Abstract
Summary: Outbreaks of the severe dengue syndrome, dengue hemorrhagic fever (DHF), emerged beginning in the 1950s, marking a dramatic change in the dengue syndrome. While intense investigations in multiple directions have been conducted for many years to elucidate the intrinsic mechanisms conducive to the development of DHF, no consensus has yet emerged. Meanwhile, relatively little attention has been paid to the occurrence of severe dengue and death prior to the 1950s. This comprehensive review was designed to evaluate outbreak records in the early dengue history to better understand the epidemiologic background and other factors that existed before the emergence of DHF outbreaks. By applying a set of stringent criteria to remove unreliable data as much as possible and by interpreting the results conservatively, a short list of etiologically more reliable outbreaks with high mortality was obtained. The results show that severe dengue syndrome, clinically very much compatible with DHF, occurred far more frequently in multiple locations than it had been assumed before; that the magnitudes of mortality in several outbreaks were not negligible; and that the epidemiologic background features shared among these outbreaks in the early period were, with the exceptions of more limited demographic changes, generally similar to the post-1950 conditions.
INTRODUCTION
It has been generally accepted that outbreaks of two severe dengue syndromes often causing a high rate of mortality, dengue hemorrhagic fever DHF) and dengue shock syndrome, emerged in Southeast Asia after 1950 (56), although hemorrhagic manifestations had been reported as early as in the late 18th century (159). The four major topics of dengue research since then have been elucidation of the mechanism(s) leading to the development of the severe syndromes, discovery of effective methods of medical intervention, disease control, and prevention, including vaccine development. For the first research question, at least several hypotheses have been proposed, and highly valuable data were accumulated to corroborate some of them. However, one area of research that has received much less attention thus far but which may shed light to improve our understanding of the evolution of these dangerous syndromes is examination of the transitional process prior to 1950 in the context of the epidemiologic conditions in the background.
Analyses of historical documents of most infectious diseases (including dengue), however, suffer from severe limitations due to the absence of absolute etiologic certainty given the unavailability of specific confirmation techniques in the earlier period. Furthermore, for documentation of a severe dengue syndrome, a unique set of data that were identified to be essential only after the 1950s, such as a physical condition meeting any of the criteria for grading disease severity, the immunologic status of patients, and the virus strain or serotype involved, must be obtained. Other additional supplemental data that are highly desirable include pathophysiologic conditions in fatal cases, genetic factors, and other miscellaneous host factors. None of them are found in the old medical literature. Furthermore, historic data contain a variety of problems, such as ambiguities of records, puzzling observations, contradictions, and other uncertainties.
Nevertheless, more reliable pre-1950 epidemiologic conditions and other background information can be obtained by navigating through historic data with the application of a set of stringent criteria as a tool and by interpreting the filtered data more conservatively. This approach of critical evaluation facilitates a better understanding of the shared epidemiologic attributes and other useful information recorded in the outbreaks of the period prior to the emergence of DHF in the 1950s.
Accordingly, the major objectives of this review are to analyze the occurrence of severe dengue syndrome resulting in death before the emergence of DHF outbreaks in the 1950s, to examine the epidemiologic background (including virologic history) and shared features among these outbreaks, and to analyze the sources contributing to inaccuracy in documenting or reporting severe dengue syndrome or death. For topics that are controversial due to conflicting reports or variation in interpretation, samples of the views or reports representing each are cited to aid readers in evaluating the issues by themselves.
As a by-product of these analyses, selected relevant data or observations are drawn from the early medical literature, not to evaluate their quality but to examine their compatibility with some of the current hypotheses proposed for the mechanism of DHF pathogenesis.
METHODS USED IN THE REVIEW PROCESS
Terms
Throughout this review, the word “dengue” is used to denote “dengue or dengue-like illness” because of the absence of confirmatory laboratory tests in much of the early period. “Confirmed dengue” is used exclusively to refer to the cases or outbreaks for which authentic dengue etiology was definitely established with specific laboratory tests, as in the latter half of the covered period. For the sake of simplicity, throughout this review only the term DHF (as defined by the World Health Organization [WHO] [166]) is applied to the post-1950 syndrome whether or not dengue shock syndrome developed.
Covered Period
Because interpretation of the dengue outbreak records before 1860s was generally considered more difficult (61) and that of the 1870s similarly was problematic, as described below, the 60-year period between 1890 and 1950 was selected, for the following reasons. First, reliable clinical records of dengue syndrome most compatible with DHF began to appear in the medical literature in the last decade of the 19th century, as described below. Second, at around the turn of the 20th century, the concept of vector-borne transmission of dengue began to crystallize for the first time, at least in the minds of a very small number of physicians investigating dengue. This reflected the strong influence of earlier discoveries of mosquito-borne transmission of filaria, malaria, and yellow fever (YF) beginning in the 1870s (by Patrick Manson, Ronald Ross, and Carlos Finlay, respectively). Third, more data on the biology of dengue vectors became available toward the end of the 19th century, and entomologic surveys of the geographic distribution of dengue vectors in tropical, semitropical, and temperate regions of the world were conducted frequently by multiple groups in this period. Fourth, the etiologic uncertainty and confusion over the definition of dengue, which were very intense before 1890s, diminished considerably toward the end of the 19th century, although problems still persisted thereafter. Fifth, after the 1920s, etiologies of some outbreaks were on more solid ground because they were serologically or virologically confirmed. However, in this review a small number of relevant articles published before or after the study period are referenced to evaluate whether the data obtained or observations made during the 60-year period represented a transition or something unique to that period. Conversely, the situation and the problems of dengue today are better understood by comparing them with the conditions in the two earlier periods.
Source of References
The original documents of the early references that had been published before 1950 and that are listed in the dengue database (77) were the major source of the documents analyzed.
Indicator of Severity
Although dengue severity has been graded since the 1970s according to the criteria established and subsequently revised by the WHO (166), the criteria did not exist in the early period. Furthermore, a consensus regarding the adequacy of the WHO criteria has not yet been obtained (52, 116, 124). Accordingly, death was arbitrarily selected as the indicator of severity in the early period.
Stringent Criteria for Selecting Etiologically More Reliable Records
A set of six stringent criteria was used in the first of two stages of the selection process to exclude as much as possible poorly documented reports of infections or outbreaks of questionable etiology. (i) The clinical syndrome of acute febrile illness recorded was generally compatible with classic dengue fever as defined by the WHO (166), allowing a range of variation in frequency of such symptoms as biphasic pyrexia and myalgia and of such signs as rash. (ii) The etiology of the outbreak was identified independently by multiple physicians. Thus, determination by consensus, relying on collective wisdom rather than the judgment of single or a few physicians, was considered to be more reliable. (iii) The clinical syndrome of representative fatal cases, as well as the number of deaths, was described. (iv) Epidemiologically, the outbreak occurred typically in human population centers with a sizable population size, where the initial foci of clustered cases diffused extensively and quickly into many other areas later. (v) The outbreak occurred during the warm season (when mosquitoes were active) and affected a large number of individuals in a relatively short period (3 to 8 months) rather than sporadically in a small number of people over a longer period. (vi) There was no obvious evidence of involvement of the following vector-borne arboviral diseases manifesting similar syndromes: chikungunya (CHIK), Rift Valley fever, Ross River fever, sandfly fever, West Nile fever, and mild YF. The kinds of data in the reports used for exclusion were as follows: outbreaks of equivocal or questionable etiology including mention of wrong vectors (such as sandflies as in sandfly fever); symptoms, signs, and/or syndromes rare in dengue but more frequent in other diseases (such as persistent arthralgia long after the acute phase, as in CHIK and Ross River fever); and reports of concurrent epidemics in unusual hosts (such as sheep, cattle, and/or birds, as in Rift Valley fever or West Nile fever). For exclusion of YF involvement, the theoretical possibility of YF in Asia and the Pacific and more serious concern about equivocal etiology in the history of outbreaks of dengue-like illness in North America were two important considerations. In fact, outbreaks of YF occurred more frequently than outbreaks of dengue in North America in the 19th century, and “black vomit” associated with gastrointestinal bleeding in dengue was not uncommon in the 1920s (121, 138). The retrospective YF-specific neutralization test conducted by Sawyer et al. conclusively demonstrated a total absence of antibody to YF virus in tropical Asia, the Pacific, Oceania, and the Middle East as well as in the patients involved in the aforementioned dengue outbreaks of the 1920s that occurred in southern states of the United States (136). The exclusion of other infectious diseases presenting similar syndromes, such as measles, influenza, typhoid fever, leptospirosis, scarlet fever, and malaria, was solely by the judgment of the original authors reporting the outbreaks. The outbreaks with mortality that fulfilled the above six stringent criteria or those meeting five of these criteria but which were serologically confirmed were classified as “probable dengue.”
In the second stage, the following two criteria were added to the requirement: (vii) minimal sets of entomologic data during the outbreak were provided, including vector distribution and biting activity during the outbreak, and (viii) retrospective serologic confirmation by a specific neutralization test or virus isolation. When these two additional criteria were also met, the outbreak was classified as “confirmed dengue.”
ANALYSES OF VIROLOGIC DATA
Virus Serotypes in the Early Period
No virologic information is available for the first half of this early period, but the data confirming the involvement of three serotypes (dengue virus serotype 1 [DENV-1], DENV-2, and DENV-4) in the confirmed outbreaks in the second half of the covered period are arranged chronologically in Table 1. The data in Table 1 may be interpreted to suggest either that DENV-1 was more active than the other serotypes or that, in the absence of systematic and comprehensive virus surveys during the early period, DENV-1 merely was more often detected or isolated than DENV-2 and DENV-4 in that period (Table 1).
TABLE 1.
Laboratory confirmation of DENV serotypes involved in the outbreaks in the early period
| Year | Location | Isolated virus | Identification by retrospective serologya | Reference(s) |
|---|---|---|---|---|
| 1924-1925 | Philippines | DENV-4 | 54 | |
| 1925-1926 | Australia | DENV-1 | 36 | |
| 1927 | South Africa | DENV-1 | 72 | |
| 1927-1928 | Greece | DENV-1 (DENV-2)b | 113 | |
| 1929-1930 | Philippines | DENV-1 | 54 | |
| 1929-1933 | Greece | DENV-2c | 128 | |
| 1941-1942 | Panamá | DENV-2 | 129 | |
| 1942-1944 | Australia | DENV-2 (DENV-1) | 36 | |
| 1942-1944 | Japan (Nagasaki/Osaka) | DENV-1 (DENV-2)b | 40, 152 | |
| 1943 | Singapore | DENV-1 | 132 | |
| 1943 | Japan (Nagasaki) | DENV-1 | 68 | |
| 1943-1944 | Hawaii | DENV-1 | DENV-1 | 65, 132 |
| 1944 | French Polynesia | DENV-1 | 129 | |
| 1944 | New Guinea | DENV-1, DENV-2 | 132 | |
| 1944 | Guam | DENV-1 (DENV-2) | 65, 132 | |
| 1945 | India | DENV-1 | 132 |
Only the serotypes that were laboratory confirmed are listed. The list does not necessarily indicate that these were the only serotypes involved in particular outbreaks.
A monotypic reaction to the serotype in parentheses was less often detected but indicates a strong possibility of concurrent activity of two serotypes.
No other document corroborating a dengue outbreak in this period was found in the early literature.
