Abstract
Purpose
The purpose of this study is to determine whether amifostine can induce elevated manganese superoxide dismutase (SOD2) in mouse tissues and a transplantable SA-NH tumor resulting in a delayed tumor cell radioprotective effect.
Methods and Materials
SA-NH tumor-bearing C3H mice were treated with a single 400 mg/kg or three daily 50 mg/kg doses of amifostine administered i.p. At selected time intervals following the last injection, heart, liver, lung, pancreas, small intestine, spleen and SA-NH tumor were removed and analyzed for SOD2, catalase, and glutathione peroxidase (GPx) enzymatic activity. The effect of elevated SOD2 enzymatic activity on the radiation response of SA-NH cells was determined.
Results
SOD2 activity was significantly elevated in selected tissues and a tumor 24 h following amifostine treatment. Catalase and GPx activities remained unchanged except for significant elevations in the spleen. GPx was also elevated in the pancreas. SA-NH tumor cells exhibited a 2-fold elevation in SOD2 activity and a 27% elevation in radiation resistance. Amifostine administered in 3 daily fractions of 50 mg/kg each also resulted in significant elevations of these anti-oxidant enzymes.
Conclusions
Amifostine can induce a delayed radioprotective effect that correlates with elevated levels of SOD2 activity in SA-NH tumor. If limited to normal tissues, this delayed radioprotective effect offers an additional potential for overall radiation protection. However, amifostine-induced elevation of SOD2 activity in tumors could have an unanticipated deleterious effect on tumor responses to fractionated radiation therapy given that the radioprotector is administered daily just prior to each 2 Gy fractionated dose.
Keywords: Amifostine, Manganese superoxide dismutase, Catalase, Glutathione peroxidase, Tumor
INTRODUCTION
Amifostine (Ethyol) is currently the only radioprotector drug approved by the United States Food and Drug Administration (FDA) for use in the protection against radiation therapy-induced moderate-to-severe xerostomia in patients undergoing postoperative radiation therapy for the treatment of head-and-neck cancer (1). Amifostine is a prodrug that requires dephosphorylation by alkaline phosphatase to its active thiol form, e.g., 2-[(aminopropyl)amino]ethanethiol designated WR1065 (2). The underlying mechanisms of action are free radical scavenging, hydrogen atom donation, and the induction of intracellular hypoxia by auto-oxidation (3). A novel fourth mechanism recently described is the induction of manganese superoxide dismutase (SOD2) (4,5). SOD2 is localized in the mitochondria where it dismutates superoxide into hydrogen peroxide (H2O2) which is then converted to water and oxygen by catalase and/or glutathione peroxidase (GPx). SOD2 protects against radiation-induced reactive oxygen species (ROS) damage that can lead to cell death (6–8). SOD2 is recognized as an effective normal tissue radioprotector (9–11).
WR1065, the free thiol form of amifostine, can reduce the cysteine disulfide bonds in the p50 and p65 subunits of nuclear transcription factor κB (NFκB) (12,13) which results in its activation and migration into the nucleus where it binds to an intronic NFκB element in the SOD2 gene that results in its elevated expression (14). Active SOD2 protein levels can rise 10- to 20-fold 24 h later (15–18) giving rise to a 20 to 40% increase in cellular radiation resistance (15–18). This increased resistance can be maintained with chronic daily or once every second day dosing for periods up to 14 days (19). This amifostine-induced “delayed radioprotective effect” can be completely inhibited using NFκB inhibitors (16,17) or SOD2-siRNA (18,19).
In the present study we investigate whether amifostine can induce an elevation of SOD2 activity in normal and malignant tissues and if this can affect the radiation response of a SA-NH tumor.
METHODS AND MATERIALS
Animal and Tumor Models
A murine sarcoma designated SA-NH was grown in the legs of C3H mice. The clonogenic response can be assayed both in culture and in vivo (15,16,20,21).
Cells and Culture Conditions
SA-NH mouse sarcoma cells were cultured in McCoy’s 5A medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), penicillin (100 units/ml) and streptomycin (100 μg/ml) (Invitrogen Life Technologies). Cells immediately derived from solid tumors having an average plating efficiency of 7% were maintained in a humidified 5% CO2 incubator at 37 °C (15,16).
Drug Treatment Conditions
Amifostine was supplied by the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute. Amifostine was dissolved in phosphate-buffered saline (PBS, Invitrogen Life Technologies) and administered i.p. as a single dose at a concentration of 400 mg/kg or 50 mg/kg daily for three days to C3H/HeNHsd female mice. Viable SA-NH cells, 1 × 107, were injected into the right hind leg of each mouse and tumors were grown to 8 mm in diameter (20,21). Tumor-bearing animals were then treated with 400 mg/kg or 50 mg/kg amifostine and sacrificed 8 h, 16 h, 24 h, 32 h and 48 h later and the small intestine, pancreas, lungs, liver, heart, spleen and tumor removed. The tissues were flash frozen in liquid nitrogen and stored at − 80 °C.
