first of which are maximal torque level (strength) and muscle mass. It has been suggested that males are more fatigable than females33,34 and adults are more fatigable than children35 and older adults36 because of greater strength and/or greater muscle mass. In our study, the maximum peak torque values of both muscle groups obtained during the fatigue test were 50% lower in the group with CP than the comparison group. Perhaps the lower capacity for force production in the participants with CP lowers the rate of decline in torque. However, it should be noted that sex and age differences in fatigue caused by absolute strength differences have been refuted by some researchers.37,38 Rather, some authors claim that weakness leads to disuse atrophy and increased levels of muscle fatigue39 because of the recruitment of more motor units or the greater frequency of excitation required to perform a given task. As a consequence of recruiting more motor units, the overall fatigability will likely increase because increased numbers of upper threshold units will be recruited, which include more fast fatiguing fibers. Although these are all plausible theories, the relationship between absolute strength and muscle fatigue is not fully understood and warrants further investigation.
Second, predominance of a particular fiber type can influence muscle fatigue, because type I (slow twitch) fibers are more fatigue resistant, whereas type II (fast twitch) fibers are more fatigable. Although there has been no consensus on fiber type predominance in CP and other spastic disorders, there are several reports of increased type I fibers in CP or atrophy of type II fibers.16,18–20 The main results of this study could partially be explained if secondary impairments in CP, such as spasticity, do result in muscle adaptations, which include increased proportions of type I fibers with a greater oxidative capacity.
Third, the issue of voluntary muscle activation must be considered. Recent evidence revealed that children are more susceptible to central fatigue than adults and that the lower peripheral fatigue of the knee extensors was associated with a lower degree of voluntary activation.40 Similarly, Stackhouse et al13 observed 33% lower voluntary muscle activation of the quadriceps in children with CP ages 7 to 13 years than age-matched typically developing children. Therefore, despite the fact that the subjects with CP were giving a maximal voluntary effort, it is possible that they were not activating all of their motor units secondary to impaired motor pathways. As a result, type I fibers may be preferentially recruited with lower firing rates, thereby contributing to greater fatigue resistance.
Study Limitations
In addition, other potential but unstudied physiologic mechanisms in CP that may affect muscle fatigue are energy metabolism,41 muscle activation strategies such as cocontraction, and alternate patterns of motor unit recruitment and rate modulation, such as motor unit rotation42 and substitution.43 A potential limiting factor is that both participant groups performed the task within their available passive ROM, which varied across subjects, particularly in the group with CP. Therefore, the time to task completion may have been different across subjects and could have influenced results. Clearly more research needs to be done to examine the role of these potential mechanisms and perhaps others on muscle fatigue.
Clinical Significance
Our observations of lower muscle fatigue in CP were contrary to our initial hypothesis and seem to contradict the prevalent complaint or perception of greater fatigue in this population. Measures of muscle fatigue presented here may or may not be related to subjective reports of fatigue. One difference is that this study did not measure the cardiorespiratory contribution to fatigue or perceived exertion. However, it is possible for persons to have greater endurance capacity during voluntary muscle activities and still experience greater subjective fatigue. The physiologic demands of walking and other daily activities require a larger percentage of the muscle’s force generating capacity in people with CP and others who are weaker than normal,44 increasing the perceived effort of the task. For example, walking may require 80% of a muscle’s physiologic capacity in a person with CP compared with 10% in a person without CP. It is the increased load relative to maximum capacity, rather than the absolute load, that results in physiologic burn-out in people with CP as first described by Pimm45 and in prevalent complaints of fatigue.5,6 Therefore, instead of designing protocols to increase endurance directly, therapeutic strategies should perhaps instead be targeted at increasing strength and thereby reducing the relative effort.
CONCLUSIONS
Participants with CP demonstrated lower fatigue rates for the knee flexors and extensors compared with age-matched peers without motor disability. In addition, lower levels of knee extensor muscle fatigue were associated with lower levels of function and participation. These results raise the question of whether the fatigue resistance observed in this population is the result of inherent muscle weakness, central fatigue, disordered motor control, or greater reliance on type I or oxidative fibers. More research is needed to explore these mechanisms and the potential effect on muscle fatigue. Furthermore, the influence of weakness on task effort and perceived fatigue warrants greater consideration and may lead to new treatment strategies for reducing the complaints of fatigue in this population, which are strongly linked to walking cessation with advancing age.
Acknowledgments
Supported by the American Physical Therapy Association Section on Pediatrics Clinical Research Grant, the Louisiana Board of Regents Fellowship, and the National Institute of Child Health and Human Development (grant no. T32HD007434).