Multiple Infections, Existence of Four Serotypes, and Endemicity
Although long-term immunity conferred after dengue infection was reported by many physicians, multiple episodes of dengue per person at intervals of longer than a few months between episodes were also documented in numerous reports. Not only was this contrast the source of controversy over the length of dengue immunity, but multiple infection was sometimes used to argue against the development of a dengue vaccine. Significantly, some of the patients with multiple episodes were examined by the same physicians at each episode. In a smaller number of the cases in locations such as Indonesia, interestingly, the highest number of such episodes was either three or four (94, 145). More convincingly, in the most meticulously documented study of serologically confirmed dengue among U.S. military servicemen in the Philippines, where medical histories of all servicemen regarding dengue infection were carefully maintained, it was determined mathematically that 0.315% of the servicemen would be likely to experience dengue infection as many as four times during a 2-year assignment (142). Thus, although DENV-3 was not isolated or detected in the early period (Table 1), the existence of four serotypes could be retrospectively suspected. This eventually led to the emergence of the concept of multiple immunologic types (91), which are roughly equivalent to the present-day serotypes. Accordingly, the theory that assumes that dengue outbreaks were caused by only one serotype per tropical location in the early period and that the coexistence of multiple serotypes per location emerged only after World War II (WWII) (146) does not accurately reflect the historical data.
In many of the early documents in the covered period, little was known about dengue endemicity in urban areas. According to a theory proposed by Gordon Smith, dengue had existed for years in Asia and had been transmitted in rural areas by the indigenous Aedes mosquitoes, which did not include A. aegypti, and endemicity in urban areas was established only after the latter vector was introduced later to the rapidly growing human population centers in the 19th century (47). Thus, by the early 20th century, repeated epidemics were already explained on the basis of endemicity in urban areas (98). Also, in semitropical locations, where the seasonality of dengue was clearer (such as parts of Queensland, Australia), an interepidemic period (or lack of endemicity) was recognized (105). Possible evidence of endemicity, rather than repeated introductions of virus, was revealed in the Philippines, where dengue cases among indigenous Filipino soldiers without a history of travel outside the country were recorded every year between 1902 and 1925 (142). Elsewhere in tropical regions of the world, due to the absence of published documents, early historical records of dengue activity in several countries where dengue is currently endemic appear “blank” for considerable lengths of time. However, a more comprehensive literature search reveals signs of dengue activity during those “blank” periods. While it was sometimes mentioned in short paragraphs in articles on dengue (47), most often the data suggesting endemicity were included in types of publications normally considered atypical and hence were difficult to retrieve (45, 94, 141, 167). Collectively, these observations support a strong probability of dengue endemicity established in multiple urban centers with a sufficiently large human population.
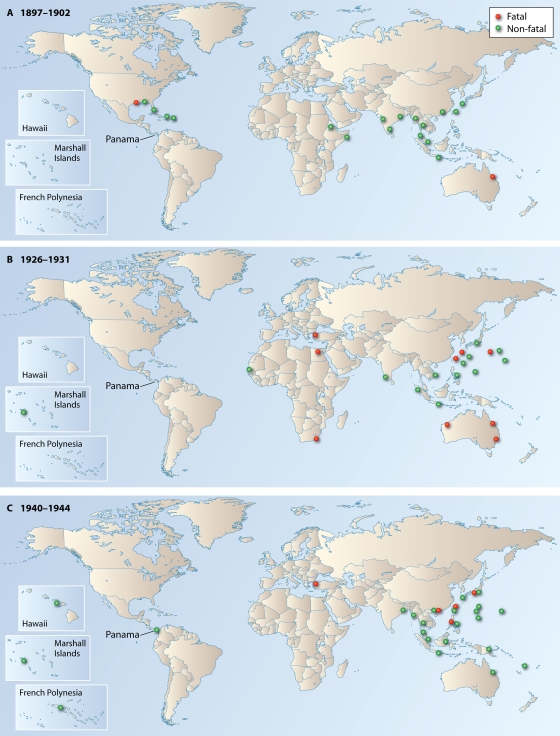
Table 2 lists outbreaks recording mortality that were identified in the literature without the application of stringent criteria designed for selecting more reliable dengue etiology. The data reveal that mortality was reported not just from particular locations but widely from many geographic areas (Fig. 1). Furthermore, the numbers of deaths in several outbreaks (Table 2) were not negligible and even exceeded the annual mortality figures registered in some tropical countries after the 1950s.
TABLE 2.
List of outbreaks of dengue or dengue-like illness resulting in mortality, prepared without application of the stringent criteria for improved etiology
| Country (subdivision) | Period | Yr | Total no. of deaths | Reference(s) |
|---|---|---|---|---|
| Australia (Queensland) | 1895-1926a | 816 | 105 | |
| 1895 | 26 | |||
| 1897 | 97 | |||
| 1898 | 87 | |||
| 1905 | 201 | |||
| 1906 | 24 | |||
| 1907 | 18 | |||
| 1910 | 19 | |||
| 1911 | 85 | |||
| 1916 | 65 | |||
| 1925-1926 | 147 | 1 | ||
| 1926 | 116 | 96 | ||
| Australia (Western Australia) | 1913-1930 | 105 | ||
| 1913 | 81 | |||
| 1914 | 26 | |||
| 1920 | 38 | |||
| 1921 | 21 | |||
| 1923 | 30 | |||
| 1927 | 29 | |||
| Australia (New South Wales) | 1926 | 31 | 96 | |
| China | 1942 | 1 | 34 | |
| Egypt | 1928 | 70 | 162 | |
| 1937 | 50 | 162 | ||
| Greece | 1927-1928 | 1,061-1,559b | 20, 30 | |
| India | 1913 | 1 | 33 | |
| Japan (Okinawa) | 1904 | 16 | 38 | |
| 1923-1931 | 38 | |||
| 1923 | 5 | |||
| 1924 | 5 | |||
| 1931 | 468-508b | 69, 101 | ||
| Japan (Nagasaki/Okinawa) | 1943-1944 | 4 | 66, 69, 171 | |
| Japan (Bonin Islands) | 1936 | 3 | 169 | |
| Lebanon | 1945 | Unspecified | 62 | |
| Northern Mariana | 1927-1929 | 3 | 100 | |
| Philippines | 1943 | 9 | 153 | |
| South Africa | 1927 | 60 | 37, 83 | |
| Taiwan | 1927 | 1 | 60 | |
| 1931 | 20-26b | 70, 111 | ||
| 1942-1944 | ||||
| 1942 | 1 | 85 | ||
| 1943 | 3 | 4, 41 | ||
| 1944 | 5 | 134 | ||
| United States (Texas) | 1897 | 1 | 140 | |
| 1922-1923 | 1 | 140 | ||
| United States (Florida) | 1934 | 3 | 48 | |
| Vietnam | 1895-1909 | 4 | 23, 160 |
Excluding 1922 to 1924, and the data for 1926 are incomplete. Only the individual years in which 15 or more deaths were recorded are listed.
The number of deaths varied among multiple reports. The values shown indicate the range.
FIG. 1.
Geographic locations of outbreaks of dengue and/or dengue-like illness, clustered in three 5-year periods. The fatal outbreak data used for this figure are based on Table 2, while the outbreaks without a fatality were derived from four sources (21, 49, 78, 137). Clustered outbreaks are shown in panels A (1897 to 1902), B (1926 to 1931), and C (1940 to 1944). The pattern of dengue spread and magnitude of morbidity/mortality in cluster C are most likely “unnatural” because of WWII and disrupted epidemiologic surveillance/reporting in many locations. Between clusters, dengue outbreaks occurred, but the numbers of fatal outbreaks were small in these intercluster periods. Red circles, outbreaks with mortality; green circles, outbreaks without a fatality. One circle (red or green) represents one or more than one outbreak superimposed, but the number of circles per location is not an indicator of the magnitude of outbreaks.
ANALYSES OF THE OUTBREAKS DOCUMENTING FATALITY
Clinical Characterization and Disease Nomenclature
The major interest in examining the clinical definition (or characterization), colloquial disease nomenclature practice, and position of dengue in disease classification is to find out if any of these factors, either alone or in combination, could have adversely affected recognition of atypical severe dengue syndrome and its fatal outcome in the early period.
Although establishing a clinical definition is an integral component of case definition in most epidemiologic investigations today, currently, the WHO does not recommend adoption of a detailed clinical definition (166). The early dengue history reveals ample reasons why the current recommendation has become necessary.
In much of the early dengue history, in particular before the 1870s, physicians relied on the characterization of dengue described earlier by authoritative figures of their choice. Variation in characterization by those authors was a frequent source of confusion or disagreement among practicing physicians over the exact clinical definition of dengue. Some of the critics came to conclude that dengue was a collection of multiple diseases, each with a different etiology but sharing many clinical manifestations of dengue (29). The definition of dengue by the Joint Committee of the Royal College of Physicians (London) in 1869 was the first attempt to standardize the clinical characterization of dengue. However, it was based primarily on clinical observations in the 1820s in the West Indies and India, where the severe syndrome was then rare and mortality almost nil (144, 147). The subsequent criticism of this definition was partially valid because the outbreaks, at least in India (1824 to 1825) and the West Indies (1827 to 1828) were later interpreted to be misidentification of CHIK (21). Carey's examination of old outbreak records revealed a significant shift in dengue characterization in the early period. In fact, the characterizations of dengue by such respected figures in medicine as T. Edmonston Charles and Leonard Rogers, whom many physicians in India relied on throughout much of the 19th century, could be, in retrospect, interpreted to be most likely those of CHIK for the description of its uniquely persistent arthralgia long after recovery from acute illness (21). On the other hand, the “seven-day fever” described by L. Rogers was most likely authentic dengue, according to Megaw (97). By the end of the 1940s, because of so much variation in dengue manifestation, it was clear to some that clinical diagnosis alone was inadequate to diagnose dengue (35).
In the early part of the 20th century, clinical characterizations based on human experiments (29, 142, 143) were often used as reliable guides in clinical diagnosis of dengue. The syndromes described in these studies fell within the typical classic dengue syndrome, and the occasional signs of hemorrhage included were mostly limited to mild epistaxis and petechiae. However, it is noted that the volunteers in those human experiments were all adults, and none died. This is an important point regarding the adequacy of using these characterizations for children in the early period, because, as is well known, most of the victims of post-1950 DHF have been children. Because of the prevailing characterization of dengue as nonfatal illness, fatal cases were sometimes dismissed as not dengue (61). Accordingly, confusion over the variation in disease characterization and the shift in definition over time were thought to have contributed to inaccurate reporting of dengue severity (91). Unfortunately, the extent of the problems of dengue characterization in morbidity and mortality statistics is not measurable due to the absence of reliable quantitative data or to the inadequate quality of epidemiologic surveys and recording in most locations in this period.
In regard to the problems associated with disease nomenclature and classification, the colloquial name “break-bone disease,” with its origin in the Caribbean in the 18th century (123), was criticized because it was considered an exaggeration of the symptom, as the proportions of patients complaining of such intensity of pain in the lumbar region, joints, and/or limbs were found to be rather small (12). Changes in disease classification also had an adverse effect. For example, in Australia, dengue-associated death records for a few years in the 1920s were lost, when those fatal cases were lumped together with of other infectious diseases (105).
Recognition of Atypical Severe Dengue Syndrome and Associated Death
Recognition of an atypical syndrome, in terms of sensitivity and rapidity of disseminating information on severe dengue, varied greatly from place to place and changed over time. It also depended on many other variables, including the level of medical practice, density and geographic distribution of physicians (affecting efficacy of detection), economic conditions, and a variety of cultural and societal traditions unique to the location. Another variables were interest in studying “atypical” syndrome and prevalent medical practice or tradition related to segregating “atypical” from “typical.” They were both influenced by the degree of freedom to debate or to challenge established medical dogma or authorities among medical professions. In Kolkata, India, a large number of physicians in the 1910s were vigilant in regard to the emergence of a new dengue-related syndrome, even though etiology of dengue was still debated and differential diagnosis problematic (6). However, this level of epidemiologic vigilance and liberal debate was rather exceptional in the tropics at that time.