Flow Cytometry
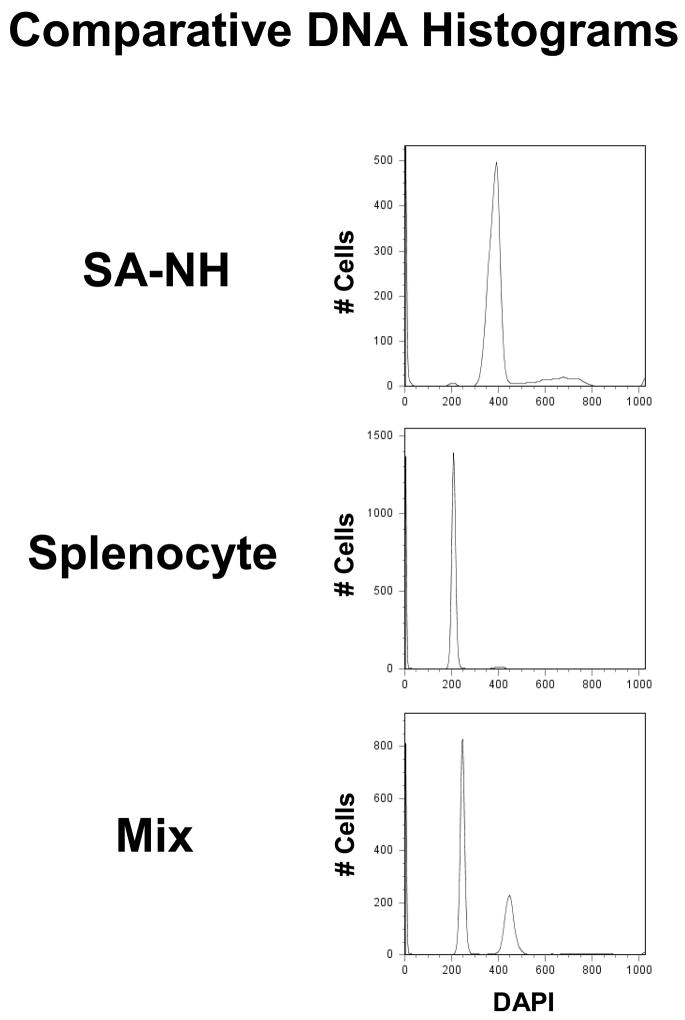
Cell suspensions were made from spleens and SA-NH tumors and mixed together. Suspensions were stained with DAPI (1 μg/ml 4′, 6-diamidino-2-phenylindole dihydrochloride hydrate; Sigma, St. Louis, MO) and were analyzed for their DNA contents using a LSRII flow cytometer (Becton Dickinson, San Jose, CA). Aneuploid SA-NH cells contain an increased amount of DNA as compared to normal diploid cells (see Fig. 1). An estimate of the normal cell contamination in each tumor suspension for the cloning efficiency assay was made by determining the area under the G1 normal peak and dividing it by the area under the total DNA histogram (22).
Fig. 1.
Comparative DNA histograms of cells isolated from spleens and SA-NH tumors in C3H mice as determined by flow cytometry.
Preparation of SA-NH Single Cell Suspensions from Tumor
Tumor-bearing mice were sacrificed, tumors aseptically harvested and minced with scissors in sterile PBS following a method described in detail elsewhere (20,21). Each cell suspension was divided into three aliquots: one used as an unirradiated control, one exposed to 2 Gy, and one for flow cytometry analysis (22). The tumor cells were plated to give rise to approximately 100 surviving colonies.
Irradiation Conditions
SA-NH tumor cells were irradiated at room temperature using a Philips X-ray generator operating at 250 kVp and 15 mA at a dose rate of 1.65 Gy/min.
In Vivo SOD2, Catalase and GPx Enzyme Activity Assays
All tissue samples were coded prior to being sent to The Radiation and Free Radical Research core lab in the Holden Comprehensive Cancer Center at The University of Iowa where the activity analyses were performed for SOD2, catalase and GPx using methods described in detail elsewhere (23–25). SOD2 activity was determined using a competitive inhibition spectrophotometric assay where increasing quantities of whole tissue homogenates (1–500 μg of protein homogenized in 50 mM potassium phosphate buffer pH 7.8 containing 1.34 mM diethylenetriaminepenta-acetic acid) are assayed for their ability to suppress superoxide-mediated NBT reduction (at 560 ηm) in the presence of 5 mM cyanide (23). The % inhibition of NBT reduction was plotted vs. protein concentration for each sample assay reaction series and one unit of SOD2 activity was defined as that amount of protein that results in 50% of maximum inhibition for each tissue of interest (23). For purified SOD2, one unit of activity equals 30–50 ng of SOD2 protein (23) and the SOD2 activity of tissue homogenates was normalized to per mg of protein. Catalase activity (calculated as κ units and normalized to protein content) was determined spectrophotometrically from aliquots of the same whole tissue homogenates (20–200 μg) used for SOD analysis by measuring the disappearance of 10 mM H2O2 at 240 ηm in 3 ml reaction volumes of potassium phosphate buffer pH 7.0 (24). Se-dependent GPx activity (normalized per mg protein) was determined spectrophotometrically from whole homogenates using 250 μM H2O2 as the substrate by determining the amount of NADPH consumed in 3 min in a reaction mixture containing glutathione, glutathione reductase, and azide to inhibit catalase activity. One unit of GPx activity was defined as the amount of enzyme required to produce 1 μmol of NADPH oxidized per min (25).
Statistics
All analyses were performed using Stata statistical software (26). Significance of relevant regression parameters were tested using a two-sided significance level of 0.05 (27). A simple linear regression was performed on the data generated from comparing the effects of amifostine on SOD2, catalase, and GPx enzymatic activities as a function of time following treatment. Significance of effect of time was also tested using a one-way ANOVA.