Moreau submitted this research as partial fulfillment of the PhD requirements at Louisiana State University.
List of Abbreviations
- CP
cerebral palsy
- GMFCS
Gross Motor Function Classification System
- V̇O2max
maximal oxygen consumption
- PODCI
Pediatric Outcomes Data Collection Instrument
- QOL
quality of life
- ROM
range of motion
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Presented to the American Academy of Cerebral Palsy and Developmental Medicine, September 14, 2006, Boston, MA.
Supplier
Biodex Medical Systems Inc, 20 Ramsay Rd, Shirley, NY 11967–4704.
References
- 1.Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–25. doi: 10.1016/s0003-9993(98)90287-8. [DOI] [PubMed] [Google Scholar]
- 2.Damiano DL, Kelly LE, Vaughn CL. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995;75:658–67. doi: 10.1093/ptj/75.8.658. [DOI] [PubMed] [Google Scholar]
- 3.Kramer JF, MacPhail HE. Relationships among measures of walking efficiency, gross motor ability, and isokinetic strength in adolescents with cerebral palsy. Pediatr Phys Ther. 1994;6:3–8. [Google Scholar]
- 4.Pirpiris M, Gates PE, McCarthy JJ, et al. Function and well-being in ambulatory children with cerebral palsy. J Pediatr Orthop. 2006;26:119–24. doi: 10.1097/01.bpo.0000191553.26574.27. [DOI] [PubMed] [Google Scholar]
- 5.Jahnsen R, Villien L, Stanghelle JK, Holm I. Fatigue in adults with cerebral palsy in Norway compared with the general population. Dev Med Child Neurol. 2003;45:296–303. doi: 10.1017/s0012162203000562. [DOI] [PubMed] [Google Scholar]
- 6.Jahnsen R, Villien L, Egeland T, Stanghelle JK, Holm I. Locomotion skills in adults with cerebral palsy. Clin Rehabil. 2004;18:309–16. doi: 10.1191/0269215504cr735oa. [DOI] [PubMed] [Google Scholar]
- 7.Bottos M, Feliciangeli A, Sciuto L, Gericke C, Vianello A. Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516–28. doi: 10.1017/s0012162201000950. [DOI] [PubMed] [Google Scholar]
- 8.Murphy KP, Molnar GE, Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075–84. doi: 10.1111/j.1469-8749.1995.tb11968.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoofwijk M, Unnithan V, Bar-Or O. Maximal treadmill performance of children with cerebral palsy. Pediatr Exerc Sci. 1995;7:305–13. [Google Scholar]
- 10.Lundberg A. Maximal aerobic capacity of young people with spastic cerebral palsy. Dev Med Child Neurol. 1978;20:205–10. doi: 10.1111/j.1469-8749.1978.tb15205.x. [DOI] [PubMed] [Google Scholar]
- 11.Rose J, Haskell WL, Gamble JG. A comparison of oxygen pulse and respiratory exchange ratio in cerebral palsied and nondisabled children. Arch Phys Med Rehabil. 1993;74:702–5. doi: 10.1016/0003-9993(93)90029-a. [DOI] [PubMed] [Google Scholar]
- 12.Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50:313–24. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- 13.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31:594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McComas AJ, Miller RG, Gandevia SC. Fatigue brought on by malfunction of the central and peripheral nervous systems. Adv Exp Med Biol. 1995;384:495–512. doi: 10.1007/978-1-4899-1016-5_38. [DOI] [PubMed] [Google Scholar]
- 15.Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001;43:314–20. doi: 10.1017/s0012162201000597. [DOI] [PubMed] [Google Scholar]
- 16.Castle ME, Reyman TA, Schneider M. Pathology of spastic muscle in cerebral palsy. Clin Orthop Relat Res. 1979 July-Aug;:223–32. [PubMed] [Google Scholar]
- 17.Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–64. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 18.Ito J, Araki A, Tanaka H, Tasaki T, Cho K, Yamazaki R. Muscle histopathology in spastic cerebral palsy. Brain Dev. 1996;18:299–303. doi: 10.1016/0387-7604(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 19.Marbini A, Ferrari A, Cioni G, Bellanova MF, Fusco C, Gemignani F. Immunohistochemical study of muscle biopsy in children with cerebral palsy. Brain Dev. 2002;24:63–6. doi: 10.1016/s0387-7604(01)00394-1. [DOI] [PubMed] [Google Scholar]
- 20.Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12:758–68. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- 21.Pincivero DM, Gear WS, Sterner RL. Assessment of the reliability of high-intensity quadriceps femoris muscle fatigue. Med Sci Sports Exerc. 2001;33:334–8. doi: 10.1097/00005768-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson NP, Mercer TH. Reproducibility of isokinetic leg strength and endurance characteristics of adult men and women. Eur J Appl Physiol Occup Physiol. 1992;65:221–8. doi: 10.1007/BF00705085. [DOI] [PubMed] [Google Scholar]