For many years, dengue had been characterized essentially as a self-limiting, nonfatal acute febrile illness (13, 61). When investigations of the transmission mechanisms and etiologic agents of YF and dengue were launched by the U.S. government at the turn of the 20th century, for the investigation of deadly YF in Havana, Cuba, informed consent was required and a monetary reward ($100 per volunteer and an additional $100 if the volunteer developed YF) was offered. On the other hand, for the dengue investigation in Manila in the Philippines, no consent was required and the monetary reward was far smaller (84). As late as 1935, it was felt that public health officials in the government would not fund measures for dengue control, because dengue, unlike YF, was perceived as a nonfatal illness (141). Hare (58) criticized this prevalent notion among medical professionals, because he believed that the mortality rate (<0.1%) recognized for dengue at that time should not have been used to dismiss the importance of fatal cases. His feeling was shared by others elsewhere (48, 63, 97). It was thought that the excessive emphasis only on classic benign signs and symptoms deemed “typical” allowed severe forms of dengue and death to be overlooked (63, 97). The 1897 outbreak in Queensland, Australia, most likely marked the first organized recognition of the seriousness of dengue by many physicians because of a large number of severe cases resulting in mortality, which hardly resembled typical cases beyond the development of a rash (29).
Recognition of the importance of atypical symptoms and death, however, varied geographically. In the areas where the significance of Hare's report was recognized early (70), elucidation of the mechanism of extensive hemorrhage in animal models (38a) and in-depth studies of the hemorrhagic manifestation in dengue were conducted. In the latter studies, quantitative changes in vascular permeability and in the concentrations of coagulation factors were the subjects of interest (41, 59).
When a new syndrome evolves in an infectious disease, at first the new manifestation may occur only sporadically for a period, rendering its early recognition difficult, until a large number of the cases manifesting the new syndrome erupt in the form of an outbreak. The pre-1950 documentation of extensive dengue hemorrhagic manifestations with subsequent development of “heart failure” and coma before death is of interest. An extensive search of the past records documenting evidence of DHF-like syndrome in the Philippines before 1954 at first failed to uncover any precedent except for questionable cases of acute idiopathic thrombocytopenic purpura (18). However, another study suggested that before the outbreak in 1954, sporadic cases of fever with hemorrhagic manifestation had been recognized there under the name of “hemorrhagic influenza with circulatory failure” (154). In Thailand, although cases compatible with DHF were not discovered in hospital autopsy records prior to 1958 (17), compatible hospital records beginning in 1950 were indeed discovered (56). Interestingly, a local physician had a recollection of “fever with rash and collapse” or “influenza with circulatory failure” observed since 1935 (139). Although the exact etiologies of these puzzling earlier cases in the Philippines and Thailand remain unknown, they are nonetheless intriguing, because dengue had been also known in Europe as “tropical influenza” in the early period.
Reporting of Severe Syndrome and Death
The quality of reporting in health statistics and demographic registries in each location and of dissemination of medical information affects the level of recognition of the importance attached to severe dengue and mortality among physicians nationally and internationally. Historically, dengue was classified for the first time as a notifiable disease in Western Australia in 1912. However, because dengue cases were filed under multiple colloquial names, the value and accuracy of notification were seriously questioned (15). Even in a location with a far-advanced public health reporting system, such as the United States in 1934, it was estimated that only one in five dengue cases was officially reported at best (48). Another disturbing problem in the recorded mortality data in Australia in the first two decades of the 20th century was that some of the data most likely represented either an overreporting or an underreporting problem, because not all cases reported were examined by physicians (96).
When the cause of death was not immediately known, it is probable that the death certificate was used in some locations for preparing mortality statistics. However, very little was described in most publications regarding the criteria used in death certificates. Thus, it is not possible to assess the impact on the quality of reporting and recording practices or systems with regard to occurrence of severe dengue and death. In the International List of Causes of Death based on the Bertillon Classification (Third Decennial Revision of 1920), death due to dengue was not listed in an independent clause unless an epidemic reached the level of prevalence set by the List. In Australia, this problem was reflected in inaccuracies of cause-of-death classification in parts of the early period (96). Even when cause of death was filed in sufficient detail in such countries as Australia, generally it was found difficult to improve the sensitivity based on postmortem studies alone without the assistance of virus-specific laboratory tests which were not available before 1950 (96, 126, 130).
In the wake of the tragic 1928 outbreak in Greece, the 1935 International Convention (International Sanitary Convention) of the League of Nations required notification of dengue and quarantine of imported cases, but this was totally ineffective, as few countries in the tropics where dengue was endemic were invited to ratify, and some countries with strong interests in dengue (such as the United States) were not even members of the League (8, 105, 158).
Dissemination of Epidemiologic Information
Dissemination of information on the occurrence of severe dengue syndrome and death through regular channels of publication was also problematic, given the limited opportunities for publication for many physicians unaffiliated with prestigious institutions and the fewer number of medical periodicals then available. It has been recognized that inefficient dissemination often facilitates perpetuation of the lack of awareness regarding atypical syndrome among medical professions, resulting in fewer reports. In early dengue history, with the exception of the 1928 outbreak in Greece, most outbreaks of dengue-like illness resulting in hemorrhagic manifestation and a considerable number of fatalities (Tables 2 and 3) were generally not communicated sufficiently and rapidly enough to draw attention in many countries. Thus, when DHF outbreaks with high mortality occurred beginning in 1954 in the Philippines and thereafter in other Southeast Asian countries, the etiology was first sought among a list of viral hemorrhagic fevers that did not then include dengue (87a, 118).
TABLE 3.
List of etiologically more reliable “dengue” outbreaks
| Country (subdivision) | Yr | No. of deathsa | CFR/1,000 | Age group(s) | Reference(s) |
|---|---|---|---|---|---|
| Australia (Queensland) | 1897 | 60b | 1.0 | Children, 50%; adults, 50% | 58 |
| Australia (Brisbane) | 1905 | 201 | 1.0-1.5 | <5 yr, 37.6%; >60 yr, 35.5% | 11 |
| Egypt | 1937 | 50 | 19.3 | ? | 162 |
| Greece | 1927-1928 | 1,210, 1,061 | 1.6, 1.6 | <16 yr, 6.62%c; >59 yr, 59.8%c | 30, 113 |
| Japan (Okinawa) | 1931 | 468 | 4.3 | ? | 112, 170 |
| South Africa | 1927 | 60 | 1.2 | ? | 37, 64, 83 |
| Taiwan | 1931 | 26 | ? | Unknown, but 9 of 11 dead were children | 3, 111 |
| United States | 1934 | 3 | 1.8 | Adults, 100% | 48 |
The total numbers of patients correspond to the numbers in the references cited but are not necessarily the highest reported numbers shown in Table 2.
Number of patients personally examined out of a total of 97 deaths.
Mortality figures are for 2 months (August and September 1928).
International information dissemination regarding the occurrence of severe dengue syndrome and death was most likely hampered not only by the less advanced communication technology then available but also by the difficulty of accessing publications in many tropical locations and by language barriers, because publications in non-European languages (Table 2) were rarely translated. Even when epidemiologic news of high mortality in Asia was translated, the very brief and anonymous news items hardly caught the attention of most dengue specialists (9). The interruption of international communication due to WWII did not help to improve dissemination either.
Problems Associated with Exclusion of Nondengue Diseases Sharing Dengue Syndrome
No specific laboratory confirmation tests were performed in dengue diagnosis in the early period to exclude involvement of other viral diseases presenting similar disease syndromes, and the tests performed to exclude parasitic and bacterial infections were primarily blood smear examination and a limited number of bacteriologic tests (including agglutination tests) for excluding malaria and bacterial infections, respectively. In the absence of reliable laboratory techniques in much of the early period, exclusion of the involvement of other viral diseases depended heavily on a combination of differential diagnosis, epidemiologic data, vector data, and accumulated knowledge obtained through experience.
Among many infectious diseases that manifest dengue-like syndrome, possible misidentification of CHIK in the early dengue records, which was raised by Carey (21), presented the most serious etiologic question. Indeed, many “dengue” outbreaks identified as probable cases of misidentification in his study fit the clinical characterization of CHIK. However, it is important to recognize the limitation of differential diagnosis of dengue and CHIK. The most important differential clinical feature used by Carey was persistent arthralgia long after the acute phase of illness, which is rare in dengue but highly prevalent in CHIK. Although persistent arthralgia unquestionably is a characteristic of CHIK, strictly speaking it is not an absolute criterion for differentiation from dengue. For example, in one of the few such studies of adult DHF patients confirmed serologically, nearly 10% of the former patients still complained persistent arthralgia 6 months after the acute phase (46). In another laboratory-confirmed dengue case, arthralgia persisted for over a year (159). Also, in the 1905 outbreak in Australia and in the 1912 outbreak in India, which were both classified as true dengue by Carey (21), patients with persistent synovitis were documented in the former outbreak and persistent arthralgia 5 months after the attack was described in the latter outbreak (11, 12, 63).
The other possible source of confusion is concurrent outbreaks of CHIK and dengue. Concurrent outbreaks of these two viruses have occurred more than several times in tropical Asia and Africa after the 1950s alone, and sometimes both viruses could be isolated from the same patient (21, 24, 106, 107, 109, 120). It is noted that all these epidemics occurred in locations in the tropics where dengue was endemic and CHIK virus was introduced temporarily. This is based on the fact that the latter virus, unlike DENV, is not known to establish perpetual endemicity year after year in a fixed urban area (80). It is uncertain whether concurrent outbreaks of those two viruses similarly occurred in North America in the past, but temporal overlap of CHIK and dengue outbreaks in 1827 in Charleston, South Carolina (according to the interpretation by Carey [21]), is intriguing.
If the reclassification of many early “dengue outbreaks” as “CHIK outbreaks” by Carey (21) was entirely correct, the negative impact would be felt even in etymology, since it must be assumed that the word “dengue” was adopted in Cuba (123) during a CHIK outbreak in 1827 to 1828. Based on the aforementioned problematic issues, however, caution should be exercised in generalizing the conclusion made by Carey to all outbreaks covered in his report. The significance of the concern by Carey for differential diagnosis between these two viruses is considerable in tropical countries, because both diseases are hemorrhagic and because concurrent outbreaks of the two viruses occasionally occur. Under such an epidemiologic condition, a careful examination of hemorrhagic hospitalized patients was necessary (109). This concern is no less significant even in the United States because the numbers of imported cases of the two viral infections increased recently. Furthermore, in Europe, where the number of imported cases of dengue has also been rising similarly, autochthonous transmission of CHIK was reported recently for the first time in modern history.
Short List of Etiologically More Reliable Outbreaks
When the aforementioned set of stringent criteria was applied to exclude the outbreaks of questionable etiology, a short list of “probable dengue” or “confirmed dengue” was obtained (Table 3). According to the criteria set forth, however, only the 1928 outbreak in Greece is classified as confirmed dengue, while the others are classified as probable dengue. Among the notable outbreaks that were not selected in Table 3 are the 1926 outbreak in Australia with a large number of fatalities, the 1945 outbreak in Lebanon, and numerous and almost yearly mortality figures registered in health statistics in Australia between 1906 and 1926, all because of lack of information in regard to one or more stringent criteria.
CFR.
Evaluation of case fatality rate (CFR) data in the early period for studying a variety of research questions in infectious disease investigations, such as effectiveness of medical care and variation of or temporal change in virulence of a virus, is difficult because of the aforementioned inaccuracy of epidemiologic reporting. Even today, there are many kinds of variables that affect the computation and significance of dengue CRFs, including the location, age group, choice of denominator (all dengue infections versus DHF cases only), year of sampling, virus serotype/genotype involved, level of medical care provided, economic level of the community, and others.