RESULTS
Effects of Amifostine on Enzyme Activities in Mouse Tissues
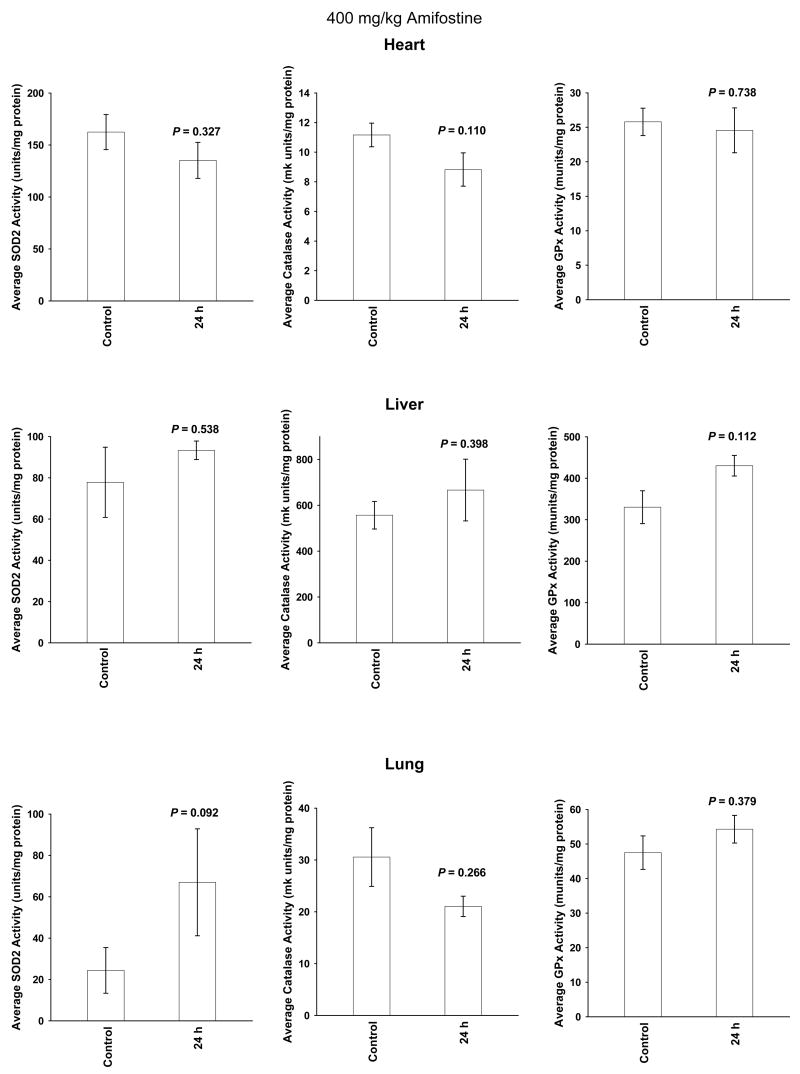
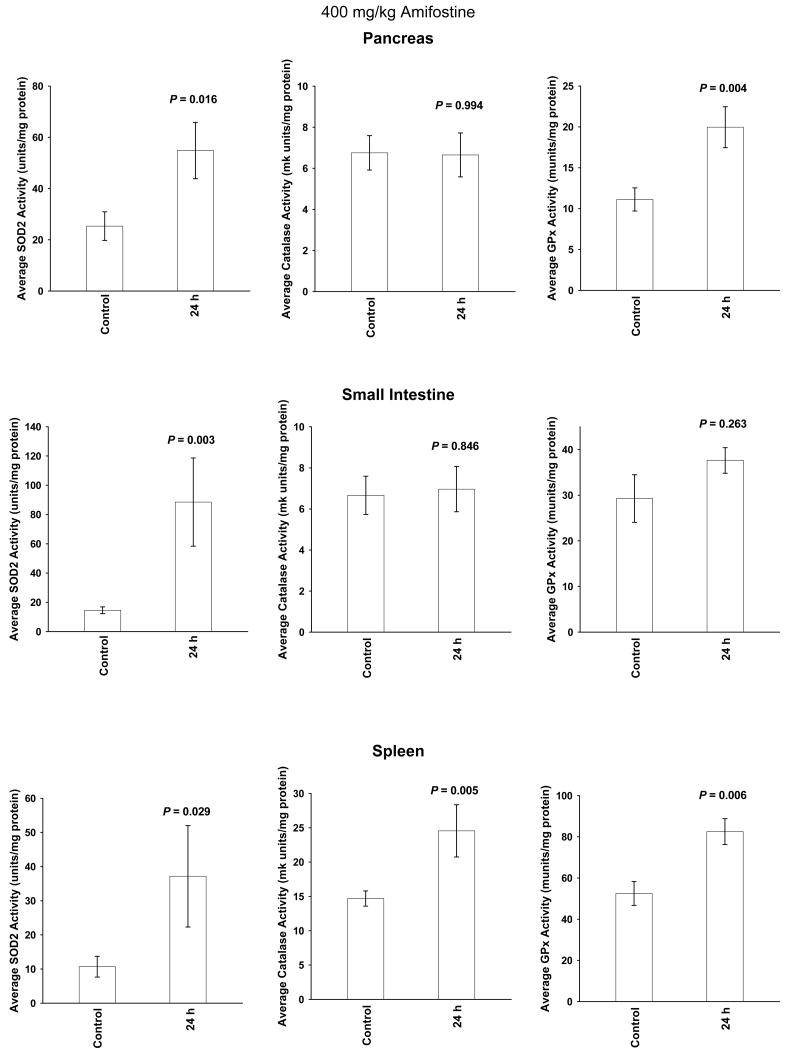
A single amifostine dose of 400 mg/kg was chosen for study because it is not toxic and has been routinely used in radiation protection studies in mice (28). Enzymatic activities were measured at baseline, e.g. 0 h, as well as 24 h later. The 24 h time point was chosen because in previous studies SOD2 activity was maximally elevated at this time (15–19) and in clinical radiotherapy trials amifostine is routinely administered on a daily basis just prior to the delivery of each succeeding fractionated dose of radiation. Presented in Figures 2 and 3 are comparative bar graphs describing the changes in SOD2, catalase and GPx activities in the six normal tissues. Amifostine dosing did not significantly affect these enzymes in heart, liver, and lung tissues (see Figure 2). SOD2 activity was significantly elevated in pancreas, small intestine and spleen. GPx activity was elevated in pancreas and spleen. Catalase activity was only elevated in spleen (see Figure 3).
