Abstract
Summary: Biologics are increasingly becoming part of routine disease management. As more agents are developed, the challenge of keeping track of indications and side effects is growing. While biologics represent a milestone in targeted and specific therapy, they are not without drawbacks, and the judicious use of these “magic bullets” is essential if their full potential is to be realized. Infectious complications in particular are not an uncommon side effect of therapy, whether as a direct consequence of the agent or because of the underlying disease process. With this in mind, we have reviewed and summarized the risks of infection and the infectious disease-related complications for all FDA-approved monoclonal antibodies and some related small molecules, and we discuss the probable mechanisms involved in immunosuppression as well as recommendations for prophylaxis and treatment of specific disease entities.
INTRODUCTION
Biologics in the Treatment of Disease
Modern therapeutics is experiencing an explosion in the development of drugs directed at specific molecular targets for the treatment of disease. As our knowledge of molecular pathogenesis grows, the fabrication of “designer molecules,” such as monoclonal antibodies and small molecules, against key steps in disease pathways has led to astounding clinical responses, remission, and cure of previously untreatable or intractable illnesses (67, 166, 192). The term “biologic” or “biological” in a broader sense applies to any biologically derived product, but for the purposes of this review, it refers to the class of drugs which includes monoclonal antibodies, receptor analogues, and chimeric small molecules designed to bind to or mimic their molecular targets (67). These drugs have obvious advantages over conventional therapy in terms of potency, specificity, and theoretically decreased side effects, since the product is engineered to bind to or interfere with a distinct molecular target (107, 166). While biologics for the most part represent a considerable boon to physicians and patients alike, they are not without their own unique set of problems, which clinicians must be aware of in order to utilize them with maximum benefit.
A Brief History of Monoclonal Antibodies and Receptor Analogues
Ever since Köhler and Milstein (91) first described the production of hybridomas producing specific antibodies, monoclonal antibodies have become a cornerstone of both basic and clinical research and have since made the transition to the clinical arena (107). The first monoclonal antibody used in the treatment of disease was muromonab, a murine anti-CD3 immunoglobulin G2a (IgG2a) antibody used for prophylaxis or treatment of allograft rejection (161). Advances in DNA recombinant technology also led to the development of the first chimeric receptor analog protein, etanercept (130). Further advances in molecular techniques allowed for the manipulation of genetic sequences, producing chimeric murine-human constructs, such as infliximab and rituximab, which contain the human effector portion of the antibody and the murine antigen binding portion, and fully “humanized” monoclonal antibodies with only the antigen binding site sequences derived from mouse genes, such as daclizumab and omalizumab, which are less likely to induce an immune reaction than purely murine antibodies (166). Since then, more than 20 monoclonal antibodies have been approved for therapeutic use in humans, along with other biologic products, from recombinant receptors to fusion proteins with higher potencies and specificities (67, 181). Tables 1, 2, and 3 give summaries of the agents and their effects.
TABLE 1.
Summary of FDA-approved monoclonal antibodies and selected small molecules and their risks of infection
| Drug name (trade name, manufacturer) | Type | Target | Indication | Risk of serious infection | Serious infections | Comments |
|---|---|---|---|---|---|---|
| Adalimumab (Humira, Abbott) | Human IgG1 | TNF-α | RA, JRA, AS, PA, PP, CD | Yes for granulomatous disease | Serious pulmonary bacterial infections, TB, candidiasis, CMV infection, toxoplasmosis, and nocardiosis | Black box for infections |
| Infliximab (Remicade, Centocor Inc.) | Chimeric IgG1 | TNF-α | RA, AS, PA, PP, CD, UC | Yes for granulomatous disease | Pneumonia, sepsis, TB, infections with MOTT, listeriosis, histoplasmosis, candidiasis, aspergillosis, cryptococcosis, salmonellosis, toxoplasmosis, brucellosis, bartonellosis, leishmaniasis, coccidiomycoses, leprosy, CMV infection, HBV reactivation | Black box for infections |
| Certolizumab pegol (Cimzia, UCB Inc.) | Humanized Fab′ antibody fragment conjugated to polyethylene glycol | TNF-α | CD | Yes for granulomatous disease | Serious pulmonary bacterial infections, TB, viral and fungal infections | Black box for infections |
| Etanercept (Enbrel, Immunex) | Human dimeric fusion protein | TNF-α | RA, JRA, AS, PA, PP | Yes for granulomatous disease but less than that with monoclonal antibodies, yes in combination with anakinra | Serious bacterial infections, TB, infections with MOTT, listeriosis, histoplasmosis, candidiasis, aspergillosis, cryptococcosis, nocardiasis, protozoal and VZV infection | Black box for infections |
| Abatacept (Orencia, Bristol-Meyers Squibb) | Fusion protein | T-cell costimulation inhibitor | RA, JRA | Yes, in combination with etanercept and other DMARDs, less clear for monotherapy | Pneumonia, sepsis, aspergillosis, sinusitis, candidiasis, bronchitis, skin and soft tissue infections, viral infections with HSV and VZV | |
| Anakinra (Kineret, Amgen) | Nonglycosylated IL-1 receptor | IL-1 | RA | Yes | Pneumonia, cellulitis, TB, unspecified mycobacterial and fungal infections | |
| Rilonacept (Arcalyst, Regeneron) | Dimeric fusion protein IL-1 inhibitor | IL-1 | CAPS | Yes | Meningitis, MOTT infection, severe bronchitis | |
| Efalizumab (Raptiva, Genentech) | Humanized IgG1 | CD11a | PP | Yes, although most infections tend to be mild | Pneumonia, cellulitis, abscess, sepsis, Legionella pneumonia, necrotizing fasciitis, TB, PML | Black box for infections |
| Alefacept (Amevive, Astellas) | Fusion protein | Inhibits T-cell activation | PP | Probable, inconsistent association from clinical trials | Cellulitis, abscess, wound infections, toxic shock, pneumonia, appendicitis, cholecystitis, gastroenteritis, MOTT infection, influenza virus, HCV, and herpesvirus infections | Causes decrease in CD4+ and CD8+ cells |
| Alemtuzamab (Campath, Genzyme Corp.) | Humanized IgG1 | CD52 | CLL, TPL, NHL | Yes, causes prolonged lymphopenia | Overwhelming bacteremia, pneumonia, meningitis, CMV, VZV, and HSV infections, PCP, PML, adenovirus infection, acanthamebiasis, toxoplasmosis, histoplasmosis, cryptococcosis, aspergillosis, Fusarium infection, Scedosporium infection, BK virus infection, HHV-6 infection, candidiasis, parvovirus infection, mucormycosis, TB, Balamuthia mandrillaris infection, MOTT infection, BCGosis, Rhodococcus infection, HBV reactivation | Black box for infections, prophylaxis for PCP and herpesviruses |
| 90Y-ibritumomab tiuxetan (Zevalin, Biogen Idec Pharmaceuticals Corp.) | Radioconjugated murine IgG1 | CD20 | NHL | Yes, causes prolonged cytopenias | pneumonia, sepsis, cellulitis, colitis, diarrhea, empyema, osteomyelitis, pericarditis, viral pneumonia and viral hepatitis | Blackbox for prolonged and severe cytopenias |
| Rituximab (Rituxan, Genentech) | Chimeric human-murine IgG1 | CD20 | RA, NHL | Yes for PML and hepatitis B, less clear for other infections | Serious bacterial infections, PML, parvovirus, CMV, HSV, and disseminated VZV infections, HBV and HCV reactivation | Black box for PML |
| 131I-tositumomab (Bexxar, Corixa Corp.) | Radioconjugated murine IgG2 | CD20 | NHL | Yes, causes prolonged cytopenias | Pneumonia, septicemia, bronchitis, skin infections, viral infections | Black box for prolonged and severe cytopenias |
| Gemtuzumab ozogamicin (Mylotarg, Wyeth Pharmaceuticals Inc.) | Humanized IgG4 conjugated to calicheamicin | CD33 | AML | Yes, from prolonged neutropenia | Sepsis, pneumonia, HSV infection, usual opportunistic infections with neutropenia, unusual pathogens include Staphylococcus hominis, Agrobacterium radiobacter, Acinetobacter lwoffii, Rhodococcus, and Pantoea agglomerans | |
| Bevacizumab (Avastin, Genentech) | Humanized IgG1 | VEGF | CRCa, NSCLCa, BrCa | Yes, severe neutropenia in combination with chemotherapy; intraocular injection without evidence of increased risk | Sepsis, anaerobic liver abscess with Bacteroides fragilis, Fusarium nasal septal infection, endophthalmitis, intraocular injection including Bacillus cereus and coagulase-negative Staphylococcus | Black box for intestinal perforation, sometimes with abscess |
| Cetuximab (Erbitux, Bristol-Meyers Squibb and Imclone) | Chimeric human-murine IgG1 | ErbB1 | CRCa, HNCa | Yes, dermatologic toxicity with infectious sequelae | Paronychia caused by Staphylococcus aureus, abscess, sepsis | |
| Panitumumab (Vectibix, Amgen) | Human IgG2 | ErbB1 | CRCa | Yes, dermatologic toxicity with infectious sequelae | Paronychia, abscess, sepsis | |
| Trastuzumab (Herceptin, Genentech) | Human IgG1 | HER2 | BrCa | Yes, in combination with certain chemotherapeutic regimens | Febrile neutropenia | |
| Basiliximab (Simulect, Novartis) | Chimeric human-murine IgG1 | CD25 | Renal transplant rejection prophylaxis | Increased risk with triple regimens for CMV, otherwise no difference versus placebo | Bacterial, CMV, and HSV infections, aspergillosis, nocardiosis, candidiasis, and protozoal infections | |
| Daclizumab (Zenapax, Hoffman-la Roche) | Humanized IgG1 | CD25 | Renal transplant rejection prophylaxis | Probable, inconsistent association from clinical trials, with overall incidence no different from that with placebo, although severe infections were more common | Nocardiosis, legionellosis, MOTT infection, TB, viral infection with CMV, BK virus, adenovirus, HSV, RSV, or influenza virus, fungal infections with Aspergillus, Scedosporium, Cunninghamella, and Candida | |
| Muromonab (Orthoclone-OKT3, Ortho Biotech) | Murine IgG2 | CD3 | Solid organ transplant rejection | Yes, but no different from that with high-dose steroids | Bacterial infections, including Listeria, Nocardia, and MOTT infections; infections with Aspergillus, Candida, Cryptococcus, or dermatophytes; PCP; infections with Toxoplasma gondii, CMV, EBV, HSV, hepatitis viruses, VZV, adenovirus, enterovirus, RSV, and parainfluenza virus | |
| Abciximab (Reopro, Centocor Inc.) | Fab′ fragment of chimeric human-murine IgG1 | GPIIb/IIIa | Adjunct for PCI | No, incidence of pneumonia is slightly higher than that with placebo (P value not reported) | Pneumonia | |
| Natalizumab (Tysabri, Biogen Idec Pharmaceuticals Corp.) | Humanized IgG4 | α4-Integrin | MS, CD | Yes with PML, less clear for other infections | PML, herpesvirus infections, influenza, Cryptosporidium diarrhea, bacterial pneumonias and UTIs, PCP, Mycobacterium avium-intracellulare infection, Aspergillus and Burkholderia cepacia infections | Black-box warning for PML, restricted distribution |
| Omalizumab (Xolair, Genentech) | Humanized IgG1 | IgE | Asthma | Theoretical increased risk of parasitic infection, not substantiated in clinical trials | None | |
| Palivizumab (Synagis, Medimmune) | Humanized IgG1 | F protein of RSV | RSV prophylaxis | No | Otitis media | |
| Arcitumomab (CEA-Scan, Immunomedics) | Fab′ fragment of murine IgG1 radioconjugated to 99mTc | CEA | Detection of CRCa | No | None | |
| Fanolesomab (NeutroSpec, Palatin Technologies) | 99mTc-radiolabeled murine IgM | CD15 | Equivocal appendicitis | No | None | Transient decrease in neutrophil count; withdrawn from market |
| Capromab pendetide (Prostascint, Cytogen) | 111In-radiolabeled murine IgG | PSMA | Detection of recurrence of prostate cancer | No | None | |
| 99mTc-nofetumomab merpentan (Verluma, Boehringer Ingelheim) | 99mTc-radiolabeled murine IgG2 | CAA | SCLCa staging | No | None |
a TNF-α, tumor necrosis factor alpha; RA, rheumatoid arthritis; JRA, juvenile rheumatoid arthritis; AS, ankylosing spondylitis; PA, psoriatic arthritis; PP, plaque psoriasis; CD, Crohn's disease; CMV, cytomegalovirus; UC, ulcerative colitis; MOTT, Mycobacterium species other than M. tuberculosis; HBV, hepatitis B virus; IL-1, interleukin-1; HSV, herpes simplex virus; VZV, varicella zoster virus; CAPS, cryopyrin-associated periodic syndrome; PML, progressive multifocal leukoencephalopathy; HCV, hepatitis C virus; CLL, B-cell chronic lymphocytic leukemia; TPL, T-cell prolymphocytic leukemia; NHL, non-Hodgkin's lymphoma; PCP, Pneumocystis jirovecii pneumonia; AML, acute myelogenous leukemia; VEGF, vascular endothelial growth factor; CRCa, colorectal cancer; NSCLCa, non-small-cell lung cancer; BrCa, breast cancer; HNCa, head-and-neck cancer; RSV, respiratory syncytial virus; EBV, Epstein-Barr virus; PCI, percutaneous coronary intervention; MS, multiple sclerosis; CEA, carcinoembryonic antigen; PSMA, prostate-specific membrane antigen; CAA, carcinoma-associated glycoprotein antigen; SCLCa, small-cell lung cancer.
TABLE 2.