The issue debated in the early period was at what level of mortality a CFR for dengue would be considered truly negligible. Hare (58) criticized the insufficient recognition of severe dengue just because the generally accepted CFR (per 1,000), which had been estimated by Patrick Manson, was less than 1.0. The highest estimate was 5.0 (86). Table 3 shows that although all CFRs were greater than 1.0, except for the outbreak in Egypt in 1937 (at 19.3), all other CFRs among the outbreaks in the short list (Table 3) fell within the pre-1950 range. As far as the importance of the CFR (per 1,000) in recognizing the seriousness of dengue-associated death is concerned, the post-1950 data also provide interesting information, even though the impact of improved medical care in the post-1950 outbreaks needs to be considered in such a comparative study. Available data based on total dengue infections (sum of DHF and classic dengue fever cases) in locations experiencing various degrees of established occurrence of DHF were selected for comparison. The results show that CFRs (per 1,000) in Malaysia in 1987 to 1992, in Puerto Rico in 1992 to 1996, and in Rio de Janeiro (Brazil) in 2001 to 1002 were 4.3, 0.73, and 0.73, all falling within the pre-1950 range (22, 117, 126).
Age group.
Table 3 shows that fatalities occurred in all age groups. However, in much of the early period, occurrences of severe cases of dengue and mortality in children and the aged were notable (11, 61). This pattern was consistently observed in the outbreaks not selected for Table 3 (such as the 1926 outbreak in Australia, where children and elderly patients represented 22.4 and 41.5% of fatal cases, respectively [96]). In contrast, in the post-1950 outbreaks, DHF-associated death occurred predominantly in children, although death in adult patients was also recognized (108, 154). However, in the 1990s and thereafter, the median age of DHF patients and the proportion of fatality in certain age groups (>15 years) both rose in many countries where the disease is endemic (43, 155).
Symptoms or syndromes in severe dengue.
The major symptoms in the outbreaks with fatal cases listed in Table 3 were a variety of hemorrhagic manifestations, ranging from milder epistaxis to extensive gastrointestinal bleeding. They were sometimes (but not always) followed by rapid “heart failure” and/or irreversible collapse. Convulsion associated with hyperpyrexia was observed more often in children than in adults, and some of the patients subsequently fell into a state of somnolence or coma (4, 7, 10-12, 111).
The report of the 1897 outbreak in Charters Towers, Queensland (58), has often been credited as the first documentation of severe dengue syndrome most compatible with the currently recognized DHF (1, 51), except that a few measurements (such as platelet count and narrowing of pulse pressure) required in the current WHO definition of DHF (166) were never obtained in this period. A similar syndrome was also described elsewhere (37, 111, 134). Deaths associated with hemorrhagic manifestation and heart failure were also documented in other outbreaks that were not selected for the Table 3 due to the absence of documentation of a few stringent criteria required (31, 62). Heart problems, often characterized as irregular and/or slow heartbeat, were also reported in severe hemorrhagic (but nonfatal) cases as well (31). In other cases, extensive blood loss necessitated blood transfusion to save lives (67, 135, 170). Upon autopsy, an enlarged liver, extensive hemorrhage in the cardiovascular system, and degeneration of the myocardium were observed (66, 99, 115).
Like DHF, pathologies and dysfunctions of the central nervous system (CNS), such as cerebral hemorrhage and paraparesis, were documented in some of the severe cases listed in Table 3 (7, 11, 12, 92, 96, 101) as well as in the outbreaks not selected for the short list (153). Pulmonary complications in fatal cases were recognized particularly in children and elderly patients (11). In the outbreak in Egypt, 11 of the 50 fatal cases presented a “pneumonia-like” syndrome (162).
Comorbidity.
Development of a severe syndrome in elderly patients suffering from chronic illness was recognized early in dengue history (61). During the 1897 outbreak in Australia, Hare (58) observed that many fatal cases in elderly patients were associated with underlying illness or addiction, such as diabetes, chronic bronchitis, and alcoholism. Elsewhere, manifestations of the deleterious effects of dengue was thought to develop more frequently in elderly patients with such chronic illnesses as cardiac conditions, tuberculosis, or nephritis (71, 86, 140). This is interesting because, as mentioned earlier, higher mortality in elderly patients was considerable in the early period. In the post-1950 period, too, chronic illnesses (such as diabetes and asthma) were found to be associated with dengue-associated deaths (73, 82, 126). The major uncertainty regarding the importance of comorbidity, however, has always been whether dengue was the primary or secondary cause of death.
Human population size, demographic shift, and heavy human traffic.
Historically, dengue outbreaks in the early period occurred in human populations of considerable size that were located along coastal areas served by ships, the primary means of long-distance travel at that time. A large population size, a rapid increase in the human population in tropical countries (47, 164), and heavy human traffic with tropical countries where dengue was endemic are three important demographic factors useful for analyzing the epidemiologic background behind the more frequent occurrence of a large number of fatal cases associated with severe dengue outbreaks in the early dengue history (Tables 2 and 3).
The numerous outbreaks that occurred in Queensland, Australia, beginning in the 1890s were preceded by a few decades of rapid increase in population, as large numbers of settlers began to move in during the second half of the 19th century, stimulated by the discoveries of mineral deposits and other new economic opportunities. The population of Brisbane, often the epicenter of repeated outbreaks, increased sharply from 28,000 around 1860 to over 126,000 by 1905, when a huge outbreak with many deaths occurred (Table 3). The population of Queensland similarly increased from 213,000 around 1880 to far more than 425,000 in 1926, when another severe outbreak claimed 116 casualties (Table 2). In Greece, beginning in 1923, major cities (such as Athens and Piraeus, in particular) experienced an influx of more than one million (out of a few million) repatriates immigrating from Turkey in the aftermath of the loss in the Greco-Turkish War, which ended an year earlier. This influx severely taxed the drinking water supply and sanitation capacity of the Greek cities. Within only 5 years the tragic outbreak with more than 1,000 fatalities struck the region (Table 3). Large population size was a shared condition in other major outbreaks with significant fatalities as well. Cairo, Egypt, already had more than 1.4 million inhabitants during the major outbreak in 1937, and the population in Okinawa, Japan, during the 1931 outbreak was 482,000. The southern region of Taiwan, which was hit repeatedly by dengue outbreaks, had a population of only 170,000 in 1931 but was a busy trading center with heavy human traffic with tropical countries. It is also emphasized that the DHF outbreaks in the 1950s were separated by only about a decade from WWII, in which a very complicated human movement of enormous size (including military personnel and displaced civilians) and in many directions took place in the tropical and semitropical regions of Asia and the Pacific, facilitating virus dispersal, as reviewed earlier (78).
This strong association between heavy human traffic and occurrence of dengue pandemics had been recorded even before the 60-year period of this study. The 1870 to 1873 pandemic is a good example. This pandemic began in east Africa and swept through the Indian Ocean islands, tropical Asia, and even Oceania. Some of the outbreaks during this pandemic are now strongly suspected to be CHIK, as described elsewhere in this review. Regardless, it is important to note that both diseases are hemorrhagic and transmitted by the same mosquito vector (A. aegypti) and that the completion of the Suez Canal in 1869 immediately opened the floodgate of human migration from Europe to tropical Asia in the days of colonialism. This event was followed by the establishment of dengue endemicity in the colonized Asian tropics, as revealed by Gordon Smith (47). Regarding the conspicuous absence of dengue outbreaks during this pandemic in Thailand, which was surrounded by the countries ravaged by the outbreak, one of the possible explanations is that Thailand was one of the very few countries in the tropics not colonized by the Western powers (and which hence had much less trade and human traffic with the neighboring countries), because of the strong resistance to Western domination by King Chulalongkorn (Rama V).
When the outbreak records in Table 2 are examined chronologically, another important set of information is obtained. In Fig. 1, for the purpose of clear understanding of dengue spread, the geographic locations of the fatal outbreaks are grouped (clustered) in three 5-year periods (A, B, and C) and are superimposed on the maps of the locations of nonfatal outbreaks that occurred in the corresponding period. Grouping of fatal outbreaks in three cluster periods is possible because most (if not all) such outbreaks (Table 2) occurred within a short span of time (within about 5 years) over a vast geographic area in a pattern resembling a pandemic, disappeared, and again occurred in clusters in another short time frame. Figure 1 reveals that the geographic locations of fatal outbreaks in tropical areas varied from cluster to cluster and showed a lack of persistent occurrence in any fixed location. Also, the intervals between clusters became shorter over time, since there was approximately 24 years between periods A and B and 9 years between periods B and C (Fig. 1). In the post-1950 outbreaks, this interval became even shorter. Also, in contrast to the pre-1950 outbreaks, fatal outbreaks after 1950 began to occur more regularly in fixed tropical locations, indicating established persistence in urban centers.
Historically, outbreaks of dengue-like illness occurred repeatedly in the pattern of a pandemic and spread over a wide geographic area, sometimes in two continents or over the Old and New Worlds in a short span of time. The basically nonfatal pandemics were recorded in 1779 to 1784, 1823 to 1829, 1844 to 1856, and 1870 to 1875 (27, 49). When an urban outbreak pattern is closely examined, even within one large population center (such as Bangkok, Thailand), the spread of a DHF outbreak can be captured mathematically as waves spreading at much shorter intervals from a presumed epicenter (32).
COMPATIBILITY OF INFORMATION FOUND IN THE OLD DENGUE LITERATURE WITH CURRENT THEORIES ON MECHANISMS INVOLVED IN EMERGENCE OF SEVERE DENGUE SYNDROME BEGINING IN THE 1950s
For elucidating the mechanisms involved in the occurrence of DHF outbreaks, two groups of interacting mechanisms have been proposed. The first group includes all intrinsic mechanisms that occur in humans after viral infection. The second group includes extrinsic mechanisms entailing epidemiologic predisposition and/or eco-virologic modifications. They facilitate dynamic qualitative changes in the composition of the viral population, frequency of human exposure to virus, host factors, and perpetual occurrence of DHF outbreaks in urban areas. The lack of relevant data in the early dengue documents precludes discussion of most intrinsic mechanisms, in particular those that require isolated viruses and knowledge of the immunologic status of the patients. Other missing data are induction of pathophysiologic modulators, a variety of host factors (including genetic traits), and interactions between virus and host, including antibody-dependent enhancement. Nevertheless, the following data that have been extracted from early documents are quite useful for evaluating the compatibility of some intrinsic and extrinsic mechanisms.
Broad Spectrum of the Clinical Manifestation of Dengue
One of the questions immediately raised after the sudden emergence of fatal outbreaks in the 1950s was whether it was absolutely a new disease. Once the dengue etiology was established, the second question raised was whether emergence of the severe syndrome was a consequence of a change in viral virulence or a manifestation of a previously poorly recognized syndrome which had always existed within a broad spectrum of the clinical characteristics but which had not been conspicuous before the 1950s (a comment by Albert B. Sabin, quoted by Hammon et al. [57]). If this theory is valid, then our natural curiosity is to identify what changes facilitated the dramatic increase in the expression of this previously infrequently recognized viral trait.
In flavivirus research, two contrasting sets of close correlations among virus group, vector group, and disease syndrome were recognized. In one set, comprising YFV and DENV, which present hemorrhagic manifestations, the viruses are transmitted by Aedes mosquitoes. In the other set, comprising the Japanese encephalitis virus group of viruses, which cause a CNS syndrome, the viruses are transmitted by Culex mosquitoes (95, 133). This early impression was more recently confirmed phylogenetically (42).