Fig. 2.
The effects of a single 400 mg/kg dose of amifostine on manganese superoxide dismutase (SOD2), catalase and glutathione peroxidase (GPx) enzymatic activity levels measured 24 h later in heart, liver and lung tissues (n = 6 per experimental point). Levels of significance (P values) were determined using a Student’s two-tailed t test. Error bars represent the Standard Error of the Mean (± S.E.M.).
Fig. 3.
The effects of a single 400 mg/kg dose of amifostine on SOD2, catalase and GPx enzymatic activity levels measured 24 h later in pancreas, small intestine and spleen tissues (n = 6 per experimental point). Levels of significance were determined using a two-tailed Student’s t test. Error bars = S.E.M. SOD2 activity was significantly elevated in all three tissues, while GPx activity was significantly elevated in pancreas and spleen, and catalase activity elevated only in spleen tissue.
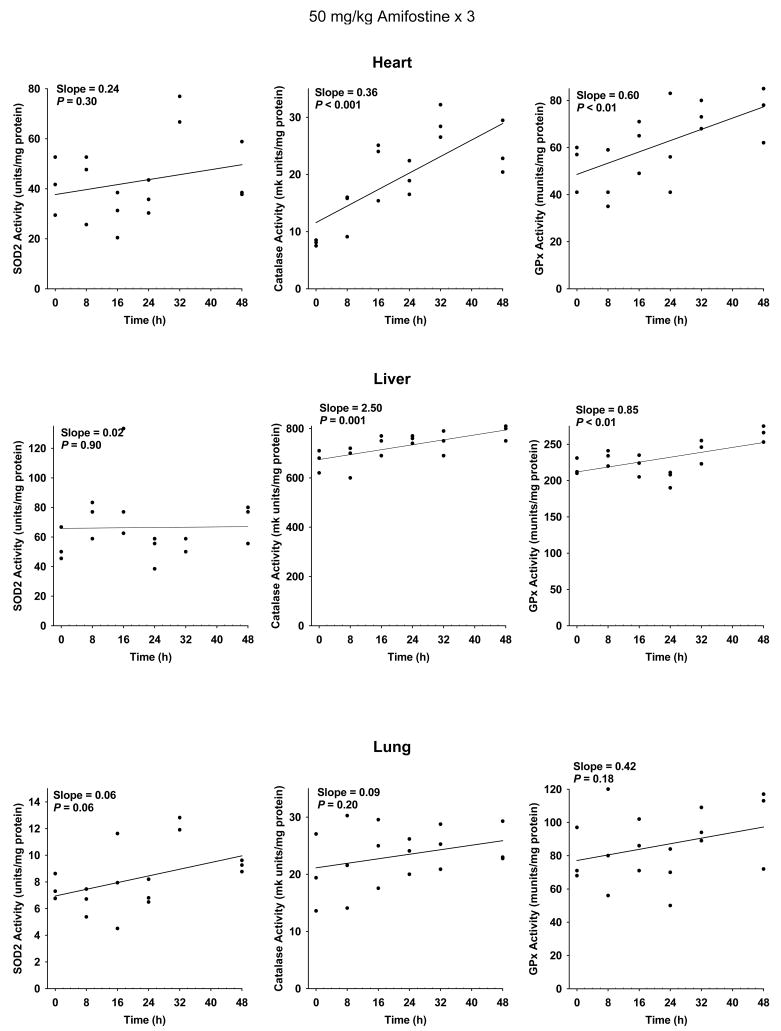
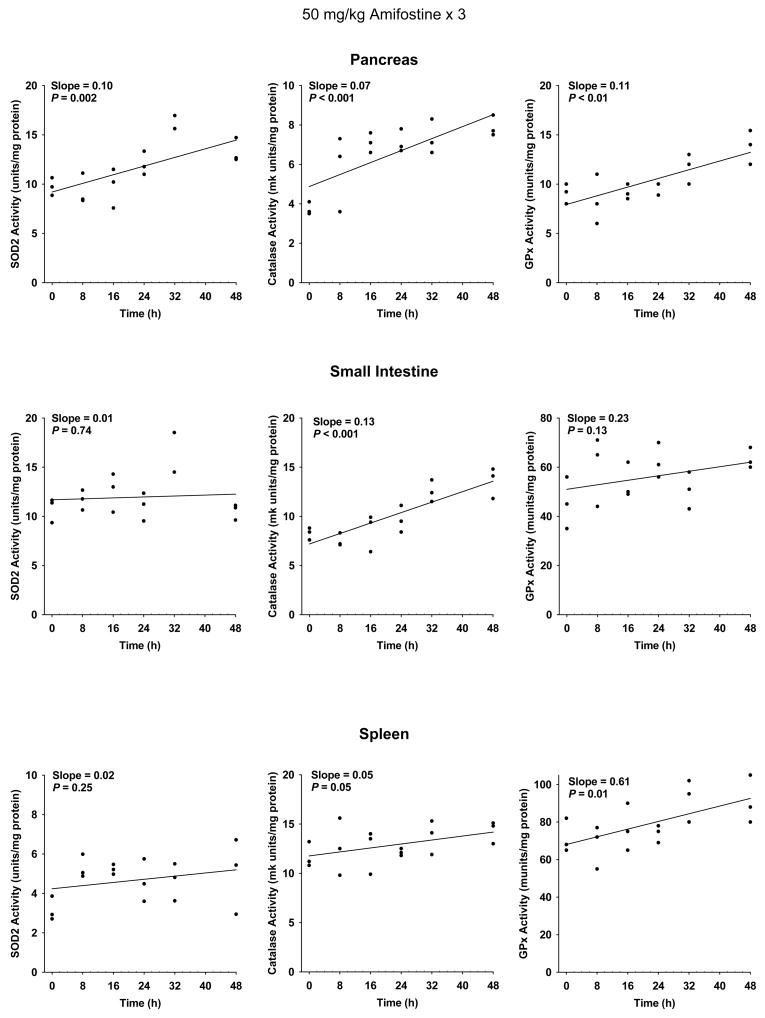
At a dose of 400 mg/kg, however, cumulative toxicity prevents daily dosing of mice (28). A dose of 50 mg/kg is well tolerated in mice. Scatter plots describing the change in SOD2, catalase and GPx activities as a function of time following the last of 3 daily 50 mg/kg doses of amifostine are presented for heart, liver and lung in Figure 4, and pancreas, small intestine and spleen in Figure 5. A simple linear regression was performed to describe the effect of organ enzyme activity over time. No significant change in slope representing SOD2 activity was observed in heart, liver, lung, small intestine and spleen (see Figures 4 and 5). Catalase and GPx activities were significantly elevated in heart and liver, but not lung (see Figure 4). Pancreas exhibited a significant elevation in SOD2, catalase and GPx activities. A significant increase in catalase and GPx activities was observed in spleen, and catalase only in the small intestine (see Figure 5).
