Abstract
Summary: The innate immune system constitutes the first line of defense against invading microbial pathogens and relies on a large family of pattern recognition receptors (PRRs), which detect distinct evolutionarily conserved structures on pathogens, termed pathogen-associated molecular patterns (PAMPs). Among the PRRs, the Toll-like receptors have been studied most extensively. Upon PAMP engagement, PRRs trigger intracellular signaling cascades ultimately culminating in the expression of a variety of proinflammatory molecules, which together orchestrate the early host response to infection, and also is a prerequisite for the subsequent activation and shaping of adaptive immunity. In order to avoid immunopathology, this system is tightly regulated by a number of endogenous molecules that limit the magnitude and duration of the inflammatory response. Moreover, pathogenic microbes have developed sophisticated molecular strategies to subvert host defenses by interfering with molecules involved in inflammatory signaling. This review presents current knowledge on pathogen recognition through different families of PRRs and the increasingly complex signaling pathways responsible for activation of an inflammatory and antimicrobial response. Moreover, medical implications are discussed, including the role of PRRs in primary immunodeficiencies and in the pathogenesis of infectious and autoimmune diseases, as well as the possibilities for translation into clinical and therapeutic applications.
INTRODUCTION
The innate immune system constitutes the first line of host defense during infection and therefore plays a crucial role in the early recognition and subsequent triggering of a proinflammatory response to invading pathogens (242). The adaptive immune system, on the other hand, is responsible for elimination of pathogens in the late phase of infection and in the generation of immunological memory. Whereas the adaptive immune response is characterized by specificity developed by clonal gene rearrangements from a broad repertoire of antigen-specific receptors on lymphocytes, the innate immune response is mediated primarily by phagocytic cells and antigen-presenting cells (APCs), such as granulocytes, macrophages, and dendritic cells (DCs), and has been regarded as relatively nonspecific (151).
The innate immune response relies on recognition of evolutionarily conserved structures on pathogens, termed pathogen-associated molecular patterns (PAMPs), through a limited number of germ line-encoded pattern recognition receptors (PRRs), of which the family of Toll-like receptors (TLRs) has been studied most extensively (7, 242). PAMPs are characterized by being invariant among entire classes of pathogens, essential for the survival of the pathogen, and distinguishable from “self” (153). However, in certain cases, PRRs also recognize host factors as “danger” signals, when they are present in aberrant locations or abnormal molecular complexes as a consequence of infection, inflammation, or other types of cellular stress (32, 236) (Fig. 1). Upon PAMP recognition, PRRs present at the cell surface or intracellularly signal to the host the presence of infection and trigger proinflammatory and antimicrobial responses by activating a multitude of intracellular signaling pathways, including adaptor molecules, kinases, and transcription factors (6). PRR-induced signal transduction pathways ultimately result in the activation of gene expression and synthesis of a broad range of molecules, including cytokines, chemokines, cell adhesion molecules, and immunoreceptors (7), which together orchestrate the early host response to infection and at the same time represent an important link to the adaptive immune response.
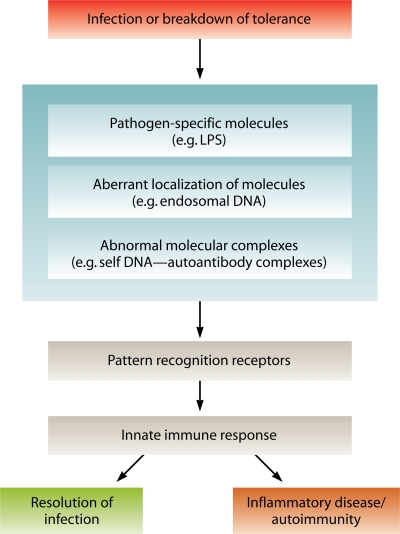
FIG. 1.
Principles in innate immune recognition by PRRs. During microbial infection or breakdown of tolerance, pathogen-specific molecules, aberrant localization of foreign or self molecules, or abnormal molecular complexes are recognized by PRRs. This event triggers PRR-mediated signaling and induction of an innate immune response, which ultimately results in resolution of infection but also may cause inflammatory diseases or autoimmunity.
The relatively recent understanding of the nature of pathogen recognition and signaling mechanisms in innate immune defenses has significantly changed previous ideas about this system. Janeway was the first to propose the existence of a class of innate immune receptors recognizing conserved microbial structures or “patterns,” even prior to the molecular identification of such a system (153). However, the immunostimulatory activity of nucleic acids had long been recognized. Already in 1963, two separate groups reported the observation that DNA and RNA derived from pathogens or host cells were capable of inducing interferon (IFN) production in fibroblasts (156, 309), but cellular receptors for nucleic acids, as well as for other microbial components, have remained unknown until a few years ago (12, 76, 119, 121, 243, 357, 393). Accordingly, the current view on pathogen recognition has been shaped only during the last two decades, initiated by Janeway's hypothesis and further stimulated by the identification of TLRs in 1997 (154, 243). Given the fact that sensing and defeating microbial infection is essential for mammalian species, PRRs and the signal transduction pathways they activate belong to an old and evolutionarily conserved system (211, 242). Pathogens of quite different biochemical composition and with entirely different life cycles, including viruses, bacteria, fungi, and protozoa, are recognized by slightly different yet surprisingly similar and overlapping mechanisms by host PRRs (7), and the unraveling of these common principles has contributed significantly to the understanding of these systems. Thus, recent studies have demonstrated that the innate immune system has a greater specificity than was previously thought and that it appears to be highly developed in its ability to discriminate between self and foreign. The innate immune system is therefore no longer regarded as a primitive, nonspecific system involved only in destroying and presenting antigen to cells of the adaptive immune system. Moreover, it now appears that innate and adaptive immune responses are much more intimately connected than initially believed, and several important findings support the idea that the innate immune system, besides being essential for early pathogen recognition, is also involved in the activation and shaping of adaptive immunity (151).
In this review, mechanisms of pathogen recognition and proinflammatory signal transduction in innate immune defenses are presented. To illustrate the complexity, yet similarities in overall principles, of these signaling pathways, viral, bacterial, fungal, and protozoan recognition by PRRs will be covered. Moreover, interference with pathogen-induced inflammatory responses by endogenous mechanisms and by pathogens is described. Finally, medical implications, including understanding of the role of PRRs in primary immunodeficiencies and in the pathogenesis of infectious diseases and autoimmunity, as well as the possibilities of therapeutic intervention with pathogen recognition and innate immune signaling are discussed.
INNATE IMMUNE DEFENSES AND PRRs
The innate immune system is based principally on physical and chemical barriers to infection, as well as on different cell types recognizing invading pathogens and activating antimicrobial immune responses (31, 242). Physical and chemical defense mechanisms are represented by epidermis, ciliated respiratory epithelium, vascular endothelium, and mucosal surfaces with antimicrobial secretions (31). Likewise, the cellular components of innate immunity include antigen-presenting DCs, phagocytic macrophages and granulocytes, cytotoxic natural killer (NK) cells, and γδ T lymphocytes (31). The important ability of the innate immune system to recognize and limit microbes early during infection is based primarily on employment of complement activation, phagocytosis, autophagy, and immune activation by different families of PRRs (Fig. 2). Whereas the focus in this review is on pathogen recognition and PRR-mediated proinflammatory signal transduction pathways, other components of innate immune defenses have been reviewed elsewhere, for instance, by Basset et al. (31). The different families of PRRs are shown in Fig. 2, and general principles of pathogen recognition by PRRs are presented below.
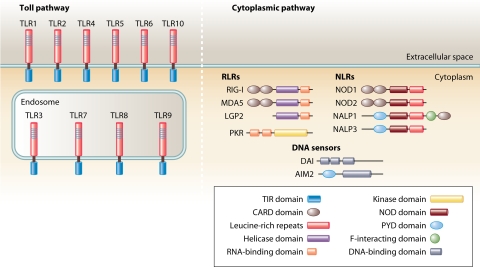
FIG. 2.
Cellular PRRs. TLRs are membrane-bound receptors localized at the cellular or endosomal membranes, recognizing PAMPs via the LRR domain and transducing signals to the intracellular environment through the TIR domain. RLRs with a C-terminal helicase domain bind RNA and become activated to transduce CARD-dependent signaling. The dsRNA-activated kinase PKR is an intracellular PRR that senses RNA through binding to two N-terminal dsRNA-binding domains. DAI and AIM2 are intracellular DNA sensors. NLRs are a class of intracellular proteins characterized by a central NOD domain and a C-terminal LRR domain, the latter of which serves as a pattern recognition domain. Signals are transduced through N-terminal domains, including CARD and pyrin (PYD) domains.
TLRs
The family of TLRs is the major and most extensively studied class of PRRs. TLRs derived their name and were originally discovered based on homology to the Drosophila melanogaster Toll protein (243), which plays a role in dorso-ventral patterning during embryogenesis as well as in the antifungal response in Drosophila (211). Structurally, TLRs are integral glycoproteins characterized by an extracellular or luminal ligand-binding domain containing leucine-rich repeat (LRR) motifs and a cytoplasmic signaling Toll/interleukin-1 (IL-1) receptor homology (TIR) domain (280). Ligand binding to TLRs through PAMP-TLR interaction induces receptor oligomerization, which subsequently triggers intracellular signal transduction. To date, 10 TLRs have been identified in humans, and they each recognize distinct PAMPs derived from various microbial pathogens, including viruses, bacteria, fungi, and protozoa (7) (Table 1).
TABLE 1.
Recognition of microbial components by PRRs
| Receptor | Cellular localization | Microbial component(s) | Origin(s) |
|---|---|---|---|
| TLRs | |||
| TLR1/TLR2 | Cell surface | Triacyl lipopeptides | Bacteria |
| TLR2/TLR6 | Cell surface | Diacyl lipopeptides | Mycoplasma |
| Lipoteichoic acid | Gram-positive bacteria | ||
| TLR2 | Cell surface | Lipoproteins | Various pathogens |
| Peptidoglycan | Gram-positive and -negative bacteria | ||
| Lipoarabinomannan | Mycobacteria | ||
| Porins | Neisseria | ||
| Envelope glycoproteins | Viruses (e.g., measles virus, HSV, cytomegalovirus) | ||
| GPI-mucin | Protozoa | ||
| Phospholipomannan | Candida | ||
| Zymosan | Fungi | ||
| β-Glycan | Fungi | ||
| TLR3 | Cell surface/endosomes | dsRNA | Viruses |
| TLR4 | Cell surface | LPS | Gram-negative bacteria |
| Envelope glycoproteins | Viruses (e.g., RSV) | ||
| Glycoinositolphospholipids | Protozoa | ||
| Mannan | Candida | ||
| HSP70 | Host | ||
| TLR5 | Cell surface | Flagellin | Flagellated bacteria |
| TLR7/8 | Endosome | ssRNA | RNA viruses |
| TLR9 | Endosome | CpG DNA | Viruses, bacteria, protozoa |
| RLRs | |||
| RIG-I | Cytoplasm | dsRNA (short), 5′-triphosphate RNA | Viruses (e.g., influenza A virus, HCV, RSV) |
| MDA5 | Cytoplasm | dsRNA (long) | Viruses (picorna- and noroviruses) |
| NLRs | |||
| NOD1 | Cytoplasm | Diaminopimelic acid | Gram-negative bacteria |
| NOD2 | Cytoplasm | MDP | Gram-positive and -negative bacteria |
| NALP1 | Cytoplasm | MDP | Gram-positive and -negative bacteria |
| NALP3 | Cytoplasm | ATP, uric acid crystals, RNA, DNA, MDP | Viruses, bacteria, and host |
| Miscellaneous | |||
| DAI | Cytoplasm | DNA | DNA viruses, intracellular bacteria |
| AIM2 | Cytoplasm | DNA | DNA viruses |
| PKR | Cytoplasm | dsRNA, 5′-triphosphate RNA | Viruses |
TLRs can be divided into subfamilies primarily recognizing related PAMPs; TLR1, TLR2, TLR4, and TLR6 recognize lipids, whereas TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids (7). Moreover, it appears that TLRs can recognize PAMPs either through direct interaction or via an intermediate PAMP-binding molecule. Thus, TLR1/2, TLR3, and TLR9 directly bind to triacetylated lipopeptides, double-stranded RNA (dsRNA), and CpG DNA, respectively (159, 202, 216), whereas TLR4 recognizes lipopolysaccharide (LPS) through the accessory molecule MD2 (187). Intriguingly, some TLRs are endowed with the capacity to recognize structurally and biochemically unrelated ligands, as exemplified by the ability of TLR4 to recognize such divergent structures as LPS, the fusion protein of respiratory syncytial virus (RSV), and cellular heat shock proteins (HSPs) (7). The molecular basis of this phenomenon may be the ability of different regions of the extracellular portion of TLRs to bind their cognate ligands or the involvement of different PAMP-binding molecules, such as MD2 (187, 216). Further distinction between different PAMPs is accomplished through the formation of heterodimers between TLR2 and either TLR1 or TLR6 (287). Another way of grouping TLRs is based on their cellular distribution. Certain TLRs (TLR1, -2, -4, -5, -6, and -10) are expressed at the cell surface and mainly recognize bacterial products unique to bacteria and not produced by the host, whereas others (TLR3, -7, -8, and -9) are located almost exclusively in intracellular compartments, including endosomes and lysosomes, and are specialized in recognition of nucleic acids, with self versus nonself discrimination provided by the exclusive localization of the ligands rather than solely based on a unique molecular structure different from that of the host (151).
The most important cell types expressing TLRs are APCs, including macrophages, DCs, and B lymphocytes (151). In different experimental systems, however, TLRs have been identified in most cell types, expressed either constitutively or in an inducible manner in the course of infection (151, 252, 269). Although patterns of TLR expression in different cell types and anatomical tissue locations, as well as mechanisms regulating TLR gene expression in response to inflammatory mediators, are of great relevance and potential profound biological significance, many of these aspects remain poorly characterized.
As a general rule, gram-negative bacteria are recognized by TLR4 via the lipid A portion of LPS (296), whereas lipoteichoic acid, lipoproteins, and peptidoglycan of gram-positive bacteria are detected by TLR2 (328, 395). However, most gram-positive and -negative bacteria can activate additional TLRs via alternative PAMPs present in the cell membrane, cell wall, or intracellularly (262), as illustrated in Fig. 3. For instance, flagellin, the major constituent of the motility apparatus of flagellated bacteria, is recognized by TLR5 (117, 118). With respect to TLR-mediated recognition of nucleic acids in intracellular compartments, TLR3 recognizes dsRNA produced during viral replication (12), whereas TLR7 and TLR8 are activated by single-stranded RNA (ssRNA) (76, 119). Finally, TLR9 is responsible for the detection of unmethylated CpG DNA present in the genomes of both viruses and bacteria (121) as opposed to methylated DNA present in mammalian cells, which generally does not activate the immune system. A fundamental property of this system is that a given pathogen can activate several different TLRs via different PAMPs, and likewise, several structurally unrelated pathogens can activate any given TLR. The net result of TLR engagement of a relevant PAMP is the triggering of downstream signaling pathways, ultimately resulting in the generation of an antimicrobial proinflammatory response. More details on PRRs and their ligands are given in Table 1.
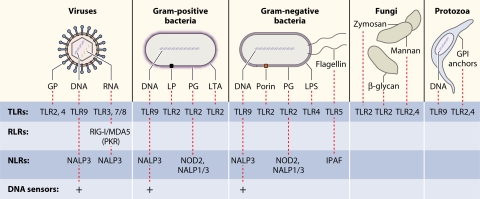
FIG. 3.
Recognition of PAMPs from different classes of microbial pathogens. Viruses, bacteria, fungi, and protozoa display several different PAMPs, some of which are shared between different classes of pathogens. Major PAMPs are nucleic acids, including DNA, dsRNA, ssRNA, and 5′-triphosphate RNA, as well as surface glycoproteins (GP), lipoproteins (LP), and membrane components (peptidoglycans [PG], lipoteichoic acid [LTA], LPS, and GPI anchors). These PAMPs are recognized by different families of PRRs.
Cytosolic PRRs
Since TLRs are expressed at either the cell surface or the luminal aspect of endo-lysosomal membranes, they do not seem capable of recognizing intracellular cytosolic pathogens and their derivatives, such as viral ssRNA, dsRNA, and DNA, as well as components of internalized or intracellular bacteria. Additionally, data from animal studies indicated the existence of other classes of PRRs. More specifically, evidence suggested that receptors other than TLR3 and TLR9 were able to induce type I IFN (IFN-α and IFN-β) production in response to RNA, DNA, or viral infections (81, 127, 149, 344). Subsequent studies revealed that TLR-independent recognition of pathogens is accomplished by a large group of cytosolic PRRs, which can be broadly divided into retinoid acid-inducible gene I (RIG-I)-like receptors (RLRs) (393) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (169).
RLRs and other cytosolic nucleotide sensors.
RIG-I and melanoma differentiation-associated gene 5 (MDA5) are IFN-inducible RNA helicases that play a pivotal role in sensing of cytoplasmic RNA (392, 393). These RNA helicases contain an N-terminal caspase recruitment domain (CARD) and a central helicase domain with ATPase activity required for RNA-activated signaling (393). Binding of dsRNA or 5′-triphosphate RNA to the C-terminal domains of RLRs (65, 356) triggers signaling via CARD-CARD interactions between the helicase and the adaptor protein IFN-β promoter stimulator 1 (IPS-1) (179, 246, 329, 383), ultimately resulting in an antiviral response mediated by type I IFN production (172, 393). The importance of CARDs is evidenced by the third helicase, LGP2, which is devoid of such domains and hence does not induce signaling but rather prevents RIG-I signaling (310), whereas the specific role of LGP2 in MDA5-activated signaling remains unresolved, with indications of a positive function (374). Although RIG-I and MDA5 function by similar mechanisms, studies have suggested differential roles of these two helicases, with RIG-I being essential for the response to paramyxoviruses and influenza virus, whereas MDA5 seems to be critical for the response to picornavirus and norovirus (174, 238). At the biochemical level, these differences may be due to length-dependent binding of dsRNA by these two RLRs (173). Specifically, RIG-I and MDA5 recognize short and long dsRNAs, respectively (173), and in addition, RIG-I detects 5′-triphosphate RNA (174, 295). Furthermore, one report has demonstrated that in addition to viral RNA, RLRs can recognize self-derived small RNAs generated by RNase L, thus amplifying the IFN response (221).
Viral RNA can also be recognized by the IFN-inducible dsRNA-activated protein kinase (PKR), which represents a major mediator of the antiviral and antiproliferative activities of IFN (270, 312, 389). Binding of dsRNA, 5′-triphosphate RNA, or poly(I-C) induces conformational changes in PKR, resulting in autophosphorylation, dimerization, and subsequent substrate phosphorylation (50, 270, 306). The best-characterized PKR substrate is the eukaryotic initiation factor eIF2α, the phosphorylation of which leads to inhibition of protein synthesis (312). Moreover, PKR has been assigned a role in proinflammatory signal transduction as an upstream kinase involved in mediating dsRNA-dependent nuclear factor (NF)-κB activation (396). However, although PKR was originally considered the principal molecule responsible for cellular recognition of dsRNA, this perception was questioned by data from PKR-deficient mice, which did not show considerable impairment in their response to viral infection (2, 131, 389). The subsequent identification of RLRs has resolved some of this paradox. The prevailing view is that the major contribution to dsRNA-activated responses is mediated by RLRs, with recent data suggesting that PKR may be able to amplify RLR signaling (237, 399), thus illustrating cross talk between these different cellular dsRNA-sensing systems involved in antiviral defense.
Cytoplasmic localization of DNA is recognized by the innate immune system independently of TLRs, RLRs, and NLRs (338, 345) and seems to be involved in mounting a response to both bacteria and DNA viruses (55, 208, 274, 301, 344). Recently, the identification of the first cytosolic DNA sensor, DAI (DNA-dependent activator of IFN-regulatory factors), was reported (357). DNAs from various sources were demonstrated to bind to DAI, thereby inducing DNA-mediated induction of type I IFN and the products of other genes involved in innate immunity (357). However, DAI is probably not the only cytosolic DNA receptor triggering the IFN response, since inhibition of DAI by small interfering RNA had little or no effect on the IFN response to different types of DNA (357, 379). This is supported by a report demonstrating induction of type I IFN by group B streptococcus via intracellular recognition of its DNA independently of DAI (55).
Although cytosolic DNA has been ascribed particularly important roles in activation of the IFN response (55, 208, 274, 301, 344), members of this class of PAMPs also activate other parts of the innate immune response (268). Recently a cytosolic DNA receptor stimulating proinflammatory signaling and maturation of pro-IL-1β has been reported and named AIM2 (absent in melanoma 2) (136). Considering the large and heterogeneous group of proteins belonging to the family of PRRs, it will not be surprising if even more cytoplasmic DNA receptors are identified.
NLRs and the inflammasome.
NLRs belong to a family of innate immune receptors which have gained increasing interest over the past few years and are now considered key sensors of intracellular microbes and danger signals and therefore believed to play an important role in infection and immunity. NLRs are defined by a centrally located NOD that induces oligomerization, a C-terminal LRR that mediates ligand sensing (in analogy with TLRs), and an N-terminal CARD that is responsible for the initiation of signaling (169). The two best-characterized members of the NLR family are NOD1 and NOD2, which sense bacterial molecules derived from the synthesis and degradation of peptidoglycan (169). Whereas NOD1 recognizes diaminopimelic acid produced primarily by gram-negative bacteria (53, 101), NOD2 is activated by muramyl dipeptide (MDP), a component of both gram-positive and -negative bacteria (102). It is currently unresolved whether NOD1 and NOD2 serve as direct receptors of PAMPs or instead detect modifications of host factors as a consequence of the presence of microbial molecules in the cytosol (169). Irrespective of the specific mechanism, activation of NOD proteins induces oligomerization and recruitment of downstream signaling molecules and transcriptional upregulation of inflammatory genes (169).