Although this conventional virus grouping based on a shared disease syndrome for a particular flavivirus group (hemorrhagic versus neurotropic viruses) proved to be generally useful, a close examination of the relationship revealed that the distinction between the two sets was not absolute. For example, hemorrhagic manifestations have been reported in a small number of patients with “neurotropic” viruses, such as Japanese encephalitis virus, St. Louis encephalitis virus, tick-borne encephalitis virus, and West Nile virus (WNV) (5, 39, 75, 103, 156). When the clinical spectrum is focused on just one virus, such as WNV, the broad spectrum becomes even more evident, since laboratory-confirmed WNV infections with additional atypical symptoms (such as hepatitis and pancreatitis) have been reported.
Conversely, among the “hemorrhagic viruses,” neurologic manifestation of YF virus has been long known, as demonstrated in cerebral pathologies and isolation of virus from the CNS (102, 114, 148, 168). The current classification of Kyasanur Forest disease virus as only a hemorrhagic virus is problematic because of high proportions (as many as 45%) of patients additionally experiencing a neurologic syndrome (2). Thus, in a recent publication, Kyasanur Forest disease virus was listed in lists of both hemorrhagic and neurotropic viruses (165). The number of publications reporting neurologic disorders, including CNS dysfunctions, in dengue patients now exceeds 200, even though no definitive evidence of viral replication in the CNS has ever been found (90, 119).
Other Viral Factors
A change in viral virulence as a cause of a shift in disease severity has been proposed for dengue. The recent dramatic increase in mortality and development of neurologic, cardiac, and other unusual syndromes in CHIK since 2005 may follow the example set by DENVs. In more recent publications, the importance of virulence variation among DENV strains was supported at least under laboratory conditions (87, 122, 163). However, early documents on dengue are basically unsuitable for locating data relevant for the discussion of this hypothesis due to a near total absence of virus isolation. Nevertheless, other related observations were subjects of interest among the early investigators.
An intraepidemic shift in the severity of dengue was recognized by physicians at multiple locations during this period. One of the reasons that led them to this suspicion was the observation that for much of the early part of an epidemic, only nonnatives (mostly temporary residents such as foreigners) became ill, but in the late stage of the epidemic, even the natives, who were thought to be immune to dengue through exposure to the virus in the early years of life, became infected (12). It should be noted that little was then known about immunity conferred by dengue or the virus serotype involved. Hossack (63) conceived the possibility that a more virulent pandemic form of dengue would suddenly emerge from the existing sporadic and endemic form. Clayton (28) also speculated about transformation from a benign to a more virulent form of the dengue pathogen through passages in humans. Interestingly, even after the 1950s, similar suspicious intraepidemic shifts in virulence were reported (25, 74, 127, 150), although the conclusion obtained in one report was negative (125). The possibility that virulence differences among serotypes may explain the differences in dengue severity observed in the early period was raised recently. According to this reanalysis of the classic human experiments conducted in the Philippines in 1924 and 1930, the severity of infection by DENV-1 was significantly greater than that of infection by DENV-4 in that period (110).
Multiple Outbreaks Preceding the Emergence of Fatal Cases
As DENV is the only arbovirus totally adapted to an urban environment for perpetual transmission between mosquito and human and which does not depend on any other hosts as reservoirs for the source of virus (80), the occurrence of severe dengue has been inseparable from demographic changes. With the exception of the 1927 outbreak in South Africa, an epidemiologic condition shared among the major outbreaks with extensive fatalities listed in Table 3 is multiple preceding outbreaks of dengue or dengue-like illness. The data in a bibliographic database (77) reveal that in Queensland, Australia, before the outbreak of 1897 at least four outbreaks had been recorded. In the 1897 outbreak in Queensland and Thursday Islands, in some patients who had experienced dengue-like illness 2 years earlier, symptoms were far more severe than in the first episode (58). In Greece, the number of outbreaks prior to the 1927 to 1928 outbreak was 4, and the corresponding numbers in Egypt before the 1937 outbreak and in Japan (Okinawa) and Taiwan before the 1931 outbreaks were at least 3, 9, and 15, respectively. Among the majority of physicians in Okinawa who had attended dengue patients in many earlier outbreaks over years, the strong consensus was that the disease severity suddenly turned worse in the 1931 outbreak (101).
In Indonesia, among the patients who experienced multiple infections (three or four infections in 4 years), the second attack was generally milder than the first attack, and, in turn, the third or fourth attack was milder than the second attack (71, 94). However, because the number of past dengue infections could not be determined scientifically in early dengue history due to lack of serologic techniques coupled with the absence of the concept of asymptomatic infection, the exact number of exposures to DENV in “second attacks” in the early history remains unknown. Nonetheless, it is interesting because the severity of at least third and fourth dengue infections was recently found to be less than that in the second attack (44).
Combination of Predisposing Demographic and Virologic-Ecologic Factors
According to a prevalent current hypothesis (50), a combination of demographic factors (the sharp increase in the number of large urban centers in the tropics and accelerated human traffic with tropical countries in the jet age, with a background of rapid economic recovery after WWII) increased the probability of the arrival of viremic travelers at vector-infested destinations. This facilitated the establishment of multiple serotype coexistence, dynamic change in the viral population, and host immunity changes through repeated introduction of viruses.
If DENVs indeed possessed the potential to induce severe disease manifestations throughout the early history, as considered earlier in terms of broad disease spectrum, the low and infrequent incidence of mortality in the early period is explained by the very small number of large urban centers, much less human traffic between areas of endemicity, and slower human movement by ship. This combination of extrinsic conditions severely limited changes in the composition of the virus population per given location and the frequency of occurrence of severe dengue. The dramatic post-WWII changes in demographic conditions are thought to have facilitated an increase in the absolute sizes of susceptible host populations, viral serotype/genotype coexistence, and increased exposure of subpopulations with previous dengue experience to dynamically changing virus populations. These factors are manifested in the dramatic increase in the number of huge human population centers, urbanization of rural communities, and sharply increased frequency of outbreaks in subtropical regions due to imported cases as a result of more frequent human movement with tropical countries. The establishment of perpetual outbreaks in inland communities is explained by improved road networks and ground transportation systems as well as accelerated air travel (in contrast to the outbreaks limited mainly to coastal port cities in early dengue history). These changes occurred dramatically after WWII, as previously studied (79, 164). Thus, these demographic changes in urban areas and accelerated human movement are compatible with and corroborate the current hypothesis. The current hypothesis is based on the combination of established hyperendemicity of virus serotypes/genotypes and explosive growth of high-density urban centers as a predisposing factor to explain the perpetual occurrence of large DHF outbreaks after 1950 (50, 81, 159). The historical records in this review further support that these conditions as predisposing factors had been operating well before the 1950s, albeit on a limited scale. The analyses of the early history show the importance of the extrinsic mechanism far more clearly through a sharper contrast between pre-1950s and post-1950s conditions than between two (early and later) periods after the 1950s, as analyzed frequently by many (81).
CONCLUDING REMARKS
Etiologically speaking, dengue history started out on shaky ground. If the etiologic question raised by Carey (21) was found to be entirely correct, it would appear that both the first substantial clinical characterization in 1779 by David Bylon in Batavia, Indonesia, and subsequent adoption of the word “dengue” for this disease in the 1820s in Cuba were seriously suspected to have been based on the observations of CHIK rather than on authentic dengue. If so, the dengue history is also paradoxical, because human experiments had been conducted without a knowledge of the etiologic agent, using acute-phase blood samples of patients presenting “dengue” symptoms, and yet retrospective neutralization tests on the participants in the earlier human experiments (142, 143) established beyond doubt that the infectious agents used were authentic DENVs (54). Similarly, Aedes aegypti was identified as the true urban vector of dengue nearly 40 years before virus isolation (16), again using infectious blood samples derived from patients with “dengue-like illness.” In all these important human experiments, the infectious blood samples used had been derived from patients presenting “dengue” syndrome, the very source of etiologic uncertainty and diagnostic controversy throughout the early period.
The other major puzzle in dengue history is the “sudden” emergence of the life-threatening syndrome DHF after 1950. When a disease syndrome changes significantly over time, it is prudent to study what the disease was like before the change, which is more or less analogous to scrutinizing the properties of “controls” in scientific experiments to better understand the differences recognized after experimentation. Thus far, reviews of historical documents have been scarce and limited to specific issues, such as geographic and chronologic information, the etiologic question, hemorrhagic manifestation similar to DHF, viral evolution deduced from phylogenetic study, or the economic cost of outbreaks (1, 19, 21, 49, 55, 108, 159). However, with the exception of the etiologic confusion raised by Carey (21), no critical analysis encompassing multiple background factors related to dengue severity and death in the early history has been published.
The importance of examining early dengue history with respect to clinical syndrome was recently raised again when a large number of observations of maculopathies were reported as new dengue syndromes that emerged after 1980 (14, 23a, 26, 88, 151). However, maculopathies, including central scotoma and total blindness, had been documented repeatedly in various locations in early dengue history (37, 76, 89, 131, 161, 172). Also, the reading of old dengue documents for this review uncovered a record of 25 deaths (including 20 children) during the outbreak of CHIK-like illness in India in 1872 (93). If the CHIK etiology of this outbreak was confirmed, this record of fatality would predate the reports of high rates of death during the recent (2005 to 2006) CHIK outbreaks.
This review revealed many troubling issues complicating the analyses of dengue severity and mortality in the early period, including the problems of clinical definition of dengue, recognition, reporting, and dissemination of medical information. However, even after discounting the value of the data in old outbreaks because of a lack of absolute certainty of etiology, inaccuracy in epidemiologic data, and many other related problems, collectively it still is highly probable that outbreaks of dengue causing a severe syndrome occurred in many locations more frequently than it had been assumed before. Also, the fatalities in some old outbreaks were not negligible. Furthermore, the clinical syndromes of the severe cases in some outbreaks, including those in Australia around the turn of the 20th century, in South Africa in 1927, in Greece in 1928, in Taiwan in 1931, and elsewhere, were clinically very much compatible with the current DHF. These data also generally agree with a recent conclusion that DHF did not emerge as a new dengue syndrome around the 1950s (53). Accordingly, miscellaneous attributes of the old outbreaks and other background data examined in this review, ranging from epidemiologic to virologic, should be valuable sources of information for more clearly understanding the mechanisms by which DHF emerged and became the most important vector-borne viral disease, affecting many millions of people annually.
Acknowledgments
I thank José G. Rigau-Pérez of San Juan, Puerto Rico, for reviewing a draft and providing me valuable comments during manuscript preparation.
Biography
 Goro Kuno (Ph.D.) developed a special interest in the diseases of insects and had his graduate training first at the University of California at Berkeley and later at the Ohio State University, Columbus, OH. Because of the degree requirements in entomology and microbiology at the two institutions, he received a broad education in both fields. He joined the Centers for Disease Control and Prevention (CDC) as a research microbiologist in San Juan, Puerto Rico, in charge of laboratory diagnosis and later continued research in Colorado, for a total of 33 years at the CDC. Recently, he donated his personal collection of dengue publications (over 11,500 references published in any language) to the Armed Forces Pest Management Board (AFPMB). Once the references are digitized, full texts of most (if not all) references are to be made accessible with a search engine to the public at the AFPMB's website (http://www.afpmb.org), a repository of references of vector biology, control, and vector-borne diseases.
Goro Kuno (Ph.D.) developed a special interest in the diseases of insects and had his graduate training first at the University of California at Berkeley and later at the Ohio State University, Columbus, OH. Because of the degree requirements in entomology and microbiology at the two institutions, he received a broad education in both fields. He joined the Centers for Disease Control and Prevention (CDC) as a research microbiologist in San Juan, Puerto Rico, in charge of laboratory diagnosis and later continued research in Colorado, for a total of 33 years at the CDC. Recently, he donated his personal collection of dengue publications (over 11,500 references published in any language) to the Armed Forces Pest Management Board (AFPMB). Once the references are digitized, full texts of most (if not all) references are to be made accessible with a search engine to the public at the AFPMB's website (http://www.afpmb.org), a repository of references of vector biology, control, and vector-borne diseases.