Fig. 4.
The effects of 50 mg/kg doses of amifostine administered each day for three consecutive days on enzymatic activities as a function of time in heart, liver, and lung (n = 3 per experimental time point). All analyses were performed using Stata statistical software. Significance of relevant regression parameters were tested using a two-sided significance level of 0.05. Both catalase and GPx activities were significantly elevated in heart and liver. Using this analysis, no changes in SOD2 activity reached statistical significance.
Fig. 5.
The effects of 50 mg/kg doses of amifostine administered each day for three consecutive days on the subsequent changes in enzymatic activities as a function of time in pancreas, small intestine and spleen (n = 3 per experimental time point). All analyses were performed using Stata statistical software. Significance of relevant regression parameters were tested using a two-sided significance level of 0.05. Elevation of SOD2 activity reached statistical significance only in pancreas. Elevation of catalase activity reached significance in all three tissues, while elevations in GPx activity reached significance only in pancreas and spleen tissues.
Effect of Amifostine on Enzyme Activity and Radiation Response in SA-NH
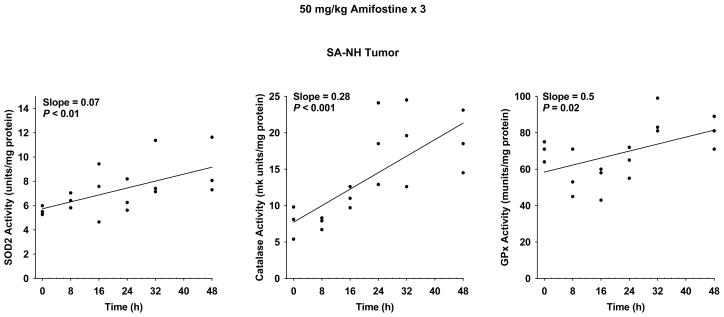
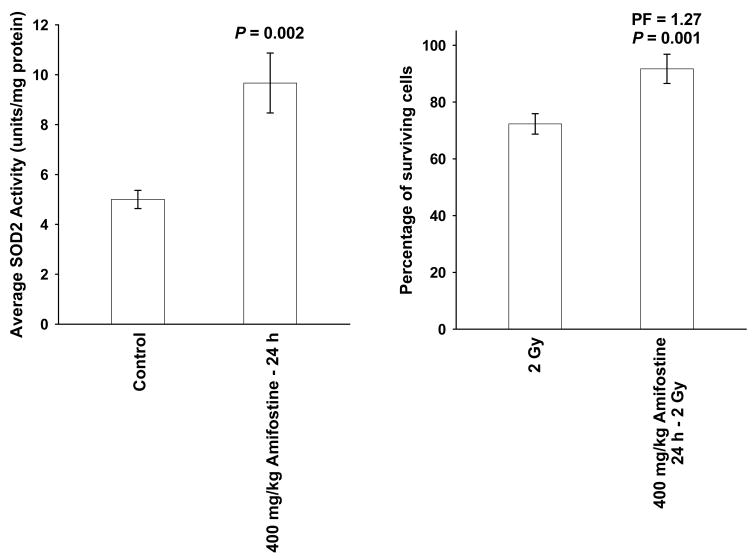
The enzymatic activities of SOD2 and catalase significantly increased in SA-NH with time (see Figure 6). SA-NH tumor-bearing mice, 26 per experimental group, were either exposed to a single 400 mg/kg dose of amifostine or were untreated. Twenty-four h later SOD2 activity increased over 2-fold (see Figure 7a). The surviving fraction of SA-NH cells with elevated SOD2 activity was 91.7% ± 5.1% as compared to 72.3% ± 3.6% in matched irradiated control cells (see Figure 7b).