Whereas NOD1 and NOD2 stimulation results primarily in activation of proinflammatory gene expression, other NLR proteins are involved in activation of caspases (169). During infection, microbes induce TLR-dependent cytosolic accumulation of inactive IL-1β precursor and activation of caspase-1, the latter of which catalyzes the cleavage of the IL-1 precursor pro-IL-1β (226, 231). A protein complex responsible for this catalytic activity has been identified by Martinon et al. and was termed the inflammasome (231). This inflammasome is composed of the adaptor ASC (apoptosis-associated speck-like protein containing a CARD), pro-caspase-1, and an NLR family member, such as Ipaf (Ice protease-activating factor), NALP (NAcht LRR protein) 1, or NALP3/Cryopyrin (226, 231). Oligomerization of these proteins through CARD-CARD interactions results in activation of caspase-1, which subsequently cleaves the accumulated IL-1 precursor, eventually resulting in secretion of biologically active IL-1 (5, 169). Several families of inflammasomes have been identified, each recognizing different danger signals or PAMPs through their respective NLR (169) (Fig. 3). For instance, NALP3 has been ascribed a role in recognition of ATP (226), uric acid crystals (232), viral RNA (168), and bacterial DNA (268), whereas both NALP3 and NALP1 have been demonstrated to mediate caspase-1 activation in response to bacterial MDP (85, 230). Intriguingly, NALP1 engages in a protein complex with NOD2 to mediate caspase-1 activation in response to MDP recognition (140), thus implying that NOD2 plays a dual role in both pro-IL-1β synthesis and caspase-1-dependent IL-1β maturation. Recent data suggest that the composition of the inflammasome may be even more complex than first anticipated, since the cytosolic DNA receptor AIM2 was found to associate with ASC and form a caspase-1-activating inflammasome (136).
PATHOGEN RECOGNITION IN INNATE IMMUNITY
The repertoire of PRRs is very extensive, and similarly, the classes of pathogens recognized by PRRs are very diverse. A central feature of innate pathogen recognition is that microbes of quite different biochemical composition and with entirely different life cycles are recognized by relatively similar mechanisms by host PRRs (7). Moreover, an important property of this system is that no single class of pathogen is sensed by only one type of PRR. Rather, a number of different PRRs are engaged by a given pathogen via various PAMPs, hence securing a rapid and potent inflammatory response and also allowing for some specificity of the response.
Viruses
The outcome of virus-mediated PRR activation can range from an antiviral response that efficiently clears the infection to the establishment of a cellular environment that favors viral replication and spread (261). Viruses possess several structurally diverse PAMPs, including surface glycoproteins, DNA, and RNA species (261). These immunostimulatory nucleotides may be present in the infecting virion or may be produced during viral replication, and the host is in possession of a broad range of viral nucleotide sensors. Whereas viral DNA is recognized by TLR9 and DAI, ssRNA is detected by TLR7 and TLR8, and finally, dsRNA and 5′-triphosphate RNA activate RLRs, TLR3, and PKR (261). Furthermore, several viral glycoproteins are recognized by TLR2 and TLR4 (7, 261). For instance, the fusion protein from RSV activates TLR4 (199), whereas TLR2 is activated by different viruses or viral components, including measles virus hemagglutinin, cytomegalovirus, and herpes simplex virus (HSV) (35, 60, 198). Prominent examples of viral PAMPs and their recognition by PRRs are listed in Table 1, and a more detailed description of HSV recognition is presented below.
HSV is an enveloped DNA virus with the two closely related subtypes HSV-1 and HSV-2. This important human pathogen can cause various diseases ranging from relatively mild illness, as in the case of gingivostomatitis, herpes labialis, and herpes genitalis, to severe and potentially fatal infections, including encephalitis, meningitis, and neonatal herpes infection (305). During an HSV infection, multiple mechanisms of pathogen recognition are operating, depending on the cell type and the stage of the viral replication cycle (301). First, HSV virions or virion surface glycoproteins interact with TLR2 on the cell surface (198). Although the molecular nature of the TLR2 agonist remains to be defined, TLR2 activation by HSV has been demonstrated to result in cytokine production (19) and to play a prominent role in HSV-1-associated immunopathology by contributing to lethal encephalitis in mice (198). Another early response evoked by the incoming virus particle is a potent type I IFN response, which is induced by viral DNA and mediated by TLR9 (194, 220, 301). This response is independent of viral replication but is cell type specific and limited to plasmacytoid DCs (pDCs) (127, 301). It is interesting that recognition of viral DNA is dependent on the endosomal location of TLR9, suggesting that virion degradation in this compartment may be required for DNA to become accessible to TLR9 (30).
The involvement of TLR-independent recognition systems in the early response to HSV infection has been strongly suggested by several individual reports (127, 223, 301) and further supported by in vivo data demonstrating that mice lacking TLR9 or myeloid differentiation primary-response gene 88 (MyD88) can still control HSV infection (194). Cell types other than pDCs may therefore have TLR-independent receptors that exert effective antiviral responses later during infection (127, 259, 301). Indeed, a recent study has demonstrated the involvement of RLRs in HSV-induced type I IFN production, which was abolished in fibroblasts unable to signal through this pathway (301). This is in agreement with the recent finding that dsRNA accumulates in the cytoplasm during infection of permissive cells with DNA viruses, including HSV, and thus represents a potential ligand for cytosolic dsRNA receptors (380). An alternative dsRNA-sensing molecule during HSV infection is PKR, which has been implicated in HSV-induced NF-κB activation and IFN production in several studies, although mainly in non-pDCs. (223, 353). Finally, a role for DNA-sensing proteins such as DAI has been suggested by data demonstrating that entry-dependent IFN production requires the presence of viral genomic DNA and proceeds through a mechanism independent of TLRs and viral replication (301). Collectively, HSV is detected by multiple cellular recognition systems, which operate in cell type- and time-dependent manners to trigger an antiviral response.
Gram-Positive Bacteria
The cell walls of gram-positive bacteria consist mainly of peptidoglycan composed of linear sugar chains of alternating N-acetylglucosamine and N-acetylmuramic acid, cross-linked by peptide bridges to form a large macromolecular structure surrounding the cytoplasmic membrane (9). Other important components include the glycolipid lipoteichoic acid anchored in the cytoplasmic membrane as well as lipoproteins embedded in the bacterial cell wall (9). As previously described, TLR2 plays a major role in the detection of gram-positive bacteria via recognition of cell wall PAMPs, including lipoteichoic acid, lipoproteins, and peptidoglycan (328, 395), although the recognition of peptidoglycan by TLR2 remains controversial due to the possibility of endotoxin contamination (366). The importance of TLR2 in host defense against gram-positive bacteria is evidenced by studies in TLR2-deficient mice, which display increased susceptibility to challenge with Streptococcus pneumoniae and Staphylococcus aureus compared to wild-type mice (80, 224, 359). In addition, bacterial CpG DNA represents an important PAMP of gram-positive bacteria and is recognized by TLR9 (121). In order to achieve a potent inflammatory response, gram-positive bacteria are also able to trigger cytosolic PRRs, including NOD2 and the NALP1 inflammasome, both activated by the peptidoglycan-derivative MDP (102) (85). Further evidence about the role of PRRs in protection from gram-positive bacterial infection is listed in Table 1 and presented below in a description of PRR-mediated recognition of S. pneumoniae.
The spectrum of diseases caused by S. pneumoniae is diverse, with invasive pneumococcal diseases such as pneumonia, sepsis, and meningitis representing a significant burden of disease in both developing and developed countries (192). S. pneumoniae is endowed with several PAMPs, and, perhaps due to the high incidence, mortality, and morbidity associated with pneumococcal diseases, recognition of this pathogen has been extensively studied (192). As is characteristic for gram-positive bacteria, TLR2 is activated by the cell wall components peptidoglycan and lipoteichoic acid (262, 327, 395). Moreover, some data suggest that the important virulence factor pneumolysin may stimulate TLR4, but controversy exists as to the importance of TLR4 in the immune response to pneumococci (40, 222, 262). Studies using preparations of live pneumococci have demonstrated activation of both TLR2 and TLR9 in vitro, whereas TLR4 did not contribute significantly to the production of proinflammatory cytokines (262). Such findings underscore the strength of using preparations of entire live organisms, which in many contexts seem to be physiologically more relevant (258, 262, 263), although at the expense of specificity in the molecular characterization of PAMP-TLR interactions. The importance of TLR9 in the generation of an inflammatory response to pneumococci is supported by detection of residual immune activation present in TLR2/TLR4 double-knockout mice (123). Further evidence of the involvement of TLR9 has been gained from studies with TLR9-deficient mice displaying increased susceptibility to pneumococcal respiratory tract infection, which was attributable to impaired pneumococcal uptake and killing by macrophages (10). Finally, NOD proteins have been demonstrated to recognize intracellular S. pneumoniae and thus to represent cytosolic innate immune receptors for this organism (281).
Gram-Negative Bacteria
The gram-negative bacterial cell wall contains a thin layer of peptidoglycan adjacent to the cytoplasmic membrane and an outer membrane consisting of LPS, phospholipids, and proteins. LPS, which is also termed endotoxin, is composed of an O-linked polysaccharide attached to the lipid A moiety via the core polysaccharide and for most bacteria is crucial for viability (11). LPS, and in particular the lipid A portion, is a prominent feature of gram-negative bacteria, being one of the most potent PAMPs known and responsible for the inflammatory response observed during endotoxic shock (7, 367). Lipid A has a mono- or biphosphorylated disaccharide backbone acetylated with fatty acids, and the levels of both phosphorylation and acylation determine the immunostimulatory potency of lipid A and LPS (9). Different bacteria produce structurally different lipid A and LPS molecules with various phosphorylations, numbers of acyl chains, and fatty acid compositions, which can profoundly affect bacterial virulence and immunogenicity and thus constitute one of the determinants of whether a bacterial strain is pathogenic or nonpathogenic (24). LPS liberated from gram-negative bacteria associates with the extracellular acute-phase protein LPS-binding protein and then binds to the coreceptor CD14 expressed at the cell surface. This event allows transfer of LPS to the accessory molecule MD2, which is associated with the extracellular domain of TLR4, and is followed by TLR4 oligomerization and signaling (7). Accordingly, C3H/HeJ mice with nonfunctional TLR4 display impaired LPS responses and are highly susceptible to infection with gram-negative bacteria, such as Neisseria meningitidis and Salmonella enterica serotype Typhimurium (296).
Furthermore, many gram-negative bacteria are simultaneously recognized by several PRRs in addition to TLR4; for instance, peptidoglycan and bacterial membrane proteins also stimulate TLR2 (88, 234, 328). Flagellin, being part of some gram-negative bacteria, is a strong activator of TLR5 (118) as demonstrated in experimental models of infection with Salmonella species, Legionella pneumophila, and Escherichia coli (15, 115, 335), and this potent PAMP also activates the Ipaf inflammasome (249). Increasing evidence suggests that TLR9 activation by unmethylated CpG DNA derived from bacterial genomes also plays an important role during infection with gram-negative bacteria, frequently in cooperation with other PRRs (26, 262). Finally, peptidoglycan derivatives of gram-negative bacteria are recognized in the cytosol by NOD1 and NOD2 (53, 102).
A complex pattern of TLR activation by a single microbe is illustrated by the gram-negative bacterium N. meningitidis, which is recognized by three different TLRs, namely, TLR2, -4, and -9 (262). N. meningitidis is responsible for conditions ranging from colonization of the nasopharynx to chronic meningococcemia or severe acute diseases, including fulminant meningococcal meningitis and sepsis (82, 307). During initial studies involving purified bacterial components from N. meningitidis, the activation of TLR2 by the outer membrane protein porin (234) as well as recognition of meningococcal lipooligosaccharide (LOS) by TLR4 was established (403). Different strains of N. meningitidis produce structurally different LOSs exhibiting varied biological activity (297), and this may be reflected in differences in the ability to induce TLR4-mediated inflammatory signaling and hence in bacterial pathogenicity (7, 263). Studies using preparations of live N. meningitidis have confirmed the involvement of TLR2 and TLR4 and additionally have established a role for TLR9 in the proinflammatory response induced by N. meningitidis (262). Moreover, it was demonstrated that only live as opposed to heat-inactivated meningococci are able to activate TLR9 (262). Meningococci have mechanisms to enter into cellular endocytic vacuoles (343), where TLR9 is also located, and this mechanism may be dependent on bacterial viability, thus possibly explaining why TLR9 activation by meningococcal DNA was observed only when cells were infected with live bacteria (262). An important role of TLR9 in vivo was recently described in a murine model of meningococcal sepsis, in which TLR9-deficient mice displayed reduced survival and elevated levels of bacteremia (336a). At the cellular level, reduced signaling to NF-κB and diminished expression of cytokines was observed in pDCs but not macrophages and bone marrow-derived DCs (336a). Collectively, these studies analyzing immune recognition of N. meningitidis demonstrate the utilization of several TLRs to recognize a pathogen and initiate an inflammatory response. Theoretically, such a strategy may enhance the immune response by engaging TLRs on multiple cell types and in a sequential manner, thus inducing a synergistic response and possibly avoiding immune evasion by the pathogen (26, 262).
Fungi
The original observation of Toll-deficient Drosophila being highly susceptible to fungal infection indicated that mammalian TLRs may also play a role in antifungal immunity (211). In addition to innate receptors such as dectin-1, pentraxin, mannose receptors, and scavenger receptors, TLR2 and TLR4 have been implicated in innate immune responses toward important fungal pathogens, such as Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, and Pneumocystis jirovecii (304), and several PAMPs located in the cell wall or at the cell surface of these fungi have been defined (Fig. 3.) First, the yeast cell wall particle zymosan activates TLR2/6 heterodimers (287), whereas C. albicans-derived mannan is detected by TLR4 (271, 352). TLR2 is also activated by phospholipomannan from C. albicans (161) and by β-glycan, the latter of which is a component of many fungal pathogens, including C. albicans and P. jirovecii (97, 210). In contrast, TLR4 is the receptor for glucuronoxylomannan, the major capsular polysaccharide from C. neoformans (333). An important contribution to the understanding of antifungal immunity was provided by Underhill et al., by their observation that TLR2 is specifically recruited to phagosomes containing fungi (371). Thus, two different classes of innate immune receptors cooperate in a process whereby phagocytic receptors, such as the mannose receptor, mediate particle internalization, after which TLRs trigger an inflammatory response (371). Subsequent studies by the same group demonstrated enhancement of TLR2-mediated NF-κB activation by dectin-1, a lectin receptor on macrophages responsible for phagocytosis of various fungi (42, 96). More recent evidence support a central role of dectin-1 in recognition of β-glycan-containing structures, including zymosan, A. fumigatus, and C. albicans (104, 248). Thus, close cooperation between TLRs and other innate immune receptors seems to be a very elaborate theme in antifungal immunity.
Protozoa
The role of PRRs during infection with protozoan pathogens is less well described than in the case of viruses and bacteria, although evidence is now accumulating, and the general principles appear to be similar to the ones described for other classes of pathogens (98). Major PAMPs identified in protozoa include glycosylphosphatidylinositol (GPI) anchors (14), which activate TLR2 and TLR4, as well as unmethylated DNA activating TLR9 (69, 98). In addition, it was originally suggested that the malaria pigment hemozoin, which represents a heme degradation product, stimulates TLR9 (58). However, a subsequent study demonstrated that hemozoin per se is immunologically inert but is coated with malarial DNA and enhances innate recognition through TLR9 by targeting DNA to the endosome (288). The intracellular protozoan Toxoplasma gondii causes asymptomatic infection in normal hosts but can be fatal in immunocompromised individuals, particularly in the absence of IL-12 production (73). In response to T. gondii infection, IL-12 is produced through a mechanism dependent on MyD88 (188, 319), hence suggesting the involvement of TLRs in recognition of this parasite. Studies further addressing this issue have now confirmed that this is indeed the case, since TLR2 and TLR4 are activated by GPIs derived from T. gondii (70). Finally, the potent IL-12 inducer profilin-like protein from T. gondii tachyzoites is recognized by murine TLR11 (390), a nonfunctional TLR in humans (7).
PRINCIPLES IN INNATE SIGNAL TRANSDUCTION
Upon engagement of TLRs by individual PAMPs, a number of different signaling pathways are triggered. Signal transduction is mediated initially by a family of adaptor molecules, which at least in part determines the specificity of the response (280). Recruitment of one or several adaptor molecules to a given TLR is followed by activation of downstream signal transduction pathways via phosphorylation, ubiquitination, or protein-protein interactions, ultimately culminating in activation of transcription factors that regulate the expression of genes involved in inflammation and antimicrobial host defenses (7) (Fig. 4). TLR-induced signaling pathways can be broadly classified on the basis of their utilization of different adaptor molecules, i.e., dependent on or independent of the adaptor MyD88 or TIR domain-containing adaptor inducing IFN-β (TRIF), and, additionally, their respective activation of individual kinases and transcription factors (6, 280). Three major signaling pathways responsible for mediating TLR-induced responses include (i) NF-κB, (ii) mitogen-activated protein kinases (MAPKs), and (iii) IFN regulatory factors (IRFs) (6, 7, 177). Whereas NF-κB and MAPKs play central roles in induction of a proinflammatory response, IRFs are essential for stimulation of IFN production (6, 177).
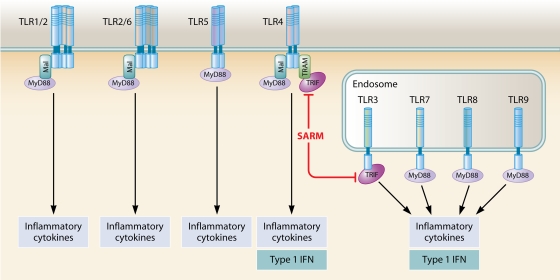
FIG. 4.
TLRs and TIR domain-containing adaptor molecules. TLR1/2 and TLR2/6 utilize MyD88 and Mal as adaptors. TLR3 is dependent on TRIF for signaling. In the case of TLR4, four different adaptors, i.e., MyD88, Mal, TRIF, and TRAM, are involved, whereas TLR5, -7, -8, and -9 utilize only MyD88. The fifth adaptor, SARM, negatively regulates TRIF-dependent signaling. Overall, MyD88-dependent signaling induces proinflammatory cytokine production, whereas TRIF-dependent signaling stimulates a type I IFN response. In pDCs, stimulation of TLR7 or TLR9 induces type I IFN production by a mechanism dependent on MyD88. See text for further details.
TLR Adaptor Molecules
Following ligand binding, TLRs dimerize and undergo conformational changes required for the subsequent recruitment of cytosolic TIR domain-containing adaptor molecules (280). MyD88, which was the first adaptor molecule to be identified (44, 381), is involved in signaling triggered by all TLRs, with the exception of TLR3, and plays a major role in TLR-induced signal transduction (176, 280). Despite the prominent role of MyD88, studies with MyD88-deficient mice revealed the existence of both MyD88-dependent and -independent pathways (176, 180), resulting in subsequent identification of additional adaptor molecules, including MyD88 adaptor-like protein (Mal) (90, 135), TRIF (283, 387), TRIF-related adaptor molecule (TRAM) (91, 284, 386), and sterile alpha- and armadillo motif-containing protein (SARM) (51, 253). Figure 4 illustrates the utilization of different adaptor molecules by individual TLRs.
Based on findings that NF-κB activation and the inflammatory cytokine response are abolished in mice deficient in MyD88 but normal in mice deficient in Mal, TRIF, or TRAM in response to agonists of TLR5, -7, and -9, it was inferred that MyD88 is used as the only adaptor by these TLRs (120, 135, 384-386). MyD88 is also utilized by TLR2, heterodimerizing with TLR1 or TLR6 (287), but in addition, Mal appears to be required for linking TLR2 and MyD88 together (135, 385). In the case of TLR4, four different adaptors, i.e., MyD88, Mal, TRIF, and TRAM, are involved in signal transduction (129, 135, 384-386), reflecting the complexity of signaling downstream of this receptor (Fig. 4). Several attempts have been made to explain how TLR4 coordinates recruitment and signaling through two potentially competing adaptor systems. Recently, Kagan et al. presented data suggesting that TLR4 activates these two pathways in a sequential manner determined by endocytosis of the TLR4 complex (162). The authors proposed a scenario in which endocytosis of TLR4 terminates an initial phase of Mal-MyD88-dependent signaling and triggers a second phase of TRAM-TRIF-dependent signal transduction originating from TLR4 molecules localized in endosomes and aided by improved access to the adaptor protein tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3) (162). Finally, TLR3 is the only TLR that does not use MyD88 but instead is dependent on TRIF for signaling (384). In contrast to these four adaptor molecules with activating potential, the fifth adaptor, SARM, appears to be a negative regulator of TRIF-dependent signaling in human cells (51), although this finding was not confirmed in studies of SARM-deficient mice (189). In summary, the existence of this family of adaptors has the important implication that the selective usage of individual adaptor molecules or combinations of molecules may explain, to some degree, the differential responses induced by different TLRs. Thus, TLR-induced signaling can be largely divided into MyD88-dependent and MyD88-independent, TRIF-dependent pathways, which are both capable of activating NF-κB, although each activates additional signaling components, including MAPKs and IRFs.
The MyD88-Dependent Signaling Pathway
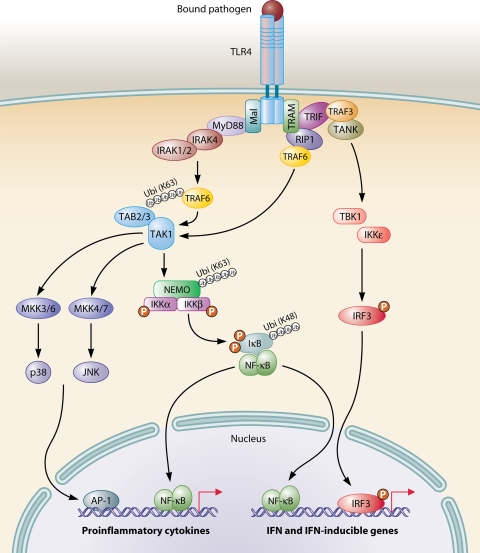
MyD88-dependent signaling is activated downstream of all TLRs except TLR3 (7). In the pathways stimulated through TLR2, -4, and -5, MyD88 primarily drives inflammatory gene expression, whereas MyD88 relays signaling to type I IFN production in pathways triggered from TLR7 and TLR9, occurring primarily in pDCs. In this review, these two MyD88-dependent signaling pathways are described separately, with TLR4 signaling representing the prototypic MyD88-dependent proinflammatory pathway as illustrated in Fig. 5.
FIG. 5.
Principles in TLR signaling. TLR4 activates both the MyD88-dependent and MyD88-independent, TRIF-dependent pathways. The MyD88-dependent pathway is responsible for early-phase NF-κB and MAPK activation, which control the induction of proinflammatory cytokines. The MyD88-independent, TRIF-dependent pathway activates IRF3, which is required for the induction of IFN-β- and IFN-inducible genes. In addition, this pathway mediates late-phase NF-κB as well as MAPK activation, also contributing to inflammatory responses.