Reported serious infections with biologics by etiologic agent
| Etiologic agent or disease | Therapeutic agent associated with serious infection |
|---|---|
| Bacteria | Adalimumab |
| Infliximab | |
| Certolizumab pegol | |
| Etanercept | |
| Abatacept | |
| Anakinra | |
| Rilonacept | |
| Efalizumab | |
| Alefacept | |
| Alemtuzamab | |
| 90Y-ibritumomab tiuxetan | |
| Rituximab | |
| 131I-tositumomab | |
| Gemtuzumab ozogamicin | |
| Bevacizumab | |
| Cetuximab | |
| Panitumumab | |
| Trastuzumab | |
| Basiliximab | |
| Daclizumab | |
| Muromonab | |
| Abciximab | |
| Natalizumab | |
| Palivizumab | |
| TB | Adalimumab |
| Infliximab | |
| Certolizumab pegol | |
| Etanercept | |
| Abatacept | |
| Anakinra | |
| Efalizumab | |
| Alemtuzamab | |
| Daclizumab | |
| MOTT | Infliximab |
| Etanercept | |
| Certolizumab pegol | |
| Anakinra | |
| Rilonacept | |
| Alefacept | |
| Alemtuzamab | |
| Daclizumab | |
| Muromonab | |
| Natalizumab | |
| Viruses | Adalimumab |
| Infliximab | |
| Certolizumab pegol | |
| Etanercept | |
| Abatacept | |
| Alefacept | |
| Alemtuzamab | |
| 90Y-ibritumomab tiuxetan | |
| Rituximab | |
| 131I-tositumomab | |
| Gemtuzumab ozogamicin | |
| Basiliximab | |
| Daclizumab | |
| Muromonab | |
| Natalizumab | |
| PML | Alemtuzamab |
| Rituximab | |
| Natalizumab | |
| Fungi | Adalimumab |
| Infliximab | |
| Etanercept | |
| Abatacept | |
| Anakinra | |
| Alemtuzamab | |
| Bevacizumab | |
| Basiliximab | |
| Daclizumab | |
| Muromonab | |
| Natalizumab | |
| Parasites | Adalimumab |
| Infliximab | |
| Etanercept | |
| Anakinra | |
| Alemtuzamab | |
| Basiliximab | |
| Muromonab | |
| Natalizumab |
TABLE 3.
Approximate levels of evidence of risk of infection
| Level of risk and druga |
|---|
| Increased risk (meta-analysis or postmarketing cases with strong pattern of risk, consistent post hoc phase III randomized controlled trial (RCT) analysis showing risk) |
| Adalimumab |
| Infliximab |
| Certolizumab pegol |
| Etanercept |
| Abatacept* |
| Anakinra |
| Rilonacept |
| Efalizumab |
| Alemtuzamab |
| 90Y-ibritumomab tiuxetan |
| Rituximab |
| 131I-tositumomab |
| Gemtuzumab ozogamicin |
| Bevacizumab** |
| Cetuximab |
| Panitumumab |
| Trastuzumab** |
| Natalizumab |
| Probable risk (most post hoc phase III RCT analysis and postmarketing studies show risk or trend to risk) |
| Alefacept |
| Basiliximab |
| Daclizumab |
| Muromonab |
| Possible risk (case reports and trend toward increased infection but inconsistent in big RCTs, reported cases with confounders, or theoretical risk) |
| Abciximab |
| Omalizumab |
| Palivizumab |
| No risk |
| Arcitumomab |
| Fanolesomab |
| Capromab pendetide |
| 99mTc-nofetumomab merpentan |
*, in combination with other agents, probable risk for monotherapy; **, in combination with chemotherapeutic agents, possible risk as monotherapy.
Biologics and Infection
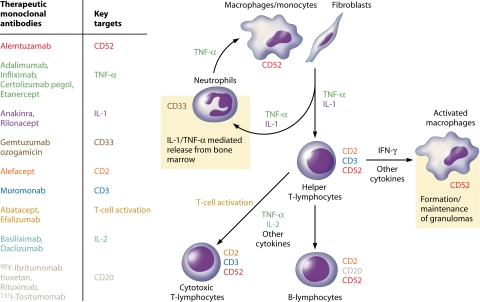
As more biologic agents become available for clinical use, certain drugs that interfere with natural immunity have led to concerns regarding increased rates of both common and unusual infections among treated patients (58). This is not surprising, particularly in the case of agents that interfere with tumor necrosis factor alpha (TNF-α) as well as for monoclonal antibodies against specific subsets of leukocytes (Fig. 1) (57, 67, 83, 189). While most monoclonal agents and related biologics are generally safe, a working knowledge of the infectious complications associated with these is essential in order to apprise the patient of the risks involved in embarking on such a course of therapy as well as to maintain vigilance for these adverse events.
FIG. 1.
Outline of human immunologic responses showing key targets of monoclonal antibodies that may suppress immune responses and enhance the risk for infection. Immune targets and therapeutic agents are color coded. CD, cluster of differentiation; TNF-α, tumor necrosis factor alpha; IL-1, interleukin-1; IFN-γ, gamma interferon; IL-2, interleukin-2.
One of the major problems in determining causality between biologic therapy and infection is the fact that, in some cases, the underlying disease process itself along with concurrent therapy can cause immunosuppression. This is the case for patients with cancer, autoimmune diseases, and inflammatory conditions such as rheumatoid arthritis (RA). In the case of rare infections, a true association can never be proven definitively due to the small number of events. Even in randomized placebo-controlled trials and in large open-label studies, it is difficult to attribute the increased risk of infection to the biologic alone due to a large number of confounders, especially since trials are powered to measure efficacy, not safety (18, 138).
The aim of this review is to present a comprehensive overview of the infectious risks of all U.S. Food and Drug Administration (FDA)-approved monoclonal antibodies. In addition, we review some related FDA-approved small molecules that are used for the treatment of inflammatory diseases and which may pose some infectious risk. The target audience includes infectious disease specialists who may be called upon to help manage infectious complications as well as general practitioners who may be involved in the care of patients treated with these agents. We have not attempted a comprehensive review of indications, dosing, or adverse events other than infections associated with the use of these agents, and specialists intending to use these for therapy are advised to review product label inserts and more comprehensive literature regarding the specific agent.
Concurrent Infections and Immune Reconstitution
In the event of a serious infectious complication, the most logical course of action is to discontinue use of the biologic. This may not always be feasible and may necessitate a longer course of treatment for a particular infection. Moreover, biologics, especially monoclonal antibodies, tend to have long half-lives and may therefore result in a substantial delay from the time of discontinuation to the time of recovery of immune function (53). Finally, the discontinuation of immunosuppressive therapy may result in a paradoxical worsening of the infection as the immune system recovers and reacts to persistent but heretofore unrecognized antigens—a phenomenon termed immune reconstitution inflammatory syndrome in the human immunodeficiency virus (HIV)/AIDS literature (149, 185). Awareness of these special problems is essential for the successful clinical application of biologics.
BIOLOGICS FOR RHEUMATOLOGIC DISEASES AND OTHER INFLAMMATORY CONDITIONS
TNF-α Inhibitors
TNF-α inhibitors represent a major breakthrough in the treatment of rheumatologic diseases and diseases of immune dysregulation (27, 123). TNF-α is a cytokine that plays a central role in establishing and maintaining the inflammatory response against infection (49, 57, 140, 189). TNF-α has both soluble and transmembrane forms, both of which are biologically active and are produced by a wide variety of cells, including macrophages, natural killer cells, granulocytes, fibroblasts, and T cells. Both forms interact with two distinct types of receptors, TNF receptor 1 (TNFR1) and TNFR2, either or both of which are found in practically all cells in the human body other than red blood cells (177). TNF-α blockade results in the interruption of TNFR-mediated functions, which include cell activation and proliferation, cytokine and chemokine production, and the formation and maintenance of granulomas (49, 57, 177, 189). It is therefore not surprising that TNF-α inhibitors have been shown to increase the risk of granulomatous infections, most importantly tuberculosis (TB) (188, 196). There are currently four TNF-α antagonists approved for treatment, including two monoclonal antibodies, one Fab′ fragment, and one soluble receptor, namely, adalimumab, infliximab, certolizumab pegol, and etanercept, respectively.
Evaluation for TB risk factors, tuberculin skin testing, and a baseline chest X-ray, as indicated, should be performed prior to initiation of therapy, and if the patient tests positive, chemoprophylaxis for latent TB should be initiated prior to the start of treatment with TNF-α inhibitors. A cutoff of 5 mm of induration for tuberculin skin testing is recommended, since patients treated with these agents are considered immunosuppresed (196). Monitoring for active TB should also be done while patients are on TNF-α inhibitors, including those who tested negative for latent TB (145, 174; Humira [adalimumab] package insert [Abbott Laboratories, North Chicago, IL, February 2008] [http://www.fda.gov/cder/foi/label/2008/125057s114lbl.pdf]; Remicade [infliximab] package insert [Centocor Inc., Malvern, PA, April 2007] [http://www.fda.gov/cder/foi/label/2007/103772s5189lbl.pdf]; Enbrel [etanercept] package insert [Immunex Corporation, Thousand Oaks, CA, March 2008] [http://www.fda.gov/medwatch/safety/2008/enbrel_pi.pdf]; Cimzia [certolizumab pegol] package insert [UCB Inc., Smyrna, GA, April 2008] [http://www.fda.gov/cder/foi/label/2008/125160s000lbl.pdf]). Newer gamma interferon release assays have been advocated to enhance screening efficacy, although the effectiveness of this approach is still being evaluated (174). Anti-TNF therapies carry different risks for TB, either as a consequence of the mechanism of action alone (infliximab versus etanercept) or as a consequence of pharmacokinetics as well (adalimumab versus etanercept) (147, 186). Saliu et al. (147) showed with whole-blood assays that the proportion of TB-responsive CD4 cells was reduced by both adalimumab and infliximab, albeit at significantly different rates (70% versus 49%), along with antigen-induced gamma interferon production, but again at significantly different rates (70% versus 64%), while etanercept had no effect on either parameter. Interleukin-10 (IL-10) production was equally suppressed by all three drugs. No significant apoptosis was induced. Murine models of chronic TB have shown that treatment with a TNF-α receptor analogue allowed for control of disease, while treatment with a TNF-α monoclonal antibody resulted in death within a month and in disruption of granulomas (33, 134). Finally, a recent review by Wallis (188) outlining the risks of TB showed a 1.3- to 5.9-fold increased risk with infliximab versus etanercept and a 1.8- to 14.6-fold increased risk with adalimumab versus etanercept. Time to developing TB was likewise shorter with infliximab than with etanercept (1:1.7 to 1:4.6). Using Markov and Monte Carlo simulation computer models, Wallis (187) attempted to determine the respective contributions of the reactivation of latent TB versus new TB infection. He found that while both infliximab and etanercept presented high risks for new TB infection, the rate of reactivation of latent TB was 12 times higher for infliximab than for etanercept.
Recently, the FDA mandated the strengthening of existing warnings on all TNF-α agents for increased risk of serious fungal infections, especially histoplasmosis. It also stressed that empirical antifungal therapy should be considered for at-risk patients, particularly those living in the Ohio and Mississippi River valleys, since delays in recognition and treatment of at least 21 cases of histoplasmosis of the 240 reported cases have led to 12 deaths (180).
Hepatitis B virus (HBV) reactivation has likewise occurred with TNF-α antagonists, and while product labels are equivocal regarding the safety of concomitant antivirals, limited data suggest that prophylaxis with lamivudine seems to be effective and well tolerated (118, 143). In general, it is not recommended that TNF-α antagonists (or any other potentially immunosuppressive agents, for that matter) be started in patients with active infections, and caution should be used for patients with a history of chronic or recurrent infections. Furthermore, while there are no data specifically pertaining to immunologic responses to live vaccines or the secondary transmission of infection from live vaccines in patients receiving TNF-α inhibitors, administration of these agents is not recommended during anti-TNF treatment (Humira package insert; Remicade package insert; Enbrel package insert; Cimzia package insert).
Adalimumab.
Adalimumab is a fully human IgG1 monoclonal antibody against TNF-α currently licensed for use against juvenile idiopathic arthritis, RA, Crohn's disease, ankylosing spondylitis, chronic plaque psoriasis, and psoriatic arthritis (27, 123; Humira package insert). Infectious complications observed while undergoing adalimumab therapy include serious pulmonary bacterial infections, TB, candidiasis, cytomegalovirus (CMV) infection, toxoplasmosis, and nocardiosis (27, 44). One case of reversal reaction with leprosy has been reported (122). Early trials and safety data analyses have been inconsistent in showing a link between adalimumab and a higher risk of serious infection (101; Humira package insert). However, pooled analysis of postmarketing reports seems to indicate an increase in the risk of infections, particularly granulomatous diseases such as TB (101, 188).
Infliximab.
Infliximab is a human-murine chimeric monoclonal antibody that binds both trimeric and monomeric forms of soluble TNF-α as well as transmembrane TNF-α and is indicated for the treatment of RA and Crohn's disease (57, 189; Remicade package insert). Infliximab has consistently been linked to an increased risk of granulomatous infection, particularly TB (21, 174, 189), as well as to an increase in overall infections (101; Remicade package insert). Other infections observed with infliximab use include infections with Mycobacterium species other than Mycobacterium tuberculosis (MOTT), Histoplasma capsulatum, Candida species, Listeria, Aspergillus species, Cryptococcus neoformans, Nocardia species, Salmonella species, Toxoplasma gondii, Brucella species, Bartonella species, Leishmania donovani, Coccidiodes immitis, CMV, and Mycobacterium leprae (12, 149, 156, 189). Immune reconstitution has been reported for TB (11, 56) and MOTT infection (149), and reversal reactions were seen in two patients with leprosy (156).
Certolizumab pegol.