REFERENCES
- 1.Aaskov, J. G. 2003. Dengue. Aust. Defense Force Health 4:66-71. [Google Scholar]
- 2.Adhikari Prabha, M. R., M. G. Prabhu, C. V. Raghuveer, B. Muktha, and M. A. Mula. 1993. Clinical study of 100 cases of Kyasanur Forest Disease with clinicopathological correlation. Indian J. Med. Sci. 47:124-130. [PubMed] [Google Scholar]
- 3.Akashi, K. 1932. An investigation of the outbreak of dengue in the southern regions of Taiwan, 1931. Taiwan-Igakukai-Zasshi 31:767-777. (In Japanese.) [Google Scholar]
- 4.Akashi, K., and E. C. Lim. 1943. Recent observations of hemorrhagic manifestations and psychiatric disturbances in dengue patients. Taiwan-no-Ikai 42:1255. (In Japanese.) [Google Scholar]
- 5.Anderson, R. C., K. B. Horn, P. Hoang, E. Gottlieb, and B. Bennin. 2004. Punctate exanthem of West Nile virus infection: report of 3 cases. J. Am. Acad. Dermatol. 51:820-823. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. 1913. Dengue or seven-days fever. Discussion at the Asiatic. Indian Med. Gaz. 48:199-202. [Google Scholar]
- 7.Anonymous. 1898. Medical society of Queensland. Discussion on dengue fever. Australas. Med. Gaz. 17:130-131. [Google Scholar]
- 8.Anonymous. 1929. Plan for international agreement regarding dengue. Publ. Hlth. Rept. 44:2106-2107. [Google Scholar]
- 9.Anonymous. 1932. The prevention of dengue fever. JAMA 98:1201. [Google Scholar]
- 10.Anonymous. 1898. Proceedings of branches. Queensland branch of the British Medical Association. Australas. Med. Gaz. 17:124-126. [Google Scholar]
- 11.Anonymous. 1905. Report on the dengue epidemic in Brisbane in 1905. J. Trop. Med. 8:355-363. [Google Scholar]
- 12.Anonymous. 1905. Reports on the dengue epidemic in Brisbane in 1905. Australas. Med. Gaz. 24:616-624. [Google Scholar]
- 13.Armstrong, C. 1923. Dengue fever, no. 856. U.S. Government Printing Office, Washington, DC.
- 14.Bacsal, K. E., C.-L. Cheng, and J. V. P. Flores. 2007. Dengue-associated maculopathy. Arch. Ophthalmol. 125:501-510. [DOI] [PubMed] [Google Scholar]
- 15.Bancroft, T. L. 1912. On a proposed technique for the prevention of dengue fever and filariasis. Australas. Med. Gaz. 31:80-81. [Google Scholar]
- 16.Bancroft, T. L. 1906. On the aetiology of dengue fever. Australas. Med. Gaz. 25:17-18. [Google Scholar]
- 17.Bhamarapravati, N. 1997. Pathology of dengue infections, p. 115-132. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 18.Caldito, J., and C. M. Tiongson. 1967. Was Philippine hemorrhagic fever present before 1954? Philippine J. Pediatr. 16:294-302. [Google Scholar]
- 19.Canyon, D. V. 2008. Historical analysis of the economic cost of dengue in Australia. J. Vector Borne Dis. 45:245-248. [PubMed] [Google Scholar]
- 20.Cardamatis, J. P. 1929. La dengue en Grece. Bull. Soc. Pathol. Exot. 22:272-292. [Google Scholar]
- 21.Carey, D. E. 1971. Chikungunya and dengue: a case of mistaken identity? J. Hist. Med. 26:243-262. [DOI] [PubMed] [Google Scholar]
- 22.Casali, C. G., M. R. Pereira, L. M. Santos, M. N. Passos, B. P. Fortes, L. I. Ortiz-Valencia, A. J. Alexandre, and R. A. Medronho. 2004. A epidemia do dengue/dengue hemorragico no municipio do Rio de Janeiro, 2001/2002. Rev. Soc. Bras. Med. Trop. 37:296-299. [DOI] [PubMed] [Google Scholar]
- 23.Caubet, P., H. Beisseige, R. Netter, and L. Carloz. 1956. Diagnostic de la dengue a Saigon. Bull. Soc. Pathol. Exot. 49:345-353. [PubMed] [Google Scholar]
- 23a.Chan, D. P., S. C. Teoh, C. S. Tan, G. K, Nah, R. Rajagopalan, M. K. Prabhakaragupta, C. K. Chee, T. H. Lim, and K. Y. Goh. 2006. Ophthalmic complications of dengue. Emerg. Infect. Dis. 12:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee, S. N., S. K. Chakravarti, A. C. Mitra, and J. K. Sarkar. 1965. Virological investigations of cases with neurological complications during the outbreak of haemorrhagic fever in Calcutta. J. Indian Med. Assoc. 45:314-316. [PubMed] [Google Scholar]
- 25.Chen, H.-L., S.-R. Lin, H.-F. Liu, C.-C. King, S.-C. Hsieh, and W.-K. Wang. 2008. Evolution of dengue virus type 2 during two consecutive outbreaks with an increase in severity in southern Taiwan in 2001-2002. Am. J. Trop. Med. Hyg. 79:495-504. [PubMed] [Google Scholar]
- 26.Chlebicki, M. P., B. Ang, T. Barkham, and A. Laude. 2005. Retinal hemorrhages in 4 patients with dengue fever. Emerg. Infect. Dis. 11:770-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie, J. 1881. On epidemics of dengue fever: their diffusion and etiology. Glasgow Med. J. 16:161-176. [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton, F. H. A. 1910. Notes on seven-day fever of the eastern ports: its occurrence in the Navy and its relationship to dengue. J. R. Army Med. Corps 14:171-198. [Google Scholar]
- 29.Cleland, J. B., B. Bradley, and W. M. McDonald. 1918. D engue fever in Australia. J. Hyg. 16:317-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copanaris, P. 1928. L'epidemie de dengue en Grece au cours de l'ete 1928. Bull. Offic. Int. Hyg. Publ. 20:1590-1601. [Google Scholar]
- 31.Cozanet. 1910. Sur deux epidemies de dengue a Noumea. Ann. d'Hyg. Med. Colon (Paris) 13:485-499. [Google Scholar]
- 32.Cummings, D. A. T., R. A. Irizarry, N. E. Huang, T. P. Endy, A. Nisalak, K. Ungchusak, and D. S. Burke. 2004. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature 427:344-347. [DOI] [PubMed] [Google Scholar]
- 33.Dalsukhram, G. 1913. Dengue in Guzrat. Indian Med. Gaz. 48:451-452. [PMC free article] [PubMed] [Google Scholar]
- 34.Dann, B. S. 1944. A dengue outbreak in Guangdon, China 1944. Taiwan-no-Ikai 2:721-726. (In Japanese.) [Google Scholar]
- 35.Dinger, J. E. 1948. Studies in dengue fever, p. 526-535. In Proc. 4th Int. Congr. Trop. Med. Malaria, vol. 1. [PubMed] [Google Scholar]
- 36.Doherty, R. L., and J. G. Carley. 1960. Studies of arthropod-borne virus infections in Queensland. II. Serological investigations of antibodies to dengue and Murray Valley encephalitis in eastern Queensland. Aust. J. Exp. Biol. 38:427-440. [DOI] [PubMed] [Google Scholar]
- 37.Edington, A. D. 1927. “Dengue,” as seen in the recent epidemic in Durban. J. Med. Assoc. S. Afr. 1:446-448. [Google Scholar]
- 38.Eshita, Y. 1982. Japanese references to the studies on dengue fever and its vectors, 1905-1979. Eisei-Dobutsu 33:77-94. (In Japanese). [Google Scholar]
- 38a.Fenner, F. 2006. Classic paper: Fenner on the exanthemata. Rev. Med. Virol. 16:353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson, D. D., K. Gerschman, A. Le Bailly, and L. R. Petersen. 2005. Characteristics of the rash associated with West Nile virus fever. Clin. Infect. Dis. 41:1204-1207. [DOI] [PubMed] [Google Scholar]
- 40.Fujita, N., and K. Yoshida. 1979. Follow-up studies on dengue endemic in Nagasaki, Japan: detection of specific antibodies in the serum taken more than 30 years after a single attack of dengue. Kobe J. Med. Sci. 25:217-224. [PubMed] [Google Scholar]
- 41.Fukui, M. 1943. An outbreak of dengue in Kaohsiung, Taiwan, 1943: clinical observations, statistical studies, early diagnosis, and re-infection. Shonika-Shinryo 9:17-38. (In Japanese). [Google Scholar]
- 42.Gaunt, M. W., A. A. Sall, X. de Lamballerie, A. K. I. Falconar, T. I. Dzhivanian, and E. A. Gould. 2001. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J. Gen. Virol. 82:1867-1876. [DOI] [PubMed] [Google Scholar]
- 43.George, R., and S. K. Lam. 1997. Dengue virus infection—the Malaysian experience. Ann. Acad. Med. Singapore 26:815-819. [PubMed] [Google Scholar]
- 44.Gibbons, R. V., S. Kalayanarooj, R. G. Jarman, A. Nisalak, D. W. Vaughn, T. P. Endy, M. P. Mammen, and A. Srikiakhachorn. 2007. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 77:910-913. [PubMed] [Google Scholar]
- 45.Glennan, A. H. 1900. Monthly report from San Juan. Public Health Rep. 14:1362-1363. [Google Scholar]
- 46.González, D., R. Martínez, O. Castro, T. Serrano, D. Portela, S. Vázquez, G. Kouri, and M. G. Guzmán. 2005. Evaluation of some clinical, humoral and immunological parameters in patients of dengue haemorrhagic fever six months after acute illness. Dengue Bull. 29:79-84. [Google Scholar]
- 47.Gordon Smith, C. E. 1956. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. Hyg. 59:243-251. [PubMed] [Google Scholar]
- 48.Griffitts, T. H. D. 1935. Dengue in Florida, 1934, and its significance. J. Florida Med. Assoc. 21:395-397. [Google Scholar]
- 49.Gubler, D. J. 1997. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem, p. 1-22. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 50.Gubler, D. J., and S. R. Zaki. 1998. Dengue and other viral hemorrhagic fevers, p. 43-71. In A. M. Nelson and C. R. Horsburgh (ed.), Pathology of emerging infections, vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 51.Halstead, S. B. 1992. Arboviruses on the Pacific and Southeast Asia, p. 1468-1488. In R. D. Feigin and J. D. Cherry (ed.), Textbook of paediatric infectious diseases. W.B. Saunders Co., Philadelphia, PA.
- 52.Halstead, S. B. 2007. Dengue—the case definition dilemma: a commentary. Pediatr. Infect. Dis. 26:291-292. [Google Scholar]
- 53.Halstead, S. B. 2008. Dengue: overview and history, p. 1-28. In S. B. Halstead (ed.), Dengue. Imperial College Press, London, United Kingdom.
- 54.Halstead, S. B. 1974. Etiologies of the experimental dengue of Siler and Simmons. Am. J. Trop. Med. Hyg. 23:974-982. [DOI] [PubMed] [Google Scholar]
- 55.Halstead, S. B. 1980. Immunological parameters of togavirus disease syndromes, p. 107-173. In R. W. Schlesinger (ed.), The togaviruses. Academic Press, New York, NY.