In response to TLR4 stimulation by an appropriate PAMP, MyD88 associates with the cytoplasmic part of the receptor and subsequently recruits members of the IL-1 receptor (IL-1R)-associated kinase (IRAK) family (44, 381). Following association with MyD88, IRAK4 and IRAK1/2 are sequentially phosphorylated, with IRAK4 being of particular importance, as it has been demonstrated to be indispensable for the response to IL-1 and various TLR ligands (348). IRAK1 was originally thought to play an essential role in TLR-induced NF-κB activation (164), but more recent data have emerged suggesting that instead IRAK2 may play a prominent role in NF-κB activation, particularly during the late phase of TLR signaling (175, 182). Further downstream, IRAK1, or alternatively IRAK2, associates with TRAF6, which acts as a ubiquitin protein ligase (E3) (214) that, together with the ubiquitination enzyme complex (E2), catalyzes the synthesis of K63-linked polyubiquitin chains on TRAF6 itself and other substrates, including transforming growth factor-activated protein kinase 1 (TAK1) and the IκB kinase (IKK) subunit NF-κB essential modifier (NEMO) (378). A central step in the downstream signaling events is the recruitment of TAK1-binding protein 2 (TAB2) and TAB3 to ubiquitinated TRAF6, which brings TAK1 into proximity to the signaling complex, leading to its activation (165). TAK1 then stimulates two distinct pathways involving the IKK complex and the MAPK pathway, respectively (378) (Fig. 5).
In the first pathway, TAK1-mediated activation of the IKK complex results in site-specific phosphorylation of the inhibitory IκB protein. Being the point of convergence for multiple NF-κB-inducible stimuli, IKK represents an essential component in many inflammatory signaling pathways (112). This high-molecular-weight kinase is composed of two structurally related kinases, IKKα and IKKβ, as well as the chaperone IKK complex-associated protein and the adaptor NEMO/IKKγ. NEMO appears to function as a signal integrator in the NF-κB pathway by receiving and transmitting converging signals from various stimuli and pathways to common downstream signaling events (112, 244, 311, 397). Despite early reports of IKK dependency upon ubiquitination for optimal kinase activity, it was only recently resolved that direct ubiquitination of NEMO is mediated by TRAF6 (346). Following phosphorylation, IκB undergoes proteasomal degradation to allow activation and translocation of NF-κB to the nucleus, where it binds to κB sites present in promoters and enhancers of a broad range of proinflammatory genes, which are then transcribed (112).
In the second pathway, TAK1 phosphorylates members of the MAPK kinase (MKK) family, including MKK3, -4, -6, and -7 (54). MKK3/6 subsequently phosphorylate and activate p38, whereas MKK4/7 activate c-Jun N-terminal kinase (JNK). However, in studies analyzing T. gondii-activated signaling, an alternative mechanism involving p38 autophosphorylation proceeding in a TAK1-dependent and MKK3/6-independent pathway has been described (188). Ultimately, these signaling pathways lead to activation of the transcription factor activator protein 1 (AP1) (54). Moreover, other members of MKK kinases, most notably MKK kinase 3 and tumor-progression locus 2, have also been implicated in MAPK activation downstream of TLR4 (28, 142). The essential and nonredundant role played by TAK1 is strongly suggested by significantly reduced NF-κB, JNK, and p38 responses to various TLR ligands in cells derived from mice deficient in this kinase (317, 332).
The MyD88-Independent, TRIF-Dependent Signaling Pathway
The existence of a MyD88-independent pathway downstream of TLR3 and TLR4 was indicated by data from MyD88-deficient mice displaying normal IFN-β production (176, 180). Extensive molecular studies by Akira and associates then led to the identification of TRIF as the adaptor responsible for signaling in the MyD88-independent pathway (384). Subsequently, the equally important discovery of two IKK-related kinases, TRAF family member-associated NF-κB activator (TANK)-binding kinase 1 (TBK1) and IKKɛ, and their essential role in induction of type I IFN were reported (89, 122, 330).
During TLR3- and TLR4-mediated signaling, TRIF (associated with TRAM in the case of TLR4) (91, 386) is responsible for initiating a signaling pathway in which TRAF3 and TANK serve to bridge to the IKK-related kinases TBK1 and IKKɛ (108, 113, 276, 318), which mediate direct phosphorylation of IRF3 and IRF7 (89, 330). Studies with cells lacking TBK1 or IKKɛ have revealed that TBK1 and, to a lesser extent, IKKɛ are responsible for TRIF-mediated IFN responses (122, 240, 292). It is notable, however, that whereas TBK1 and IKKɛ are essential for TRIF-dependent IRF3/7 phosphorylation, these kinases are not involved in TLR-mediated NF-κB activation (122). As a consequence of phosphorylation, IRF3 and IRF7 form hetero- or homodimers, translocate to the nucleus, and, in association with transcriptional coactivators such as CBP and p300, bind to target sequences in DNA, such as IFN-stimulated response elements (89, 330). Importantly, IRFs together with NF-κB and AP1 form a multiprotein complex termed the enhanceosome, which induces transcription of the IFN-β gene (364).
TRIF-dependent activation of NF-κB occurs through binding of TRAF6 to TRIF and subsequent ubiquitination-dependent recruitment and activation of TAK1 (318). In order to obtain robust NF-κB activation, a second molecule, receptor-interacting protein 1 (RIP1), involved in TNF-receptor mediated NF-κB activation, is also recruited to TRIF (245). RIP1 is polyubiquitinated to form a complex with TRAF6, and these two molecules appear to cooperate in facilitating TAK1 activation, resulting in IKK-mediated activation of NF-κB as well as activation of the MAPK pathway (66). Thus, molecular signaling mechanisms within the TRIF-dependent pathway illustrate how selective binding of different molecules, i.e., either TRAF3 or TRAF6, results in recruitment and downstream activation of TBK1-IRF versus TAK1-IKK-NF-κB, respectively (Fig. 5). An example of differential pathogen-induced activation of diverse signaling pathways emanating from a given TLR has been reported for two strains of N. meningitidis with different abilities to stimulate TLR4 through the MyD88-independent pathway (263). In molecular terms, this observation may be explained by the ability of CD14 to distinguish between different carbohydrate chains of LPS/LOS, which governs the MyD88-independent signaling pathway of TLR4 (157).
MyD88-Dependent Signaling from TLR7 and TLR9
It has been well established for some time that in addition to TLR3 and TLR4 activation, TLR7 and TLR9 activation also triggers IFN production in pDCs, a subset of DCs specialized in producing large amounts of type I IFN in response to virus infection (59). Intriguingly, however, TLR7- and TLR9-mediated IFN production requires MyD88, in contrast to TLR3- and TLR4-mediated IFN production, which is dependent on TRIF (128, 178, 384). Another notable difference between these pathways is that TLR7- and TLR9-mediated IFN production does not depend on TBK1/IKKɛ but instead proceeds through a molecular network including IRAK1, IRAK4, TRAF6, TRAF3, and IKKα (113, 132, 138, 178, 276, 370) (Fig. 5). The presence in this network of IKKα, which otherwise mediates its primary function as part of IKK, is surprising and still not fully understood. In pDCs, which are characterized by constitutive expression of IRF7, TLR7 and TLR9 signaling results in IRAK1-mediated phosphorylation of IRF7 and production of type I IFN (370). Several reports analyzing this pathway in mice deficient in relevant components have demonstrated an important and nonredundant role of MyD88, IRAK1/4, and IRF7 (178, 370), thus emphasizing that IRF7 is an essential transcription factor regulating IFN production in pDCs (133). In addition, stimulation of TLR7 and TLR9 also triggers MyD88-dependent activation of NF-κB and MAPKs in all cell types by mechanisms similar to the ones operating downstream of TLR3 and TLR4. Finally, IRF5 has been reported to interact with MyD88 in hematopoietic cells (358) and to play a role in induction of type I IFN production through TLR7 and TLR9 (63, 291, 324). IRF5 also participates in induction of inflammatory cytokines through TLR3, -4, -7, and -9 (291, 324, 358).
In summary, TLRs signal through a proinflammatory MyD88-dependent pathway mainly responsible for cytokine expression induced by potent activation of NF-κB and MAPKs. An additional MyD88-dependent pathway is triggered by TLR7 and TLR9 and induces type IFN production, particularly in pDCs. On the other hand, a MyD88-independent, TRIF-dependent pathway is essential for mounting a type I IFN response in non-pDCs as well as for contributing to activation of NF-κB.
Signaling by Cytosolic PRRs
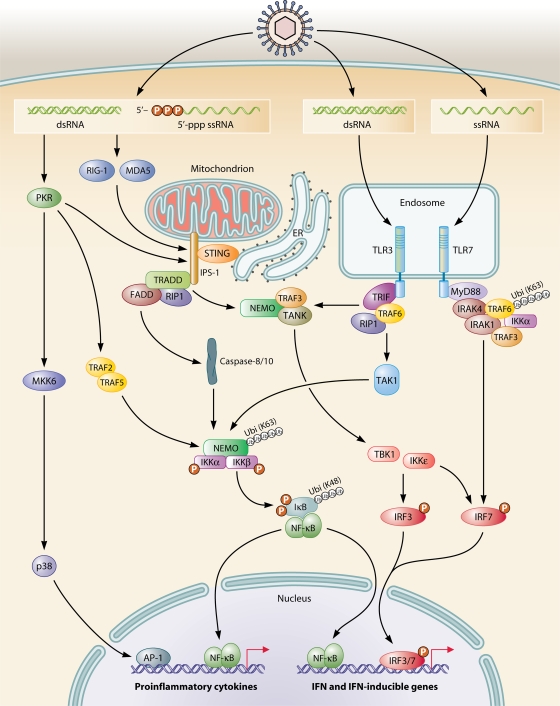
In addition to RNA detection in the endosomal compartment through TLR3- and TLR7/8-mediated recognition of dsRNA and ssRNA, respectively, several cytosolic RNA-sensing receptors have been identified, including RLRs and NLRs, as previously described (Fig. 6). Binding of dsRNA or 5′-triphosphate RNA to RLRs triggers signaling via CARD-CARD interactions between the helicase RIG-I/MDA5 and the adaptor protein IPS-1 (179). This adaptor protein, which has been independently characterized by several groups and also named, in abbreviated form, CARDIF (246), MAVS (329), or VISA (383), appears to be associated with the outer mitochondrial membrane (329), and this association is essential for the function of IPS-1 (246). Yet another adaptor protein, stimulator of IFN genes (STING, also termed MITA), was recently described (150, 402). Although also present in the mitochondrial membrane, STING resides predominantly in the endoplasmic reticulum (ER) and interacts with RIG-I and IPS-1, which are linked to the mitochondrial membrane, thus potentially allowing cross talk between these two organelles, in terms of both viral recognition and signaling (150, 402). Indeed, a central position of mitochondria in virus-induced NF-κB activation was already reported prior to the identification of RLRs, IPS-1, and STING (259). In this early study, NF-κB activation in HSV-infected murine macrophages was demonstrated to be associated with generation of mitochondrial oxidative stress and calcium release and to be dependent on activation of TAK1, MKK kinase 1, and IKK (259). Since many viruses replicate in the membranous web connecting the ER to mitochondria, the localization of dsRNA sensing and signaling pathways to these organelles may provide the host with an opportunity to sense an incoming viral challenge and subsequently coordinate an immune or apoptotic response (125). However, the precise connection of mitochondrial oxidative stress and calcium-dependent signaling to mitochondrion-associated adaptor proteins such as IPS-1 remains to be fully understood.
FIG. 6.
Intracellular RNA recognition and signaling. Cytosolic dsRNA or 5′-triphosphate ssRNA is recognized primarily by the cytoplasmic RNA helicases RIG-I and MDA5, which mediate interaction with the adaptor IPS-1, localized to mitochondria, and trigger signaling to NF-κB and IRF3 via IKK and TBK/IKKɛ, respectively. dsRNA can also be recognized by TLR3 localized in the endosomal compartment or by cytosolic PKR, but whereas TLR3 triggers signaling to NF-κB and IRF3, PKR instead activates NF-κB and MAPKs. Finally, ssRNA is recognized by TLR7/8 in endosomes and induces signaling to IRF7 as well as to NF-κB and MAPKs (not shown in the figure).
In the RLR pathway, IPS-1 appears to be the point of divergence of two different signaling pathways, involving either IRFs or NF-κB, as illustrated in Fig. 6. A central role of TNF receptor-associated death domain (TRADD) in signal transduction from IPS-1 to both of these pathways was reported by Michallet et al. (251). TRADD, which is also an essential adaptor for the TNF receptor, was demonstrated to be recruited to IPS-1 and to orchestrate complex formation with downstream effector molecules (251). Thus, the IPS-1-TRADD complex recruits the E3 ubiquitin ligase TRAF3 and the adaptor protein TANK (108, 251, 313), which subsequently activate TBK1/IKKɛ to phosphorylate IRF3 and IRF7, ultimately resulting in an antiviral response mediated by IFN-β production (172, 174, 393). Alternatively, the IPS-1-TRADD complex propagates the signal to NF-κB by a mechanism involving interaction with Fas-associated death domain, RIP1, and caspase-8/10, which were also originally identified in the signaling pathway downstream of the TNF receptor (27, 355). These molecules transduce the signal to IKK, eventually mediating NF-κB activation. NEMO has been demonstrated to participate not only in NF-κB activation but also in IRF3 activation (401), although the mechanism, by which NEMO activates IRF3 remains to be clarified. In similarity with most TLR signaling pathways, RLRs also propagate activating signals to MAPKs (329).
Intracellular sensing of RNA has also been attributed to PKR, which plays a role in IFN and stress responses (270, 312, 389). In studies of PKR signal transduction pathways, it was demonstrated that PKR-mediated NF-κB activation in response to poly(I-C) is transduced through NF-κB-inducing kinase and IKK (396). Moreover, a number of reports have described the involvement of PKR in signaling induced by diverse proinflammatory stimuli, including TNF-α (74, 396), IFN-γ (68), and LPS (109). It remains unsettled, however, whether PKR kinase activity is a prerequisite for signal transduction or whether PKR mediates its functions as a structural protein through interaction with other kinases and adaptor proteins. For instance, it has been suggested that PKR may participate in TLR3-dependent NF-κB activation by functioning as a scaffold protein as part of an intracellular complex containing TRAF6, TAK1, and TAB2 (158), and others have demonstrated that interaction of PKR with TRAF2 and TRAF5 leads to activation of IKK (100). Furthermore, one study with human keratinocytes has suggested that PKR may be involved in the dsRNA response through both TLR3 and RLR signaling pathways (163). This was supported by findings of PKR facilitating IPS-1-dependent signaling to induce IFN-β and apoptosis in response to vaccinia virus infection and 5′-triphosphate RNA (237, 399). Alternatively, PKR may be associated with the IKK complex under certain conditions or in response to specific stimuli (312, 396), thus explaining the importance of PKR in obtaining a full response under some experimental conditions, whereas in other situations PKR seems to be redundant. Such models may be a way to reconcile the apparent involvement of PKR in several proinflammatory pathways with recent data on more prominent dsRNA-sensing systems, such as TLR3 and RIG-I. Finally, several pieces of evidence suggest that PKR activates p38 in response to dsRNA by a mechanism that may include PKR-mediated phosphorylation of the p38 upstream kinase MKK6 (334).
Regarding cytosolic DNA sensors, including DAI, strong evidence suggests that these receptors signal via TBK1/IKKɛ to IRFs, whereas the pathway for NF-κB activation remains unknown (149, 344, 345, 357). Considerably more data describing signaling mechanisms have been collected in the case of NOD proteins. Signaling by these cytosolic PRRs involves recruitment of the kinase RIP2, which promotes polyubiquitination of NEMO and TAK1 (1, 382), a prerequisite for activation of NF-κB. Likewise, the adaptor molecule CARD9 is directly recruited to NOD proteins, thus allowing downstream activation of MAPK signaling (169).
INNATE AND ADAPTIVE IMMUNE RESPONSES
Proinflammatory signaling pathways induced by PRRs activate the innate immune response and also play a role in the activation, maturation, and shaping of the adaptive immune response (151). To initiate these responses, the transcription factors NF-κB, AP1, and IRF3/7 play pivotal roles (54, 177) due to their capacity to stimulate the production of proinflammatory mediators, including cytokines and IFNs. However, tight regulation is essential to ensure the strong, albeit transient, nature of these responses, and this is achieved via amplification early during infection, as well as restriction and downregulation when needed at later stages. It is notable that regulation of gene expression at the transcriptional level plays an important, although not exclusive, role in the generation of an adequate immune response; posttranscriptional mechanisms regulating the decay of mRNAs encoding various pro- and anti-inflammatory molecules also contribute to achieve this goal.
NF-κB-Inducible Proinflammatory Mediators
The central role played by NF-κB in both innate and adaptive inflammation and immunity is mediated by the coordinate expression of multiple genes essential for the immune response. The importance of NF-κB is revealed by the extensive list of NF-κB-inducible genes, including those for proinflammatory cytokines such as IL-1, IL-6, and TNF-α, as well as chemokines, including IL-8 and RANTES (99). Moreover, the expression of cell adhesion molecules, such as intercellular adhesion molecule-1 and E-selectin, is upregulated. A major class of molecules involved in many aspects of the inflammatory response and upregulated in response to cellular pathogen recognition consists of immunoreceptors, including cytokine and chemokine receptors, immunoglobulins, TLRs, major histocompatibility complex (MHC) molecules, and costimulatory molecules (99, 151, 252). Together, these NF-κB-inducible proteins participate in the activation and recruitment of leukocytes to sites of inflammation, in enhanced phagocytosis of microbes, in complement- or NK cell-mediated cellular lysis, and in enhanced antigen presentation. Finally, in some instances, it appears to be advantageous for the host to stimulate cell division and inhibit apoptosis, and in order to achieve these effects, NF-κB induces a number of growth factors and antiapoptotic proteins (99, 171).
Posttranscriptional Regulatory Mechanisms
Posttranscriptional regulation, particularly control of mRNA stability, also represents an important level of regulation of the proinflammatory response (184). In this respect, two prominent mechanisms are micro-RNAs and AU-rich element (ARE)-mediated mRNA degradation (184). RNA degradation proceeds through specific, inducible ARE-binding proteins that determine the fate of a given mRNA, i.e., degradation versus stabilization, through binding to the 3′ untranslated region (29). Many gene products involved in immunity and inflammation, especially cytokines and chemokines, harbor such destabilization sequence elements (92). ARE-binding proteins function by targeting AU-rich RNA to the exosome for degradation or by recruitment of alternative degradation factors (87, 139). Several signaling pathways with impact on mRNA stability have been described, with the p38 MAPK pathway being one of the most prominent (92).
The importance of ARE-mediated mRNA regulation in generation of an inflammatory response was described in a study of live S. pneumoniae-induced TNF-α production, which was demonstrated to be highly dependent on p38 and AREs in the 3′ untranslated region of TNF-α mRNA (257). However, despite previous findings that many purified and synthetic TLR ligands can stabilize RNA (114, 302), in this study, TNF-α mRNA stabilization was found to be independent of MyD88. An interpretation of these results is that other members of the PRR family, such as NLRs or DNA receptors, may be activated by S. pneumoniae and mediate the effect (257). The close relationship between PRRs and the mRNA-regulating machinery is suggested by other examples illustrating both cooperation and interplay between these evolutionary ancient systems. For instance, several micro-RNAs which have the ability to bind and induce degradation of specific mRNA transcripts through the enzyme Dicer (145) are encoded by LPS-responsive genes (275, 354), and likewise, specific micro-RNAs are involved in regulation of TRAF6 and IRAK1, prominent members of TLR-induced signaling pathways (354).
Leukocyte Recruitment and Activation
Overall, pathogen recognition through PRRs regulates leukocyte recruitment to sites of infection by activating several cell type subsets, including tissue stromal cells, tissue-resident innate cells (most notably DCs and macrophages), and circulating leukocytes. Leukocyte recruitment is a complex process involving a series of interactions with integrins expressed on vascular endothelium. The sequential rolling, adhesion, and migration of leukocytes into the inflamed tissue is tightly regulated through induced expression of cell adhesion molecules and integrins in response to PRR activation (141, 151, 204). The acute inflammatory cellular infiltrate consists of monocytes, DCs, neutrophils, and NK cells. Among these, neutrophils and NK cells are critical innate effector cells protecting the host by killing pathogenic microbes and infected cells, respectively (31, 151). Whereas neutrophils express mRNA for all TLRs and can be activated by most PAMPs, NK cells seem to be activated mainly by type I IFN produced by pDCs in a TLR-dependent manner. These activated NK cells in turn secrete IFN-γ, hence augmenting pathogen clearance by macrophages (229, 315). Despite the central role played by these innate immune cells, recognition of PAMPs by epithelial, endothelial, and hematopoietic cells in different tissues through TLRs is also an integral part of innate immune defenses at sites of infection (151, 236).
IFN Responses
The ability of IFNs to confer an antiviral state on cells is their defining activity and at the same time one of the fundamental properties that allowed their discovery (148). Type I IFN, which is induced by PRR signaling and plays a major role in innate antiviral defenses, consists of IFN-α and IFN-β. IFN-γ, on the other hand, belongs to type II IFN, is produced by T lymphocytes and NK cells, and plays a central role in activation of macrophages (143). Finally, type III IFN is represented by IFN-λ and shares many properties with type I IFN (18). As previously described, type I IFN can be induced through different pathways depending on the cell type involved. In pDCs, which are responsible for the majority of IFN produced during a viral infection, stimulation of TLR7 or TLR9 with relevant ligands induces type I IFN in a MyD88-dependent manner (132, 178). Conversely, in macrophages, conventional DCs, and fibroblasts, type I IFN is induced by cytoplasmic RNA primarily via RLR-mediated signaling or, alternatively, by TLR3 or TLR4 stimulation by a mechanism dependent on TRIF (172, 384).
An important aspect adding to the complexity of IFN production and regulation is the existence of a positive feedback loop, which represents a way to significantly enhance the IFN response (227). Whereas IRF3 is constitutively expressed, IRF7 expression is weak in unstimulated non-pDCs but is dramatically upregulated in response to virus infection, LPS, or type I IFN (59, 227). Initial expression of IFN-β is therefore largely dependent on phosphorylation and activation of IRF3, but the secreted IFN-β subsequently acts on neighboring cells to induce expression of IRF7. Finally, IRF7 phosphorylated during virus infection together with phosphorylated IRF3 induces the production of both IFN-β and IFN-α subtypes, thereby amplifying the response (133, 227, 316).
IFNs mediate their responses by signaling through distinct but related pathways via specific type 1 or 2 receptors that bind to Janus kinases (JAKs) and subsequently activate signal transducers and activators of transcription (STATs), resulting in expression of a broad range of IFN-stimulated genes (74, 342). Classical functions of type I IFN comprise antiviral activities mediated by PKR, 2′-5′-oligoadenylate synthase/2-5A-dependent RNase L, and Mx proteins, which together inhibit protein synthesis, induce RNA cleavage, and interfere with viral replication (342). Type I IFN also plays a prominent role in inhibition of cellular growth and control of apoptosis, although the effect may be either pro- or antiapoptotic depending on the cellular context (342).