Certolizumab pegol is a recombinant humanized Fab′ antibody fragment conjugated to polyethylene glycol which is specific for human TNF-α and indicated for the treatment of Crohn's disease (113; Cimzia package insert). It has currently completed phase III trials for the treatment of RA as well (85, 163). It is similar to the monoclonal antibodies infliximab and adalimumab in that it binds to both soluble and membrane-bound TNF-α, but it neither binds nor fixes complement and it does not induce cell-mediated toxicity in vitro, since it does not have an Fc receptor (113). Certolizumab carries the same black-box warnings for infection as do all TNF-α blockers. Controlled clinical studies have shown an increased rate of infection among patients treated with certolizumab compared to patients treated with placebo (38% versus 30%) (Cimzia package insert). Phase III data on Crohn's disease specifically show higher rates of serious infections in both the induction and maintenance phases, but these are comparable to the rates reported with other TNF-α inhibitors and are generally low (2 to 3%). These infections included bacterial, viral, and fungal infections as well as TB (151, 155). Phase III trials of certolizumab in combination with methotrexate for RA showed similar safety data, with mostly mild infections, particularly respiratory tract infections and urinary tract infections (UTIs), with serious infections ranging from 2 to 3% versus 0% for placebo (163) and from 5 to 7% versus 2% for placebo (85), respectively.
Etanercept.
Etanercept is a fully human dimeric fusion protein consisting of two molecules of the extracellular ligand-binding portion of human TNFR2 attached to the Fc domain of human IgG1 and is approved for the treatment of juvenile RA (JRA), RA, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis (53, 150, 189; Enbrel package insert). While early trials showed no definitive increased risk of overall infection compared to placebo (as opposed to infliximab) (101), a number of opportunistic infections have subsequently been observed with increased incidence, including TB, MOTT infection, and listeriosis (83, 149, 150, 189). Other granulomatous infections reported in higher numbers than those for placebo include histoplasmosis, candidiasis, aspergillosis, cryptococcosis, nocardiosis, and salmonellosis (180, 189). The use of etanercept in combination with other biologics, specifically anakinra and abatacept, has resulted in higher rates of infectious complications without added benefit in terms of therapeutic response (65, 194).
Other Biologics for Rheumatologic Diseases and Inflammatory Conditions
Abatacept.
Abatacept is a fully human soluble fusion protein made up of the extracellular domain of human cytotoxic T lymphocyte-associated antigen 4 linked to the modified Fc portion of human IgG1 and is indicated for the treatment of refractory RA and JRA (119, 194; Orencia [abatacept] package insert [Bristol-Meyers Squibb Company, Princeton, NJ, March 2007] [http://www.fda.gov/cder/foi/label/2007/125118s0016lbl.pdf]). It acts by selectively modulating the CD80/CD86-CD28 costimulatory signal required for full T-cell activation. Infections most commonly associated with abatacept use are upper respiratory tract infections, although in early trials there was no difference in the incidence of infection compared to that with placebo (194). In phase III trials, the incidence of serious infections was either the same or higher than that with placebo (119, 154). A meta-analysis showed no association between serious infections and abatacept treatment for RA (148). Pneumonia, septicemia, aspergillosis, sinusitis, candidiasis, bronchitis, skin and soft tissue infections, and infections with herpes simplex virus (HSV) and varicella zoster virus (VZV) have all been reported during treatment with abatacept (94, 119, 154, 194). It seems that combination treatment with biological disease-modifying antirheumatic drugs (DMARDs) such as etanercept is more likely to lead to an adverse event, including infection, than abatacept used in combination with a nonbiological DMARD (194). A tuberculin skin test is recommended by the manufacturer prior to the initiation of abatacept. If the test is positive, the patient should receive chemoprophylaxis for latent TB prior to the start of treatment (Orencia package insert).
Anakinra.
Anakinra is a recombinant, nonglycosylated form of the human IL-1 receptor antagonist with a single methionine residue added at the amino terminus. It is approved for the treatment of RA (35, 37; Kineret [anakinra] package insert [Amgen Manufacturing Limited, Thousand Oaks, CA, April 2004] [http://www.fda.gov/cder/foi/label/2004/103950s5039lbl.pdf]). Murine models with IL-1 knockouts have demonstrated reduced survival after challenge with several bacterial species, one fungal species, one parasitic species, and one virus (influenza virus) (182). Overall, infection-related adverse events in humans treated with anakinra were similar to those with placebo and were mostly upper respiratory tract infections (37, 68). However, there seemed to be an increased risk of serious infections compared to that with placebo (2.1% versus 0.4%) in at least one study (48), although a smaller trial did not show an increased risk (37). The product label also asserts a higher risk of serious infections in patients with asthma (4% versus 0%), although post hoc analysis of the largest phase III trial showed that this difference did not reach statistical significance (154; Kineret package insert). There is an increased risk of serious infections in combination with etanercept (3.7 to 7.4% versus 0% for placebo), so combination therapy with other biologics is not recommended (65; Kineret package insert). Only one case of reactivation of TB has been reported in the literature (160), and anakinra therapy does not generally seem to put patients at higher risk of TB or other opportunistic infections, although labeling information alludes to risks for mycobacterial and fungal infections which were not further specified (Kineret package insert). One case of visceral leishmaniasis in a child with JRA after 6 months of anakinra treatment has been reported and was treated successfully (92). Mirkinson et al. (115) observed that a patient with biopsy-proven human herpesvirus 6 (HHV-6) encephalitis who then developed JRA showed improvement in the manifestations of arthritis as well as improved neurologic symptoms after treatment with anakinra. The authors proposed a link between IL-1, JRA, and HHV-6.
Rilonacept.
Rilonacept is a fully human dimeric fusion protein made up of the extracellular component of the IL-1 receptor and the Fc portion of IgG1, and it binds circulating IL-1α and IL-1β with very high affinities, thereby acting as a long-acting IL-1 inhibitor (70, 73; Arcalyst [rilonacept] package insert [Regeneron Pharmaceuticals, Tarrytown, NY, February 2008] [http://www.fda.gov/cder/foi/label/2008/125249lbl.pdf]). It is approved for the treatment of cryopyrin-associated periodic syndromes, including familial cold autoinflammatory syndrome and Muckle-Wells syndrome (Arcalyst package insert). Two sequential placebo control phase III studies of 47 patients with cryopyrin-associated periodic syndromes showed a higher reported incidence of infection in study 1, with mostly mild upper respiratory tract infections (48% versus 17% for placebo), but not in study 2 (18% versus 22%) (73). Severe infections reported for patients using rilonacept included Streptococcus pneumoniae meningitis, MOTT infection, and severe bronchitis (73; Arcalyst package insert). No cases of TB have been reported, although the label advises testing and treatment for latent TB prior to the initiation of therapy (Arcalyst package insert).
Efalizumab.
Efalizumab is a monoclonal humanized recombinant IgG1 antibody against the α-subunit (CD 11a) of lymphocyte or leukocyte function-associated antigen type 1 (LFA-1) and is indicated for the treatment of psoriasis (97; Raptiva [efalizumab] package insert [Genentech, Inc., South San Francisco, CA, June 2005] [http://www.fda.gov/cder/foi/label/2005/125075_0031_lbl.pdf]). LFA-1 is a mediator of T-cell activation in lymph nodes, the transfer of these T cells from the circulation to cutaneous sites of inflammation, and the reactivation of T cells in the dermis and epidermis (98). Infections reported in trials during treatment with efalizumab were generally mild to moderate in severity and were mostly upper respiratory tract infections, gastroenteritis, and HSV infections (98, 126). Most trials have reported no significant difference in the incidence of infection versus that with placebo, although at least one trial and a large case series reported slightly higher incidences of infection-related adverse events (158). Serious infections requiring hospitalization during treatment with efalizumab included pneumonia, cellulitis, abscess, sepsis, and necrotizing fasciitis, while opportunistic infections reported included TB, a case of disseminated Cryptococcus infection (178), and a case of legionellosis (126; Raptiva package insert).
Alefacept.
Alefacept is a fully human fusion protein of LFA-3 and IgG1 that binds to CD2, inhibits T-cell activation and proliferation, and induces the selective apoptosis of memory T cells, which are important in the pathogenesis of psoriasis (69, 71; Amevive [alefacept] package insert [Astellas Pharma US, Inc., Deerfield, IL, October 2006] [http://www.fda.gov/cder/foi/label/2006/125036s071lbl.pdf]). Alefacept has been shown to decrease circulating CD4 and CD8 T-cell populations, and placebo-controlled studies showed a higher rate of serious infections requiring hospitalization (Amevive package insert). However, subsequent studies have failed to definitively demonstrate this association, and no opportunistic infections were reported during large clinical trials (55, 69, 71). Infections observed during treatment with alefacept include upper respiratory infections, HSV, VZV, and hepatitis C virus (HCV) infections, influenza, pneumonia, gastroenteritis, skin and soft tissue infections, and one case of MOTT infection (69, 136). Alefacept is contraindicated for patients with HIV because it decreases circulating CD4 lymphocytes. Monitoring of CD4 lymphocyte counts is recommended during therapy, and alefacept should be withheld if the circulating CD4 count decreases below 250 cells/μl (Amevive package insert).
BIOLOGICS FOR MALIGNANCIES
Antilymphocyte Antibodies
Alemtuzumab.
Alemtuzumab is a fully humanized IgG1 against CD52 and is used in the treatment of B-cell chronic lymphocytic leukemia, T-cell prolymphocytic leukemia, and non-Hodgkin's lymphoma. It forms an antibody-antigen complex which induces the lysis of lymphocytes and other cells expressing CD52 (108; Campath [alemtuzumab] package insert [Genzyme Corp., Cambridge, MA, September 2007] [http://www.fda.gov/cder/foi/label/2007/103948s5070lbl.pdf]). Infections that have occurred during the course of large studies and also in case reports include overwhelming bacteremia, pneumonia, meningitis, infections with CMV, VZV, and HSV, Pneumocystic jirovecii pneumonia (PCP), progressive multifocal leukoencephalopathy (PML), acanthamebiasis, Balamuthia mandrillaris toxoplasmosis, histoplasmosis, cryptococcosis, aspergillosis, invasive fungal infection (with Fusarium, Rhizopus, or Scedosporium), viral infection (with BK virus, HHV-6, parvovirus, or adenovirus), candidiasis, mycobacterial infection (TB, MOTT infection, or bacillus Calmette-Guérin [BCG] infection), and Rhodococcus infection (1, 30, 108, 112, 127, 175). Chronic HBV carriers have also experienced severe flare-ups. Since treatment with alemtuzumab results in severe and prolonged lymphopenia, prophylaxis against PCP and herpesviruses is indicated for a minimum of 2 months after completion of therapy or until CD4 counts are ≥200 cells/μl (Campath package insert).
Ibritumomab tiuxetan.
90Y-ibritumomab tiuxetan is a chimeric molecule composed of ibritumomab, a murine anti-CD20 IgG2a monoclonal antibody, conjugated to tiuxetan, a chelator that attaches to 90Y (106; Zevalin [ibritumomab tiuxetan] package insert [Biogen Idec Inc., Cambridge, MA, March 2008] [http://www.fda.gov/cder/foi/label/2008/125019s135lbl.pdf]). It is used as radioimmunotherapy for non-Hodgkin's lymphoma and has been shown to have superior response rates compared to rituximab (see below) (42, 45, 106), but it has a higher rate of infectious complications (197). Most infections encountered are likely a result of prolonged and severe cytopenias and include unspecified upper respiratory tract infections, UTIs, gastroenteritis, and pneumonia, and less frequently, sepsis, cellulitis, infectious diarrhea and colitis, empyema, osteomyelitis, pericarditis, viral pneumonia, and viral hepatitis (191, 197; Zevalin package insert).
Rituximab.
Rituximab is a chimeric human-mouse IgG1 anti-CD20 monoclonal antibody used in the treatment of non-Hodgkin's lymphoma and other B-cell malignancies, as well as RA (87, 148; Rituxan [rituximab] package insert [Genentech, Inc., South San Francisco, CA, January 2008] [http://www.fda.gov/cder/foi/label/2008/103705s5256lbl.pdf]). Treatment with rituximab results in rapid depletion of both malignant and nonmalignant CD20-positive cells and leads to depletion of normal B cells which can last for 2 to 6 months. However, serum immunoglobulin levels seem to remain stable after a conventional cycle of treatment (87). It is not consistently associated with an increased risk of bacterial infections (38, 86, 87, 148). However, there have been reports of reactivation of hepatitis B and exacerbation of hepatitis C (43, 46, 159). Sera et al. (159) reported an HBV surface antigen (HBsAg)-negative, anti-HBV surface antibody (anti-HBsAb)-positive patient converting to HBsAg-positive status with acute hepatitis during rituximab therapy, and they suggested prophylaxis with lamivudine in both HBsAg-positive and HBsAg-negative (with evidence of previous infection, i.e., anti-HBV core antibody) patients prior to initiation of rituximab therapy, despite the presence of anti-HBsAb. Rare opportunistic infections, such as PML (17, 28, 128) and parvovirus, CMV, HSV, and disseminated VZV infections, have been reported for rituximab used for treatment of malignancy (87; Rituxan package insert), but until recently, no opportunistic infections were observed when it was used for treatment of RA (38, 86, 148; http://www.fda.gov/medwatch/safety/2008/rituxan_DHCP_Final%209411700.pdf). A case of PML in an RA patient was recently reported but was confounded by concurrent therapy for oropharyngeal cancer as well as treatment with methotrexate and steroids (http://www.fda.gov/medwatch/safety/2008/rituxan_DHCP_Final%209411700.pdf). Patients at possibly higher risk of developing PML on rituximab therapy include those with systemic lupus erythematosus as well those who are receiving hematopoietic stem cell transplantation and highly cytotoxic chemotherapy (28, 51, 128).
Tositumomab.