Fig. 6.
The effects of 50 mg/kg doses of amifostine administered each day for three consecutive days on the subsequent changes in enzymatic activities as a function of time in 8 mm diameter SA-NH tumors growing in C3H mice (n = 3 for each experimental time point). All analyses were performed using Stata statistical software. Significance of relevant regression parameters were tested using a two-sided significance level of 0.05. Elevation of SOD2, catalase and GPx enzymatic activities all reached statistically significant levels in SA-NH tumors.
Fig. 7.
The effects of a single 400 mg/kg dose of amifostine to tumor-bearing C3H mice on SOD2 enzymatic activity and radiation response in SA-NH tumor cells measured 24 h later. Because amifostine is a potent radioprotector, baseline SOD2 activity and radiation response of SA-NH was measured immediately after irradiation of animals that were not exposed to the drug (n = 26 animals per experimental point) (7a). SA-NH tumor cell suspensions from 8 mm tumors grown in control and amifostine treated C3H mice were split into three aliquots, respectively, with one used for flow cytometry analysis to determine % normal cell contamination, one for the determination of colony forming efficiency in vitro, and one for an exposure to a dose of 2 Gy to determine a surviving fraction (7b). Significance was determined using a Student’s two-tailed t test. Error bars = S.E.M.
DISCUSSION
Amifostine’s free thiol form, WR1065, is a potent inducer of SOD2 activity in cells (16). Amifostine is rapidly cleared from the bloodstream of mice as evidenced by a 10-fold drop in concentration within 30 min of injection (29). Maximal induction of SOD2 activity in human and mouse tumor cells, however, occurs about 24 h following exposure even to concentrations of WR1065 as low as 40 μM, a concentration readily achievable in the bloodstream of patients administered amifostine during radiation therapy (30). Since amifostine is routinely administered to cancer patients about 30 min prior to their radiation treatment on a daily basis during a typical fractionated radiation therapy protocol, the potential exists for a buildup of SOD2 activity in both normal and tumor tissues on succeeding days of treatment. While this would be beneficial with regards to augmenting the direct protective effects of amifostine on dose limiting normal tissues, it could also detrimentally affect overall tumor response in a 2 Gy per fraction treatment regimen.
We chose to evaluate the effects of amifostine exposure on subsequent changes in SOD2 activity and radiation response in SA-NH tumors grown in C3H mice because of its well characterized response under in vitro conditions (15,16,19). SOD2 is localized within the mitochondria where it catalyzes the dismutation of superoxide anion to O2 and H2O2. Catalase and GPx are also important anti-oxidants whose enzymatic activities are required to convert H2O2 into H2O and O2. Failure to accomplish this can result in a toxic buildup of H2O2 that can lead to cell sensitization and death. Therefore, the potential exists for both radiation protection and sensitization following an elevation of SOD2 activity induced by amifostine treatment. For this reason catalase and GPx activities were also monitored along with SOD2 as a function of amifostine exposure. No significant inductive or repressive effects were observed on any of these three enzymatic activities in heart, liver or lung (see Figure 2). In contrast, SOD2 activities were significantly elevated in pancreas, small intestine and spleen (see Figure 3). While the change in catalase and GPx activities in tissue from the small intestine did not reach significance, there was a measured increase in catalase and/or GPx activities in pancreas and spleen. The maintenance or elevation of either of these two enzymes in these tissues could prevent the toxic buildup of H2O2.
A dose of 400 mg/kg in the mouse is highly cytoprotective but is too toxic to be administered on a daily basis (28). To assess the effect of daily dosing on these enzyme activities, animals were administered 50 mg/kg of amifostine at 24 h intervals for 3 days. The resulting data are presented and analyzed using two different approaches. Presented in Figures 4 and 5 are scatter plots describing the enzymatic activity of each of the three anti-oxidant enzymes as a function of time following the administration of the last dose of amifostine. Regression lines were fitted to the data and their respective slopes determined. Using this approach all of the calculated slopes for each of the three enzymes and tissues analyzed exhibited positive values (see Figures 4 and 5). The increase in the slope describing the change in SOD2 activity as a function of time approached significance for lung tissue (P = 0.06), and was highly significant for the pancreas (P = 0.002). Catalase and GPx activities were significantly elevated as a function of time following multi-dose amifostine treatment in heart, liver, pancreas and spleen (see Figures 4 and 5).
The data were also subjected using a one-way ANOVA test for the significance of changes in enzymatic activity as a function of time following multi-dose amifostine treatment (see Table 1). A result of this analysis indicates that a significant elevation of SOD2 activity occurred following amifostine treatment in heart, lung, pancreas and small intestine, but not in SA-NH tumor. However, if the SA-NH data are plotted and subjected to regression analysis in the manner described above for the normal tissues (see Figures 4 and 5), SOD2, catalase and GPx activities all appear to be significantly elevated as a function of time (see Figure 6). Catalase and GPx activities were both significantly elevated in SA-NH following amifostine treatment as determined by a one-way ANOVA test of the data (P <0.01), while heart, liver, pancreas and small intestine each exhibited a significant elevation in catalase and/or GPx (see Table 1). The maintenance and or elevation of catalase and GPx activities in tissues that exhibit an elevation in SOD2 could prevent any tissue sensitization due to toxic buildup of H2O2.
Table 1.