The importance of IFN in innate immunity can be extended to include a role in adaptive immune defenses as well (151). For instance, cross-presentation of viral antigens occurs via a mechanism dependent on type I IFN (209a), and B-lymphocyte isotype switching and differentiation into plasma cells have also been attributed to IFN-α/β as well as to IFN-γ (209). Finally, DC maturation is induced by IFN-α/β following stimulation with unmethylated CpG DNA, poly(I-C), LPS, or viral infection (129, 131, 137). It is notable that only a subset of TLRs, including TLR3, -4, -7, and -9, have the ability to induce IFN, although through different signaling pathways. However, since many pathogens activate several TLRs or additional PRRs, most antimicrobial responses seem to include some degree of IFN production. In regard to type II IFN, IFN-γ participates in many aspects of innate and adaptive immunity in cooperation with type I IFN, but IFN-γ also has unique roles, of which activation of macrophages and intracellular killing of microbial pathogens are among the most prominent (342). Despite the IFN system being known for decades, novel functions of these molecules have been described in recent years. For instance, accumulating evidence suggests that type I IFN may not only be involved in antiviral defenses but also may play a role in antibacterial defenses (71), representing a subject of current interest in the field.
Bridging Innate and Adaptive Immune Defenses
A number of studies have established the important link between innate and adaptive immunity provided by PRRs, particularly via TLR-mediated maturation of DCs and activation of pathogen-specific T lymphocytes (151, 303). Following antigen uptake by DCs, these cells become activated and migrate to regional lymph nodes to present antigenic peptides in the context of relevant MHC molecules. During this process, phagocytosis, upregulation of costimulatory molecules (including CD80, CD86, and CD40, and antigen-presenting MHC molecules), switches in chemokine receptor expression, and cytokine secretion are all events that are regulated through the recognition of pathogens by PRRs expressed on DCs (129, 131, 137, 151). Importantly, different subsets of DCs express different and nonoverlapping sets of TLRs, which together with the selective tissue distribution may explain some of the diverse functions carried out by these DC subsets (77). This subject has been excellently reviewed by Iwasaki and Medzhitov (151) but is not within the scope of the present review. After having undergone maturation, DCs are endowed with the ability to stimulate naïve CD4+ T lymphocytes into different T helper (Th) subsets, the differentiation of which is controlled by a variety of factors, including TLR-induced cytokines. The Th1-Th2 paradigm introduced by Mosmann et al. in 1986 states that Th cells can be separated into two distinct subsets, depending on the cytokines they produce (265). In general, Th1 responses are important for protection against viruses and intracellular bacteria, whereas Th2 responses mediate immunity to extracellular protozoa at mucosal surfaces and are involved in allergic responses. More recently, a Th17 subset with important functions in protection against certain bacterial infections and with possible roles in the development of autoimmunity was discovered (286, 372).
In the process of DC-mediated polarization of different Th subsets, IL-12 plays a critical role in inducing Th1 responses, most notably IFN-γ (368), whereas IL-23 and IL-1 in humans, and transforming growth factor β and IL-6 in mice, participate in inducing Th17 responses with preferential secretion of IL-17, IL-21, and IL-22 (225, 286, 372). In contrast, Th2 responses consisting of IL-4, IL-5, and IL-13 are largely dependent on IL-4 (265). The demonstration that mice deficient in MyD88 display severely compromised Th1 differentiation and instead undergo Th2 differentiation is consistent with the view that TLRs primarily control the induction of Th1 and Th17 responses as opposed to Th2 responses (323). The relationship between PRRs and the induction of Th17 responses has been further elaborated in a recent report demonstrating that bacteria prime DCs to stimulate IL-17 production in human memory T lymphocytes through the NOD2 ligand MDP (372). Also of interest is the demonstration of a critical role of NOD1 in synergizing with TLRs to prime Th1 and Th17 pathways in vivo (93). TLRs may also play a role in mechanisms of immune suppression by regulatory T lymphocytes and in humoral immune responses mediated by B lymphocytes (48, 289, 290). Collectively, the important coupling of pathogen recognition with the induction of costimulation and hence activation of pathogen-specific T lymphocytes, an idea originally introduced by Janeway (153), supports the view that innate immunity and adaptive immunity should be perceived as tightly connected and interacting mechanisms of defense, rather than as two separate systems.
INTERFERENCE WITH PATHOGEN-ACTIVATED SIGNALING
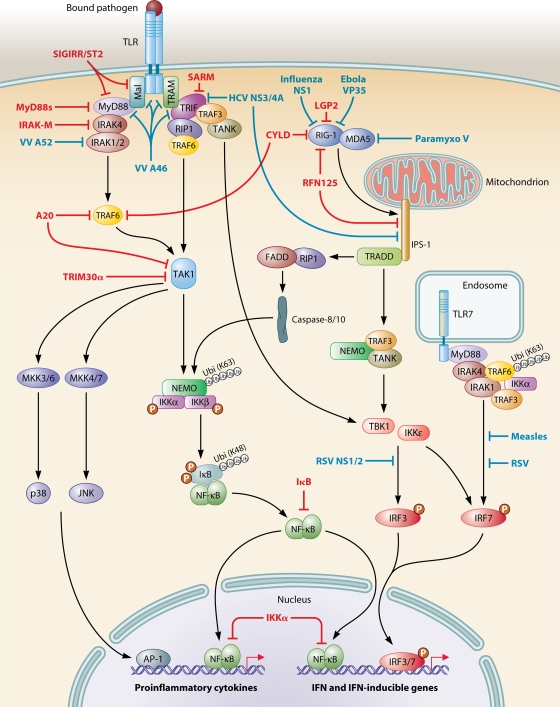
Although the generation of a potent immune response is of crucial importance for the containment and eradication of microbial infection, excessive or inappropriate inflammation may be harmful to the host and result in immunopathology or autoimmunity. The innate immune system therefore needs to be able to control inflammatory signaling during infection and, not least, to downregulate the inflammatory response once the infection has been resolved. Recent progress in understanding mechanisms of regulation of innate immunity has revealed a common theme, in which PRRs that detect microbial pathogens activate both a proinflammatory response directed against the pathogen and a specific inhibitory pathway to limit the duration and magnitude of inflammation. This implies that both activating and inhibitory signals are induced sequentially by a given PAMP during generation of the inflammatory response. Furthermore, pathogenic microbes have evolved sophisticated molecular strategies to subvert host defenses by interfering with molecules involved in pathogen recognition and signaling.
Endogenous Mechanisms
Several cellular molecules that negatively regulate PRR signaling, particularly targeting the NF-κB pathway, have been identified (Fig. 7). First, the fifth TLR adaptor molecule SARM acts as a negative regulator of TRIF-dependent signaling from TLR3 and TLR4 (51). TLR adaptor molecules are also the target of another inhibitory molecule, MyD88s, an alternatively spliced variant of MyD88, which does not bind to IRAK4 and hence does not promote IRAK1/2 phosphorylation and NF-κB activation in response to LPS (43). Similar principles are employed by TIR domain-containing orphan receptors, such as single immunoglobulin IL-1R-related molecule and ST2, which have been demonstrated to negatively regulate TLR signaling by sequestration of MyD88 and Mal (41, 377). In addition, a molecule belonging to the family of IRAKs but deficient in kinase activity, and therefore abrogating further downstream signaling to NF-κB, has been identified and termed IRAK-M (155). Importantly, IRAK-M-deficient mice secrete elevated levels of proinflammatory cytokines and are defective in induction of LPS tolerance (191). Another family of proteins with negative regulatory function is the suppressor of cytokine signaling (SOCS) proteins. These proteins, originally defined by their ability to interfere with cytokine-induced JAK-STAT signaling, are inducible by both cytokines and TLR ligands, although their action appears to be mediated indirectly by negative regulation of paracrine IFN-β signaling rather than by direct inhibition of TLR-induced signaling pathways (25, 67).
FIG. 7.
Negative regulation of PRR signaling. Signaling through PRRs is negatively regulated by endogenous and microbial proteins. Endogenous negative control of PRR signaling (molecules depicted in red) serves the purpose of mediating negative feedback on the inflammatory response, whereas microbial interference with PRRs (viral molecules shown in green) constitutes a means to evade the host antimicrobial response. See text for further details.
A key role in terminating TLR responses has been attributed to the deubiquitinating enzymes A20 and CYLD, which regulate TLR- and TNF-α-induced NF-κB activation by a mechanism involving deubiquitination of TRAF6 (38, 193, 394). Likewise, recent data suggest that A20 restricts NOD-mediated signaling to NF-κB by interfering with NOD2-induced ubiquitination of RIP2 (126). More recently, it was demonstrated that the tripartite-motif protein family member TRIM30α negatively regulates TLR4-mediated NF-κB activation by specifically inducing degradation of TAB2/3 required for TAK1 activation and function, thus preventing downstream signaling to NF-κB (331). Since TRIM30α gene expression is itself stimulated by NF-κB, this represents a negative feedback loop with potentially important implications due to the central position of TAK1 in TLR- as well as in IL-1- and TNF-α-induced signaling (331). At a level further downstream, another example of an elegant autoregulatory circuit is provided by the NF-κB-mediated induction of IκB gene expression (56). Newly synthesized IκB protein in the cytoplasm or nucleus binds to free activated NF-κB, thereby preventing NF-κB from inducing further inflammatory mediators and in this way contributing to downregulation of the inflammatory response (347). A direct role of IKKα in the degradation of the NF-κB subunits p65 and c-Rel bound to the promoter has also been suggested as a means of terminating the response and suppressing DNA-bound NF-κB (205).
Within the IFN pathway, a negative regulatory feedback circuit is provided by the ring finger protein RNF125, which induces ubiquitination and proteasomal degradation of RIG-I, MDA5, and IPS-1, resulting in termination of IFN responses (21). Since RNF125 is itself induced by IFN, this again illustrates a manner, in which the initial stimulus activating RLR signaling subsequently triggers production of a factor capable of terminating the response. Furthermore, the RNA helicase LGP2 devoid of CARDs prevents activation of antiviral responses, most likely through sequestration of dsRNA from RIG-I and by prevention of RIG-I multimerization (310, 314). In addition, CYLD inhibits the ubiquitination essential for signaling through RIG-I, and accordingly, loss of CYLD causes constitutive activation of TBK1/IKKɛ (94, 398). Finally, an important level of negative regulation in TLR-induced signaling appears to be RNA-destabilizing proteins, the absence of which leads to exaggerated inflammation in experimental animals due to overproduction of TNF-α and IL-1 as a result of impaired degradation of mRNA encoding these cytokines (219).
Viral Immune Evasion Strategies
Given the fact that mounting an inflammatory response through PRRs is a prerequisite for containment and eradication of invading pathogens, it is not surprising that most pathogens have developed mechanisms for modulating or interfering with PRR-mediated responses. Viruses are the class of pathogens that have evolved the most diverse and sophisticated molecular mechanisms for interfering with antimicrobial and proinflammatory responses. Due to the ability of viruses to exploit the cellular machinery during their replication cycle, an intricate virus-host relationship has developed throughout evolution, and this allows many types of viruses to interfere profoundly with host signaling (125) (Fig. 7). Overall, viruses can interfere in multiple ways with NF-κB and IRF pathways to inhibit induction of proinflammatory molecules and IFN. Paradoxically, however, viruses can also stimulate NF-κB activation in order to induce viral replication due to the presence of NF-κB DNA-binding sites in many viral promoters, including the human immunodeficiency virus (HIV) long terminal repeat (23, 125). Viruses with oncogenic potential can induce constitutive NF-κB activation and modulate cellular growth pathways and apoptosis, hence profoundly influencing cell cycle regulation and promoting malignant transformation, as in the case of Epstein-Barr virus (lymphoma), human herpesvirus 8 (Kaposi sarcoma), and human T-cell leukemia/lymphotropic virus type I (adult T-cell leukemia) (125).
One of the first examples of viral interference with TLR-induced signaling to be described was the identification of the vaccinia virus proteins A46R and A52R, with putative TIR domains targeting host MyD88 and TRIF and suppressing TLR- and IL-1R-induced NF-κB activation (39, 341). However, despite its sequence similarities with TIR domains, A52R appears to fold like a Bcl-2-like domain and hence is not a bona fide viral TIR family member (106). In addition, the HSV-1 immediate-early protein infected-cell protein 0 (ICP0) inhibits IRF3- and IRF7-mediated activation of IFN-stimulated genes (215). Likewise, RSV NS1/2 interferes with TBK1-mediated phosphorylation of IRF3 (339), whereas measles virus prevents phosphorylation of IRF7 (322). Moreover, as might be expected based on recent knowledge on the central role played by dsRNA-sensing systems, RLR-mediated signaling pathways are also the target of several viral components, of which the most extensively studied is the hepatitis C virus (HCV) NS3/4 serine protease. In addition to cleaving TRIF and thereby abrogating TLR3 responses to extracellular and endosomal dsRNA (212), NS3/4 also targets IPS-1 and prevents cytosolic dsRNA-induced production of type I IFN (212, 246). In this manner, HCV targets both TLR-dependent and -independent arms of the host antiviral response.
The induction of IFN-β by viral dsRNA can also be inhibited by paramyxoviruses, such as parainfluenza virus type 2, mumps virus, and Hendra virus, through association between the viral V protein and MDA5 (17). Similarly, RIG-I signaling is targeted by the influenza A virus NS1 and the Ebola virus VP35 proteins through sequestration of viral RNA and direct interaction with RIG-I/IPS-1 (49, 250, 295), the result being inhibition of downstream signaling to IRF3. Recently, a novel mechanism for prevention of RIG-I activation was described, consisting of virally induced posttranscriptional removal of 5′-triphosphate RNA from genomes of negative-strand RNA viruses, including Hantaan virus, Crimean-Congo hemorrhagic fever virus, and Borna disease virus (111). By cleavage of triphosphates from their RNA, these viruses impede RIG-I-binding and activation (268). Additionally, PKR appears to be the target of several viral proteins, including the E2 and NS5A proteins of HCV, which bind to PKR to inhibit IRF1 activation (95, 293), and the influenza A NS1 protein, which functions as an IFN antagonist in part through inhibition of PKR (213), although the precise mechanism of action is controversial (125, 250).
Finally, proinflammatory cytokine and IFN expression can also be manipulated by viral interference with the posttranscriptional machinery of the host. For instance, vesicular stomatitis virus M protein inhibits nuclear-to-cytoplasmic export of mRNAs, including those encoding IFN-α/β (124). Moreover, in studies of virus-host interactions, HSV-1 has been reported to suppress proinflammatory cytokine production by a mechanism involving destabilization of mRNA encoding proinflammatory cytokines, in a process dependent on the viral immediate-early proteins ICP4 and ICP27, thus impeding the antiviral host response to infection (260).
Bacterial, Protozoan, and Fungal Immune Evasion Strategies
Although viral evasion strategies may be the most elaborate and have been studied most extensively, other classes of pathogens have also developed several elegant evasion strategies, either by altering their PAMPs to be less immunogenic or through activation of anti-inflammatory responses induced by different virulence factors. Certain bacteria, such as Helicobacter pylori, Porphyromonas gingivalis, and L. pneumophila, synthesize modified forms of LPS, which are not recognized by TLR4 (134), and similarly, Campylobacter jejuni, H. pylori, and Bartonella bacilliformis produce subclasses of flagellin that do not activate TLR5 (16). Recently, a novel family of virulence factors was identified in Escherichia coli and Brucella melitensis (57). These proteins, which have been named TIR domain-containing proteins, impede TLR signaling through direct binding to MyD88, thus suppressing innate immunity and increasing bacterial virulence (57).
Important enteric pathogens, including Shigella, Salmonella, and Yersinia species, are endowed with a type III secretion system mediating the delivery of effector proteins into host cells, thus representing bacterial virulence factors (9). One such type III effector protein is the Yop protein of Yersinia pestis, which has been demonstrated to act as a deubiquitinating protease acting on TRAF6 and TRAF3 to inhibit NF-κB/MAPK and IRF signaling, respectively (350). Other data suggest that Yop may also act as an acetyltransferase that modifies critical residues in the activation loop of MAPK6 and IKKβ, thereby preventing their phosphorylation and further downstream signaling (267). A similar type III effector is represented by the Shigella OspG protein kinase, which appears to bind a subset of ubiquitin-conjugating enzymes, including those mediating degradation of IκB, thereby preventing nuclear translocation of NF-κB (186). Finally, another class of virulence factors is the V antigens, which in the case of Yersinia species induce an anti-inflammatory response by stimulating TLR2-mediated IL-10 production, ultimately resulting in TLR2-deficient mice being less susceptible to yersinia infection (336). Likewise, the Vi antigen of S. enterica serotype Typhi exerts an immunosuppressive effect in the intestinal mucosa that promotes bacterial dissemination and invasive disease (298). The Vi antigen is therefore considered to play a role in the differences in pathogenesis between S. enterica serotype Typhi and S. enterica serotype Typhimurium, the latter of which does not express the Vi antigen and causes only local inflammatory disease (298).
At present, much less is known about protozoan and fungal immune evasion strategies, although in particular in the case of protozoans, such strategies are likely to be of major importance for the establishment of long-term residence within their hosts (98). For instance, T. gondii induces LPS unresponsiveness mediated at least in part by activation of STAT3, a transcription factor also used by IL-10 to exert anti-inflammatory activities (45). T. gondii also negatively regulates LPS-induced MAPK activation and IL-12 production, although it remains unresolved whether the target is a p38-activating kinase or induction of a p38 MAPK phosphatase (188). Suppression of macrophage IL-12 production is also the final outcome of infection with Leishmania mexicana due to expression of amastigote-specific cysteine peptidases that proteolytically degrade IκB and NF-κB (47). Finally, a number of fungal immune evasion strategies have been described, exemplified by IL-10-mediated immunosuppression induced through TLR2 by C. albicans and A. fumigatus (272).
MEDICAL IMPLICATIONS
The pivotal role of TLRs in innate host defenses was first indicated by the finding that C3H/HeJ mice with nonfunctional TLR4 are resistant to LPS-mediated shock (296), and valuable insights into TLR-mediated pathogen recognition and signaling has since been gained from the use of knockout mice challenged with individual purified PAMPs or entire microbial pathogens. However, data from in vitro studies or mouse models may not be entirely representative of the physiological situation in the human organism with respect to understanding natural conditions of infection and immunity, primary immunodeficiencies, altered disease susceptibility, and development of autoimmunity. Therefore, it is important to translate findings from cell culture and animal studies to the human organism, where important lessons may be learned from the identification and description of associations between alterations in the function of PRRs and development of human diseases.
Primary Immunodeficiencies and Susceptibility to Infectious Diseases
To date, six Mendelian primary immunodeficiencies associated with impaired TLR signaling and NF-κB activation have been reported (52, 62, 78, 294, 376, 400) (Table 2). These genetic disorders are excessively rare, and progress has been made primarily through international collaborations directed by Casanova (196). First, anhydrotic ectodermal dysplasia with immunodeficiency (EDA-ID) is an X-linked recessive immunodeficiency caused by mutations in NEMO, a critical component of the IKK complex, and resulting in impaired NF-κB signaling (78). Affected patients display abnormalities in ectodermal development, including absent or diminished dental growth and hair, as well as various degrees of immunodeficiency. The infectious phenotype is dominated by invasive infections by encapsulated pyogenic bacteria, such as Haemophilus influenzae, S. pyogenes, and S. aureus (195). These patients can also be affected by weakly pathogenic mycobacteria, viral illnesses with herpesviruses (282), and fungal diseases, particularly P. jirovecii pneumonitis (78, 195). Laboratory investigations reveal various degrees of hyper-immunoglobulin M syndrome (152), NK cell abnormalities (282), and a poor response to LPS, IL-1β, and TNF-α (196). Moreover, a second form of EDA-ID inherited in a dominant way has been reported in a patient with a mutation in the ikba gene (62), which prevents IκBα phosphorylation and NF-κB activation.
TABLE 2.
Genetic disorders in innate immunity: associations with human diseases
| Gene producta | Disease association | Reference(s) |
|---|---|---|
| TLRs | ||
| TLR2 | Susceptibility to leprosy and tuberculosis | 33, 167 |
| TLR2 | Protection from Lyme disease; susceptibility to staphylococcal disease and tuberculosis | 217, 278, 326 |
| TLR2 | Increased severity of genital herpes | 37 |
| TLR3 | Susceptibility to HSV encephalitis | 400 |
| TLR4 | Susceptibility to infection with gram-negative bacteria, malaria, and RSV | 4, 218, 255, 360 |
| TLR4 | Susceptibility to meningococcal sepsis | 337 |
| TLR4 | Protection from Legionnaires' disease | 116 |
| TLR5 | Susceptibility to Legionnaires' disease | 117 |
| TLR7 | Protection from hepatic fibrosis in chronic HCV infection | 325 |
| TLR9 | Susceptibility to SLE | 361 |
| TLR9 | Rapid progression of HIV infection | 36 |
| TLR9 | Increased risk of low birth wt in malaria during pregnancy | 256 |
| Signaling proteins | ||
| MyD88 | Susceptibility to infection with pyogenic bacteria | 376 |
| Mal | Protection from invasive pneumococcal disease, malaria, and tuberculosis | 185 |
| IRAK1 | Increased severity of sepsis | 20 |
| IRAK4 | Susceptibility to infection with pyogenic bacteria | 294 |
| NEMO | EDA-ID; susceptibility to infection with pyogenic bacteria, mycobacteria, herpesviruses, fungi | 78, 195, 282 |
| IκBα | EDA-ID | 62 |
| IRF5 | Increased risk of SLE | 105, 183 |
| NLRs | ||
| NOD2 | Susceptibility to Crohn's disease | 144, 277 |
| NOD2 | Blau syndrome, early-onset sarcoidosis | 166 |
| NOD2 | Susceptibility to psoriatic arthritis | 299 |
| NALP1 | Susceptibility to vitiligo-associated multiple autoimmune diseases | 160 |
| NALP3 | Periodic fever syndromes | 86 |
| Miscellaneous | ||
| CD14 | Susceptibility to RSV bronchiolitis | 147 |
| UNC93B | Susceptibility to HSV encephalitis | 52 |
In cases where the same PRR gene product is listed repeatedly, each entry represents a different mutation/SNP.