131I-tositumomab is a radiolabeled murine IgG2a monoclonal antibody against CD20 used in the treatment of non-Hodgkin's lymphoma and other B-cell malignancies (Bexxar [tositumomab and iodine-131 tositumomab] package insert; Corixa Corp., Seattle, WA, December 2004). 131I labeling offers the advantage of targeting adjacent antigen-negative and nontargeted antigen-positive malignant cells because beta radiation travels about 0.4 mm and gamma radiation allows the quantification of the total radiation delivered (184). The major toxicity of tositumomab is neutropenia, which represents the major risk factor for infectious complications (42, 74, 184). Infections reported for patients treated with tositumomab include bacterial and viral upper respiratory tract infections as well as pneumonia (74).
Anti-Myeloid Receptor Antibodies
Gemtuzumab.
Gemtuzumab ozogamicin is a fully humanized anti-CD33 IgG4 monoclonal antibody conjugated with the cytotoxic antibiotic calicheamicin that is indicated for the treatment of CD33-positive acute myelogenous leukemia (8; Mylotarg [gemtuzumab ozogamicin] package insert [Wyeth Pharmaceuticals Inc., Philadelphia, PA, January 2006] [http://www.fda.gov/cder/foi/label/2006/021174s020lbl.pdf]). Infectious risk is related mostly to significant myelosuppression, since CD33 is also found on normal myeloid precursors, although this is reversible because pluripotent hematopoietic stem cells do not express CD33 and therefore are not affected (8). Infectious complications encountered during trials included sepsis from Pseudomonas, Acinetobacter, and Klebsiella infections, pneumonia, and neutropenic fever (7, 99, 164). Unusual pathogens reported with treatment include Staphylococcus hominis, Agrobacterium radiobacter, Acinetobacter lwoffii, Rhodococcus, and Pantoea agglomerans (142).
Antiangiogenesis Antibodies
Bevacizumab.
Bevacizumab is a recombinant humanized IgG1 monoclonal antibody against human vascular endothelial growth factor that is used in the treatment of advanced colorectal cancer, non-small-cell lung cancer, and breast cancer and is in trials for renal cancer (36, 144; Avastin [bevacizumab] package insert [Genentech, Inc., South San Francisco, CA, February 2008] [http://www.fda.gov/cder/foi/label/2008/125085s145lbl.pdf]). It has also been used as an intravitreal injection for the treatment of exudative age-related macular degeneration (81). Infectious risk has been demonstrated only in combination with chemotherapy as a result of severe neutropenia, while a black-box warning exists for intestinal perforation with or without abscess formation, presumably from the inhibition of endothelial cell proliferation and new blood vessel formation (36; Avastin package insert). Infectious complications associated with systemic bevacizumab include neutropenic fever, sepsis, and pneumonitis (36, 114; Avastin package insert). Case reports of anaerobic liver abscess with Bacteroides fragilis (100) and Fusarium-associated nasal septal perforation (144) have also been published. Intraocular injection has infrequently resulted in endophthalmitis (81, 109, 132), including at least one case each with Bacillus cereus (93) and coagulase-negative Staphylococcus (109), although an increased risk for endophthalmitis has not been demonstrated.
ErbB Receptor Antibodies
Cetuximab.
Cetuximab is a chimeric murine-human IgG1 monoclonal antibody against ErbB1 that is approved for colorectal and head-and-neck cancer and in trials for other ErbB1-expressing tumors. ErbB1, also known as epidermal growth factor receptor (EGFR), is constitutively expressed in many types of cancer as well as in normal epithelial tissues, including skin and hair follicles, such that blocking the activity of EGFR leads to inhibition of cell growth and induction of apoptosis and decreases matrix metalloproteinase and vascular endothelial growth factor production (52; Erbitux [cetuximab] package insert [Bristol-Meyers Squibb Company, Princeton, NJ, and Imclone Systems Incorporated, Branchburg, NJ, October 2007] [http://www.fda.gov/cder/foi/label/2007/125084s103lbl.pdf]). Infectious complications are largely related to cutaneous toxicity, resulting in acneiform eruptions and paronychia caused by Staphylococcus aureus (including methicillin-resistant S. aureus), which can lead to abscess formation and sepsis; these infections may represent secondary infection, while the primary process is unclear (19, 20, 75, 171).
Panitumumab.
Panitumumab is a fully human IgG2 monoclonal antibody against ErbB1 used in the treatment of advanced colorectal cancer (52, 105; June 2008, revision date. Vectibix [panitumumab] package insert [Amgen Inc., Thousand Oaks, CA, June 2008] [http://www.fda.gov/cder/foi/label/2008/125147s026lbl.pdf]). Infectious events observed with panitumumab treatment are similar to those with cetuximab, including acneiform eruptions and paronychia, and are generally mild. However, there seems to be an increased incidence of severe reactions when panitumumab is used in combination with other chemotherapy (105). Blocking EGFR can decrease neutrophil-driven inflammation but does not seem to interfere with antiviral pathways, particularly in the case of respiratory syncytial virus (RSV) (103).
Trastuzumab.
Trastuzumab is a humanized recombinant monoclonal antibody directed against HER2, which is overexpressed by certain types of breast tumors (Herceptin [trastuzumab] package insert; Genentech, Inc., South San Francisco, CA, May 2008 [http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf]). HER2 (also called human epidermal growth factor receptor 2 or ErbB2) is structurally related to EGFR but has no known ligand, although there is evidence that it may be autoactivated, suggesting that HER2 alone can cause transformation if overexpressed (52). Infectious complications are generally mild, such as upper respiratory tract infections and UTIs, and no opportunistic infections have been reported in the literature (3, 176, 183), although an increase in the number of overall infections and degree of febrile neutropenia has been reported for combinations with certain chemotherapeutic agents, such as doxorubicin plus cyclophosphamide followed by paclitaxel plus trastuzumab (Herceptin package insert). Life-threatening interstitial lung disease has been reported, although it was not clearly identified as infectious in nature (129). Interestingly, ErbB2 activation has been implicated in demyelination induced by Mycobacterium leprae, and ErbB2 blockade has been shown to abrogate M. leprae-induced myelin damage in both in vitro and in vivo models (172).
BIOLOGICS FOR TREATMENT OF OTHER DISEASES AND OTHER INDICATIONS
Monoclonal Antibodies for Graft-Versus-Host Disease and Transplant Rejection
Basiliximab.
Basiliximab is a chimeric murine-human IgG1 monoclonal antibody directed against the α-chain of the IL-2 receptor (IL-2Rα or CD25), is indicated as immunoprophylaxis in the prevention of rejection of renal transplants, and has been studied for prophylaxis in other solid organs (34, 84; Simulect [basiliximab] package insert [Novartis Pharmaceutical Corp., East Hanover, NJ, January 2003] [http://www.fda.gov/cder/foi/label/2003/basnov010203LB.htm]). CD25 blockade by basiliximab prevents IL-2-mediated activation of lymphocytes, a critical pathway in cell-mediated immune responses which is intimately involved in allograft rejection (Simulect package insert). No increased incidence of infection was reported with basiliximab versus placebo in initial trials for rejection (34, 82, 84). One study reported a higher incidence of CMV disease but a lower overall infection rate than that with antithymocyte globulin (ATG) (23). This increase in CMV disease was not borne out in a retrospective study involving presensitized patients (199). Infections observed during treatment with basiliximab include bacterial infections, particularly UTIs, infections with CMV and HSV, aspergillosis, nocardiosis, candidiasis, and protozoal infections (23). Among liver transplant patients, basiliximab-treated patients actually had a lower incidence of HCV recurrence and no difference in posttransplantation lymphoproliferative disease or CMV incidence (137). In cardiac transplant patients, basiliximab treatment had a lower incidence of infectious deaths than did ATG treatment (110).
Daclizumab.
Daclizumab is a recombinant humanized IgG1 anti-CD25 monoclonal antibody that affects activated T cells through the IL-2Rα chain and is indicated for induction therapy in the prevention of rejection in renal transplant recipients. It is also being studied for other solid organ transplant recipients (26, 76; Zenapax [daclizumab] package insert [Hoffman-La Roche Inc., Nutley, NJ, September 2005] [http://www.fda.gov/cder/foi/label/2005/103749s5059lbl.pdf]). The mechanism of action is the same as that for basiliximab. Infections associated with daclizumab for induction therapy are generally mild upper respiratory tract infections, and in comparison with ATG, daclizumab has a lower incidence of CMV viremia (26, 76). One retrospective study found a higher incidence of Aspergillus colonization and invasive disease than that with ATG in lung transplant patients, although the reason for this was not clear (80). When used for steroid-refractory graft-versus-host disease in combination with other immunosuppressive therapy, daclizumab was found to have an extremely high rate of opportunistic infections (95%), although there was lower mortality than that with ATG used for the same indication. These infections included the usual bacterial infections as well as Nocardia, Legionella, and MOTT infections, TB, viral infections (CMV, BK virus, adenovirus, HSV, RSV, and influenza virus), and invasive fungal infections (Aspergillus, Scedosporium, Cunninghamella, and Candida) (131). When combined with cytolytic therapy in heart transplant patients, more patients treated with daclizumab died than those receiving placebo (six versus zero) (72). Finally, in heart transplant patients treated with daclizumab in combination with other immunosuppressive drugs, a progressive decline in pneumococcal polysaccharide antibodies in previously vaccinated individuals was observed during the year after transplantation (152).
Muromonab.
Muromonab (OKT3) is a murine IgG2 monoclonal antibody against CD3 indicated for acute rejection in solid organ transplant recipients (161; Orthoclone-OKT3 [muromonab] package insert [Ortho Biotech, Raritan, NJ, November 2004] [http://www.orthobiotech.com/orthobiotech/shared/OBI/PI/OKT3_PI.pdf]). The interaction of muromonab with CD3, found in all T lymphocytes, blocks the generation and function of effector T cells and leads to a rapid decrease in the number of circulating CD3-positive cells. Binding of muromonab to T cells results in early activation, leading to cytokine release followed by the blocking of T-cell functions. T-cell function returns to normal after muromonab therapy, within about a week (Orthoclone-OKT3 package insert). Infection rates with muromonab were not significantly different from previous experience with non-monoclonal-antibody-containing regimens. However, there is evidence that the rates of posttransplantation lymphoproliferative disease and lymphoma are increased compared to those with antirejection regimens that do not employ muromonab therapy (117). Infections reported with OKT3 therapy include infections with Listeria, Nocarida, MOTT, Aspergillus, Candida, Cryptococcus, dermatophytes, PCP, Toxoplasma gondii, CMV, Epstein-Barr virus, HSV, hepatitis viruses, VZV, adenovirus, enterovirus, RSV, and parainfluenza virus but have not been significantly different in head-to-head studies from basiliximab and daclizumab or from high-dose steroids alone (29, 157; Orthoclone-OKT3 package insert). Because muromonab is usually administered with other immunosuppressive drugs, resulting in profound immunosuppression, the manufacturer suggests judicious use of anti-infective prophylaxis, including that directed against herpesviruses such as HSV and CMV (Orthoclone-OKT3 package insert).
Monoclonal Antibodies for Cardiovascular Disease
Abciximab.
Abciximab is the Fab′ fragment of a chimeric human-mouse monoclonal antibody against the glycoprotein IIb/IIIa receptor, which is responsible for platelet aggregation and is indicated as an adjunct in the treatment of acute coronary syndrome following percutaneous coronary intervention (13; Reopro [abciximab] package insert [Centocor B.V., Leiden, The Netherlands, November 2005] [http://www.centocor.com/centocor/assets/reopro.pdf]). At least one study showed a slightly higher incidence of pneumonia than that with placebo, although the mechanism and significance of this finding are unknown. Otherwise, no additional risk of infection has been associated with abciximab (Reopro package insert). In fact, there is in vitro and murine model evidence that glycoprotein IIb/IIIa inhibition protects against endothelial cell dysfunction and leukocyte adherence to the vascular wall during experimental endotoxemia (88, 190).
Monoclonal Antibodies for Neurologic Disease
Natalizumab.
Natalizumab is a humanized recombinant IgG4 monoclonal antibody that binds to the α4β1 (very late activating antigen 4) and α4β7 integrins, found in all white blood cells except for neutrophils, and is indicated for the treatment of relapsing multiple sclerosis (MS) and Crohn's disease (169, 170; Tysarbi [natalizumab] package insert [Biogen Idec Inc., Cambridge, MA, January 2008] [http://www.fda.gov/cder/foi/label/2008/125104s033lbl.pdf]). The pathogenesis of MS is thought to be driven by the egress of inflammatory cells into the central nervous system from the peripheral blood, and natalizumab interferes with this process (89, 168). Natalizumab was voluntarily withdrawn from the market in 2005 following the development of PML caused by the JC polyomavirus in three patients, but it has now been reapproved for relapsing forms of MS and Crohn's disease (168; Tysarbi package insert). Analyses of previous clinical trials indicate that the risk of development of PML with natalizumab is estimated to be around 0.1% during a mean of 17.9 monthly doses (200). Measurement of immunologic parameters in treated patients has shown that natalizumab leads to a prolonged decrease in lymphocytes in the central nervous system, particularly CD4 T cells and B cells; this deficit persists for over 6 months (natalizumab half-life = 11 days) after treatment (168, 170) and could presumably be responsible for the increased risk for PML. Other infections reported with natalizumab include upper respiratory tract infections, bacterial pneumonias, and UTIs, as well as PCP and infections with Cryptosporidium parvum, Mycobacterium avium complex, Aspergillus species, HSV, influenza virus, and Burkholderia cepacia. Most trials show that the overall risk of infection is no different from that with placebo (Tysarbi package insert).
Monoclonal Antibodies for Pulmonary Disease
Omalizumab.
Omalizumab is a chimeric humanized IgG1 monoclonal antibody that binds human IgE, is indicated for the treatment of asthma, and is in trials for allergic rhinitis and other eosinophil-associated disorders (50, 133; Xolair [omalizumab] package insert [Genentech, Inc., South San Francisco, CA, July 2007] [http://www.fda.gov/cder/foi/label/2007/103976s5102lbl.pdf]). Mild infections, including upper respiratory tract infections and viral infections, have been observed with omalizumab, but the incidence of infection was no different from that with placebo (Xolair package insert). Prolonged therapy has been shown to result in a sustained decrease in IgE levels, which could theoretically decrease immunity toward helminthic infections (58, 165). A trial in Brazil with patients at high risk for geohelminth infections showed a trend toward increased infection risk, but the risk was not statistically different from that with placebo (40).