Effects of 50 mg/kg Amifostine × 3 on Anti-Oxidant Activities in Selected Normal and SA-NH Tissues in C3H Mice
| SOD2 | Catalase | Glutathione Peroxidase | ||||
|---|---|---|---|---|---|---|
| Organ | F Statistic | P Value | F Statistic | P Value | F Statistic | P Value |
| Heart | 4.00 | 0.02 | 12.30 | < 0.001 | 2.40 | 0.09 |
| Liver | 1.90 | 0.20 | 3.30 | 0.04 | 8.22 | < 0.01 |
| Lung | 3.50 | 0.03 | 0.35 | 0.86 | 1.01 | 0.45 |
| Pancreas | 9.30 | < 0.01 | 7.65 | < 0.01 | 5.62 | < 0.01 |
| Small Intestine | 4.60 | 0.01 | 11.00 | < 0.001 | 1.90 | 0.16 |
| Spleen | 1.70 | 0.20 | 0.91 | 0.50 | 2.77 | 0.06 |
| Tumor | 1.70 | 0.22 | 5.90 | < 0.01 | 5.92 | < 0.01 |
All analyses were performed using Stata statistical software. Significance of the effect of changes in enzymatic activity in target tissues as a function of time following exposure of SA-NH tumor-bearing animals to multiple doses of amifostine was tested using a one-way ANOVA.
The ability of amifostine to induce SOD2 gene expression (4,5,13) and elevate SOD2 enzymatic activity 24 h later in both normal and tumor cells (13,15–19) presents a potential concern regarding tumor protection. A single 400 mg/kg dose of amifostine significantly elevated SOD2 activity 24 h later in SA-NH tumors (see Figure 7a) consistent with earlier published data obtained using SA-NH cells grown in culture (15,16). This increase in SOD2 activity in SA-NH was accompanied by a 27% increase in radiation resistance, also consistent with earlier reports (15,16) (see Figure 7b). If such an increase in tumor cell survival would occur following each succeeding 2 Gy fractioned dose of radiation, overall tumor response could be adversely affected. It is possible that the amifostine-mediated inductive effect of SOD2 activity might diminish over time. However, this was not observed in multiple dosing studies that were carried out under in vitro conditions that were limited to 2 weeks of duration (19). It is also possible that prolonged elevation of SOD2 activity from daily dosing might not be accompanied by a similar effective level of catalase and/or GPx activity. If the activities of these enzymes do not keep pace with an elevated SOD2 activity in the tumor throughout the course of treatment, H2O2 buildup and subsequent toxicity could then lead to tumor sensitization.
The issue of amifostine-induced tumor protection continues to plague the general acceptance of this agent for use in cancer therapy (31). This stems in part from the lack of clinical studies designed to be robust enough to evaluate the influence of amifostine on therapeutic index (32). It has been pointed out that to identify a reduction in patient survival from 45 to 40% with a significance of 0.05 and an 80% power, a total of 1,200 patients per study arm would be required (33). Another approach is to perform a meta-analyses on completed randomized studies (34,35). A meta-analysis on the effect of amifostine on response rates in locally advanced non-small-cell lung cancer patients was performed using data from seven randomized trials involving about 600 patients (35). It was concluded that amifostine had no effect on tumor response. However, in the seven studies that were evaluated, amifostine was administered using 5 different dosing regimens, two different routes of administration, and three of the studies did not exhibit a conclusive evidence of normal tissue protection. Dosing and timing are two very important parameters that are recognized as major variables in determining cytoprotection by amifostine (28). Since the rationale for amifostine’s use in the clinic is its ability to differentially protect normal from tumor tissues, it would seem prudent to require that all studies entered into a meta-analysis should demonstrate a significant level of normal tissue protection to insure that a sufficient amount of amifostine was present at the time of irradiation to exert a protective effect. The potential for amifostine mediated tumor protection would best be assessed under these conditions (35).
Before amifostine’s potential can be fully recognized and its role in radiation therapy be accepted as “The First Selective-Target and Broad-Spectrum Radioprotector” (1) studies must be completed regarding its potential effects on long-term tumor control. This is especially important if it is ever to be used in patients having a good prognosis and a relatively long life expectancy. However, the more that is understood regarding amifostine’s cytoprotective properties, the better these trials can be designed. We have identified a novel cytoprotective mechanism induced by amifostine, the induction and elevation of the potent anti-oxidant SOD2. The demonstration that SOD2 activity is significantly elevated 24 h following amifostine administration suggests that daily or every other day dosing with this drug may exert an additional protective effect that was here-to-fore unrecognized. However, if this effect is extended to tumors, the potential for adversely affecting their response to radiation therapy could be minimized by increasing the interval of amifostine administration to once every 72 h during a fractionated radiation therapy regimen.
Acknowledgments
This work was supported in part by NIH/NCI RO1 CA99005 (DJG), PO1 CA086862 (MCC, DRS) and DOE Grants DE-FG02-05ER64086 (DJG) and DE-FG02-05ER64050 (DRS).