The third primary immunodeficiency, involving IRAK4, is inherited as an autosomal recessive disorder (294). As previously described, IRAK4 plays an important role in signal propagation to NF-κB and MAPKs downstream of certain TLRs (294, 348). Accordingly, poor inflammatory responses and recurrent pyogenic bacterial infections, particularly with S. pneumoniae, have been reported in patients with this disorder (294). Blood cells from these patients fail to produce IL-1β, IL-6, IL-8, IL-12, and TNF-α in response to various TLR agonists (196), and fibroblasts are unable to activate both NF-κB and MAPK pathways following IL-1β stimulation (196). Given the central position of IRAK4 in TLR and IL-1R signaling, the relatively mild phenotype of IRAK4-deficient patients may seem surprising. However, a similar immunological and infectious phenotype was recently described in the case of MyD88-deficient patients, who are vulnerable to recurrent pyogenic infections early in life (376), supporting a narrow but nonredundant role of both IRAK4 and MyD88 in protective immunity against certain bacterial infections. The observation that both IRAK4- and MyD88-deficient patients are affected mostly in the neonatal and childhood period and then appear to improve with age may also suggest that maturation of TLR-independent innate immunity or T and B lymphocyte-specific adaptive responses progressively compensate for the poor innate immune response early in life.
The first immunodeficiency originating from a TLR mutation was identified in a recent report describing increased susceptibility to HSV encephalitis in patients with rare mutations in TLR3, indicating a possible role for this receptor in central nervous system infections with HSV and possibly other neurotropic viruses (400). Finally, a genetic predisposition to HSV encephalitis was identified in two children lacking functional UNC-93B (52), an ER protein required for delivering nucleotide-sensing TLRs (TLR3, -7, -8, and -9) from the ER to endosomes (190, 351).
The list of associations between PRR single-nucleotide polymorphisms (SNPs) and altered susceptibility to different pathogens is rapidly expanding; some examples are given below, and a more extensive overview is presented in Table 2. However, interpretation of the causal association between an SNP and alteration in disease susceptibility at the mechanistic level is frequently hampered by inadequate knowledge of the molecular consequences of the polymorphism, i.e., whether any given SNP results in diminished or increased inflammation.
Two SNPs in TLR2 have been linked to reduced NF-κB activation and human diseases (363). First, the Arg677Trp polymorphism has been associated with leprosy in a Korean population (167) and with susceptibility to tuberculosis in a Tunisian population (33). At the mechanistic level, this may be explained by the mutation causing reduced IL-12 levels in response to mycobacteria, thereby resulting in diminished IFN-γ-mediated Th1 responses. The second functional TLR2 variant, Arg753Gln, is overrepresented in patients with staphylococcal septic shock (217) and associated with an increased risk of developing tuberculosis (278), in agreement with TLR2 recognizing PAMPs from these two pathogens. The central position of TLR4 as the receptor for LPS of gram-negative bacteria has inspired several studies on the role of this receptor in human diseases. Two relatively frequent cosegregating polymorphisms in TLR4, Asp299Gly and Thr399Ile, have been linked to an increased risk of infection and septic shock with gram-negative bacteria as well as to an increased risk of severe malaria in African children (4, 218, 255). Furthermore, rare TLR4 mutations were associated with increased meningococcal susceptibility in a study involving a large collection of patients with meningococcal sepsis (337). In a case-control study, other TLR4 polymorphisms have been associated with resistance to Legionnaires' disease, suggesting a protective association (116). In contrast, TLR5 has a stop codon variant (TLR5 392STOP) that is associated with increased susceptibility to pneumonia caused by L. pneumophila (117).
Regarding TLR9, data from the Swiss HIV cohort have suggested that TLR9 polymorphisms may influence the clinical course of HIV type 1 infection, based on findings that two SNPs in TLR9 were associated with rapid progression of HIV type 1 infection (36). Although the cellular mechanism behind this association remains to be elucidated, a possible explanation may be that TLR9-mediated NF-κB activation enhances HIV replication by binding to HIV long terminal repeats or, alternatively, that nonspecific immunostimulation participates in CD4 T-lymphocyte depletion and exhaustion of cellular immunity (75, 83). A negative influence of excessive immunostimulation was also reported by Arcaroli et al., who identified a variant IRAK1 haplotype (Leu522Ser) associated with increased NF-κB activation and increased severity of sepsis (20). In a large epidemiological study, heterozygosity of a Mal Ser180Leu functional variant has been associated with protection from invasive pneumococcal disease, bacteremia, malaria, and tuberculosis (185). The authors investigated the functional consequences of this amino acid substitution and concluded that the Mal variant attenuated TLR2 signal transduction, thus implying that strong TLR2 activation may be disadvantageous in these common infectious diseases (185).
Collectively, the majority of studies strongly suggest that in humans as well as in mice, TLRs play an important role in inflammatory immune responses. However, genetic population studies seem to indicate that no strong selective pressure operates on TLRs, and thus it has been speculated that TLRs may be redundant and that other sensors of innate immunity can substitute for their functions (196). Alternatively, it may be that a more profound defect in pan-TLR signaling has not been identified in humans, simply because it would be incompatible with survival, thus supporting the current idea that TLRs are key sensors of invading pathogens in innate immune defenses.
Hereditary Periodic Fever Syndromes and Other Disorders of NLRs
Alterations in the function of NLR proteins can also cause human disease. Mutations in NALP3/cryopyrin or other subunits of the inflammasome have been linked to relatively rare hereditary periodic fever syndromes as a consequence of constitutive caspase activity and excessive production of IL-1 (233). Patients with the syndromes neonatal-onset multisystem inflammatory disease, familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and familiary Mediterranean fever suffer from periodic attacks of fever and inflammation (86, 233). Fever episodes seem to be triggered more or less spontaneously, possibly by subclinical viral infections or cellular stress activating the inflammasome, and the resultant IL-1 hyperproduction results in a clinical picture resembling a microbial infection (169, 233). Accordingly, some of the periodic fever syndromes have been successfully treated with the specific IL-1R antagonist Anakinra (130).
Other members of the family of NLRs have been implicated in aberrant NF-κB regulation, although mutations can result in either inhibition or activation of NF-κB. For instance, a missense point mutation in the human NOD2 gene resulting in defective NF-κB activation has been correlated to increased susceptibility to Crohn's disease (144, 277). In contrast, constitutive NF-κB activation is responsible for development of early-onset sarcoidosis and Blau syndrome, two closely related juvenile systemic granulomatosis syndromes, originating from other mutations in NOD2 (166). Finally, SNPs in NALP1 were demonstrated to contribute to the risk of vitiligo-associated multiple autoimmune diseases (160).
Systemic Autoimmune Disorders
Since nucleic acids of the host are generally inaccessible to RNA- and DNA-sensing receptors present in specific compartments, they do not trigger PRR signaling. However, under certain pathological conditions, such as incomplete clearance of apoptotic cells, host-derived nucleic acids may become available for TLRs, an event that may break tolerance and result in autoimmunity (228, 236). Despite TLR3, -7, -8, and -9 being originally identified as receptors specific for microbial nucleic acids, more recently some of these receptors have been linked to the detection of endogenous host RNA and DNA in systemic autoimmune diseases (228). Fundamental progress in the understanding of the pathogenesis of systemic autoimmune diseases was made when the need for dual engagement of the B-cell receptor (BCR) and TLRs by autoreactive B lymphocytes was described (207). These self-reactive B lymphocytes are responsible for the formation of immune complexes by producing antibodies recognizing a limited range of nuclear self-autoantigens, including DNA, histones, RNA, and RNA-binding proteins (207). Binding of the immune complex by the BCR, or by the FcγR in the case of pDCs, is followed by BCR-mediated endocytosis and delivery of the immune complex to an endosomal compartment, which allows ssRNA and CpG DNA motifs within the autoantigen to activate the cell through TLR7 and TLR9, respectively (203, 207, 241).
Based on findings that immune complexes associated with self-DNA and -RNA can activate pDCs to produce large amounts of IFN-α, coupled with clinical data revealing that IFN-producing pDCs accumulate in cutaneous systemic lupus erythematosus (SLE) lesions (84, 241), the common mechanism, by which TLRs play a role in SLE pathogenesis, is believed to be via production of IFN-α. This is supported by epidemiological studies, which have demonstrated an association between a TLR9 polymorphism and susceptibility to SLE in a Japanese population (361). Furthermore, an association between genetic variants of IRF5, which is involved in regulating IFN-α production, and SLE in multiple ethnic groups has now been established (105, 183, 358). Mechanistic insights into the process of immune complex formation and uptake, TLR activation, and type I IFN production may explain some fundamental observations on the nature of systemic autoimmune diseases, such as the association between infections and a triggering event in disease development, and the subsequent association between infection and disease flares once an autoimmune condition has manifested. However, despite recent progress in the understanding of autoimmune diseases such as SLE, the precise mechanism behind loss of peripheral tolerance remains unknown (228).
Finally, uncontrolled excessive or constitutive NF-κB activation has been associated with oncogenesis, particularly in the lymphoid system, and has been implicated in the development of multiple hematological malignancies as well as in several solid tumors. This subject is not within the scope of the present review but has been excellently reviewed by Courtois and Gilmore (61) and by Karin (171).
Therapeutic Implications
Since excessive inflammation plays an important role in the pathogenesis of many infectious diseases and autoimmune conditions as well as in the development of malignancy, targeting innate immune activation and signaling potentially holds great medical promise.
Glucocorticoids.
Glucocorticoids have been used extensively in the treatment of various medical conditions, where their potent immunosuppressive and anti-inflammatory effects may be desirable. However, whereas the use of adjunctive dexamethasone for the treatment of bacterial meningitis, at least in an adult population in the developed world, is currently well documented from large clinical trials (72, 273, 320), the use of high-dose glucocorticoids in patients with septic shock remains controversial and may even be harmful (340).
Several targets of glucocorticoids have been identified, but the molecular mechanisms behind these effects are very complex and remain incompletely understood. Since glucocorticoids are known to interfere with various signaling pathways and molecules involved in TLR signaling, it has been hypothesized that these pathways may be important targets of glucocorticoid action and hence may explain many of their effects (13, 266). A number of levels at which glucocorticoids can exert their effects have been defined, including interference with upstream signal transduction, direct interaction with the transcriptional machinery, and modulation of RNA stability (13, 266). Classically, glucocorticoids bind to the intracellular glucocorticoid receptor and subsequently translocate to the nucleus, where they bind to glucocorticoid response elements to activate expression of anti-inflammatory genes (3, 13, 258).
Another major mechanism of action of glucocorticoids is repression of proinflammatory genes, which is achieved mainly through direct protein-protein interactions between the glucocorticoid ligand-receptor complex and the transcription factors NF-κB and AP1 (266), thereby preventing interaction with the essential transcriptional coactivator CBP/p300 (266). Further upstream, glucocorticoids can inhibit TLR-induced proinflammatory signaling through MAPK, NF-κB, and IRF pathways (3, 239, 321). The glucocorticoid dexamethasone has been demonstrated to inhibit the MAPKs extracellular signal-regulated kinases 1/2, JNK, and p38 by a mechanism involving upregulation and decreased degradation of MAPK phosphatase 1 (34, 46, 103, 200, 349). Further insight into the mechanisms of glucocorticoid-mediated interference with the inflammatory response during microbial infection was provided by a study demonstrating that dexamethasone inhibits TLR signaling induced by N. meningitidis and S. pneumoniae by targeting the NF-κB pathway at several levels, including inhibition of IKK-mediated IκB phosphorylation and NF-κB DNA-binding activity as well as upregulation of IκB resynthesis (258). Additionally, dexamethasone has been reported to inhibit IRF3 and the IFN response by targeting the IKK-related kinase TBK1 (239).
Given the central position of the molecules targeted by glucocorticoids (258, 266), their potent and broad range of effects are understandable, although their use may often seem like a double-edged sword. The long list of adverse effects of glucocorticoids and their profound interference with almost any physiological system in the organism is the rationale behind the effort to identify and develop therapeutic molecules with more specific cellular targets and thus theoretically a more narrow profile of adverse effects.
NF-κB and p38 inhibitors.
Specific inhibitors of the NF-κB pathway have been the focus of interest for a number of years (388). More than a decade ago, nonsteroidal anti-inflammatory drugs, such as aspirin and sodium salicylate, were reported to prevent IκBα phosphorylation and NF-κB nuclear translocation by direct inhibition of IKK kinase activity (388, 391). Novel specific inhibitors of IKK or inhibitors of the ubiquitin-proteasome pathway that interferes with IκB degradation and NF-κB activation are being developed and tested in clinical trials (254). However, significant safety problems related to total inhibition of all NF-κB-mediated functions, including regulation of inflammation and apoptosis, remain important matters of concern and the subject of ongoing studies.
Turning to the MAPK pathway, p38 inhibitors have received much attention. Several chemically diverse compounds potently inhibit p38α/β in an ATP-competitive manner, and specific and selective p38α/β inhibitors have been demonstrated to block the production of IL-1, TNF-α, and IL-6 both in vitro and in vivo, with potential implications for the treatment of inflammatory diseases (197). A number of p38 inhibitors have reached clinical trials in humans, but adverse effects, including liver toxicity and neurological manifestations, have been reported. One of the reasons for these undesirable side effects may be cross-reactivity against other cellular kinases, implying that the development of inhibitors that are non-ATP competitive may be advantageous.
TLR agonists.
Triggering TLR activation during vaccination, either as part of the immunogen or as an adjuvant, is the most extensively explored application of TLR agonists (373). Direct TLR activation has been demonstrated in the case of the bacillus Calmette-Guérin (BCG) vaccine for the prevention of tuberculosis, in which cell wall components of BCG, including arabinogalactomannan and peptidoglycan, activate TLR2 and TLR4 in a MyD88-dependent manner (369). In contrast, an adjuvant effect of TLR activation has been demonstrated in an H. influenzae type B vaccine conjugated with a meningococcal protein (201), consistent with the activation of TLR2 by Neisseria outer membrane proteins (8, 234, 262). Furthermore, two hepatitis B virus vaccines using TLR4 agonists as adjuvants have been approved (79). One concern is whether a TLR4 agonist may mimic the effects of LPS and induce endotoxic shock (177). However, the TLR4 agonist monophosphoryl lipid A exhibits low toxicity compared to LPS and possess only weak ability to induce proinflammatory cytokines, which may be explained by its selective activation of the TRAM-TRIF pathway as opposed to the Mal-MyD88 pathway (235). Allergy and asthma, both of which are characterized by inappropriate Th2 responses to environmental allergens, have also been the targets of TLR agonist therapies. Given their ability to induce strong Th1 responses, TLR4 and TLR9 agonists are being developed for the treatment of allergic diseases, and such agonists have been administered along with an allergen (170). A successful example is the report of immunotherapy with a ragweed-TLR9 agonist vaccine for allergic rhinitis, with an observed shift in the Th2/Th1 ratio together with significant clinical benefits (64). In the treatment of infectious diseases, agonists of TLR3, -7, -8, and -9 have shown some promise, particularly against chronic viral infections, where the mechanism seem to involve production of type I IFN and generation of IFN-dependent antiviral responses (170). For example, the TLR7 agonist imiquimod has been approved for topical use against genital warts caused by human papillomavirus, whereas other TLR7 and TLR9 agonists are undergoing clinical trials for potential use during chronic infection with HCV and genital HSV infection (170). Finally, a potentially very important use of TLR agonists is in the treatment of cancer, which has been reviewed elsewhere (170, 373).
TLR antagonists.
Modulation of the inflammatory response by TLR antagonists may be of clinical benefit in order to dampen exaggerated responses observed during some infectious diseases, thus avoiding immunopathology. Indeed, this is the strategy behind the development of TLR4 antagonists to prevent LPS-induced endotoxic shock during gram-negative bacterial sepsis. Two lipid A analogues, TAK-242 and Eritoran, have been approved for clinical trials involving patients with sepsis (308), and their potential benefits are awaited with interest, since previous efforts to modulate the pathophysiology of sepsis by inhibiting TNF-α or LPS have been disappointing (107). The small molecule TAK-242 selectively inhibits TLR4-activated cytokine production by targeting the intracellular domain of the receptor, thus preventing signaling to NF-κB through both the MyD88-dependent and -independent pathways (181). Finally, TLR antagonists have recently been suggested to possess desirable properties in the treatment of autoimmune diseases, most notably SLE (170, 373). Given that TLR7 and TLR9 have been implicated in the generation of autoantibodies directed against endogenous TLR ligands (228) and to be essential for pDC-mediated production of type I IFN, (84, 241), targeting of these TLRs may theoretically prevent the development and progression of autoimmune pathophysiology.
In conclusion, as knowledge on the role of inflammation in the pathogenesis of infectious, allergic, autoimmune, and malignant conditions increases, the list of targets for therapeutic interference with TLRs or TLR signaling components expands accordingly. The vast number of preclinical and clinical approaches, of which only the most prominent examples are described above, seem to indicate that it is possible to translate the current understanding of TLRs and innate immunity into clinically relevant therapies. However, much experimental animal work, and not least clinical studies involving patients, is needed to evaluate possible advantageous effects as well as toxicity and side effects, such as the consequences of TLR inhibition in terms of enhanced susceptibility to infections or, alternatively, the consequences of TLR activation with regard to the development of malignancy. Some of these scenarios cannot be predicted at present due to our inadequate insight into the complexities and full biological perspectives of the TLR system and proinflammatory signaling in innate immune defenses. The great challenge remains to inhibit the detrimental components of the immune response while leaving untouched an appropriate and beneficial immune response essential for the resolution of infection and elimination of the invading pathogen.
CONCLUDING REMARKS AND PERSPECTIVES
In this review, important aspects of pathogen recognition and activation of signaling pathways ultimately resulting in a proinflammatory innate immune response have been presented. Rapid and efficient mechanisms for detection of invading pathogens and triggering of proinflammatory signaling are of pivotal importance for the initial containment and control of infection. To achieve this, the organism is in possession of PRRs, which recognize evolutionarily conserved structures on pathogens, PAMPs, and trigger innate immune responses, including production of proinflammatory cytokines and type I IFN (7). In addition, pathogen recognition by PRRs is a prerequisite for the activation and shaping of adaptive immune responses through maturation of antigen-presenting DCs and activation of antigen-specific T lymphocytes to resolve the infection and induce immunological memory (151). The development of the concept of pattern recognition and the identification of a large family of receptors with this ability has significantly changed the entire perception of the innate immune system, which is now recognized to have a high degree of specificity and complexity. Moreover, current insight into common principles of cell-cell interactions and intracellular signaling suggests that innate immunity and adaptive immunity are much more intimately connected than previously believed (242).
Despite enormous progress in this field of research during recent years, many areas of uncertainty remain. While TLRs represent the most extensively studied group of innate immune receptors and have been assigned essential roles in the induction of proinflammatory and antimicrobial responses, accumulating evidence has revealed that they are part of a large and diverse family of PRRs. Thus, in addition to TLRs, cytosolic PRRs with the ability to sense intracellular PAMPs also seem to be important for both immediate innate protection and bridging to the adaptive immune response (93, 174, 372). Recent insight into the structure and function of these PRRs highlights the fascinating complexity of cellular pattern recognition. During a microbial infection, several different families of PRRs are activated in a cell type- and time-dependent manner by a given pathogen, and the PRR-derived signals appear to cooperate in the generation of a proinflammatory response. The relationship between TLRs and cytosolic PRRs and the principles after which they interact during infection are important issues that remain to be further elucidated.
Although the primary function of PRRs is to sense foreign microbial material in the form of “infectious nonself,” in certain cases of “danger,” such as cellular injury or stress, PRRs stimulate inflammation in response to recognition of self molecules. These danger signals originate from host molecules with aberrant locations, structures, or interactions induced by, e.g., necrosis or oxidative damage of cells or tissues, as exemplified by uric acid, ATP, potassium, hyaluronan fragments, and HSPs (146, 236, 247, 362, 365). In this respect, HSPs, which are highly conserved immunodominant proteins with central roles in protein folding and transport, illustrate the intimate relationship between danger and pathogen recognition, which is also underscored by the fact that these proteins are shared between microbial pathogens and eukaryotes. Indeed, endogenous HSPs are upregulated and released by stressed, infected, necrotic, or malignant cells and mediate activation of APCs through TLR2 and TLR4/CD14 either directly or in association with PAMPs (22, 110, 279). Additionally, a role for HSPs as endogenous danger signals recognized by APCs independently of TLRs has been proposed (285). Recognition of endogenous danger signals is particularly evident in the case of the inflammasome, but it also seems to be a common theme for TLRs. Therefore, PRRs may be perceived as evolutionarily ancient detectors of cellular danger invoked not only by pathogens but also by other types of cellular stress. In line with this idea, it has been proposed that PRRs be regarded as sensors of both “microbe-associated molecular patterns” and “danger-associated molecular patterns” (236, 247). This concept places PRRs in a central position in the regulation of immunity and other aspects of cellular physiology. At present, the study of innate immune recognition and signaling remains an area of intensive investigation, with new ideas and concepts being proposed and challenged at an impressing pace. Entirely new and unpredicted functions of PRRs are emerging. For instance, work by Medzhitov and associates has suggested that TLRs are also involved in central aspects of cellular physiology, as demonstrated by the requirement for TLR recognition of commensal microflora in intestinal homeostasis (300).
The great versatility of PRRs is also apparent when considering the variability in the microbial PAMPs recognized by these receptors. Indeed, pathogens with quite different biochemical compositions and with entirely different life cycles are recognized by slightly different yet surprisingly similar and overlapping mechanisms (7). Likewise, the signaling pathways they activate are shared to a great extent and regulated by the same overall molecular mechanisms. In the future, it may become apparent that PRR-activated signaling is far more tightly connected to other cellular signaling pathways than is currently realized. For instance, recent studies have demonstrated that RLR signaling utilizes some of the same molecules as the TNF-α signaling pathway (251), which may seem surprising given the diverse functions mediated by these receptors. Describing these signaling pathways in more depth, including aspects concerning PRR expression and signaling in different cell types and in different anatomical locations within the organism, may hold the key to a more profound understanding of some of the central questions as to how specificity, complexity, and regulation of both innate and adaptive immunity is achieved.
Due to the potency of the inflammatory response, tight regulation to limit the magnitude and duration of this response is crucial in order to avoid the development of immunopathology. Recent progress in understanding mechanisms of regulation of innate immunity has revealed a common theme, in which PRRs detecting microbial pathogens activate both a proinflammatory response directed against the pathogen and a specific inhibitory pathway to downregulate the inflammatory response once the infection has been resolved. The emerging picture reveals intricate molecular mechanisms by which innate inflammatory immune responses are regulated by tightly connected activating and inhibitory circuits to fine-tune and modulate an appropriate response over time. Given the extreme complexity of this system, it may seem surprising that it does not fail more frequently. Moreover, many pathogens, and viruses in particular, have developed sophisticated molecular strategies to subvert host defenses by targeting individual components of PRR-mediated signaling. In this way, interactions between host and pathogen have developed throughout evolution and continue to fascinate by their great ingenuity. Importantly, the molecular mechanisms of signal transduction pathways elicited by a given pathogen and the interactions between host and pathogen serve to illustrate and reveal important aspects of how the immune system functions in defense and disease.