Palivizumab.
Palivizumab is a humanized monoclonal antibody (IgG1) against an epitope in the A antigenic site of the F protein of RSV and is indicated for the prevention of serious lower respiratory tract infection due to RSV in pediatric patients at high risk (173; Synagis [palivizumab] package insert [MedImmune Inc., Gaithersburg, MD, February 2007] [http://www.fda.gov/cder/foi/label/2007/103770_5096lbl.pdf]). The incidences of upper respiratory tract infections, rhinitis, and otitis media were slightly increased compared to those with placebo in initial trials (Synagis package insert), although subsequent studies did not bear this out (146, 173). In a trial involving a second course of palivizumab in the subsequent year, an increased incidence of otitis media was noted in the group receiving a second course compared to the group receiving a single course and placebo, although this was judged not to be related to palivizumab by the investigators (121). At present, there is no evidence that palivizumab increases susceptibility to infection.
Monoclonal Antibodies for Imaging
Arcitumomab.
Arcitumomab is the 99mTc-labeled Fab′ fragment of the anti-CEA IgG1 murine monoclonal antibody IMMU-4 and is indicated for detection of advanced colorectal cancer in conjunction with other diagnostic modalities (47; CEA-Scan [arcitumomab] package insert [Immunomedics, Inc., Morris Plains, NJ, August 1999] [http://www.cea-scan.com/inserts/pckginstn.htm]). Initial trials for approval, using mostly single-dose infusions, as well as serial administration in subsequent trials did not show any associated infection-related adverse events (54, 193). While arcitumomab has been shown to have similar accuracy to that of fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) in detecting local recurrence of cancer, FDG-PET is clearly superior in detecting distant metastasis and so the use of the anti-CEA scan is less preferred (195).
Technitium fanolesomab.
99mTc-fanolesumab is a murine anti-CD15 IgM monoclonal antibody labeled with 99mTc which was approved for imaging of equivocal cases of acute appendicitis and was in trials for use in imaging osteomyelitis and occult infection (6, 90, 104, 162; Neutro-Spec [fanolesomab] package insert [Palatin Technologies, Inc., Cranbury, NJ, July 2004] [http://www.fda.gov/cder/foi/label/2004/103928lbl.pdf]). The drug was withdrawn from the market in 2005 following two fatalities and reports of serious cardiovascular complications during postmarketing surveillance (90). Shortly after infusion, a transient drop in the absolute neutrophil number has been observed, but no infectious complications have been attributed to Tc-fanolesumab (102, 104).
Capromab pendetide.
Capromab pendetide is an indium-111-radiolabeled murine monoclonal antibody (7E11-C5.3) covalently joined to a linker-chelator molecule directed against human prostate-specific membrane antigen and is used in the detection of recurrence in patients with prostate cancer (95, 116; Prostascint [capromab pendetide] package insert [Cytogen Corporation, Princeton, NJ, October 1996] [http://www.fda.gov/cder/foi/label/1996/capcyt102896lab.pdf]). No infections have been associated with capromab use during single and repeated infusions, although some patients have developed fever and local site irritation (95, 135).
Nofetumomab.
99mTc-nofetumomab merpentan is the Fab′ fragment of the IgG2 murine antibody NR-LU-10 against carcinoma-associated glycoprotein antigen radioconjugated with 99mTc and is indicated as a diagnostic imaging agent for staging patients with lung cancer, particularly as an adjunct when results from other studies are equivocal; it has also been evaluated for use with other carcinomas (10, 22, 167; Verluma [nofetumomab] package insert [Dr. Karl Thomae GmbH, Ingelheim, Germany, August 1996] [http://www.fda.gov/cder/foi/label/1996/nofedrk082096lb.pdf]). No infectious complications have been associated with this agent.
THE FUTURE: PANDORA'S BOX OR HORN OF PLENTY?
Based on the human genome, it is estimated that there are about 6,000 to 8,000 targets of pharmaceutical interest, with around 300 currently targeted by existing drugs (96). The science of monoclonal antibodies and the development of small molecules that target important cellular steps or factors, coupled with further advances in recombinant protein technology, create nearly infinite possibilities to manipulate disease pathways. The ongoing development of drugs that target specific pathways of the immune system, such as the JAK/STAT pathway and other immunoreceptors such as those involved in antigen recognition and processing, as well as those targeting complement, will give rise to agents with profound effects on immune function and risk for infections (96, 166). As more and more biologics find their way into our therapeutic armamentarium, the challenge becomes not whether a drug exists to treat a disease but which is the most appropriate with the lowest number of side effects, not the least of which are infectious complications.
Biography
 Edsel Maurice T. Salvana (M.D., D.T.M.H.) received his medical degree from the University of the Philippines. He trained in internal medicine at the Medical College of Wisconsin and originated the International Infectious Diseases Fellowship Track at the University Hospitals Case Medical Center and the Case Western Reserve University. He returned to his native country, the Philippines, as a Balik (Tagalog for “returning”) Scientist awardee of the Department of Science and Technology and is a clinical associate professor of medicine at the University of the Philippines College of Medicine. He is also the clinical research coordinator for the Section of Infectious Diseases at the Philippine General Hospital and is the assistant director of the Institute of Molecular Biology and Biotechnology at the National Institutes of Health-University of the Philippines, Manila. Dr. Salvana's interests include tropical and travel medicine, global health, and infections in the immunocompromised host.
Edsel Maurice T. Salvana (M.D., D.T.M.H.) received his medical degree from the University of the Philippines. He trained in internal medicine at the Medical College of Wisconsin and originated the International Infectious Diseases Fellowship Track at the University Hospitals Case Medical Center and the Case Western Reserve University. He returned to his native country, the Philippines, as a Balik (Tagalog for “returning”) Scientist awardee of the Department of Science and Technology and is a clinical associate professor of medicine at the University of the Philippines College of Medicine. He is also the clinical research coordinator for the Section of Infectious Diseases at the Philippine General Hospital and is the assistant director of the Institute of Molecular Biology and Biotechnology at the National Institutes of Health-University of the Philippines, Manila. Dr. Salvana's interests include tropical and travel medicine, global health, and infections in the immunocompromised host.
 Robert A. Salata (M.D.) is Professor of Medicine, International Health and Epidemiology/Biostatistics at Case Western Reserve University. He is Executive Vice-Chair of the Department of Medicine and Chief of the Division of Infectious Diseases and HIV Medicine. Dr. Salata has research interests in clinical trials of antiretrovirals, including those in resource-limited settings, as well as the epidemiology and prevention of human immunodeficiency virus (HIV) in women, including the use of topical microbicides. Dr. Salata's special clinical interests are in infections in immunocompromised patients (HIV/AIDS and transplant recipients).
Robert A. Salata (M.D.) is Professor of Medicine, International Health and Epidemiology/Biostatistics at Case Western Reserve University. He is Executive Vice-Chair of the Department of Medicine and Chief of the Division of Infectious Diseases and HIV Medicine. Dr. Salata has research interests in clinical trials of antiretrovirals, including those in resource-limited settings, as well as the epidemiology and prevention of human immunodeficiency virus (HIV) in women, including the use of topical microbicides. Dr. Salata's special clinical interests are in infections in immunocompromised patients (HIV/AIDS and transplant recipients).
REFERENCES
- 1.Abad, S., E. Gyan, L. Moachon, D. Bouscary, D. Sicard, F. Dreyfus, and P. Blanche. 2003. Tuberculosis due to Mycobacterium bovis after alemtuzumab administration. Clin. Infect. Dis. 37:e27-e28. [DOI] [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Adamo, V., T. Franchina, B. Adamo, G. Ferraro, R. Rossello, M. Maugeri Saccà, C. Scibilia, M. R. Valerio, and A. Russo. 2007. Safety and activity of trastuzumab-containing therapies for the treatment of metastatic breast cancer: our long-term clinical experience (GOIM study). Ann. Oncol. 18(Suppl.):vi11-vi15. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Reference deleted.
- 6.Anonymous. 2002. LeuTech. Biodrugs 16:319-320. [DOI] [PubMed] [Google Scholar]
- 7.Aplenc, R., T. A. Alonzo, R. B. Gerbing, B. J. Lange, C. A. Hurwitz, R. J. Wells, I. Bernstein, P. Buckley, K. Krimmel, F. O. Smith, E. L. Sievers, R. J. Arceci, et al. 2008. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children's Oncology Group. J. Clin. Oncol. 26:2390-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arceci, R. J., J. Sande, B. Lange, K. Shannon, J. Franklin, R. Hutchinson, T. A. Vik, D. Flowers, R. Aplenc, M. S. Berger, M. L. Sherman, F. O. Smith, I. Bernstein, and E. L. Sievers. 2005. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 106:1183-1188. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Balaban, E. P., B. S. Walker, J. V. Cox, R. P. Bordlee, D. Salk, P. G. Abrams, R. G. Sheehan, and E. P. Frenkel. 1992. Detection and staging of small cell lung carcinoma with a technetium-labeled monoclonal antibody. A comparison with standard staging methods. Clin. Nucl. Med. 17:439-445. [DOI] [PubMed] [Google Scholar]
- 11.Belknap, R., R. Reves, and W. Burman. 2005. Immune reconstitution to Mycobacterium tuberculosis after discontinuing infliximab. Int. J. Tuberc. Lung Dis. 9:1057-1058. [PubMed] [Google Scholar]
- 12.Bergstrom, L., D. E. Yocum, N. M. Ampel, I. Villanueva, J. Lisse, O. Gluck, J. Tesser, J. Posever, M. Miller, J. Araujo, D. M. Kageyama, M. Berry, L. Karl, and C. M. Yung. 2004. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum. 50:1959-1966. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand, O. F., J. Rodés-Cabau, E. Larose, C. M. Nguyen, L. Roy, J. P. Déry, J. Courtis, I. Nault, P. Poirier, O. Costerousse, and R. de Larochellière. 2008. One-year clinical outcome after abciximab bolus-only compared with abciximab bolus and 12-hour infusion in the randomized Early Discharge after Transradial Stenting of Coronary Arteries (EASY) study. Am. Heart J. 156:135-140. [DOI] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Reference deleted.
- 16.Reference deleted.
- 17.Bonavita, S., R. Conforti, A. Russo, R. Sacco, A. Tessitore, A. Gallo, M. Della Corte, M. R. Monsurrò, and G. Tedeschi. 2008. Infratentorial progressive multifocal leukoencephalopathy in a patient treated with fludarabine and rituximab. Neurol. Sci. 29:37-39. [DOI] [PubMed] [Google Scholar]
- 18.Bongartz, T., A. J. Sutton, M. J. Sweeting, I. Buchan, E. L. Matteson, and V. Montori. 2006. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295:2275-2285. [DOI] [PubMed] [Google Scholar]
- 19.Boucher, K. W., K. Davidson, B. Mirakhur, J. Goldberg, and W. R. Heymann. 2002. Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J. Am. Acad. Dermatol. 47:632-633. [DOI] [PubMed] [Google Scholar]
- 20.Bragg, J., and M. K. Pomeranz. 2007. Papulopustular drug eruption due to epidermal growth factor receptor inhibitors, erlotinib and cetuximab. Dermatol. Online J. 13:1. [PubMed] [Google Scholar]
- 21.Brassard, P., A. Kezouh, and S. Suissa. 2006. Antirheumatic drugs and the risk of tuberculosis. Clin. Infect. Dis. 43:717-722. [DOI] [PubMed] [Google Scholar]
- 22.Breitz, H. B., A. Tyler, M. J. Bjorn, T. Lesley, and P. L. Weiden. 1997. Clinical experience with Tc-99m nofetumomab merpentan (Verluma) radioimmunoscintigraphy. Clin. Nucl. Med. 22:615-620. [DOI] [PubMed] [Google Scholar]
- 23.Brennan, D. C., J. A. Daller, K. D. Lake, D. Cibrik, D. del Castillo, et al. 2006. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 355:1967-1977. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Reference deleted.
- 26.Buggage, R. R., G. Levy-Clarke, H. N. Sen, R. Ursea, S. K. Srivastava, E. B. Suhler, C. Altemare, G. Velez, J. Ragheb, C. C. Chan, R. B. Nussenblatt, A. T. Bamji, P. Sran, T. Waldmann, and D. J. Thompson. 2007. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behçet's disease. Ocul. Immunol. Inflamm. 15:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burmester, G. R., X. Mariette, C. Montecucco, I. Monteagudo-Sáez, M. Malaise, A. G. Tzioufas, J. W. Bijlsma, K. Unnebrink, S. Kary, and H. Kupper. 2007. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann. Rheum. Dis. 66:732-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calabrese, L. H., and E. S. Molloy. 2008. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann. Rheum. Dis. 67:iii64-ii65. [DOI] [PubMed] [Google Scholar]
- 29.Campos, A., E. Lage, R. Hinojosa, A. Ordóñez, J. M. Cisneros, S. Cabezón, S. Gómez, A. Aguilera, E. Arana, and A. Cayuela. 2005. Comparative study of muromonab-CD3 (OKT3) versus daclizumab (Zenapax) in cardiac transplantation at our center. Transplant. Proc. 37:1548-1549. [DOI] [PubMed] [Google Scholar]
- 30.Cavalli-Björkman, N., E. Osby, J. Lundin, M. Kalin, A. Osterborg, and A. Gruber. 2002. Fatal adenovirus infection during alemtuzumab (anti-CD52 monoclonal antibody) treatment of a patient with fludarabine-refractory B-cell chronic lymphocytic leukemia. Med. Oncol. 19:277-280. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Reference deleted.