- 56.Halstead, S. B., and C. Yamarat. 1965. Recent epidemics of hemorrhagic fever in Thailand. Observations related to pathogenesis of a “new” dengue disease. Am. J. Public Health 55:1386-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammon, W. M., A. Rudnick, G. E. Sather, and K. D. Rogers. 1960. New hemorrhagic fevers of children in the Philippines and Thailand. Trans. Assoc. Am. Physicians 73:140-155. [PubMed] [Google Scholar]
- 58.Hare, F. E. 1898. The 1897 epidemic of dengue in North Queensland. Australas. Med. Gaz. 17:98-107. [Google Scholar]
- 59.Hattori, J. 1946. The causes of hemorrhagic manifestations in dengue infection. Rinsho-to-Kenkyu 23:305-308. (In Japanese.) [Google Scholar]
- 60.Hayashi, T. 1929. A dengue epidemic among the soldiers of a Cavalry Division of the Japanese Army. Gun-Idan-Zasshi 191:715-764. (In Japanese.) [Google Scholar]
- 61.Hirsch, A. 1883. A comparatively new disease: its symptoms (translation of the original German version of 1881), p. 55-81. In A. Hirsch (ed.), Handbook of geogrpahical and historical pathology, vol. 1. Sydenham Society, London, United Kingdom. [Google Scholar]
- 62.Hitti, J. K., and A. A. Khairallah. 1946. A report on the recnt epidemic of dengue in Beirut, Lebanon and some of its complications. J. Palestine Arab Med. Assoc. 1:150-153. [Google Scholar]
- 63.Hossack, W. L. 1913. The problem of dengue, three day and seven-day fever. Indian Med. Gaz. 48:49-52. [PMC free article] [PubMed] [Google Scholar]
- 64.Howe, G. M. 1977. A world geography of human diseases. Academic Press, New York, NY.
- 65.Imrie, A., J. Meeks, A. Gurady, M. Sukhbaatar, T. T. Truong, C. B. Cropp, and P. Effler. 2007. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 20:672-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneko, T. 1943. A histopathological investigation of a fatal case of dengue infection during the outbreak in Nagasaki, 1943. Nippon Igaku 3329:748. (In Japanese.) [Google Scholar]
- 67.Kawaishi, K. 1943. Surgery necessitated in severe complications of dengue. Nettai-Igaku 1:618. (In Japanese.) [Google Scholar]
- 68.Kimura, R., and S. Hotta. 1944. Research on dengue. Report 6: inoculation of mice with dengue virus. Nippon Igaku 3379:629-633. (In Japanese.) [Google Scholar]
- 69.Kinosita, R., and T. Miyaji. 1944. Fatal cases of dengue infection: a study of the cause. Nippon-Rinsho 2:999-1002. (In Japanese.) [Google Scholar]
- 70.Kitano, M. 1931. An epidemiologic review of the dengue infections among military servicemen in the Japanese Army: an outbreak in Okinawa, 1931. Gun-Idan-Zasshi 220:1951-1972. (In Japanese.) [Google Scholar]
- 71.Koizumi, M., K. Yamaguchi, and K. Tonomura. 1917. Dengue fever. Nisshin-Igaku 6:955-1004. (In Japanese.) [Google Scholar]
- 72.Kokernot, R. H., K. C. Smithburn, and M. P. Weinbren. 1956. Neutralizing antibodies to arthropod-borne viruses in human beings and animals in the Union of South Africa. J. Immunol. 77:313-323. [PubMed] [Google Scholar]
- 73.Kouri, G. P., M. G. Guzmán, and J. R. Bravo. 1987. Why dengue haemorrhagic fever in Cuba? 2. An integral analysis. Trans. R. Soc. Trop. Med. Hyg. 81:821-823. [DOI] [PubMed] [Google Scholar]
- 74.Kouri, G. P., M. G. Guzmán, J. R. Bravo, and C. Triana. 1989. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull. W. H. O. 67:375-380. [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar, R., P. Tripathi, S. Singh, and G. Bannerji. 2006. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin. Infect. Dis. 43:123-131. [DOI] [PubMed] [Google Scholar]
- 76.Kunitomo, N., and L. Y. Khoo. 1943. Maculopathies associated with dengue infection. Nettai-Igaku 1:614. (In Japanese.) [Google Scholar]
- 77.Kuno, G. 1993. A bibliographic database on dengue. Centers for Disease Control, San Juan, PR.
- 78.Kuno, G. 2007. Research on dengue and dengue-like illness in East Asia and the Western Pacific during the first half of the 20th century. Rev. Med. Virol. 17:327-341. [DOI] [PubMed] [Google Scholar]
- 79.Kuno, G. 1995. Review of the factors modulating dengue transmission. Epidemiol. Rev. 17:321-335. [DOI] [PubMed] [Google Scholar]
- 80.Kuno, G., and G.-J. J. Chang. 2005. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 18:608-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kyle, J. L., and E. Harris. 2008. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62:71-92. [DOI] [PubMed] [Google Scholar]
- 82.Lahiri, M., D. Fisher, and P. A. Tambyah. 2008. Dengue mortality: reassessing the risks in transition countries. Trans. R. Soc. Trop. Med. Hyg. 102:1011-1016. [DOI] [PubMed] [Google Scholar]
- 83.League of Nations. 1928. The dengue epidemic in Greece. League of Nations Month. Epidemiol. Rep. 7:335-338. [Google Scholar]
- 84.Lederer, S. E. 1997. Subjected to science: human experimentation in America before the Second World War. The Johns Hopkins University Press, Baltimore, MD. [PubMed]
- 85.Lee, T. L. 1942. Stomach bleeding associated with dengue infection and a cumulative analysis of clinical observations. Taiwan-no-Ikai 1:383-397. (In Japanese.) [Google Scholar]
- 86.Leichtenstern, O. 1905. Malaria, influenza and dengue, p. 719-742. In R. Ross, J. W. W. Stephens, and A. S. Grunbaum (ed.), Nothnagel's practice. W. B. Saunders & Co., Philadelphia, PA.
- 87.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos de Chacón, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87a.Lim, L. E., and E. Stransky. 1956. On the infectious acute thrombocytopenic purpura (hemorrhagic fever) observed in children in the Philippines. Ann. Paediatr. 187:309-320. [PubMed] [Google Scholar]
- 88.Lim, W.-K., R. Mathur, A. Koh, R. Yeoh, and S.-P. Chee. 2004. Ocular manifestations of dengue fever. Ophthalmology 111:2057-2064. [DOI] [PubMed] [Google Scholar]
- 89.Lockhart-Gibson, J. 1898. Acute inflammatory glaucoma induced in a susceptible patient by dengue. Australas. Med. Gaz. 17:339. [Google Scholar]
- 90.Lum, L. C. S., S. K. Lam, Y. S. Choy, R. George, and F. Harun. 1996. Dengue encephalitis: a true entity? Am. J. Trop. Med. Hyg. 54:256-257. [DOI] [PubMed] [Google Scholar]
- 91.Lumley, G. F., and F. H. Taylor. 1943. Dengue, vol. 1, p. 1-171. School of Pulic Health and Tropical Medicine, Sydney, Australia.
- 92.Marks, A. H. 1905. Dengue fever. Dublin J. Med. Sci. 120:95-99. [Google Scholar]
- 93.Martialis, M. 1874. La dengue: d'apres des documents anglais de Madras et de Calcutta et les observations recueilles dans les possessions francaises. Arch. Med. Naval (Paris) 21:21-49. [Google Scholar]
- 94.Matsuura, T. 1920. Dengue fever. Nippon-Biseibutsugaku-Zasshi 14:143-150. (In Japanese.) [Google Scholar]
- 95.Mattingly, P. F. 1960. Ecological aspects of the evolution of mosquito-borne virus diseases. Trans. R. Soc. Trop. Med. Hyg. 54:97-112. [Google Scholar]
- 96.McCallum, F., and J. P. Dwyer. 1927. Dengue as a cause of death. Med. J. Aust. 1:10-15. [Google Scholar]
- 97.Megaw, J. W. D. 1909. Are “seven-day fever” and “three-day fever” forms of dengue? Indian Med. Gaz. 44:9-20. [PMC free article] [PubMed] [Google Scholar]
- 98.Megaw, J. W. D. 1906. Is Calcutta seven-day fever a form of dengue? Indian Med. Gaz. 41:429-432. [PMC free article] [PubMed] [Google Scholar]
- 99.Melissinos, J. 1937. Pathologisch-anatomische Untersuchungen bei Denguefieber. Arch. Schiffs. Tropen-Hyg. 41:321-331. [Google Scholar]
- 100.Misaizu, H. 1932. A clinical examination of dengue patients. Hifuka-Kiyo 19:54-55. (In Japanese.) [Google Scholar]
- 101.Miyao, T. 1931. The dengue outbreak in Okinawa, 1931. Kaigun-Gun-Ikai-Zasshi 20:564-580. (In Japanese.) [Google Scholar]
- 102.Monath, T. P. 2001. Yellow fever: an update. Lancet Infect. Dis. 1:11-20. [DOI] [PubMed] [Google Scholar]
- 103.Mondini, A., I. L. S. Cardeal, E. Lázaro, S. H. Nunes, C. C. Moreira, and P. Rahal. 2007. Saint Louis encephalitis virus, Brazil. Emerg. Infect. Dis. 13:176-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reference deleted.
- 105.Murray, G. A. 1931. Dengue fever. Health (Australia) 9:105-111. [Google Scholar]
- 106.Myers, R. M., and D. E. Carey. 1967. Concurrent isolation from patient of two arboviruses, chikungunya and dengue type 2. Science 157:1307-1308. [DOI] [PubMed] [Google Scholar]
- 107.Nayar, S. K., O. Noridah, V. Paranthaman, K. Ranjit, I. Norizah, Y. K. Chen, B. Mustafa, and K. B. Chua. 2007. Co-infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med. J. Malaysia 62:335-336. [PubMed] [Google Scholar]
- 108.Nelson, E. R., and H. R. Bierman. 1964. Dengue fever, a thrombocytopenic disease? JAMA 190:99-103. [DOI] [PubMed] [Google Scholar]
- 109.Nimmannitya, S., S. B. Halstead, S. N. Cohen, and M. R. Margiotta. 1969. Dengue and chikungunya virus infections in man in Thailand, 1962-1964. I. Observations of hospitalized patients with hemorrhagic fever. Am. J. Trop. Med. Hyg. 18:954-971. [DOI] [PubMed] [Google Scholar]
- 110.Nishiura, H., and S. B. Halstead. 2007. Natural history of dengue virus (DENV)-1 and DENV-4 infections: reanalysis of classic studies. J. Infect. Dis. 195:1007-1013. [DOI] [PubMed] [Google Scholar]
- 111.Nomura, S., and K. Akashi. 1931. Hemorrhagic death associated with dengue infection. Taiwan-Igakukai-Zasshi 30:1154-1156. (In Japanese.) [Google Scholar]
- 112.Osajima, S. 1970. Clinical manifestations of dengue fever in Nagasaki, Japan during 1942-1944. Fujiki Hakueisha Printing Co., Nagasaki, Japan. (In Japanese.)