The importance of PRRs in regulating inflammatory responses is underscored by their involvement in a wide range of medical conditions. Primary immunodeficiencies as a consequence of impaired TLR signaling and NF-κB activation have been identified (196), and accumulating evidence also links genetic polymorphisms in PRRs to altered susceptibility to infectious diseases. In addition, genetic polymorphisms resulting in increased IFN production have recently been associated with the development of systemic autoimmune diseases, particularly SLE (228), and rare mutations in NLR components of the inflammasome resulting in constitutive IL-1 production cause hereditary inflammatory fever syndromes (233). Thus, insight into pathogen recognition and proinflammatory signaling in infection and immunity is a prerequisite for understanding the nature of primary immunodeficiencies and the pathogenesis of infectious diseases and inflammatory disorders and hence for the development of novel therapeutic intervention strategies.
Acknowledgments
I thank Søren Riis Paludan for discussions and critical reading of the manuscript.
I thank the Danish Cancer Society, the Kong Christian IX og Dronning Louises Mindelegat, the Katrine og Vigo Skovgaards Fond, the Beckett Fonden, and the Leo Pharma Research Foundation for supporting part of my work referenced in this review.
Biography
 Trine Mogensen conducted her medical studies at Aarhus University (AU), Denmark, between 1993 and 2002. During this time, she spent 1 year as a research fellow at the Cleveland Clinic Foundation in the laboratory of Professor Bryan Williams, Ph.D., studying mechanisms of double-stranded RNA-activated signaling to NF-κB. After receiving her medical degree from AU, Trine Mogensen continued research on virus-induced inflammatory signaling at the Department of Medical Microbiology and Immunology, AU, in the laboratory of Professor D.M.Sc. Per Höllsberg, and earned her Ph.D. degree in 2003. Since then, Trine Mogensen has pursued a clinical career specializing in infectious diseases at Skejby Hospital, Denmark. Her current research interest is pathogen recognition and signaling in the innate immune system, focusing on the role of Toll-like receptors in the pathogenesis of bacterial meningitis and human immunodeficiency virus infection. Trine Mogensen is the recipient of a Clinical Research Fellowship funded by The Danish Medical Research Council.
Trine Mogensen conducted her medical studies at Aarhus University (AU), Denmark, between 1993 and 2002. During this time, she spent 1 year as a research fellow at the Cleveland Clinic Foundation in the laboratory of Professor Bryan Williams, Ph.D., studying mechanisms of double-stranded RNA-activated signaling to NF-κB. After receiving her medical degree from AU, Trine Mogensen continued research on virus-induced inflammatory signaling at the Department of Medical Microbiology and Immunology, AU, in the laboratory of Professor D.M.Sc. Per Höllsberg, and earned her Ph.D. degree in 2003. Since then, Trine Mogensen has pursued a clinical career specializing in infectious diseases at Skejby Hospital, Denmark. Her current research interest is pathogen recognition and signaling in the innate immune system, focusing on the role of Toll-like receptors in the pathogenesis of bacterial meningitis and human immunodeficiency virus infection. Trine Mogensen is the recipient of a Clinical Research Fellowship funded by The Danish Medical Research Council.
REFERENCES
- 1.Abbott, D. W., A. Wilkins, J. M. Asara, and L. C. Cantley. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 14:2217-2227. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, N., D. F. Stojdl, P. I. Duncan, N. Methot, T. Ishii, M. Dube, B. C. Vanderhyden, H. L. Atkins, D. A. Gray, M. W. McBurney, A. E. Koromilas, E. G. Brown, N. Sonenberg, and J. C. Bell. 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274:5953-5962. [DOI] [PubMed] [Google Scholar]
- 3.Adcock, I. M., and G. Caramori. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79:376-384. [DOI] [PubMed] [Google Scholar]
- 4.Agnese, D. M., J. E. Calvano, S. J. Hahm, S. M. Coyle, S. A. Corbett, S. E. Calvano, and S. F. Lowry. 2002. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J. Infect. Dis. 186:1522-1525. [DOI] [PubMed] [Google Scholar]
- 5.Agostini, L., F. Martinon, K. Burns, M. F. McDermott, P. N. Hawkins, and J. Tschopp. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20:319-325. [DOI] [PubMed] [Google Scholar]
- 6.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 7.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 8.Al Bader, T., M. Christodoulides, J. E. Heckels, J. Holloway, A. E. Semper, and P. S. Friedmann. 2003. Activation of human dendritic cells is modulated by components of the outer membranes of Neisseria meningitidis. Infect. Immun. 71:5590-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albiger, B., S. Dahlberg, B. Henriques-Normark, and S. Normark. 2007. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J. Intern. Med. 261:511-528. [DOI] [PubMed] [Google Scholar]
- 10.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9:633-644. [DOI] [PubMed] [Google Scholar]
- 11.Albiger, B., L. Johansson, and A. B. Jonsson. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 13.Almawi, W. Y., and O. K. Melemedjian. 2002. Negative regulation of nuclear factor-kappaB activation and function by glucocorticoids. J. Mol. Endocrinol. 28:69-78. [DOI] [PubMed] [Google Scholar]
- 14.Almeida, I. C., and R. T. Gazzinelli. 2001. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J. Leukoc. Biol. 70:467-477. [PubMed] [Google Scholar]
- 15.Andersen-Nissen, E., T. R. Hawn, K. D. Smith, A. Nachman, A. E. Lampano, S. Uematsu, S. Akira, and A. Aderem. 2007. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178:4717-4720. [DOI] [PubMed] [Google Scholar]
- 16.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ank, N., H. West, and S. R. Paludan. 2006. IFN-lambda: novel antiviral cytokines. J. Interferon Cytokine Res. 26:373-379. [DOI] [PubMed] [Google Scholar]
- 19.Aravalli, R. N., S. Hu, T. N. Rowen, J. M. Palmquist, and J. R. Lokensgard. 2005. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189-4193. [DOI] [PubMed] [Google Scholar]
- 20.Arcaroli, J., E. Silva, J. P. Maloney, Q. He, D. Svetkauskaite, J. R. Murphy, and E. Abraham. 2006. Variant IRAK-1 haplotype is associated with increased nuclear factor-kappaB activation and worse outcomes in sepsis. Am. J. Respir. Crit. Care Med. 173:1335-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arimoto, K., H. Takahashi, T. Hishiki, H. Konishi, T. Fujita, and K. Shimotohno. 2007. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. USA 104:7500-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asea, A., S. K. Kraeft, E. A. Kurt-Jones, M. A. Stevenson, L. B. Chen, R. W. Finberg, G. C. Koo, and S. K. Calderwood. 2000. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6:435-442. [DOI] [PubMed] [Google Scholar]
- 23.Bachelerie, F., J. Alcami, F. Arenzana-Seisdedos, and J. L. Virelizier. 1991. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature 350:709-712. [DOI] [PubMed] [Google Scholar]
- 24.Backhed, F., S. Normark, E. K. Schweda, S. Oscarson, and A. Richter-Dahlfors. 2003. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes. Infect. 5:1057-1063. [DOI] [PubMed] [Google Scholar]
- 25.Baetz, A., M. Frey, K. Heeg, and A. H. Dalpke. 2004. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J. Biol. Chem. 279:54708-54715. [DOI] [PubMed] [Google Scholar]
- 26.Bafica, A., C. A. Scanga, C. G. Feng, C. Leifer, A. Cheever, and A. Sher. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balachandran, S., E. Thomas, and G. N. Barber. 2004. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432:401-405. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee, A., R. Gugasyan, M. McMahon, and S. Gerondakis. 2006. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc. Natl. Acad. Sci. USA 103:3274-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreau, C., L. Paillard, and H. B. Osborne. 2005. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 33:7138-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton, G. M., J. C. Kagan, and R. Medzhitov. 2006. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 7:49-56. [DOI] [PubMed] [Google Scholar]
- 31.Basset, C., J. Holton, R. O'Mahony, and I. Roitt. 2003. Innate immunity and pathogen-host interaction. Vaccine 21(Suppl. 2):S12-S23. [DOI] [PubMed] [Google Scholar]
- 32.Beg, A. A. 2002. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23:509-512. [DOI] [PubMed] [Google Scholar]
- 33.Ben Ali, M., M. R. Barbouche, S. Bousnina, A. Chabbou, and K. Dellagi. 2004. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab Immunol. 11:625-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharyya, S., D. E. Brown, J. A. Brewer, S. K. Vogt, and L. J. Muglia. 2007. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109:4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bochud, P. Y., M. Hersberger, P. Taffe, M. Bochud, C. M. Stein, S. D. Rodrigues, T. Calandra, P. Francioli, A. Telenti, R. F. Speck, and A. Aderem. 2007. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS 21:441-446. [DOI] [PubMed] [Google Scholar]
- 37.Bochud, P. Y., A. S. Magaret, D. M. Koelle, A. Aderem, and A. Wald. 2007. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus type 2 infection. J. Infect. Dis. 196:505-509. [DOI] [PubMed] [Google Scholar]
- 38.Boone, D. L., E. E. Turer, E. G. Lee, R. C. Ahmad, M. T. Wheeler, C. Tsui, P. Hurley, M. Chien, S. Chai, O. Hitotsumatsu, E. McNally, C. Pickart, and A. Ma. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5:1052-1060. [DOI] [PubMed] [Google Scholar]
- 39.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Speelman, S. Florquin, and P. T. van der. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brint, E. K., D. Xu, H. Liu, A. Dunne, A. N. McKenzie, L. A. O'Neill, and F. Y. Liew. 2004. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5:373-379. [DOI] [PubMed] [Google Scholar]
- 42.Brown, G. D., P. R. Taylor, D. M. Reid, J. A. Willment, D. L. Williams, L. Martinez-Pomares, S. Y. Wong, and S. Gordon. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns, K., S. Janssens, B. Brissoni, N. Olivos, R. Beyaert, and J. Tschopp. 2003. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns, K., F. Martinon, C. Esslinger, H. Pahl, P. Schneider, J. L. Bodmer, F. Di Marco, L. French, and J. Tschopp. 1998. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273:12203-12209. [DOI] [PubMed] [Google Scholar]
- 45.Butcher, B. A., L. Kim, P. F. Johnson, and E. Y. Denkers. 2001. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J. Immunol. 167:2193-2201. [DOI] [PubMed] [Google Scholar]
- 46.Caelles, C., J. M. Gonzalez-Sancho, and A. Munoz. 1997. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 11:3351-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron, P., A. McGachy, M. Anderson, A. Paul, G. H. Coombs, J. C. Mottram, J. Alexander, and R. Plevin. 2004. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. J. Immunol. 173:3297-3304. [DOI] [PubMed] [Google Scholar]
- 48.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardenas, W. B., Y. M. Loo, M. Gale, Jr., A. L. Hartman, C. R. Kimberlin, L. Martinez-Sobrido, E. O. Saphire, and C. F. Basler. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpick, B. W., V. Graziano, D. Schneider, R. K. Maitra, X. Lee, and B. R. Williams. 1997. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 272:9510-9516. [DOI] [PubMed] [Google Scholar]
- 51.Carty, M., R. Goodbody, M. Schroder, J. Stack, P. N. Moynagh, and A. G. Bowie. 2006. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7:1074-1081. [DOI] [PubMed] [Google Scholar]
- 52.Casrouge, A., S. Y. Zhang, C. Eidenschenk, E. Jouanguy, A. Puel, K. Yang, A. Alcais, C. Picard, N. Mahfoufi, N. Nicolas, L. Lorenzo, S. Plancoulaine, B. Senechal, F. Geissmann, K. Tabeta, K. Hoebe, X. Du, R. L. Miller, B. Heron, C. Mignot, T. B. de Villemeur, P. Lebon, O. Dulac, F. Rozenberg, B. Beutler, M. Tardieu, L. Abel, and J. L. Casanova. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308-312. [DOI] [PubMed] [Google Scholar]
- 53.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 54.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 55.Charrel-Dennis, M., E. Latz, K. A. Halmen, P. Trieu-Cuot, K. A. Fitzgerald, D. L. Kasper, and D. T. Golenbock. 2008. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe 4:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiao, P. J., S. Miyamoto, and I. M. Verma. 1994. Autoregulation of I kappa B alpha activity. Proc. Natl. Acad. Sci. USA 91:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cirl, C., A. Wieser, M. Yadav, S. Duerr, S. Schubert, H. Fischer, D. Stappert, N. Wantia, N. Rodriguez, H. Wagner, C. Svanborg, and T. Miethke. 2008. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14:399-406. [DOI] [PubMed] [Google Scholar]
- 58.Coban, C., K. J. Ishii, T. Kawai, H. Hemmi, S. Sato, S. Uematsu, M. Yamamoto, O. Takeuchi, S. Itagaki, N. Kumar, T. Horii, and S. Akira. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 60.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courtois, G., and T. D. Gilmore. 2006. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25:6831-6843. [DOI] [PubMed] [Google Scholar]
- 62.Courtois, G., A. Smahi, J. Reichenbach, R. Doffinger, C. Cancrini, M. Bonnet, A. Puel, C. Chable-Bessia, S. Yamaoka, J. Feinberg, S. Dupuis-Girod, C. Bodemer, S. Livadiotti, F. Novelli, P. Rossi, A. Fischer, A. Israel, A. Munnich, F. Le Deist, and J. L. Casanova. 2003. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J. Clin. Investig. 112:1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Couzinet, A., K. Tamura, H. M. Chen, K. Nishimura, Z. Wang, Y. Morishita, K. Takeda, H. Yagita, H. Yanai, T. Taniguchi, and T. Tamura. 2008. A cell-type-specific requirement for IFN regulatory factor 5 (IRF5) in Fas-induced apoptosis. Proc. Natl. Acad. Sci. USA 105:2556-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Creticos, P. S., J. T. Schroeder, R. G. Hamilton, S. L. Balcer-Whaley, A. P. Khattignavong, R. Lindblad, H. Li, R. Coffman, V. Seyfert, J. J. Eiden, and D. Broide. 2006. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 355:1445-1455. [DOI] [PubMed] [Google Scholar]
- 65.Cui, S., K. Eisenacher, A. Kirchhofer, K. Brzozka, A. Lammens, K. Lammens, T. Fujita, K. K. Conzelmann, A. Krug, and K. P. Hopfner. 2008. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 29:169-179. [DOI] [PubMed] [Google Scholar]
- 66.Cusson-Hermance, N., S. Khurana, T. H. Lee, K. A. Fitzgerald, and M. A. Kelliher. 2005. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-κB activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280:36560-36566. [DOI] [PubMed] [Google Scholar]
- 67.Dalpke, A. H., S. Opper, S. Zimmermann, and K. Heeg. 2001. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 166:7082-7089. [DOI] [PubMed] [Google Scholar]
- 68.Deb, A., S. J. Haque, T. Mogensen, R. H. Silverman, and B. R. Williams. 2001. RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. J. Immunol. 166:6170-6180. [DOI] [PubMed] [Google Scholar]
- 69.Debierre-Grockiego, F., N. Azzouz, J. Schmidt, J. F. Dubremetz, H. Geyer, R. Geyer, R. Weingart, R. R. Schmidt, and R. T. Schwarz. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 278:32987-32993. [DOI] [PubMed] [Google Scholar]
- 70.Debierre-Grockiego, F., M. A. Campos, N. Azzouz, J. Schmidt, U. Bieker, M. G. Resende, D. S. Mansur, R. Weingart, R. R. Schmidt, D. T. Golenbock, R. T. Gazzinelli, and R. T. Schwarz. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179:1129-1137. [DOI] [PubMed] [Google Scholar]
- 71.Decker, T., M. Muller, and S. Stockinger. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5:675-687. [DOI] [PubMed] [Google Scholar]
- 72.de Gans, J., D. van de Beek, and European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549-1556. [DOI] [PubMed] [Google Scholar]
- 73.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Derdeyn, C. A., and G. Silvestri. 2005. Viral and host factors in the pathogenesis of HIV infection. Curr. Opin. Immunol. 17:366-373. [DOI] [PubMed] [Google Scholar]
- 76.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 77.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doffinger, R., A. Smahi, C. Bessia, F. Geissmann, J. Feinberg, A. Durandy, C. Bodemer, S. Kenwrick, S. Dupuis-Girod, S. Blanche, P. Wood, S. H. Rabia, D. J. Headon, P. A. Overbeek, F. Le Deist, S. M. Holland, K. Belani, D. S. Kumararatne, A. Fischer, R. Shapiro, M. E. Conley, E. Reimund, H. Kalhoff, M. Abinun, A. Munnich, A. Israel, G. Courtois, and J. L. Casanova. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat. Genet. 27:277-285. [DOI] [PubMed] [Google Scholar]
- 79.Dupont, J., J. Altclas, A. Lepetic, M. Lombardo, V. Vazquez, C. Salgueira, M. Seigelchifer, N. Arndtz, E. Antunez, K. von Eschen, and Z. Janowicz. 2006. A controlled clinical trial comparing the safety and immunogenicity of a new adjuvanted hepatitis B vaccine with a standard hepatitis B vaccine. Vaccine 24:7167-7174. [DOI] [PubMed] [Google Scholar]
- 80.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 81.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 82.Emonts, M., J. A. Hazelzet, R. de Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3:565-577. [DOI] [PubMed] [Google Scholar]
- 83.Equils, O., M. L. Schito, H. Karahashi, Z. Madak, A. Yarali, K. S. Michelsen, A. Sher, and M. Arditi. 2003. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170:5159-5164. [DOI] [PubMed] [Google Scholar]
- 84.Farkas, L., K. Beiske, F. Lund-Johansen, P. Brandtzaeg, and F. L. Jahnsen. 2001. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faustin, B., L. Lartigue, J. M. Bruey, F. Luciano, E. Sergienko, B. Bailly-Maitre, N. Volkmann, D. Hanein, I. Rouiller, and J. C. Reed. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25:713-724. [DOI] [PubMed] [Google Scholar]
- 86.Feldmann, J., A. M. Prieur, P. Quartier, P. Berquin, S. Certain, E. Cortis, D. Teillac-Hamel, A. Fischer, and B. G. de Saint. 2002. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71:198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fenger-Gron, M., C. Fillman, B. Norrild, and J. Lykke-Andersen. 2005. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20:905-915. [DOI] [PubMed] [Google Scholar]
- 88.Fisette, P. L., S. Ram, J. M. Andersen, W. Guo, and R. R. Ingalls. 2003. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 278:46252-46260. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 90.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- 91.Fitzgerald, K. A., D. C. Rowe, B. J. Barnes, D. R. Caffrey, A. Visintin, E. Latz, B. Monks, P. M. Pitha, and D. T. Golenbock. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frevel, M. A., T. Bakheet, A. M. Silva, J. G. Hissong, K. S. Khabar, and B. R. Williams. 2003. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23:425-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritz, J. H., L. Le Bourhis, G. Sellge, J. G. Magalhaes, H. Fsihi, T. A. Kufer, C. Collins, J. Viala, R. L. Ferrero, S. E. Girardin, and D. J. Philpott. 2007. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 26:445-459. [DOI] [PubMed] [Google Scholar]
- 94.Gack, M. U., Y. C. Shin, C. H. Joo, T. Urano, C. Liang, L. Sun, O. Takeuchi, S. Akira, Z. Chen, S. Inoue, and J. U. Jung. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916-920. [DOI] [PubMed] [Google Scholar]
- 95.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gazzinelli, R. T., and E. Y. Denkers. 2006. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat. Rev. Immunol. 6:895-906. [DOI] [PubMed] [Google Scholar]
- 99.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 100.Gil, J., M. A. Garcia, P. Gomez-Puertas, S. Guerra, J. Rullas, H. Nakano, J. Alcami, and M. Esteban. 2004. TRAF family proteins link PKR with NF-kappa B activation. Mol. Cell. Biol. 24:4502-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 102.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez, M. V., J. M. Gonzalez-Sancho, C. Caelles, A. Munoz, and B. Jimenez. 1999. Hormone-activated nuclear receptors inhibit the stimulation of the JNK and ERK signalling pathways in endothelial cells. FEBS Lett. 459:272-276. [DOI] [PubMed] [Google Scholar]
- 104.Goodridge, H. S., R. M. Simmons, and D. M. Underhill. 2007. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178:3107-3115. [DOI] [PubMed] [Google Scholar]
- 105.Graham, R. R., S. V. Kozyrev, E. C. Baechler, M. V. Reddy, R. M. Plenge, J. W. Bauer, W. A. Ortmann, T. Koeuth, M. F. Gonzalez Escribano, B. Pons-Estel, M. Petri, M. Daly, P. K. Gregersen, J. Martin, D. Altshuler, T. W. Behrens, and M. E. Alarcon-Riquelme. 2006. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38:550-555. [DOI] [PubMed] [Google Scholar]
- 106.Graham, S. C., M. W. Bahar, S. Cooray, R. A. Chen, D. M. Whalen, N. G. Abrescia, D. Alderton, R. J. Owens, D. I. Stuart, G. L. Smith, and J. M. Grimes. 2008. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS. Pathog. 4:e1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grau, G. E., and D. N. Maennel. 1997. TNF inhibition and sepsis—sounding a cautionary note. Nat. Med. 3:1193-1195. [DOI] [PubMed] [Google Scholar]
- 108.Guo, B., and G. Cheng. 2007. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J. Biol. Chem. 282:11817-11826. [DOI] [PubMed] [Google Scholar]
- 109.Gusella, G. L., T. Musso, S. E. Rottschafer, K. Pulkki, and L. Varesio. 1995. Potential requirement of a functional double-stranded RNA-dependent protein kinase (PKR) for the tumoricidal activation of macrophages by lipopolysaccharide or IFN-alpha beta, but not IFN-gamma. J. Immunol. 154:345-354. [PubMed] [Google Scholar]
- 110.Habich, C., K. Kempe, Z. R. van der, R. Rumenapf, H. Akiyama, H. Kolb, and V. Burkart. 2005. Heat shock protein 60: specific binding of lipopolysaccharide. J. Immunol. 174:1298-1305. [DOI] [PubMed] [Google Scholar]
- 111.Habjan, M., I. Andersson, J. Klingstrom, M. Schumann, A. Martin, P. Zimmermann, V. Wagner, A. Pichlmair, U. Schneider, E. Muhlberger, A. Mirazimi, and F. Weber. 2008. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS One 3:e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hacker, H., and M. Karin. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 113.Hacker, H., V. Redecke, B. Blagoev, I. Kratchmarova, L. C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Hacker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204-207. [DOI] [PubMed] [Google Scholar]