- 33.Chakravarty, S. D., G. Zhu, M. C. Tsai, V. P. Mohan, S. Marino, D. E. Kirschner, L. Huang, J. Flynn, and J. Chan. 2008. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect. Immun. 76:916-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman T. M., and G. M. Keating. 2003. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs 63:2803-2835. [DOI] [PubMed] [Google Scholar]
- 35.Clark, W., P. Jobanputra, P. Barton, and A. Burls. 2004. The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis. Health Technol. Assess. 8:iii-iv, ix, x, 1-105. [DOI] [PubMed] [Google Scholar]
- 36.Cohen, M. H., J. Gootenberg, P. Keegan, and R. Pazdur. 2007. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 12:356-361. [DOI] [PubMed] [Google Scholar]
- 37.Cohen, S. B., L. W. Moreland, J. J. Cush, M. W. Greenwald, S. Block, W. J. Shergy, P. S. Hanrahan, M. M. Kraishi, A. Patel, G. Sun, M. B. Bear, et al. 2004. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann. Rheum. Dis. 63:1062-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen, S. B., P. Emery, M. W. Greenwald, M. Dougados, R. A. Furie, M. C. Genovese, E. C. Keystone, J. E. Loveless, G. R. Burmester, M. W. Cravets, E. W. Hessey, T. Shaw, M. C. Totoritis, et al. 2006. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54:2793-2806. [DOI] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Cruz, A. A., F. Lima, E. Sarinho, G. Ayre, C. Martin, H. Fox, and P. J. Cooper. 2007. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clin. Exp. Allergy 37:197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.Dillman, R. O. 2006. Radioimmunotherapy of B-cell lymphoma with radiolabelled anti-CD20 monoclonal antibodies. Clin. Exp. Med. 6:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dizdar, O., U. Tapan, S. Aksoy, H. Harputluoglu, S. Kilickap, and I. Barista. 2008. Liver dysfunction after chemotherapy in lymphoma patients infected with hepatitis C. Eur. J. Haematol. 80:381-385. [DOI] [PubMed] [Google Scholar]
- 44.Doraiswamy, V. A. 2008. Nocardia infection with adalimumab in rheumatoid arthritis. J. Rheumatol. 35:542-543. [PubMed] [Google Scholar]
- 45.Emmanouilides, C. 2007. Radioimmunotherapy for non-Hodgkin lymphoma: historical perspective and current status. J. Clin. Exp. Hematop. 47:43-60. [DOI] [PubMed] [Google Scholar]
- 46.Ennishi, D., Y. Terui, M. Yokoyama, Y. Mishima, S. Takahashi, K. Takeuchi, H. Okamoto, M. Tanimoto, and K. Hatake. 2008. Monitoring serum hepatitis C virus (HCV) RNA in patients with HCV-infected CD20-positive B-cell lymphoma undergoing rituximab combination chemotherapy. Am. J. Hematol. 83:59-62. [DOI] [PubMed] [Google Scholar]
- 47.Erb, D. A., and H. A. Nabi. 2000. Clinical and technical considerations for imaging colorectal cancers with technetium 99m-labeled antiCEA Fab′ fragment. J. Nucl. Med. Technol. 28:12-18. [PubMed] [Google Scholar]
- 48.Fleischmann, R. M., J. Schechtman, R. Bennett, M. L. Handel, G. R. Burmester, J. Tesser, D. Modafferi, J. Poulakos, and G. Sun. 2003. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 48:927-934. [DOI] [PubMed] [Google Scholar]
- 49.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 50.Foroughi, S., B. Foster, N. Kim, L. B. Bernardino, L. M. Scott, R. G. Hamilton, D. D. Metcalfe, P. J. Mannon, and C. Prussin. 2007. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J. Allergy Clin. Immunol. 120:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freim Wahl, S. G., M. R. Folvik, and S. H. Torp. 2007. Progressive multifocal leukoencephalopathy in a lymphoma patient with complete remission after treatment with cytostatics and rituximab: case report and review of the literature. Clin. Neuropathol. 26:68-73. [DOI] [PubMed] [Google Scholar]
- 52.Friedländer, E., M. Barok, J. Szöllősi, and G. Vereb. 2008. ErbB-directed immunotherapy: antibodies in current practice and promising new agents. Immunol. Lett. 116:126-140. [DOI] [PubMed] [Google Scholar]
- 53.Furst, D. E., R. Wallis, M. Broder, and D. O. Beenhouwer. 2006. Tumor necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin. Arthritis Rheum. 36:159-167. [DOI] [PubMed] [Google Scholar]
- 54.Fuster, D., J. Maurel, A. Muxí, X. Setoain, C. Ayuso, F. Martín, M. L. Ortega, S. Fuertes, and F. Pons. 2003. Is there a role for (99m)Tc-anti-CEA monoclonal antibody imaging in the diagnosis of recurrent colorectal carcinoma? Q. J. Nucl. Med. 47:109-115. [PubMed] [Google Scholar]
- 55.Gade, J. N. 2004. Clinical update on alefacept: consideration for use in patients with psoriasis. J. Manag. Care. Pharm. 10:s33-s37. [PubMed] [Google Scholar]
- 56.Garcia Vidal, C., S. Rodríguez Fernández, J. Martínez Lacasa, M. Salavert, R. Vidal, M. Rodríguez Carballeira, and J. Garau. 2005. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin. Infect. Dis. 40:756-759. [DOI] [PubMed] [Google Scholar]
- 57.Gardam, M. A., E. C. Keystone, R. Menzies, S. Manners, E. Skamene, R. Long, and D. C. Vinh. 2003. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect. Dis. 3:148-155. [DOI] [PubMed] [Google Scholar]
- 58.Gea-Banacloche, J. C., and G. A. Weinberg. 2007. Monoclonal antibody therapeutics and risk for infection. Pediatr. Infect. Dis. J. 26:1049-1052. [DOI] [PubMed] [Google Scholar]
- 59.Reference deleted.
- 60.Reference deleted.
- 61.Reference deleted.
- 62.Reference deleted.
- 63.Reference deleted.
- 64.Reference deleted.
- 65.Genovese, M. C., S. Cohen, L. Moreland, D. Lium, S. Robbins, R. Newmark, P. Bekker, et al. 2004. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50:1412-1419. [DOI] [PubMed] [Google Scholar]
- 66.Reference deleted.
- 67.Gerber, D. E. 2008. Targeted therapies: a new generation of cancer treatments. Am. Fam. Physician 77:311-319. [PubMed] [Google Scholar]
- 68.Giles, J. T., and J. M. Bathon. 2004. Serious infections associated with anticytokine therapies in the rheumatic diseases. J. Intensive Care Med. 19:320-334. [DOI] [PubMed] [Google Scholar]
- 69.Goffe, B., K. Papp, D. Gratton, G. G. Krueger, M. Darif, S. Lee, C. Bozic, M. T. Sweetser, and B. Ticho. 2005. An integrated analysis of thirteen trials summarizing the long-term safety of alefacept in psoriasis patients who have received up to nine courses of therapy. Clin. Ther. 27:1912-1921. [DOI] [PubMed] [Google Scholar]
- 70.Goldbach-Mansky, R., S. D. Shroff, M. Wilson, C. Snyder, S. Plehn, B. Barham, T. H. Pham, F. Pucino, R. A. Wesley, J. H. Papadopoulos, S. P. Weinstein, S. J. Mellis, and D. L. Kastner. 2008. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum. 58:2432-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottlieb, A. B., T. B. Casale, E. Frankel, B. Goffe, N. Lowe, H. D. Ochs, J. L. Roberts, K. Washenik, A. K. Vaishnaw, and K. B. Gordon. 2003. CD4+ T-cell-directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized study. J. Am. Acad. Dermatol. 49:816-825. [DOI] [PubMed] [Google Scholar]
- 72.Hershberger, R. E., R. C. Starling, H. J. Eisen, C. H. Bergh, R. L. Kormos, R. B. Love, A. van Bakel, R. D. Gordon, R. Popat, L. Cockey, and R. D. Mamelok. 2005. Daclizumab to prevent rejection after cardiac transplantation. N. Engl. J. Med. 352:2705-2713. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman, H. M., M. L. Throne, N. J. Amar, M. Sebai, A. J. Kivitz, A. Kavanaugh, S. P. Weinstein, P. Belomestnov, G. D. Yancopoulos, N. Stahl, and S. J. Mellis. 2008. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 58:2443-2452. [DOI] [PubMed] [Google Scholar]
- 74.Horning, S. J., A. Younes, V. Jain, S. Kroll, J. Lucas, D. Podoloff, and M. Goris. 2005. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J. Clin. Oncol. 23:712-719. [DOI] [PubMed] [Google Scholar]
- 75.Hu, J. C., P. Sadeghi, L. C. Pinter-Brown, S. Yashar, and M. W. Chiu. 2007. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J. Am. Acad. Dermatol. 56:317-326. [DOI] [PubMed] [Google Scholar]
- 76.Huurman, V. A., J. S. Kalpoe, P. van de Linde, N. Vaessen, J. Ringers, A. C. Kroes, B. O. Roep, and J. W. de Fijter. 2006. Choice of antibody immunotherapy influences cytomegalovirus viremia in simultaneous pancreas-kidney transplant recipients. Diabetes Care 29:842-847. [DOI] [PubMed] [Google Scholar]
- 77.Iannitto, E., V. Minardi, G. Calvaruso, A. Mulè, E. Ammatuna, R. di Trapani, D. Ferraro, V. Abbadessa, A. Craxí, and R. di Stefano. 2005. Hepatitis B virus reactivation and alemtuzumab therapy. Eur. J. Haematol. 74:254-258. [DOI] [PubMed] [Google Scholar]
- 78.Reference deleted.
- 79.Reference deleted.
- 80.Iversen, M., C. M. Burton, S. Vand, L. Skovfoged, J. Carlsen, N. Milman, C. B. Andersen, M. Rasmussen, and M. Tvede. 2007. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur. J. Clin. Microbiol. Infect. Dis. 26:879-886. [DOI] [PubMed] [Google Scholar]
- 81.Jonas, J. B., U. H. Spandau, F. Rensch, S. von Baltz, and F. Schlichtenbrede. 2007. Infectious and noninfectious endophthalmitis after intravitreal bevacizumab. J. Ocul. Pharmacol. Ther. 23:240-242. [DOI] [PubMed] [Google Scholar]
- 82.Jorge, S., J. Guerra, S. Silva, A. Santana, C. Mil-Homens, and M. M. Prata. 2008. Induction immunosuppressive therapy in renal transplantation: does basiliximab make the difference? Transplant. Proc. 40:693-696. [DOI] [PubMed] [Google Scholar]
- 83.Keane, J. 2005. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology 44:714-720. [DOI] [PubMed] [Google Scholar]
- 84.Keown, P., R. Balshaw, S. Khorasheh, M. Chong, C. Marra, Z. Kalo, and A. Korn. 2003. Meta-analysis of basiliximab for immunoprophylaxis in renal transplantation. Biodrugs 17:271-279. [DOI] [PubMed] [Google Scholar]
- 85.Keystone, E., D. Heijde, D. Mason, Jr., R. Landewé, R. V. Vollenhoven, B. Combe, P. Emery, V. Strand, P. Mease, C. Desai, and K. Pavelka. 2008. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 58:3319-3329. [DOI] [PubMed] [Google Scholar]
- 86.Keystone, E., R. Fleischmann, P. Emery, D. E. Furst, R. van Vollenhoven, J. Bathon, M. Dougados, A. Baldassare, G. Ferraccioli, A. Chubick, J. Udell, M. W. Cravets, S. Agarwal, S. Cooper, and F. Magrini. 2007. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 56:3896-3908. [DOI] [PubMed] [Google Scholar]
- 87.Kimby, E. 2005. Tolerability and safety of rituximab (MabThera). Cancer Treat. Rev. 31:456-473. [DOI] [PubMed] [Google Scholar]
- 88.Kirschenbaum, L. A., D. Adler, M. E. Astiz, R. S. Barua, D. Saha, and E. C. Rackow. 2002. Mechanisms of platelet-neutrophil interactions and effects on cell filtration in septic shock. Shock 17:508-512. [DOI] [PubMed] [Google Scholar]
- 89.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353:369-374. [DOI] [PubMed] [Google Scholar]
- 90.Klingensmith, W. C., III, D. Perlman, and K. Baum. 2007. Intrapatient comparison of 2-deoxy-2-[F-18]fluoro-d-glucose with positron emission tomography/computed tomography to Tc-99m fanolesomab (NeutroSpec) for localization of infection. Mol. Imaging Biol. 9:295-299. [DOI] [PubMed] [Google Scholar]