- 113.Papaevangelou, G., and S. B. Halstead. 1977. Infections with two dengue viruses in Greece in the 20th century. Did dengue hemorrhagic fever occur in the 1928 epidemic? J. Trop. Med. Hyg. 80:46-51. [PubMed] [Google Scholar]
- 114.Parent, M., P. Hantson, P. Honore, R. Colebunders, J. Rahier, and F. Bonbled. 2005. Correlations anatomo-biologiques dans un cas de fievre jaune imposte de Gambie. Ann. Pathol. 25:393-397. [DOI] [PubMed] [Google Scholar]
- 115.Photakis, B. A. 1929. Die klinischen Ausserungen des Dengue Fiebers in Lichite der Obdaktionbefunde. Arch. Schiffs. Tropen-Hyg. 3:333-335. [Google Scholar]
- 116.Phuong, C. X. T., N. T. Nhan, R. Kneen, P. T. T. Thuy, C. V. Thien, N. T. T. Nga, T. T. Thuy, T. Solomon, K. Stepniewska, and B. Wills. 2004. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the World Health Organization classification system helpful? Am. J. Trop. Med. Hyg. 70:172-179. [PubMed] [Google Scholar]
- 117.Poovaneswari, S. 1993. Dengue situation in Malaysia. Malaysian J. Pathol. 15:3-7. [PubMed] [Google Scholar]
- 118.Quintos, F. N., L. E. Lim, L. Juliano, A. Reyes, and P. Lacson. 1954. Hemorrhagic fever observed among children in the Philippines. Philippine J. Pediatr. 3:1-18. [Google Scholar]
- 119.Ramos, C., G. Sánchez, R. Hernández-Pando, J. Boquera, D. Hernández, J. Mota, J. Ramos, A. Flores, and E. Llausas. 1998. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 4:465-468. [DOI] [PubMed] [Google Scholar]
- 120.Ratsitorahina, M., J. Harisoa, J. Ratovonjato, S. Biacabe, J.-M. Reynes, and H. Zeller. 2008. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg. Infect. Dis. 14:1135-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rice, L. 1923. Dengue fever. Am. J. Trop. Med. 3:73-90. [Google Scholar]
- 122.Rico-Hesse, R. 2003. Microevolution and virulence of dengue viruses, p. 316-341. In T. J. Chambers and T. P. Monath (ed.), The flaviviruses: structure, replication and evolution. Elsevier-Academic Press, Amsterdam, The Netherlands.
- 123.Rigau-Pérez, J. G. 1998. The early use of break-bone fever (Quebrada huesos, 1771) and dengue (1801) in Spanish. Am. J. Trop. Med. Hyg. 59:272-274. [DOI] [PubMed] [Google Scholar]
- 124.Rigau-Pérez, J. G. 2006. Severe dengue: the need for new case definition. Lancet Infect. Dis. 6:297-302. [DOI] [PubMed] [Google Scholar]
- 125.Rigau-Pérez, J. G., A. Aguso-Lamadrid, D. R. Wolff, P. Reiter, and G. Kuno. 1994. Dengue severity throughout seasonal changes in incidence in Puerto Rico. Am. J. Trop. Med. Hyg. 51:408-415. [PubMed] [Google Scholar]
- 126.Rigau-Pérez, J. G., and M. K. Laufer. 2006. Dengue-related deaths in Puerto Rico, 1992-1996: diagnosis and clinical alarm signals. Clin. Infect. Dis. 42:1241-1246. [DOI] [PubMed] [Google Scholar]
- 127.Rodríguez-Roche, R., M. Alvarez, T. Gritsun, S. B. Halstead, G. Kouri, E. A. Gould, and M. G. Guzmán. 2005. Virus evolution during a severe dengue epidemic in Cuba, 1997. Virology 334:154-159. [DOI] [PubMed] [Google Scholar]
- 128.Rosen, L. 1986. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. Am. J. Trop. Med. Hyg. 35:642-653. [DOI] [PubMed] [Google Scholar]
- 129.Rosen, L. 1958. Observations on the epidemiology of dengue in Panama. Am. J. Hyg. 68:45-58. [DOI] [PubMed] [Google Scholar]
- 130.Rosenberg, H. M. 1999. Cause of death as a contemporary problem. J. Hist. Med. 54:133-153. [DOI] [PubMed] [Google Scholar]
- 131.Roy, B. R. N. 1873. Affections of the eyes following dengue. Indian Med. Gaz. 8:42-43. [PMC free article] [PubMed] [Google Scholar]
- 132.Sabin, A. B. 1950. The dengue group of viruses and its family relationships. Bacteriol. Rev. 14:225-232. [PubMed] [Google Scholar]
- 133.Sabin, A. B. 1959. Survey of knowledge and problems in field of arthropod-borne virus infections. Arch. G es amte Virusforsch. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 134.Sawada, H. 1944. Clinical observations of dengue infections in Taiwan, 1944, with an emphasis on four fatal cases. Taiwan-Igakukai-Zasshi 43:893-894. (In Japanese.) [Google Scholar]
- 135.Sawada, T., H. Sato, K.-E. Liao, G. Arai, and C.-H. Chhoa. 1943. Clinical observations of dengue patients. Nettai-Igaku 1:615. (In Japanese.) [Google Scholar]
- 136.Sawyer, W. A., J. H. Bauer, and L. Whitman. 1937. The distribution of yellow fever immunity in North America, Central America, the West Indies, Europe, Asia, and Australia, with special reference to the specificity of the protection test. Am. J. Trop. Med. 17:137-161. [Google Scholar]
- 137.Schlesinger, R. W. 1977. Dengue viruses. Springer-Verlag, New York, NY.
- 138.Scott, L. C. 1923. Dengue fever in Louisiana. JAMA 80:387-393. [Google Scholar]
- 139.SEATO. 1961. Round table discussion. Symposium on haemorrhagic fever. SEATO Med. Res. Monogr. 2:129-137. [Google Scholar]
- 140.Sharp, W. B., and E. Hollar. 1955. Immunity in dengue fever. Am. J. Trop. Med. 15:247-264. [Google Scholar]
- 141.Siler, J. F. 1935. Dengue fever, p. 402-408 In E. B. McKinley (ed.), A geography of disease. The George Washington University Press, Washington, DC.
- 142.Siler, J. F., M. W. Hall, and A. P. Hitchen. 1926. Dengue, its history, mechanism of transmission, etiology, clinical manifestation, immunity, and prevention. Philippine J. Sci. 29:1-304. [Google Scholar]
- 143.Simmons, J. S., J. H. St. John, and F. H. K. Reynolds. 1931. Experimental studies of dengue. Philippine J. Sci. 44:1-251. [Google Scholar]
- 144.Sircar, M. L. 1872. The present epidemic of dengue and its treatment. Calcutta J. Med. 5:127-144. [Google Scholar]
- 145.Snijders, E. P., E. J. Dinger, and W. A. P. Schuffner. 1931. On the transmission of dengue in Sumatra. Am. J. Trop. Med. 11:171-197. [Google Scholar]
- 146.Snowden, F. M. 2008. Emerging and reemerging diseases: a historical perspective. Immunol. Rev. 225:9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stedman, G. W. 1828. Some account of an anomalous disease which raged in the islands of St. Thomas and Santa Cruz, in the West Indies, during the months of Sept., Oct., Nov., Dec., and Jan., 1827-1828. Edingburgh Med. Surg. J. 30:227-248. [PMC free article] [PubMed] [Google Scholar]
- 148.Stevenson, L. D. 1939. Pathologic changes in the nervous system in yellow fever. Arch. Pathol. 27:249-266. [Google Scholar]
- 149.Reference deleted.
- 150.Streatfield, R., G. Bielby, and D. Sinclair. 1993. A primary dengue 2 epidemic with spontaneous haemorrhagic manifestations. Lancet 342:560-561. [DOI] [PubMed] [Google Scholar]
- 151.Su, D. H.-W., K. Bascal, S.-P. Chee, J. V. P. Flores, W.-K. Lim, B. C.-L. Cheng, and A. H.-E. Jap. 2007. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology 114:1743-1747. [DOI] [PubMed] [Google Scholar]
- 152.Tadano, M., Y. Okuno, T. Fukunaga, and K. Fukai. 1983. Retrospective serological studies on dengue epidemics in Osaka and Okinawa. Biken J. 26:165-167. [PubMed] [Google Scholar]
- 153.Takasaki, H., and T. Okada. 1958. Seven necropsy cases of dengue associated with the cerebral hemorrhage, especially as to the specificity of hemorrhagic focus. Mie Igaku 2:554-559. (In Japanese.) [Google Scholar]
- 154.Tan, J. S., L. Espiritu-Campos, A. P. Caviles, and P. C. Campos. 1968. Haemorrhagic fever in adults in the Philippines. Far East Med. J. 4:245-259. [Google Scholar]
- 155.Teixeira, M. G., M. C. N. Costa, G. Coelho, and M. L. Barreto. 2008. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. Emerg. Infect. Dis. 14:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ternovoi, V. A., G. P. Kurzhukov, Y. V. Sokolov, G. Y. Ivanov, V. A. Ivanisenko, A. V. Loktev, R. W. Ryder, S. V. Netesov, and V. B. Loktev. 2003. Tick-borne encephalitis with hemorrhagic syndrome, Novosibirsk Region, Russia, 1999. Emerg. Infect. Dis. 9:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reference deleted.
- 158.Van Rooyen, C. F., and A. J. Rhodes. 1948. Virus diseases of man, p. 437-454. Thomas Nelson, New York, NY.
- 159.Vasilakis, N., and S. C. Weaver. 2008. The history and evolution of human dengue emergence. Adv. Virus Res. 72:1-76. [DOI] [PubMed] [Google Scholar]
- 160.Vassal, J. J., and A. Brochet. 1909. Dengue in indo-Chine: epidemic on board the “Manche.” Philippine J. Sci. 4:21-36. [Google Scholar]
- 161.Wakamatsu, C. 1943. Two cases of central scotoma in dengue patients. Sogo-Igaku-Shi 111:1115-1116. (In Japanese.) [Google Scholar]
- 162.Wakil, A. W., and F. Hilmy. 1938. The dengue epidemic of 1937 in Cairo. J. Egyptian Med. Assoc. 21:716-737. [Google Scholar]
- 163.Watts, D. M., K. R. Porter, P. Putvatana, B. Vázquez, C. Calampa, C. G. Hayes, and S. B. Halstead. 1999. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 354:1431-1434. [DOI] [PubMed] [Google Scholar]
- 164.Wellmer, H. 1983. Dengue haemorrhagic fever in Thailand. Springer-Verlag, Berlin, Germany.
- 165.Whitley, R. J., and J. W. Gnann. 2002. Viral encephalitis: familiar infections and emerging pattern. Lancet 359:507-514. [DOI] [PubMed] [Google Scholar]
- 166.WHO. 1997. Dengue haemorrhagic fever: diagnosis, treatment, and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 167.Wilcocks, C. 1944. Medical organization and diseases of Thailand. Trop. Dis. Bull. 41:791-802. [Google Scholar]
- 168.Williams, M. C., J. P. Woodall, and D. I. H. Simpson. 1964. Yellow fever in Central Uganda, 1964. Part III. Virus isolation from man and laboratory studies. Trans. R. Soc. Trop. Med. Hyg. 59:444-448. [DOI] [PubMed] [Google Scholar]
- 169.Yamamoto, S. 1936. Dengue outbreak in the Ogasawara Islands (Bonin Islands). Saishin-Igaku 12:32-34. (In Japanese.) [Google Scholar]
- 170.Yoneda, M., and M. Shiroma. 1943. Clinical observations of dengue infections in Okinawa, 1943, part II. Nippon-Iji-Shimpo 1105:2165. (In Japanese.) [Google Scholar]
- 171.Yoneda, M., and M. Shiroma. 1943. A clinical study of dengue fever in Okinawa, Japan, 1943. Nippon-Iji-Shimpo 1105:2129. (In Japanese.) [Google Scholar]
- 172.Yuguchi, K. 1943. A case of maculopathy associated with dengue infection. Kaigun-Gun-Ikai-Zasshi 32:627-630. (In Japanese.) [Google Scholar]