- 114.Han, J., T. Brown, and B. Beutler. 1990. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J. Exp. Med. 171:465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawn, T. R., W. R. Berrington, I. A. Smith, S. Uematsu, S. Akira, A. Aderem, K. D. Smith, and S. J. Skerrett. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 179:6981-6987. [DOI] [PubMed] [Google Scholar]
- 116.Hawn, T. R., A. Verbon, M. Janer, L. P. Zhao, B. Beutler, and A. Aderem. 2005. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires' disease. Proc. Natl. Acad. Sci. USA 102:2487-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J. Exp. Med. 198:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 119.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 120.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 121.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 122.Hemmi, H., O. Takeuchi, S. Sato, M. Yamamoto, T. Kaisho, H. Sanjo, T. Kawai, K. Hoshino, K. Takeda, and S. Akira. 2004. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169:3970-3977. [DOI] [PubMed] [Google Scholar]
- 124.Her, L. S., E. Lund, and J. E. Dahlberg. 1997. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 276:1845-1848. [DOI] [PubMed] [Google Scholar]
- 125.Hiscott, J., T. L. Nguyen, M. Arguello, P. Nakhaei, and S. Paz. 2006. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 25:6844-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hitotsumatsu, O., R. C. Ahmad, R. Tavares, M. Wang, D. Philpott, E. E. Turer, B. L. Lee, N. Shiffin, R. Advincula, B. A. Malynn, C. Werts, and A. Ma. 2008. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 28:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 129.Hoebe, K., E. M. Janssen, S. O. Kim, L. Alexopoulou, R. A. Flavell, J. Han, and B. Beutler. 2003. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat. Immunol. 4:1223-1229. [DOI] [PubMed] [Google Scholar]
- 130.Hoffman, H. M., S. Rosengren, D. L. Boyle, J. Y. Cho, J. Nayar, J. L. Mueller, J. P. Anderson, A. A. Wanderer, and G. S. Firestein. 2004. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364:1779-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W. C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 101:15416-15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 134.Hornef, M. W., M. J. Wick, M. Rhen, and S. Normark. 2002. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 3:1033-1040. [DOI] [PubMed] [Google Scholar]
- 135.Horng, T., G. M. Barton, R. A. Flavell, and R. Medzhitov. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329-333. [DOI] [PubMed] [Google Scholar]
- 136.Hornung, V., A. Ablasser, M. Charrel-Dennis, F. Bauernfeind, G. Horvath, D. R. Caffrey, E. Latz, and K. A. Fitzgerald. 21 January 2009, posting date. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed]
- 137.Hoshino, K., T. Kaisho, T. Iwabe, O. Takeuchi, and S. Akira. 2002. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14:1225-1231. [DOI] [PubMed] [Google Scholar]
- 138.Hoshino, K., T. Sugiyama, M. Matsumoto, T. Tanaka, M. Saito, H. Hemmi, O. Ohara, S. Akira, and T. Kaisho. 2006. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440:949-953. [DOI] [PubMed] [Google Scholar]
- 139.Houseley, J., J. LaCava, and D. Tollervey. 2006. RNA-quality control by the exosome. Nat. Rev. Mol. Cell Biol. 7:529-539. [DOI] [PubMed] [Google Scholar]
- 140.Hsu, L. C., S. R. Ali, S. McGillivray, P. H. Tseng, S. Mariathasan, E. W. Humke, L. Eckmann, J. J. Powell, V. Nizet, V. M. Dixit, and M. Karin. 2008. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA 105:7803-7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 142.Huang, Q., J. Yang, Y. Lin, C. Walker, J. Cheng, Z. G. Liu, and B. Su. 2004. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol. 5:98-103. [DOI] [PubMed] [Google Scholar]
- 143.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 144.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 145.Hutvagner, G., and P. D. Zamore. 2002. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297:2056-2060. [DOI] [PubMed] [Google Scholar]
- 146.Imai, Y., K. Kuba, G. G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, M. Ermolaeva, R. Veldhuizen, Y. H. Leung, H. Wang, H. Liu, Y. Sun, M. Pasparakis, M. Kopf, C. Mech, S. Bavari, J. S. Peiris, A. S. Slutsky, S. Akira, M. Hultqvist, R. Holmdahl, J. Nicholls, C. Jiang, C. J. Binder, and J. M. Penninger. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Inoue, Y., N. Shimojo, Y. Suzuki, E. J. Campos Alberto, A. Yamaide, S. Suzuki, T. Arima, T. Matsuura, M. Tomiita, M. Aoyagi, A. Hoshioka, A. Honda, A. Hata, and Y. Kohno. 2007. CD14-550 C/T, which is related to the serum level of soluble CD14, is associated with the development of respiratory syncytial virus bronchiolitis in the Japanese population. J. Infect. Dis. 195:1618-1624. [DOI] [PubMed] [Google Scholar]
- 148.Isaacs, A., and J. Lindemann. 1957. Virus interference I. The interferon. Proc. R. Soc. London B 147:258-267. [DOI] [PubMed] [Google Scholar]
- 149.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 150.Ishikawa, H., and G. N. Barber. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 152.Jain, A., C. A. Ma, S. Liu, M. Brown, J. Cohen, and W. Strober. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat. Immunol. 2:223-228. [DOI] [PubMed] [Google Scholar]
- 153.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp. Quant. Biol. 54:1-13. [DOI] [PubMed] [Google Scholar]
- 154.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 155.Janssens, S., and R. Beyaert. 2003. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11:293-302. [DOI] [PubMed] [Google Scholar]
- 156.Jensen, K. E., A. L. Neal, R. E. Owens, and J. Warren. 1963. Interferon responses of chick embryo fibroblasts to nucleic acids and related compounds. Nature 200:433-434. [DOI] [PubMed] [Google Scholar]
- 157.Jerala, R. 2007. Structural biology of the LPS recognition. Int. J. Med. Microbiol. 297:353-363. [DOI] [PubMed] [Google Scholar]
- 158.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. Williams, and X. Li. 2003. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278:16713-16719. [DOI] [PubMed] [Google Scholar]
- 159.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S. G. Paik, H. Lee, and J. O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- 160.Jin, Y., C. M. Mailloux, K. Gowan, S. L. Riccardi, G. LaBerge, D. C. Bennett, P. R. Fain, and R. A. Spritz. 2007. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356:1216-1225. [DOI] [PubMed] [Google Scholar]
- 161.Jouault, T., S. Ibata-Ombetta, O. Takeuchi, P. A. Trinel, P. Sacchetti, P. Lefebvre, S. Akira, and D. Poulain. 2003. Candida albicans phospholipomannan is sensed through Toll-like receptors. J. Infect. Dis. 188:165-172. [DOI] [PubMed] [Google Scholar]
- 162.Kagan, J. C., T. Su, T. Horng, A. Chow, S. Akira, and R. Medzhitov. 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 9:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kalali, B. N., G. Kollisch, J. Mages, T. Muller, S. Bauer, H. Wagner, J. Ring, R. Lang, M. Mempel, and M. Ollert. 2008. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 181:2694-2704. [DOI] [PubMed] [Google Scholar]
- 164.Kanakaraj, P., P. H. Schafer, D. E. Cavender, Y. Wu, K. Ngo, P. F. Grealish, S. A. Wadsworth, P. A. Peterson, J. J. Siekierka, C. A. Harris, and W. P. Fung-Leung. 1998. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J. Exp. Med. 187:2073-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kanayama, A., R. B. Seth, L. Sun, C. K. Ea, M. Hong, A. Shaito, Y. H. Chiu, L. Deng, and Z. J. Chen. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 15:535-548. [DOI] [PubMed] [Google Scholar]
- 166.Kanazawa, N., I. Okafuji, N. Kambe, R. Nishikomori, M. Nakata-Hizume, S. Nagai, A. Fuji, T. Yuasa, A. Manki, Y. Sakurai, M. Nakajima, H. Kobayashi, I. Fujiwara, H. Tsutsumi, A. Utani, C. Nishigori, T. Heike, T. Nakahata, and Y. Miyachi. 2005. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105:1195-1197. [DOI] [PubMed] [Google Scholar]
- 167.Kang, T. J., and G. T. Chae. 2001. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 31:53-58. [DOI] [PubMed] [Google Scholar]
- 168.Kanneganti, T. D., M. Body-Malapel, A. Amer, J. H. Park, J. Whitfield, L. Franchi, Z. F. Taraporewala, D. Miller, J. T. Patton, N. Inohara, and G. Nunez. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560-36568. [DOI] [PubMed] [Google Scholar]
- 169.Kanneganti, T. D., M. Lamkanfi, and G. Nunez. 2007. Intracellular NOD-like receptors in host defense and disease. Immunity. 27:549-559. [DOI] [PubMed] [Google Scholar]
- 170.Kanzler, H., F. J. Barrat, E. M. Hessel, and R. L. Coffman. 2007. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 13:552-559. [DOI] [PubMed] [Google Scholar]
- 171.Karin, M. 2006. Nuclear factor-kappaB in cancer development and progression. Nature 441:431-436. [DOI] [PubMed] [Google Scholar]
- 172.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 173.Kato, H., O. Takeuchi, E. Mikamo-Satoh, R. Hirai, T. Kawai, K. Matsushita, A. Hiiragi, T. S. Dermody, T. Fujita, and S. Akira. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 175.Kawagoe, T., S. Sato, K. Matsushita, H. Kato, K. Matsui, Y. Kumagai, T. Saitoh, T. Kawai, O. Takeuchi, and S. Akira. 2008. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 9:684-691. [DOI] [PubMed] [Google Scholar]
- 176.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 177.Kawai, T., and S. Akira. 2007. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 13:460-469. [DOI] [PubMed] [Google Scholar]
- 178.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 179.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 180.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 181.Kawamoto, T., M. Ii, T. Kitazaki, Y. Iizawa, and H. Kimura. 2008. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 584:40-48. [DOI] [PubMed] [Google Scholar]
- 182.Keating, S. E., G. M. Maloney, E. M. Moran, and A. G. Bowie. 2007. IRAK-2 participates in multiple Toll-like receptor signaling pathways to NFkappaB via activation of TRAF6 ubiquitination. J. Biol. Chem. 282:33435-33443. [DOI] [PubMed] [Google Scholar]
- 183.Kelly, J. A., J. M. Kelley, K. M. Kaufman, J. Kilpatrick, G. R. Bruner, J. T. Merrill, J. A. James, S. G. Frank, E. Reams, E. E. Brown, A. W. Gibson, M. C. Marion, C. D. Langefeld, Q. Z. Li, D. R. Karp, E. K. Wakeland, M. Petri, R. Ramsey-Goldman, J. D. Reveille, L. M. Vila, G. S. Alarcon, R. P. Kimberly, J. B. Harley, and J. C. Edberg. 2008. Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes Immun. 9:187-194. [DOI] [PubMed] [Google Scholar]
- 184.Khabar, K. S. 2007. Rapid transit in the immune cells: the role of mRNA turnover regulation. J. Leukoc. Biol. 81:1335-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khor, C. C., S. J. Chapman, F. O. Vannberg, A. Dunne, C. Murphy, E. Y. Ling, A. J. Frodsham, A. J. Walley, O. Kyrieleis, A. Khan, C. Aucan, S. Segal, C. E. Moore, K. Knox, S. J. Campbell, C. Lienhardt, A. Scott, P. Aaby, O. Y. Sow, R. T. Grignani, J. Sillah, G. Sirugo, N. Peshu, T. N. Williams, K. Maitland, R. J. Davies, D. P. Kwiatkowski, N. P. Day, D. Yala, D. W. Crook, K. Marsh, J. A. Berkley, L. A. O'Neill, and A. V. Hill. 2007. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat. Genet. 39:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim, D. W., G. Lenzen, A. L. Page, P. Legrain, P. J. Sansonetti, and C. Parsot. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 102:14046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim, H. M., B. S. Park, J. I. Kim, S. E. Kim, J. Lee, S. C. Oh, P. Enkhbayar, N. Matsushima, H. Lee, O. J. Yoo, and J. O. Lee. 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906-917. [DOI] [PubMed] [Google Scholar]
- 188.Kim, L., L. Del Rio, B. A. Butcher, T. H. Mogensen, S. R. Paludan, R. A. Flavell, and E. Y. Denkers. 2005. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 174:4178-4184. [DOI] [PubMed] [Google Scholar]
- 189.Kim, Y., P. Zhou, L. Qian, J. Z. Chuang, J. Lee, C. Li, C. Iadecola, C. Nathan, and A. Ding. 2007. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J. Exp. Med. 204:2063-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim, Y. M., M. M. Brinkmann, M. E. Paquet, and H. L. Ploegh. 2008. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:234-238. [DOI] [PubMed] [Google Scholar]
- 191.Kobayashi, K., L. D. Hernandez, J. E. Galan, C. A. Janeway, Jr., R. Medzhitov, and R. A. Flavell. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191-202. [DOI] [PubMed] [Google Scholar]
- 192.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 193.Kovalenko, A., C. Chable-Bessia, G. Cantarella, A. Israel, D. Wallach, and G. Courtois. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424:801-805. [DOI] [PubMed] [Google Scholar]
- 194.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 195.Ku, C. L., S. Dupuis-Girod, A. M. Dittrich, J. Bustamante, O. F. Santos, I. Schulze, Y. Bertrand, G. Couly, C. Bodemer, X. Bossuyt, C. Picard, and J. L. Casanova. 2005. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics 115:e615-e619. [DOI] [PubMed] [Google Scholar]
- 196.Ku, C. L., K. Yang, J. Bustamante, A. Puel, H. von Bernuth, O. F. Santos, T. Lawrence, H. H. Chang, H. Al Mousa, C. Picard, and J. L. Casanova. 2005. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol. Rev. 203:10-20. [DOI] [PubMed] [Google Scholar]
- 197.Kumar, S., J. Boehm, and J. C. Lee. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2:717-726. [DOI] [PubMed] [Google Scholar]
- 198.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 200.Lasa, M., M. Brook, J. Saklatvala, and A. R. Clark. 2001. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 21:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Latz, E., J. Franko, D. T. Golenbock, and J. R. Schreiber. 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol. 172:2431-2438. [DOI] [PubMed] [Google Scholar]
- 202.Latz, E., A. Verma, A. Visintin, M. Gong, C. M. Sirois, D. C. Klein, B. G. Monks, C. J. McKnight, M. S. Lamphier, W. P. Duprex, T. Espevik, and D. T. Golenbock. 2007. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 8:772-779. [DOI] [PubMed] [Google Scholar]
- 203.Lau, C. M., C. Broughton, A. S. Tabor, S. Akira, R. A. Flavell, M. J. Mamula, S. R. Christensen, M. J. Shlomchik, G. A. Viglianti, I. R. Rifkin, and A. Marshak-Rothstein. 2005. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Laudanna, C., J. Y. Kim, G. Constantin, and E. Butcher. 2002. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186:37-46. [DOI] [PubMed] [Google Scholar]
- 205.Lawrence, T., M. Bebien, G. Y. Liu, V. Nizet, and M. Karin. 2005. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434:1138-1143. [DOI] [PubMed] [Google Scholar]
- 206.Reference deleted.
- 207.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- 208.Leber, J. H., G. T. Crimmins, S. Raghavan, N. P. Meyer-Morse, J. S. Cox, and D. A. Portnoy. 2008. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461-470. [DOI] [PubMed] [Google Scholar]
- 209a.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 210.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kappaB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 211.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 212.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102:2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13-21. [DOI] [PubMed] [Google Scholar]
- 214.Li, S., A. Strelow, E. J. Fontana, and H. Wesche. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 99:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu, L., I. Botos, Y. Wang, J. N. Leonard, J. Shiloach, D. M. Segal, and D. R. Davies. 2008. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science 320:379-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lorenz, E., J. P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68:6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lorenz, E., J. P. Mira, K. L. Frees, and D. A. Schwartz. 2002. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch. Intern. Med. 162:1028-1032. [DOI] [PubMed] [Google Scholar]
- 219.Lu, J. Y., N. Sadri, and R. J. Schneider. 2006. Endotoxic shock in AUF1 knockout mice mediated by failure to degrade proinflammatory cytokine mRNAs. Genes Dev. 20:3174-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Malathi, K., B. Dong, M. Gale, Jr., and R. H. Silverman. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 224.Mancuso, G., A. Midiri, C. Beninati, C. Biondo, R. Galbo, S. Akira, P. Henneke, D. Golenbock, and G. Teti. 2004. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J. Immunol. 172:6324-6329. [DOI] [PubMed] [Google Scholar]
- 225.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 226.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 227.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marshak-Rothstein, A. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6:823-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Martinez, J., X. Huang, and Y. Yang. 2008. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol. 180:1592-1597. [DOI] [PubMed] [Google Scholar]
- 230.Martinon, F., L. Agostini, E. Meylan, and J. Tschopp. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14:1929-1934. [DOI] [PubMed] [Google Scholar]
- 231.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10:417-426. [DOI] [PubMed] [Google Scholar]
- 232.Martinon, F., V. Petrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237-241. [DOI] [PubMed] [Google Scholar]
- 233.Martinon, F., and J. Tschopp. 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117:561-574. [DOI] [PubMed] [Google Scholar]
- 234.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 235.Mata-Haro, V., C. Cekic, M. Martin, P. M. Chilton, C. R. Casella, and T. C. Mitchell. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628-1632. [DOI] [PubMed] [Google Scholar]
- 236.Matzinger, P. 2002. The danger model: a renewed sense of self. Science 296:301-305. [DOI] [PubMed] [Google Scholar]
- 237.McAllister, C. S., and C. E. Samuel. 2009. The RNA-activated protein kinase enhances the induction of interferon-β and apoptosis mediated by cytoplasmic RNA sensors. J. Biol. Chem. 284:1644-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McCartney, S. A., L. B. Thackray, L. Gitlin, S. Gilfillan, H. W. Virgin Iv, and M. Colonna. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McCoy, C. E., S. Carpenter, E. M. Palsson-McDermott, L. J. Gearing, and L. A. O'Neill. 2008. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J. Biol. Chem. 283:14277-14285. [DOI] [PubMed] [Google Scholar]
- 240.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Means, T. K., E. Latz, F. Hayashi, M. R. Murali, D. T. Golenbock, and A. D. Luster. 2005. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Investig. 115:407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 243.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 244.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 245.Meylan, E., K. Burns, K. Hofmann, V. Blancheteau, F. Martinon, M. Kelliher, and J. Tschopp. 2004. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 5:503-507. [DOI] [PubMed] [Google Scholar]
- 246.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 247.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature 442:39-44. [DOI] [PubMed] [Google Scholar]
- 248.Mezger, M., S. Kneitz, I. Wozniok, O. Kurzai, H. Einsele, and J. Loeffler. 2008. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 197:924-931. [DOI] [PubMed] [Google Scholar]
- 249.Miao, E. A., R. K. Ernst, M. Dors, D. P. Mao, and A. Aderem. 2008. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA 105:2562-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cardenas, M. Gale, Jr., and A. Garcia-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Michallet, M. C., E. Meylan, M. A. Ermolaeva, J. Vazquez, M. Rebsamen, J. Curran, H. Poeck, M. Bscheider, G. Hartmann, M. Konig, U. Kalinke, M. Pasparakis, and J. Tschopp. 2008. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28:651-661. [DOI] [PubMed] [Google Scholar]
- 252.Miettinen, M., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2:349-355. [DOI] [PubMed] [Google Scholar]
- 253.Mink, M., B. Fogelgren, K. Olszewski, P. Maroy, and K. Csiszar. 2001. A novel human gene (SARM) at chromosome 17q11 encodes a protein with a SAM motif and structural similarity to Armadillo/beta-catenin that is conserved in mouse, Drosophila, and Caenorhabditis elegans. Genomics 74:234-244. [DOI] [PubMed] [Google Scholar]
- 254.Mitchell, B. S. 2003. The proteasome—an emerging therapeutic target in cancer. N. Engl. J. Med. 348:2597-2598. [DOI] [PubMed] [Google Scholar]
- 255.Mockenhaupt, F. P., J. P. Cramer, L. Hamann, M. S. Stegemann, J. Eckert, N. R. Oh, R. N. Otchwemah, E. Dietz, S. Ehrhardt, N. W. Schroder, U. Bienzle, and R. R. Schumann. 2006. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc. Natl. Acad. Sci. USA 103:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mockenhaupt, F. P., L. Hamann, C. von Gaertner, G. Bedu-Addo, C. von Kleinsorgen, R. R. Schumann, and U. Bienzle. 2006. Common polymorphisms of toll-like receptors 4 and 9 are associated with the clinical manifestation of malaria during pregnancy. J. Infect. Dis. 194:184-188. [DOI] [PubMed] [Google Scholar]
- 257.Mogensen, T. H., R. S. Berg, L. Ostergaard, and S. R. Paludan. 2008. Streptococcus pneumoniae stabilizes tumor necrosis factor alpha mRNA through a pathway dependent on p38 MAPK but independent of Toll-like receptors. BMC Immunol. 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mogensen, T. H., R. S. Berg, S. R. Paludan, and L. Ostergaard. 2008. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect. Immun. 76:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mogensen, T. H., J. Melchjorsen, P. Hollsberg, and S. R. Paludan. 2003. Activation of NF-kappa B in virus-infected macrophages is dependent on mitochondrial oxidative stress and intracellular calcium: downstream involvement of the kinases TGF-beta-activated kinase 1, mitogen-activated kinase/extracellular signal-regulated kinase kinase 1, and I kappa B kinase. J. Immunol. 170:6224-6233. [DOI] [PubMed] [Google Scholar]
- 260.Mogensen, T. H., J. Melchjorsen, L. Malmgaard, A. Casola, and S. R. Paludan. 2004. Suppression of proinflammatory cytokine expression by herpes simplex virus type 1. J. Virol. 78:5883-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mogensen, T. H., and S. R. Paludan. 2005. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J. Mol. Med. 83:180-192. [DOI] [PubMed] [Google Scholar]
- 262.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80:267-277. [DOI] [PubMed] [Google Scholar]
- 263.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Two Neisseria meningitidis strains with different ability to stimulate toll-like receptor 4 through the MyD88-independent pathway. Scand. J. Immunol. 64:646-654. [DOI] [PubMed] [Google Scholar]
- 264.Reference deleted.