- 91.Köhler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 92.Koné-Paut, I., K. Retornaz, J. M. Garnier, and B. Bader-Meunier. 2007. Visceral leishmaniasis in a patient with systemic juvenile arthritis treated by IL-1RA agonist (anakinra). Clin. Exp. Rheumatol. 25:119. [PubMed] [Google Scholar]
- 93.Kopel, A. C., P. E. Carvounis, and E. R. Holz. 2008. Bacillus cereus endophthalmitis following intravitreous bevacizumab injection. Ophthalmic Surg. Lasers Imaging 39:153-154. [DOI] [PubMed] [Google Scholar]
- 94.Kremer, J. M., H. K. Genant, L. W. Moreland, A. S. Russell, P. Emery, C. Abud-Mendoza, J. Szechinski, T. Li, Z. Ge, J. C. Becker, and R. Westhovens. 2006. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 144:865-876. [DOI] [PubMed] [Google Scholar]
- 95.Lamb, H. M., and D. Faulds. 1998. Capromab pendetide. A review of its use as an imaging agent in prostate cancer. Drugs Aging 12:293-304. [DOI] [PubMed] [Google Scholar]
- 96.Landry, Y., and J. P. Gies. 2008. Drugs and their molecular targets: an updated overview. Fundam. Clin. Pharmacol. 22:1-18. [DOI] [PubMed] [Google Scholar]
- 97.Langley, R. G., V. Ho, C. Lynde, K. A. Papp, Y. Poulin, N. Shear, J. Toole, and C. Zip. 2006. Recommendations for incorporating biologicals into management of moderate to severe plaque psoriasis: individualized patient approaches. J. Cutan. Med. Surg. 9(Suppl. 1):18-25. [DOI] [PubMed] [Google Scholar]
- 98.Langley, R. G., W. P. Carey, E. S. Rafal, S. K. Tyring, I. Caro, X. Wang, G. Wetherill, and K. B. Gordon. 2005. Incidence of infection during efalizumab therapy for psoriasis: analysis of the clinical trial experience. Clin. Ther. 27:1317-1328. [DOI] [PubMed] [Google Scholar]
- 99.Larson, R. A., E. L. Sievers, E. A. Stadtmauer, B. Löwenberg, E. H. Estey, H. Dombret, M. Theobald, D. Voliotis, J. M. Bennett, M. Richie, L. H. Leopold, M. S. Berger, M. L. Sherman, M. R. Loken, J. J. van Dongen, I. D. Bernstein, and F. R. Appelbaum. 2005. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 104:1442-1452. [DOI] [PubMed] [Google Scholar]
- 100.Lieuw-a-Fa, M., J. Peringa, O. Leeksma, and W. Terpstra. 2008. Sepsis from liver abscesses in metastatic colorectal carcinoma after chemoimmunotherapy. J. Clin. Oncol. 26:1381-1382. [DOI] [PubMed] [Google Scholar]
- 101.Lin, J., D. Ziring, S. Desai, S. Kim, M. Wong, Y. Korin, J. Braun, E. Reed, D. Gjertson, and R. R. Singh. 2008. TNFα blockade in human diseases: an overview of efficacy and safety. Clin. Immunol. 126:13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Line, B. R., R. J. Breyer, K. D. McElvany, D. C. Earle, and M. B. Khazaeli. 2004. Evaluation of human anti-mouse antibody response in normal volunteers following repeated injections of fanolesomab (NeutroSpec), a murine anti-CD15 IgM monoclonal antibody for imaging infection. Nucl. Med. Commun. 25:807-811. [DOI] [PubMed] [Google Scholar]
- 103.Liu, K., R. C. Gualano, M. L. Hibbs, G. P. Anderson, and S. Bozinovski. 2008. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J. Biol. Chem. 283:9977-9985. [DOI] [PubMed] [Google Scholar]
- 104.Love, C., G. G. Tronco, and C. J. Palestro. 2006. Imaging of infection and inflammation with 99mTc-fanolesomab. Q. J. Nucl. Med. Mol. Imaging 50:113-120. [PubMed] [Google Scholar]
- 105.Mano, M., and Y. Humblet. 2008. Drug insight: panitumumab, a human EGFR-targeted monoclonal antibody with promising clinical activity in colorectal cancer. Nat. Clin. Pract. Oncol. 5:415-425. [DOI] [PubMed] [Google Scholar]
- 106.Marcus, R. 2005. Use of 90Y-ibritumomab tiuxetan in non-Hodgkin's lymphoma. Semin. Oncol. 32:s36-s43. [DOI] [PubMed] [Google Scholar]
- 107.Margulies, D. H. 2005. Monoclonal antibodies: producing magic bullets by somatic cell hybridization. J. Immunol. 174:2451-2452. [DOI] [PubMed] [Google Scholar]
- 108.Martin, S. I., F. M. Marty, K. Fiumara, S. P. Treon, J. G. Gribben, and L. R. Baden. 2006. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin. Infect. Dis. 43:16-24. [DOI] [PubMed] [Google Scholar]
- 109.Mason, J. O., III, M. F. White, R. M. Feist, M. L. Thomley, M. A. Albert, T. O. Persaud, J. J. Yunker, and R. S. Vail. 2008. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina 28:564-567. [DOI] [PubMed] [Google Scholar]
- 110.Mattei, M. F., M. Redonnet, I. Gandjbakhch, A. M. Bandini, A. Billes, E. Epailly, R. Guillemain, B. Lelong, A. Pol, M. Treilhaud, E. Vermes, R. Dorent, D. Lemay, A. S. Blanc, and P. Boissonnat. 2007. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti-thymocyte globulin as induction therapy. J. Heart Lung Transplant. 26:693-699. [DOI] [PubMed] [Google Scholar]
- 111.Reference deleted.
- 112.Meeuse, J. J., H. G. Sprenger, S. van Assen, D. Leduc, S. M. Daenen, J. P. Arends, and T. S. van der Werf. 2007. Rhodococcus equi infection after alemtuzumab therapy for T-cell prolymphocytic leukemia. Emerg. Infect. Dis. 13:1942-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Melmed, G. Y., S. R. Targan, U. Yasothan, D. Hanicq, and P. Kirkpatrick. 2008. Certolizumab pegol. Nat. Rev. Drug Discov. 7:641-642. [DOI] [PubMed] [Google Scholar]
- 114.Miller, K., M. Wang, J. Gralow, M. Dickler, M. Cobleigh, E. A. Perez, T. Shenkier, D. Cella, and N. E. Davidson. 2007. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357:2666-2676. [DOI] [PubMed] [Google Scholar]
- 115.Mirkinson, L. J., D. Nagle, N. Kadom, and O. Y. Jones. 2006. Anakinra therapy in a child with systemic-onset juvenile rheumatoid arthritis after human herpesvirus 6 encephalitis. J. Clin. Rheumatol. 12:83-86. [DOI] [PubMed] [Google Scholar]
- 116.Nagda, S. N., N. Mohideen, S. S. Lo, U. Khan, G. Dillehay, R. Wagner, S. Campbell, and R. Flanigan. 2007. Long-term follow up of 111In-capromab pendetide (ProstaScint) scan as pretreatment assessment in patients who undergo salvage radiotherapy for rising prostate-specific antigen after radical prostatectomy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 67:834-840. [DOI] [PubMed] [Google Scholar]
- 117.Nashan, B. 2005. Antibody induction therapy in renal transplant patients receiving calcineurin-inhibitor immunosuppressive regimens: a comparative review. Biodrugs 19:39-46. [DOI] [PubMed] [Google Scholar]
- 118.Nathan, D. M., P. W. Angus, and P. R. Gibson. 2006. Hepatitis B and C virus infections and anti-tumor necrosis factor-α therapy: guidelines for clinical approach. J. Gastroenterol. Hepatol. 21:1366-1371. [DOI] [PubMed] [Google Scholar]
- 119.Nogid, A., and D. Q. Pham. 2006. Role of abatacept in the management of rheumatoid arthritis. Clin. Ther. 28:1764-1778. [DOI] [PubMed] [Google Scholar]
- 120.Reference deleted.
- 121.Null, D., Jr., B. Pollara, P. H. Dennehy, J. Steichen, P. J. Sánchez, L. B. Givner, D. Carlin, B. Landry, F. H. Top, Jr., and E. Connor. 2005. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr. Infect. Dis. J. 24:1021-1023. [DOI] [PubMed] [Google Scholar]
- 122.Oberstein, E. M., O. Kromo, and E. C. Tozman. 2008. Type I reaction of Hansen's disease with exposure to adalimumab: a case report. Arthritis Rheum. 59:1040-1043. [DOI] [PubMed] [Google Scholar]
- 123.Ormerod, A. D. 2008. Adalimumab: a new alternative biologic agent for chronic plaque psoriasis. Br. J. Dermatol. 158:435-436. [DOI] [PubMed] [Google Scholar]
- 124.Reference deleted.
- 125.Reference deleted.
- 126.Papp, K. A., C. Camisa, S. P. Stone, I. Caro, X. Wang, P. Compton, P. A. Walicke, and A. B. Gottlieb. 2005. Safety of efalizumab in patients with moderate to severe chronic plaque psoriasis: review of clinical data. J. Cutan. Med. Surg. 9:313-323. [DOI] [PubMed] [Google Scholar]
- 127.Peleg, A. Y., S. Husain, E. J. Kwak, F. P. Silveira, M. Ndirangu, J. Tran, K. A. Shutt, R. Shapiro, N. Thai, K. Abu-Elmagd, K. R. McCurry, A. Marcos, and D. L. Paterson. 2007. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin. Infect. Dis. 44:204-212. [DOI] [PubMed] [Google Scholar]
- 128.Pelosini, M., D. Focosi, F. Rita, S. Galimberti, F. Caracciolo, E. Benedetti, F. Papineschi, and M. Petrini. 2008. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann. Hematol. 87:405-412. [DOI] [PubMed] [Google Scholar]
- 129.Pepels, M. J., K. A. Boomars, R. van Kimmenade, and P. S. Hupperets. 16 March 2008, posting date. Life-threatening interstitial lung disease associated with trastuzumab: case report. Breast Cancer Res. Treat. doi: 10.1007/s10549-008-9966-8. [DOI] [PubMed]
- 130.Peppel, K., D. Crawford, and B. Beutler. 1991. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J. Exp. Med. 174:1483-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perales, M. A., N. Ishill, W. A. Lomazow, D. M. Weinstock, E. B. Papadopoulos, H. Dastigir, M. Chiu, F. Boulad, H. R. Castro-Malaspina, G. Heller, A. A. Jakubowski, R. J. O'Reilly, T. N. Small, J. W. Young, and N. A. Kernan. 2007. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transplant. 40:481-486. [DOI] [PubMed] [Google Scholar]
- 132.Pilli, S., A. Kotsolis, R. F. Spaide, J. Slakter, K. B. Freund, J. Sorenson, J. Klancnik, and M. Cooney. 2008. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am. J. Ophthalmol. 145:879-882. [DOI] [PubMed] [Google Scholar]
- 133.Plaut, M. 2001. Immune-based, targeted therapy for allergic diseases. JAMA 286:3005-3006. [DOI] [PubMed] [Google Scholar]
- 134.Plessner, H. L., P. L. Lin, T. Kohno, J. S. Louie, D. Kirschner, J. Chan, and J. L. Flynn. 2007. Neutralization of tumor necrosis factor (TNF) by antibody but not TNF receptor fusion molecule exacerbates chronic murine tuberculosis. J. Infect. Dis. 195:1643-1650. [DOI] [PubMed] [Google Scholar]
- 135.Plut, E. M., and G. H. Hinkle. 2000. 111In-capromab pendetide: the evolution of prostate specific membrane antigen and the nuclear imaging of its 111In-labelled murine antibody in the evaluation of prostate cancer. Biodrugs 13:437-447. [DOI] [PubMed] [Google Scholar]
- 136.Prasertsuntarasai, T., and E. F. Bello. 2005. Mycobacterium avium complex olecranon bursitis in a patient treated with alefacept. Mayo Clin. Proc. 80:1532-1533. [DOI] [PubMed] [Google Scholar]
- 137.Ramirez, C. B., C. Doria, F. di Francesco, M. Iaria, Y. Kang, and I. R. Marino. 2006. Basiliximab induction in adult liver transplant recipients with 93% rejection-free patient and graft survival at 24 months. Transplant. Proc. 38:3633-3635. [DOI] [PubMed] [Google Scholar]
- 138.Reddy, J. G., and E. V. Loftus, Jr. 2006. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol. Clin. N. Am. 35:837-855. [DOI] [PubMed] [Google Scholar]
- 139.Reference deleted.
- 140.Reimold, A. M. 2002. TNFα as therapeutic target: new drugs, more applications. Curr. Drug Targets Inflamm. Allergy 1:377-392. [DOI] [PubMed] [Google Scholar]
- 141.Reference deleted.