- 23.De Ste Croix MB, Armstrong N, Welsman JR. The reliability of an isokinetic knee muscle endurance test in young children. Pediatr Exerc Sci. 2003;15:313–23. [Google Scholar]
- 24.Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc. 2001;33:1613–9. doi: 10.1097/00005768-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Jones, Stratton G. Muscle function assessment in children. Acta Paediatr. 2000;89:753–61. [PubMed] [Google Scholar]
- 26.Moreau NG, Li L, Damiano DL. A feasible and reliable muscle fatigue protocol for individuals with cerebral palsy. Pediatr Phys Ther. 2008;20:59–65. doi: 10.1097/PEP.0b013e31815e410c. [DOI] [PubMed] [Google Scholar]
- 27.Lindstrom B, Karlsson S, Gerdle B. Knee extensor performance of dominant and non-dominant limb throughout repeated isokinetic contractions, with special reference to peak torque and mean frequency of the EMG. Clin Physiol. 1995;15:275–86. doi: 10.1111/j.1475-097x.1995.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindstrom B, Kristensen B, Gerdle B. Dynamic strength and endurance of the thigh muscles in patients with minimum sequel after ischaemic stroke. Neurorehabilitation. 1999;12:157–67. [Google Scholar]
- 29.Jahnsen R, Aamodt G, Rosenbaum P. Gross Motor Function Classification System used in adults with cerebral palsy: agreement of self-reported versus professional rating. Dev Med Child Neurol. 2006;48:734–8. doi: 10.1017/S0012162206001575. [DOI] [PubMed] [Google Scholar]
- 30.McCormick A, Brien M, Plourde J, Wood E, Rosenbaum P, McLean J. Stability of the Gross Motor Function Classification System in adults with cerebral palsy. Dev Med Child Neurol. 2007;49:265–9. doi: 10.1111/j.1469-8749.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 31.Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. J Pediatr Orthop. 1998;18:561–71. doi: 10.1097/00004694-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Phys Ther. 2005;85:134–41. [PubMed] [Google Scholar]
- 33.Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol. 2001;91:2686–94. doi: 10.1152/jappl.2001.91.6.2686. [DOI] [PubMed] [Google Scholar]
- 34.Pincivero DM, Gandaio CM, Ito Y. Gender-specific knee extensor torque, flexor torque, and muscle fatigue responses during maximal effort contractions. Eur J Appl Physiol. 2003;89:134–41. doi: 10.1007/s00421-002-0739-5. [DOI] [PubMed] [Google Scholar]
- 35.Kanehisa H, Okuyama H, Ikegawa S, Fukunaga T. Fatigability during repetitive maximal knee extensions in 14-year-old boys. Eur J Appl Physiol Occup Physiol. 1995;72:170–4. doi: 10.1007/BF00964133. [DOI] [PubMed] [Google Scholar]
- 36.Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–75. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- 37.Lindstrom B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997;52:B59–66. doi: 10.1093/gerona/52a.1.b59. [DOI] [PubMed] [Google Scholar]
- 38.Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004;96:2125–32. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- 39.Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am J Phys Med Rehabil. 2002;81(Suppl 11):S127–47. doi: 10.1097/00002060-200211001-00014. [DOI] [PubMed] [Google Scholar]
- 40.Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle Nerve. 2007;36:357–63. doi: 10.1002/mus.20816. [DOI] [PubMed] [Google Scholar]
- 41.Ratel S, Duche P, Williams CA. Muscle fatigue during high-intensity exercise in children. Sports Med. 2006;36:1031–65. doi: 10.2165/00007256-200636120-00004. [DOI] [PubMed] [Google Scholar]
- 42.Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–48. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- 43.Westgaard RH, de Luca CJ. Motor unit substitution in long-duration contractions of the human trapezius muscle. J Neurophysiol. 1999;82:501–4. doi: 10.1152/jn.1999.82.1.501. [DOI] [PubMed] [Google Scholar]
- 44.Binder-Macleod SA, Snyder-Mackler L. Muscle fatigue: clinical implications for fatigue assessment and neuromuscular electrical stimulation. Phys Ther. 1993;73:902–10. doi: 10.1093/ptj/73.12.902. [DOI] [PubMed] [Google Scholar]
- 45.Pimm P. Cerebral palsy: a non progressive disorder? Educ Child Psychol. 1992;9:27–33. [Google Scholar]