- 265.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 266.Moynagh, P. N. 2003. Toll-like receptor signalling pathways as key targets for mediating the anti-inflammatory and immunosuppressive effects of glucocorticoids. J. Endocrinol. 179:139-144. [DOI] [PubMed] [Google Scholar]
- 267.Mukherjee, S., G. Keitany, Y. Li, Y. Wang, H. L. Ball, E. J. Goldsmith, and K. Orth. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211-1214. [DOI] [PubMed] [Google Scholar]
- 268.Muruve, D. A., V. Petrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103-107. [DOI] [PubMed] [Google Scholar]
- 269.Muzio, M., D. Bosisio, N. Polentarutti, G. D'amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 270.Nallagatla, S. R., J. Hwang, R. Toroney, X. Zheng, C. E. Cameron, and P. C. Bevilacqua. 2007. 5′-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 318:1455-1458. [DOI] [PubMed] [Google Scholar]
- 271.Netea, M. G., C. A. Van der Graaf, A. G. Vonk, I. Verschueren, J. W. van der Meer, and B. J. Kullberg. 2002. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185:1483-1489. [DOI] [PubMed] [Google Scholar]
- 272.Netea, M. G., C. Van der Graaf, J. W. van der Meer, and B. J. Kullberg. 2004. Recognition of fungal pathogens by Toll-like receptors. Eur. J. Clin. Microbiol. Infect. Dis. 23:672-676. [DOI] [PubMed] [Google Scholar]
- 273.Nguyen, T. H., T. H. Tran, G. Thwaites, V. C. Ly, X. S. Dinh, T. N. Ho Dang, Q. T. Dang, D. P. Nguyen, H. P. Nguyen, S. D. To, V. C. Nguyen, M. D. Nguyen, J. Campbell, C. Schultsz, C. Parry, M. E. Torok, N. White, T. C. Nguyen, T. H. Tran, K. Stepniewska, and J. J. Farrar. 2007. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N. Engl. J. Med. 357:2431-2440. [DOI] [PubMed] [Google Scholar]
- 274.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.O'Connell, R. M., K. D. Taganov, M. P. Boldin, G. Cheng, and D. Baltimore. 2007. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 104:1604-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 277.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 278.Ogus, A. C., B. Yoldas, T. Ozdemir, A. Uguz, S. Olcen, I. Keser, M. Coskun, A. Cilli, and O. Yegin. 2004. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 23:219-223. [DOI] [PubMed] [Google Scholar]
- 279.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 280.O'Neill, L. A., and A. G. Bowie. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353-364. [DOI] [PubMed] [Google Scholar]
- 281.Opitz, B., A. Puschel, B. Schmeck, A. C. Hocke, S. Rosseau, S. Hammerschmidt, R. R. Schumann, N. Suttorp, and S. Hippenstiel. 2004. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 279:36426-36432. [DOI] [PubMed] [Google Scholar]
- 282.Orange, J. S., S. R. Brodeur, A. Jain, F. A. Bonilla, L. C. Schneider, R. Kretschmer, S. Nurko, W. L. Rasmussen, J. R. Kohler, S. E. Gellis, B. M. Ferguson, J. L. Strominger, J. Zonana, N. Ramesh, Z. K. Ballas, and R. S. Geha. 2002. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J. Clin. Investig. 109:1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4:161-167. [DOI] [PubMed] [Google Scholar]
- 284.Oshiumi, H., M. Sasai, K. Shida, T. Fujita, M. Matsumoto, and T. Seya. 2003. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J. Biol. Chem. 278:49751-49762. [DOI] [PubMed] [Google Scholar]
- 285.Osterloh, A., and M. Breloer. 2008. Heat shock proteins: linking danger and pathogen recognition. Med. Microbiol. Immunol. 197:1-8. [DOI] [PubMed] [Google Scholar]
- 286.Ouyang, W., J. K. Kolls, and Y. Zheng. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Parroche, P., F. N. Lauw, N. Goutagny, E. Latz, B. G. Monks, A. Visintin, K. A. Halmen, M. Lamphier, M. Olivier, D. C. Bartholomeu, R. T. Gazzinelli, and D. T. Golenbock. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. USA 104:1919-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033-1036. [DOI] [PubMed] [Google Scholar]
- 290.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364-368. [DOI] [PubMed] [Google Scholar]
- 291.Paun, A., J. T. Reinert, Z. Jiang, C. Medin, M. Yaseen, K. A. Fitzgerald, and P. M. Pitha. 2008. Functional characterization of murine interferon regulatory factor (IRF-5) and its role in the innate antiviral response. J. Biol. Chem. 283:14295-14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Perry, A. K., E. K. Chow, J. B. Goodnough, W. C. Yeh, and G. Cheng. 2004. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J. Exp. Med. 199:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pflugheber, J., B. Fredericksen, R. Sumpter, Jr., C. Wang, F. Ware, D. L. Sodora, and M. Gale, Jr. 2002. Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc. Natl. Acad. Sci. USA 99:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Picard, C., A. Puel, M. Bonnet, C. L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, C. Elbim, R. Hitchcock, D. Lammas, G. Davies, A. Al Ghonaium, H. Al Rayes, S. Al Jumaah, S. Al Hajjar, I. Z. Al Mohsen, H. H. Frayha, R. Rucker, T. R. Hawn, A. Aderem, H. Tufenkeji, S. Haraguchi, N. K. Day, R. A. Good, M. A. Gougerot-Pocidalo, A. Ozinsky, and J. L. Casanova. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076-2079. [DOI] [PubMed] [Google Scholar]
- 295.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 296.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 297.Pridmore, A. C., G. A. Jarvis, C. M. John, D. L. Jack, S. K. Dower, and R. C. Read. 2003. Activation of Toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect. Immun. 71:3901-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Raffatellu, M., D. Chessa, R. P. Wilson, C. Tukel, M. Akcelik, and A. J. Baumler. 2006. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 74:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rahman, P., S. Bartlett, F. Siannis, F. J. Pellett, V. T. Farewell, L. Peddle, C. T. Schentag, C. A. Alderdice, S. Hamilton, M. Khraishi, Y. Tobin, D. Hefferton, and D. D. Gladman. 2003. CARD15: a pleiotropic autoimmune gene that confers susceptibility to psoriatic arthritis. Am. J. Hum. Genet. 73:677-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 301.Rasmussen, S. B., L. N. Sorensen, L. Malmgaard, N. Ank, J. D. Baines, Z. J. Chen, and S. R. Paludan. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81:13315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Reddy, K. V., G. Bhattacharjee, G. Schabbauer, A. Hollis, K. Kempf, M. Tencati, M. O'Connell, M. Guha, and N. Mackman. 2004. Dexamethasone enhances LPS induction of tissue factor expression in human monocytic cells by increasing tissue factor mRNA stability. J. Leukoc. Biol. 76:145-151. [DOI] [PubMed] [Google Scholar]
- 303.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 304.Roeder, A., C. J. Kirschning, R. A. Rupec, M. Schaller, and H. C. Korting. 2004. Toll-like receptors and innate antifungal responses. Trends Microbiol. 12:44-49. [DOI] [PubMed] [Google Scholar]
- 305.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex viruses, p. 2501. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott, Williams, and Wilkins, Philadelphia, PA.
- 306.Romano, P. R., S. R. Green, G. N. Barber, M. B. Mathews, and A. G. Hinnebusch. 1995. Structural requirements for double-stranded RNA binding, dimerization, and activation of the human eIF-2 alpha kinase DAI in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 308.Rossignol, D. P., and M. Lynn. 2005. TLR4 antagonists for endotoxemia and beyond. Curr. Opin. Investig. Drugs 6:496-502. [PubMed] [Google Scholar]
- 309.Rotem, Z., R. A. Cox, and A. Isaacs. 1963. Inhibition of virus multiplication by foreign nucleic acid. Nature 197:564-566. [DOI] [PubMed] [Google Scholar]
- 310.Rothenfusser, S., N. Goutagny, G. DiPerna, M. Gong, B. G. Monks, A. Schoenemeyer, M. Yamamoto, S. Akira, and K. A. Fitzgerald. 2005. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175:5260-5268. [DOI] [PubMed] [Google Scholar]
- 311.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 312.Sadler, A. J., and B. R. Williams. 2007. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316:253-292. [DOI] [PubMed] [Google Scholar]
- 313.Saha, S. K., E. M. Pietras, J. Q. He, J. R. Kang, S. Y. Liu, G. Oganesyan, A. Shahangian, B. Zarnegar, T. L. Shiba, Y. Wang, and G. Cheng. 2006. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Saito, T., R. Hirai, Y. M. Loo, D. Owen, C. L. Johnson, S. C. Sinha, S. Akira, T. Fujita, and M. Gale, Jr. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. USA 104:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Salio, M., A. O. Speak, D. Shepherd, P. Polzella, P. A. Illarionov, N. Veerapen, G. S. Besra, F. M. Platt, and V. Cerundolo. 2007. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. USA 104:20490-20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 317.Sato, S., H. Sanjo, K. Takeda, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, K. Matsumoto, O. Takeuchi, and S. Akira. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6:1087-1095. [DOI] [PubMed] [Google Scholar]
- 318.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171:4304-4310. [DOI] [PubMed] [Google Scholar]
- 319.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 320.Scarborough, M., S. B. Gordon, C. J. Whitty, N. French, Y. Njalale, A. Chitani, T. E. Peto, D. G. Lalloo, and E. E. Zijlstra. 2007. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N. Engl. J. Med. 357:2441-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schaaf, M. J., and J. A. Cidlowski. 2002. Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 83:37-48. [DOI] [PubMed] [Google Scholar]
- 322.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 324.Schoenemeyer, A., B. J. Barnes, M. E. Mancl, E. Latz, N. Goutagny, P. M. Pitha, K. A. Fitzgerald, and D. T. Golenbock. 2005. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 280:17005-17012. [DOI] [PubMed] [Google Scholar]
- 325.Schott, E., H. Witt, K. Neumann, S. Taube, D. Y. Oh, E. Schreier, S. Vierich, G. Puhl, A. Bergk, J. Halangk, V. Weich, B. Wiedenmann, and T. Berg. 2007. A Toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J. Hepatol. 47:203-211. [DOI] [PubMed] [Google Scholar]
- 326.Schroder, N. W., I. Diterich, A. Zinke, J. Eckert, C. Draing, V. von Baehr, D. Hassler, S. Priem, K. Hahn, K. S. Michelsen, T. Hartung, G. R. Burmester, U. B. Gobel, C. Hermann, and R. R. Schumann. 2005. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J. Immunol. 175:2534-2540. [DOI] [PubMed] [Google Scholar]
- 327.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 328.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 329.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 330.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 331.Shi, M., W. Deng, E. Bi, K. Mao, Y. Ji, G. Lin, X. Wu, Z. Tao, Z. Li, X. Cai, S. Sun, C. Xiang, and B. Sun. 2008. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat. Immunol. 9:369-377. [DOI] [PubMed] [Google Scholar]
- 332.Shim, J. H., C. Xiao, A. E. Paschal, S. T. Bailey, P. Rao, M. S. Hayden, K. Y. Lee, C. Bussey, M. Steckel, N. Tanaka, G. Yamada, S. Akira, K. Matsumoto, and S. Ghosh. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19:2668-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 334.Silva, A. M., M. Whitmore, Z. Xu, Z. Jiang, X. Li, and B. R. Williams. 2004. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 279:37670-37676. [DOI] [PubMed] [Google Scholar]
- 335.Simon, R., and C. E. Samuel. 2007. Activation of NF-kappaB-dependent gene expression by Salmonella flagellins FliC and FljB. Biochem. Biophys. Res. Commun. 355:280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336a.Sjolinder, H., T. H. Mogensen, M. Kilian, A. B. Jonsson, and S. R. Paludan. 2008. An important role for Toll-like receptor 9 in host-defense against meningococcal sepsis. Infect. Immun. 76:5421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Smirnova, I., N. Mann, A. Dols, H. H. Derkx, M. L. Hibberd, M. Levin, and B. Beutler. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. USA 100:6075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Soulat, D., A. Bauch, S. Stockinger, G. Superti-Furga, and T. Decker. 2006. Cytoplasmic Listeria monocytogenes stimulates IFN-beta synthesis without requiring the adapter protein MAVS. FEBS Lett. 580:2341-2346. [DOI] [PubMed] [Google Scholar]
- 339.Spann, K. M., K. C. Tran, and P. L. Collins. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-κB, and proinflammatory cytokines. J. Virol. 79:5353-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sprung, C. L., D. Annane, D. Keh, R. Moreno, M. Singer, K. Freivogel, Y. G. Weiss, J. Benbenishty, A. Kalenka, H. Forst, P. F. Laterre, K. Reinhart, B. H. Cuthbertson, D. Payen, and J. Briegel. 2008. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 358:111-124. [DOI] [PubMed] [Google Scholar]
- 341.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 343.Stephens, D. S., L. H. Hoffman, and Z. A. McGee. 1983. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J. Infect. Dis. 148:369-376. [DOI] [PubMed] [Google Scholar]
- 344.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 345.Stockinger, S., B. Reutterer, B. Schaljo, C. Schellack, S. Brunner, T. Materna, M. Yamamoto, S. Akira, T. Taniguchi, P. J. Murray, M. Muller, and T. Decker. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 173:7416-7425. [DOI] [PubMed] [Google Scholar]
- 346.Sun, L., L. Deng, C. K. Ea, Z. P. Xia, and Z. J. Chen. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14:289-301. [DOI] [PubMed] [Google Scholar]
- 347.Sun, S. C., P. A. Ganchi, D. W. Ballard, and W. C. Greene. 1993. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259:1912-1915. [DOI] [PubMed] [Google Scholar]
- 348.Suzuki, N., S. Suzuki, G. S. Duncan, D. G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, J. M. Penninger, H. Wesche, P. S. Ohashi, T. W. Mak, and W. C. Yeh. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416:750-756. [DOI] [PubMed] [Google Scholar]
- 349.Swantek, J. L., M. H. Cobb, and T. D. Geppert. 1997. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sweet, C. R., J. Conlon, D. T. Golenbock, J. Goguen, and N. Silverman. 2007. YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell. Microbiol. 9:2700-2715. [DOI] [PubMed] [Google Scholar]
- 351.Tabeta, K., K. Hoebe, E. M. Janssen, X. Du, P. Georgel, K. Crozat, S. Mudd, N. Mann, S. Sovath, J. Goode, L. Shamel, A. A. Herskovits, D. A. Portnoy, M. Cooke, L. M. Tarantino, T. Wiltshire, B. E. Steinberg, S. Grinstein, and B. Beutler. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156-164. [DOI] [PubMed] [Google Scholar]
- 352.Tada, H., E. Nemoto, H. Shimauchi, T. Watanabe, T. Mikami, T. Matsumoto, N. Ohno, H. Tamura, K. Shibata, S. Akashi, K. Miyake, S. Sugawara, and H. Takada. 2002. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46:503-512. [DOI] [PubMed] [Google Scholar]
- 353.Taddeo, B., T. R. Luo, W. Zhang, and B. Roizman. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA 100:12408-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Taganov, K. D., M. P. Boldin, K. J. Chang, and D. Baltimore. 2006. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 103:12481-12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Takahashi, K., T. Kawai, H. Kumar, S. Sato, S. Yonehara, and S. Akira. 2006. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 176:4520-4524. [DOI] [PubMed] [Google Scholar]
- 356.Takahasi, K., M. Yoneyama, T. Nishihori, R. Hirai, H. Kumeta, R. Narita, M. Gale, Jr., F. Inagaki, and T. Fujita. 2008. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell 29:428-440. [DOI] [PubMed] [Google Scholar]
- 357.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 358.Takaoka, A., H. Yanai, S. Kondo, G. Duncan, H. Negishi, T. Mizutani, S. Kano, K. Honda, Y. Ohba, T. W. Mak, and T. Taniguchi. 2005. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434:243-249. [DOI] [PubMed] [Google Scholar]
- 359.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 360.Tal, G., A. Mandelberg, I. Dalal, K. Cesar, E. Somekh, A. Tal, A. Oron, S. Itskovich, A. Ballin, S. Houri, A. Beigelman, O. Lider, G. Rechavi, and N. Amariglio. 2004. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J. Infect. Dis. 189:2057-2063. [DOI] [PubMed] [Google Scholar]
- 361.Tao, K., M. Fujii, S. Tsukumo, Y. Maekawa, K. Kishihara, Y. Kimoto, T. Horiuchi, H. Hisaeda, S. Akira, S. Kagami, and K. Yasutomo. 2007. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann. Rheum. Dis. 66:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Taylor, K. R., J. M. Trowbridge, J. A. Rudisill, C. C. Termeer, J. C. Simon, and R. L. Gallo. 2004. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 279:17079-17084. [DOI] [PubMed] [Google Scholar]
- 363.Texereau, J., J. D. Chiche, W. Taylor, G. Choukroun, B. Comba, and J. P. Mira. 2005. The importance of Toll-like receptor 2 polymorphisms in severe infections. Clin. Infect. Dis. 41(Suppl. 7):S408-S415. [DOI] [PubMed] [Google Scholar]
- 364.Thanos, D., and T. Maniatis. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091-1100. [DOI] [PubMed] [Google Scholar]
- 365.Tian, J., A. M. Avalos, S. Y. Mao, B. Chen, K. Senthil, H. Wu, P. Parroche, S. Drabic, D. Golenbock, C. Sirois, J. Hua, L. L. An, L. Audoly, G. La Rosa, A. Bierhaus, P. Naworth, A. Marshak-Rothstein, M. K. Crow, K. A. Fitzgerald, E. Latz, P. A. Kiener, and A. J. Coyle. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8:487-496. [DOI] [PubMed] [Google Scholar]
- 366.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Trent, M. S., C. M. Stead, A. X. Tran, and J. V. Hankins. 2006. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 12:205-223. [DOI] [PubMed] [Google Scholar]
- 368.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 369.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of Toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K. J. Ishii, T. Kawai, O. Takeuchi, and S. Akira. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 372.van Beelen, A. J., Z. Zelinkova, E. W. Taanman-Kueter, F. J. Muller, D. W. Hommes, S. A. Zaat, M. L. Kapsenberg, and E. C. de Jong. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660-669. [DOI] [PubMed] [Google Scholar]
- 373.van Duin, D., R. Medzhitov, and A. C. Shaw. 2006. Triggering TLR signaling in vaccination. Trends Immunol. 27:49-55. [DOI] [PubMed] [Google Scholar]
- 374.Venkataraman, T., M. Valdes, R. Elsby, S. Kakuta, G. Caceres, S. Saijo, Y. Iwakura, and G. N. Barber. 2007. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178:6444-6455. [DOI] [PubMed] [Google Scholar]
- 375.Reference deleted.
- 376.von Bernuth, H., C. Picard, Z. Jin, R. Pankla, H. Xiao, C. L. Ku, M. Chrabieh, I. B. Mustapha, P. Ghandil, Y. Camcioglu, J. Vasconcelos, N. Sirvent, M. Guedes, A. B. Vitor, M. J. Herrero-Mata, J. I. Arostegui, C. Rodrigo, L. Alsina, E. Ruiz-Ortiz, M. Juan, C. Fortuny, J. Yague, J. Anton, M. Pascal, H. H. Chang, L. Janniere, Y. Rose, B. Z. Garty, H. Chapel, A. Issekutz, L. Marodi, C. Rodriguez-Gallego, J. Banchereau, L. Abel, X. Li, D. Chaussabel, A. Puel, and J. L. Casanova. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wald, D., J. Qin, Z. Zhao, Y. Qian, M. Naramura, L. Tian, J. Towne, J. E. Sims, G. R. Stark, and X. Li. 2003. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4:920-927. [DOI] [PubMed] [Google Scholar]
- 378.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 379.Wang, Z., M. K. Choi, T. Ban, H. Yanai, H. Negishi, Y. Lu, T. Tamura, A. Takaoka, K. Nishikura, and T. Taniguchi. 2008. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA 105:5477-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wesche, H., W. J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 7:837-847. [DOI] [PubMed] [Google Scholar]
- 382.Windheim, M., C. Lang, M. Peggie, L. A. Plater, and P. Cohen. 2007. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem. J. 404:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 384.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 385.Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, K. Takeda, and S. Akira. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324-329. [DOI] [PubMed] [Google Scholar]
- 386.Yamamoto, M., S. Sato, H. Hemmi, S. Uematsu, K. Hoshino, T. Kaisho, O. Takeuchi, K. Takeda, and S. Akira. 2003. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4:1144-1150. [DOI] [PubMed] [Google Scholar]
- 387.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 388.Yamamoto, Y., and R. B. Gaynor. 2001. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 107:135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Yarovinsky, F., D. Zhang, J. F. Andersen, G. L. Bannenberg, C. N. Serhan, M. S. Hayden, S. Hieny, F. S. Sutterwala, R. A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626-1629. [DOI] [PubMed] [Google Scholar]
- 391.Yin, M. J., Y. Yamamoto, and R. B. Gaynor. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396:77-80. [DOI] [PubMed] [Google Scholar]
- 392.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]
- 393.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 394.Yoshida, H., H. Jono, H. Kai, and J. D. Li. 2005. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for Toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J. Biol. Chem. 280:41111-41121. [DOI] [PubMed] [Google Scholar]
- 395.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 396.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 398.Zhang, M., X. Wu, A. J. Lee, W. Jin, M. Chang, A. Wright, T. Imaizumi, and S. C. Sun. 2008. Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J. Biol. Chem. 283:18621-18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhang, P., and C. E. Samuel. 2008. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J. Biol. Chem. 283:34580-34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Zhang, S. Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, C. Picard, A. Chapgier, S. Plancoulaine, M. Titeux, C. Cognet, H. von Bernuth, C. L. Ku, A. Casrouge, X. X. Zhang, L. Barreiro, J. Leonard, C. Hamilton, P. Lebon, B. Heron, L. Vallee, L. Quintana-Murci, A. Hovnanian, F. Rozenberg, E. Vivier, F. Geissmann, M. Tardieu, L. Abel, and J. L. Casanova. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522-1527. [DOI] [PubMed] [Google Scholar]
- 401.Zhao, T., L. Yang, Q. Sun, M. Arguello, D. W. Ballard, J. Hiscott, and R. Lin. 2007. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat. Immunol. 8:592-600. [DOI] [PubMed] [Google Scholar]
- 402.Zhong, B., Y. Yang, S. Li, Y. Y. Wang, Y. Li, F. Diao, C. Lei, X. He, L. Zhang, P. Tien, and H. B. Shu. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538-550. [DOI] [PubMed] [Google Scholar]
- 403.Zughaier, S. M., Y. L. Tzeng, S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]