- 142.Roman, E., E. Cooney, L. Harrison, O. Militano, K. Wolownik, R. Hawks, S. Foley, P. Satwani, E. Unal, M. Bhatia, B. Bradley, G. Del Toro, D. George, J. Garvin, C. van de Ven, and M. S. Cairo. 2005. Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin. Cancer Res. 11:7164s-7170s. [DOI] [PubMed] [Google Scholar]
- 143.Roux, C. H., O. Brocq, V. Breuil, C. Albert, and L. Euller-Ziegler. 2006. Safety of anti-TNF-α therapy in rheumatoid arthritis and spondylarthropathies with concurrent B or C chronic hepatitis. Rheumatology (Oxford) 45:1294-1297. [DOI] [PubMed] [Google Scholar]
- 144.Ruiz, N., C. Fernandez-Martos, I. Romero, A. Pla, J. Maiquez, A. Calatrava, and V. Guillem. 2007. Invasive fungal infection and nasal septum perforation with bevacizumab-based therapy in advanced colon cancer. J. Clin. Oncol. 25:3376-3377. [DOI] [PubMed] [Google Scholar]
- 145.Saag, K. G., G. G. Teng, N. M. Patkar, J. Anuntiyo, C. Finney, J. R. Curtis, H. E. Paulus, A. Mudano, M. Pisu, M. Elkins-Melton, R. Outman, J. J. Allison, M. Suarez Almazor, S. L. Bridges, Jr., W. W. Chatham, M. Hochberg, C. MacLean, T. Mikuls, L. W. Moreland, J. O'Dell, A. M. Turkiewicz, D. E. Furst, et al. 2008. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 59:762-784. [DOI] [PubMed] [Google Scholar]
- 146.Sáez-Llorens, X., M. T. Moreno, O. Ramilo, P. J. Sánchez, F. H. Top, Jr., E. M. Connor, et al. 2004. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 23:707-712. [DOI] [PubMed] [Google Scholar]
- 147.Saliu, O. Y., C. Sofer, D. S. Stein, S. K. Schwander, and R. S. Wallis. 2006. Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. J. Infect. Dis. 194:486-492. [DOI] [PubMed] [Google Scholar]
- 148.Salliot, C., M. Dougados, and L. Gossec. 18 January 2008, posting date. Risk of serious infections during rituximab, abatacept and anakinra therapies for rheumatoid arthritis: meta-analyses of randomized placebo-controlled trials. Ann. Rheum. Dis. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed]
- 149.Salvana, E. M., G. S. Cooper, and R. A. Salata. 2007. Mycobacterium other than tuberculosis (MOTT) infection: an emerging disease in infliximab-treated patients. J. Infect. 55:484-487. [DOI] [PubMed] [Google Scholar]
- 150.Sánchez Carazo, J. L., L. Mahiques Santos, and V. Oliver Martinez. 2006. Safety of etanercept in psoriasis: a critical review. Drug Saf. 29:675-685. [DOI] [PubMed] [Google Scholar]
- 151.Sandborn, W. J., B. G. Feagan, S. Stoinov, P. J. Honiball, P. Rutgeerts, D. Mason, R. Bloomfield, S. Schreiber, et al. 2007. Certolizumab pegol for the treatment of Crohn's disease. N. Engl. J. Med. 357:228-238. [DOI] [PubMed] [Google Scholar]
- 152.Sarmiento, E., C. Rodríguez-Hernández, J. Rodríguez-Molina, J. Fernández-Yánez, J. Palomo, J. Anguita, J. L. Pérez, N. Lanio, E. Fernández-Cruz, and J. Carbone. 2006. Impaired anti-pneumococcal polysaccharide antibody production and invasive pneumococcal infection following heart transplantation. Int. Immunopharmacol. 6:2027-2030. [DOI] [PubMed] [Google Scholar]
- 153.Schiff, M., M. Keiserman, C. Codding, S. Songcharoen, A. Berman, S. Nayiager, C. Saldate, T. Li, R. Aranda, J. C. Becker, C. Lin, P. L. Cornet, and M. Dougados. 2008. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann. Rheum. Dis. 67:1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schiff, M. H., G. DiVittorio, J. Tesser, R. Fleischmann, J. Schechtman, S. Hartman, T. Liu, and A. M. Solinger. 2004. The safety of anakinra in high-risk patients with active rheumatoid arthritis: six-month observations of patients with comorbid conditions. Arthritis Rheum. 50:1752-1760. [DOI] [PubMed] [Google Scholar]
- 155.Schreiber, S., M. Khaliq-Kareemi, I. C. Lawrance, O. Ø. Thomsen, S. B. Hanauer, J. McColm, R. Bloomfield, W. J. Sandborn, et al. 2007. Maintenance therapy with certolizumab pegol for Crohn's disease. N. Engl. J. Med. 357:239-250. [DOI] [PubMed] [Google Scholar]
- 156.Scollard, D. M., M. P. Joyce, and T. P. Gillis. 2006. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of 2 cases. Clin. Infect. Dis. 43:e19-e22. [DOI] [PubMed] [Google Scholar]
- 157.Segovia, J., J. L. Rodríguez-Lambert, M. G. Crespo-Leiro, L. Almenar, E. Roig, M. A. Gómez-Sánchez, E. Lage, N. Manito, and L. Alonso-Pulpón. 2006. A randomized multicenter comparison of basiliximab and muromonab (OKT3) in heart transplantation: SIMCOR study. Transplantation 81:1542-1548. [DOI] [PubMed] [Google Scholar]
- 158.Selenko-Gebauer, N., F. Karlhofer, and G. Stingl. 2007. Efalizumab in routine use: a clinical experience. Br. J. Dermatol. 156(Suppl. 2):1-6. [DOI] [PubMed] [Google Scholar]
- 159.Sera, T., Y. Hiasa, K. Michitaka, I. Konishi, K. Matsuura, Y. Tokumoto, B. Matsuura, T. Kajiwara, T. Masumoto, N. Horiike, and M. Onji. 2006. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern. Med. 45:721-724. [DOI] [PubMed] [Google Scholar]
- 160.Settas, L. D., G. Tsimirikas, G. Vosvotekas, E. Triantafyllidou, and P. Nicolaides. 2007. Reactivation of pulmonary tuberculosis in a patient with rheumatoid arthritis during treatment with IL-1 receptor antagonists (anakinra). J. Clin. Rheumatol. 13:219-220. [DOI] [PubMed] [Google Scholar]
- 161.Sgro, C. 1995. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review. Toxicology 105:23-29. [DOI] [PubMed] [Google Scholar]
- 162.Shanthly, N., M. R. Aruva, K. Zhang, B. Mathew, and M. L. Thakur. 2006. 99mTc-fanolesomab: affinity, pharmacokinetics and preliminary evaluation. Q. J. Nucl. Med. Mol. Imaging 50:104-112. [PubMed] [Google Scholar]
- 163.Smolen, J. S., R. B. Landewé, P. J. Mease, J. Brzezicki, D. Mason, K. Luijtens, R. F. van Vollenhoven, A. Kavanaugh, M. H. Schiff, G. R. Burmester, V. Strand, J. Vencovsky, and D. M. van der Heijde. 17 November 2008, posting date. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. Ann. Rheum. Dis. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed]
- 164.Specchia, G., D. Pastore, P. Carluccio, G. Spinosa, M. Giannoccaro, R. Rizzi, A. Mestice, and V. Liso. 2007. Gemtuzumab ozogamicin with cytarabine and mitoxantrone as a third-line treatment in a poor prognosis group of adult acute myeloid leukemia patients: a single-center experience. Ann. Hematol. 86:425-428. [DOI] [PubMed] [Google Scholar]
- 165.Steiss, J. O., P. Strohner, K. P. Zimmer, and H. Lindemann. 2008. Reduction of the total IgE level by omalizumab in children and adolescents. J. Asthma 45:233-236. [DOI] [PubMed] [Google Scholar]
- 166.Stowell, C. P. 2006. Therapy with immunoglobulin: applications for monoclonal antibodies. J. Infus. Nurs. 29:s29-s44. [DOI] [PubMed] [Google Scholar]
- 167.Straka, M. R., J. M. Joyce, and D. T. Myers. 2000. Tc-99m nofetumomab merpentan complements an equivocal bone scan for detecting skeletal metastatic disease from lung cancer. Clin. Nucl. Med. 25:54-55. [DOI] [PubMed] [Google Scholar]
- 168.Stüve O. 12 May 2008, posting date. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J. Neurol. Sci. doi: 10.1016/j.jns.2008.03.022. [DOI] [PubMed]
- 169.Stüve, O., and J. L. Bennett. 2007. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev. 13:79-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stüve, O., C. M. Marra, K. R. Jerome, L. Cook, P. D. Cravens, S. Cepok, E. M. Frohman, J. T. Phillips, G. Arendt, B. Hemmer, N. L. Monson, and M. K. Racke. 2006. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 59:743-747. [DOI] [PubMed] [Google Scholar]
- 171.Suh, K.-Y., H. L. Kindler, M. Medenica, and M. Lacouture. 2006. Doxycycline for the treatment of paronychia induced by the epidermal growth factor receptor inhibitor cetuximab. Br. J. Dermatol. 154:191-192. [DOI] [PubMed] [Google Scholar]
- 172.Tapinos, N., M. Ohnishi, and A. Rambukkana. 2006. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat. Med. 12:961-966. [DOI] [PubMed] [Google Scholar]
- 173.The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 174.Theis, V. S., and J. M. Rhodes. 2008. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 27:19-30. [DOI] [PubMed] [Google Scholar]
- 175.Thursky, K. A., L. J. Worth, J. F. Seymour, H. M. Prince, and M. A. Slavin. 2006. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab. Br. J. Haematol. 132:3-12. [DOI] [PubMed] [Google Scholar]
- 176.Tokunaga, E., E. Oki, K. Nishida, T. Koga, A. Egashira, M. Morita, Y. Kakeji, and Y. Maehara. 2006. Trastuzumab and breast cancer: developments and current status. Int. J. Clin. Oncol. 11:199-208. [DOI] [PubMed] [Google Scholar]
- 177.Tracey, D., L. Klareskog, E. H. Sasso, J. G. Salfeld, and P. P. Tak. 2008. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117:244-279. [DOI] [PubMed] [Google Scholar]
- 178.Tuxen, A. J., M. K. Yong, A. C. Street, and C. Dolianitis. 2007. Disseminated cryptococcal infection in a patient with severe psoriasis treated with efalizumab, methotrexate and ciclosporin. Br. J. Dermatol. 157:1067-1068. [DOI] [PubMed] [Google Scholar]
- 179.Reference deleted.
- 180.U.S. Food and Drug Administration. 30 December 2008, accession date. Manufacturers of TNF-blocker drugs must highlight risk of fungal infections. U.S. FDA, Silver Spring, MD. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01879.html.
- 181.U.S. Food and Drug Administration. 9 March 2008, accession date. Therapeutic biological products. U.S. FDA, Silver Spring, MD. http://www.fda.gov/cder/biologics/default.htm.
- 182.van de Vosse, E., and M. A. van Agtmael. 2007. Targets of anticytokine therapy and the risk of infections in humans and mice. Curr. Opin. Rheumatol. 19:626-635. [DOI] [PubMed] [Google Scholar]
- 183.Vogel, C. L., M. A. Cobleigh, D. Tripathy, J. C. Gutheil, L. N. Harris, L. Fehrenbacher, D. J. Slamon, M. Murphy, W. F. Novotny, M. Burchmore, S. Shak, S. J. Stewart, and M. Press. 2002. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20:719-726. [DOI] [PubMed] [Google Scholar]
- 184.Vose, J. M., R. L. Wahl, M. Saleh, A. Z. Rohatiner, S. J. Knox, J. A. Radford, A. D. Zelenetz, G. F. Tidmarsh, R. J. Stagg, and M. S. Kaminski. 2000. Multicenter phase II study of iodine-131 tositumomab for chemotherapy relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin's lymphomas. J. Clin. Oncol. 18:1316-1323. [DOI] [PubMed] [Google Scholar]
- 185.Wallis, R. S. 2005. Reconsidering adjuvant immunotherapy for tuberculosis. Clin. Infect. Dis. 41:201-208. [DOI] [PubMed] [Google Scholar]
- 186.Wallis, R. S. 2007. Reactivation of latent tuberculosis by TNF blockade: the role of interferon gamma. J. Investig. Dermatol. Symp. Proc. 12:16-21. [DOI] [PubMed] [Google Scholar]
- 187.Wallis, R. S. 2008. Mathematical modeling of the cause of tuberculosis during tumor necrosis factor blockade. Arthritis Rheum. 58:947-952. [DOI] [PubMed] [Google Scholar]
- 188.Wallis, R. S. 2008. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect. Dis. 8:601-611. [DOI] [PubMed] [Google Scholar]
- 189.Wallis, R. S., M. S. Broder, J. Y. Wong, M. E. Hanson, and D. O. Beenhouwer. 2004. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38:1261-1265. [DOI] [PubMed] [Google Scholar]
- 190.Walther, A., M. Czabanka, M. M. Gebhard, and E. Martin. 2004. Glycoprotein IIB/IIIA-inhibition and microcirculatory alterations during experimental endotoxemia—an intravital microscopic study in the rat. Microcirculation 11:79-88. [DOI] [PubMed] [Google Scholar]
- 191.Watanabe, T., S. Terui, K. Itoh, T. Terauchi, T. Igarashi, N. Usubuchi, M. Nakata, S. Nawano, N. Sekiguchi, S. Kusumoto, K. Tanimoto, Y. Kobayashi, K. Endo, T. Seriu, M. Hayashi, and K. Tobinai. 2005. Phase I study of radioimmunotherapy with an anti-CD20 murine radioimmunoconjugate (90Y-ibritumomab tiuxetan) in relapsed or refractory indolent B-cell lymphoma. Cancer Sci. 96:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weber, R. W. 2004. Adverse reactions to biological modifiers. Curr. Opin. Allergy Clin. Immunol. 4:277-283. [DOI] [PubMed] [Google Scholar]
- 193.Wegener, W. A., N. Petrelli, A. Serafini, and D. M. Goldenberg. 2000. Safety and efficacy of arcitumomab imaging in colorectal cancer after repeated administration. J. Nucl. Med. 41:1016-1020. [PubMed] [Google Scholar]
- 194.Weinblatt, M., M. Schiff, A. Goldman, J. Kremer, M. Luggen, T. Li, D. Chen, and J. C. Becker. 2007. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Willkomm, P., H. Bender, M. Bangard, P. Decker, F. Grünwald, and H. J. Biersack. 2000. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J. Nucl. Med. 41:1657-1663. [PubMed] [Google Scholar]
- 196.Winthrop, K. L., J. N. Siegel, J. Jereb, Z. Taylor, and M. F. Iademarco. 2005. Tuberculosis associated with therapy against tumor necrosis factor alpha. Arthritis Rheum. 52:2968-2974. [DOI] [PubMed] [Google Scholar]
- 197.Witzig, T. E., C. A. White, L. I. Gordon, G. A. Wiseman, C. Emmanouilides, J. L. Murray, J. Lister, and P. S. Multani. 2003. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-Hodgkin's lymphoma. J. Clin. Oncol. 21:1263-1270. [DOI] [PubMed] [Google Scholar]
- 198.Reference deleted.
- 199.Yang, S. L., D. Wang, W. Z. Wu, W. H. Lin, T. Z. Xu, J. Q. Cai, and J. M. Tan. 2008. Comparison of single bolus ATG and basiliximab as induction therapy in presensitized renal allograft recipients receiving tacrolimus-based immunosuppressive regimen. Transpl. Immunol. 18:281-285. [DOI] [PubMed] [Google Scholar]
- 200.Yousry, T. A., E. O. Major, C. Ryschkewitsch, G. Fahle, S. Fischer, J. Hou, B. Curfman, K. Miszkiel, N. Mueller-Lenke, E. Sanchez, F. Barkhof, E. W. Radue, H. R. Jäger, and D. B. Clifford. 2006. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354:924-933. [DOI] [PMC free article] [PubMed] [Google Scholar]