Footnotes
Conflict of Interest Notification
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: The first selective-target and broad-spectrum radioprotector. The Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 2.Smoluk GD, Fahey RC, Calabro-Jones, et al. Radioprotection of cells in culture by WR2721 and derivatives: Form of the drug responsible for protection. Cancer Res. 1988;48:3641–3647. [PubMed] [Google Scholar]
- 3.Giambarresi L, Jacobs AJ. Radioprotectants. In: Conklin JJ, Walker RI, editors. Military radiobiology. Orlando, FL: Academic Press; 1987. pp. 265–301. [Google Scholar]
- 4.Das KC, Lewis-Molock Y, White CW. Activation of NF-κb and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am J Physiol. 1995;269:L588–L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- 5.Antras-Ferry J, Maheo K, Chevanne M, et al. Oltipraz stimulates the transcription of the manganese superoxide dismutase gene in rat hepatocytes. Carcinogenesis. 1997;18:2113–2117. doi: 10.1093/carcin/18.11.2113. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Chen Y, Li M, et al. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–593. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 7.Epperly MW, Gretton JE, Sikora CA, et al. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 8.Guo G, Yan-Sanders Y, Lyn-Cook BD, et al. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23(7):2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberger JS, Epperly MW. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo. 2007;21(2):141–146. [PubMed] [Google Scholar]
- 10.Niu Y, Epperly MW, Shen H, et al. Intraesophageal MnSOD-plasmid liposome enhances engraftment and self-renewal of bone marrow derived progenitors of esophageal squamous epithelium. Gene Ther. 2008;15(5):347–356. doi: 10.1038/sj.gt.3303089. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Epperly MW, Kay MA, et al. Radioprotection In Vitro and In Vivo by mini circle plasmid containing the human manganese superoxide dismutase (MnSOD) transgene. Hum Gene Ther. 2008 doi: 10.1089/hum.2007.141. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews JR, Wakasugi N, Virelizier JL, et al. Thioredoxin regulates the DNA binding activity of NFκB by reduction of the disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murley JS, Kataoka Y, Hallahan DE, et al. Activation of NFκB and MnSOD gene expression by free radical scavengers in human microvascular endothelial cells. Free Radic Biol Med. 2001;30(12):1426–1439. doi: 10.1016/s0891-5849(01)00554-8. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Kiningham KK, Devalaraja MN, et al. An intronic NFκB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin 1β. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 15.Murley JS, Kataoka Y, Weydert CJ, et al. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of Amifostine. Radiat Res. 2002;158:101–109. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Murley JS, Kataoka Y, Cao D, et al. Delayed radioprotection by NF-κb-mediated induction of Sod2 (MnSOD) in SA-NH tumor cells after exposure to clinically used thiol-containing drugs. Radiat Res. 2004;162:536–546. doi: 10.1667/rr3256. [DOI] [PubMed] [Google Scholar]
- 17.Murley JS, Kataoka Y, Weydert CJ, et al. Delayed radioprotection by nuclear transcription factor κb-mediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to the free radical scavenger WR1065. Free Radic Biol Med. 2006;40:1004–1016. doi: 10.1016/j.freeradbiomed.2005.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murley JS, Kataoka Y, Baker KL, et al. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of Amifostine and tumor necrosis factor α. Radiat Res. 2007;167:465–474. doi: 10.1667/RR0758.1. [DOI] [PubMed] [Google Scholar]
- 19.Murley JS, Nantajit D, Baker KL, et al. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of Amifostine. Radiat Res. 2008;169:494–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpe JP, Hunter B, Basic I, et al. Metastatic properties of murine sarcomas and carcinomas. I. Positive correlation with lung colonization and lack of correlation with s.c. tumor take. Clin Exp Met. 1985;3:281–294. doi: 10.1007/BF01585082. [DOI] [PubMed] [Google Scholar]
- 21.Grdina DJ, Kataoka Y, Murley JS, et al. Inhibition of spontaneous metastases formation by Amifostine. Int J Cancer. 2002;97:135–141. doi: 10.1002/ijc.1592. [DOI] [PubMed] [Google Scholar]
- 22.Grdina DJ, Linde S, Mason K. Response of selected tumor cell populations separated from a fibrosarcoma following irradiation in situ with fast neutrons. Br J Radiol. 1978;51:291–301. doi: 10.1259/0007-1285-51-604-291. [DOI] [PubMed] [Google Scholar]
- 23.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 26.Statcorp. Stata Statistical Software: Release 10.0. College Station, TX: Stata Corporation; 2007. [Google Scholar]
- 27.Daly LE, Bourke GJ, editors. Comparison of more than two independent groups. Intrepretation and uses of medical statistics. 5. Blackwell Science; NJ: 2000. pp. 296–338. [Google Scholar]
- 28.Grdina DJ, Kataoka Y, Murley JS. Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Inter. 2000;16(4):237–279. doi: 10.1515/dmdi.2000.16.4.237. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LM, Bonner HS, Brown DQ. Metabolic pathways of WR-2721 (Ethyol, Amifostine) in the BALB/c mouse. Drug Metabol Dispos. 1994;22:895–902. [PubMed] [Google Scholar]
- 30.Shaw LM, Bonner HS, Schuchter L, et al. Pharmacokinetics of Amifostine: Effects of dose and method of administration. Sem Oncol. 1999;26(2Suppl 7):34–36. [PubMed] [Google Scholar]
- 31.Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000;57:113–118. doi: 10.1016/s0167-8140(00)00235-8. [DOI] [PubMed] [Google Scholar]
- 32.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 33.Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol. 2007;25(26):4084–4089. doi: 10.1200/JCO.2007.11.5816. [DOI] [PubMed] [Google Scholar]
- 34.Sasse AD, Oliveira Clark LG, Sasse EC, et al. Amifostine reduces side effects and improves complete response rate during radiotherapy: Results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2006;64(3):784–791. doi: 10.1016/j.ijrobp.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Mell LK, Malik R, Komaki R, et al. Effect of amifostine on response rates in locally advanced non-small-cell lung cancer patients treated on randomized controlled trials: A meta-analysis. Int J Radiat Oncol Biol Phys. 2007;68(1):111–118. doi: 10.1016/j.ijrobp.2006.11.043. [DOI] [PubMed] [Google Scholar]