Summary
HEK293 cells expressing wild-type CXCR2 recruit PH-Akt-GFP to the leading edge of the cell in response to chemokine. However, in cells expressing mutant CXCR2 defective in AP-2 and HIP binding, i.e. with a mutation in the LLKIL motif, PH-Akt-GFP does not localize to the leading edge in response to ligand. Inhibition of Akt/PKB by transfection of HEK 293 cells with a dominant negative (kinase defective) Akt/PKB inhibits CXCR2 mediated chemotaxis. FRET analysis reveals that membrane-bound activated Cdc42 and Rac1 localize to the leading edge of cells expressing wild-type CXCR2 receptor, but not in cells expressing mutant CXCR2. By contrast, when the activation of Cdc42 and Rac1 are monitored by affinity precipitation assay, cells expressing either wild-type or LLKIL mutant receptors show equivalent ligand induction. Altogether, these data suggest that restricted localized activation of Akt/PKB, Rac1 and Cdc42 is crucial for chemotactic responses and that events mediated by the LLKIL motif are crucial for chemotaxis.
Keywords: CXCR2, AP-2, Internalization, Chemotaxis, PH-Akt-GFP, FRET
Introduction
One of the characteristics of many G-protein-coupled receptors is that they undergo phosphorylation, desensitization and internalization upon agonist stimulation. The internalized receptors are enclosed in the transporting vesicles and enter the endosomal compartment for recycling or are transferred to the lysosome for degradation. During these processes, several intracellular molecules are recruited to the internalized receptor, such as β-arrestin, adaptor protein-2 (AP-2), heat shock 70-interacting protein (HIP) and protein phosphatase 2A (PP2A) (Ferguson et al., 1996; Laporte et al., 1999; Fan et al., 2001a; Fan et al., 2001b; Fan et al., 2002). These proteins may further recruit kinases such as Src, Erk1/2 (extracellular signal-regulated kinase 1 and 2) and Jnk (c-Jun N-terminal kinase), to modulate the signaling pathways that is activated when a ligand binds to the receptor (Luttrell et al., 1999; DeFea et al., 2000; McDonald et al., 2000). The finding of new roles of these adaptor proteins through interaction with the components of MAP kinase (mitogen activated protein kinase) cascades during receptor endocytosis implies important functional roles for receptor internalization. We previously reported that the internalization of CXCR2, an angiogenic CXC chemokine receptor, could be required for chemotactic response to the ligands, CXCL1 and CXCL8 (Yang et al., 1999; Fan et al., 2001b). However, the effect of this receptor internalization on intracellular signal transduction pathways remains unknown.
The mechanism by which a cell amplifies and translates its detection of the shallow extracellular chemoattractant gradient into a polarization of internal signals to facilitate chemotaxis remains an intriguing and unsolved issue. The simple scenario would be that in response to the detection of a chemokine gradient, chemoattractant receptors concentrate at the edge of the cell with the highest concentration of chemoattractant, and this polarized focal distribution of receptors then triggers the polarization of intracellular signals. Some reports have shown that CCR2 and CCR5 in T lymphocytes, as well as CXCR4 in B lymphocytes and hematopoietic progenitor cells, relocalize to the leading edge of chemotaxing cells when these cells are exposed to their cognate chemokine gradient (Nieto et al., 1997; van Buul et al., 2003). However, the data from experiments using GFP-tagged cAMP receptor in Dictyostelium discoideum and from the C5a receptor in neutrophils showed the opposite results, and the groups performing these experiments concluded there is no biased distribution of chemoattractant receptors during chemotaxis (Xiao et al., 1997; Servant et al., 1999). It was reported that a shallow gradient of the G protein β-subunit was found from the anterior to the posterior of polarized Dictyostelium cells. However, this shallow G protein gradient cannot completely account for the very steep gradient of intracellular signaling molecules, such as pleckstrin homology (PH)-domain containing proteins (Jin et al., 2000). Considerable controversy remains around this point. Current data suggest that receptor relocalization to the leading edge of chemotaxing cells might vary with different cell types and/or receptors. Increasing evidence suggest that the phophoinositide-3 kinase (PI 3 kinase) signal cascade plays a key role in chemotaxis and the signal molecules in this cascade are recruited to the leading edge of chemotaxing cells in a polarized manner. In Dictyostelium, a PH-domain containing protein, cytosolic regulator of adenylyl cyclase (CRAC), is recruited to the leading edge of chemotaxing cells (Parent et al., 1998). Another PH-domain containing protein, protein kinase B (Akt/PKB), is recruited to the leading edge of the cells during chemotaxis in Dictyostelium and in mammalian neutrophils (Meili et al., 1999; Servant et al., 2000). The recruitment of these PH-domain containing proteins is directly influenced by the localized cell membrane accumulation of phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3], a direct product of phosphorylation by PI 3 kinase, when cells are exposed to a chemoattractant gradient. The polarized accumulation of PtdIns(3,4,5)P3 and PH-domain containing proteins can be used as readout for the polarization of intracellular signaling molecules in cells responding to a chemokine gradient.
Rho family GTPases play a very important role in cell cytoskeleton reorganization and therefore are crucial to cell morphology and motility. There are more than 25 members of the Rho GTPase family, and RhoA, Rac1 and Cdc42 are the three best studied. Rac1 is involved in the membrane ruffling, crucial for the formation of pseudopodia. Cdc42 plays a role in formation of the filapodia that facilitate matrix attachment when the pseudopod extends to a new spot during cell movement. RhoA functions in uropod retraction in moving cells and in stress fiber formation in resting cells. The usual technique of using the GFP-tagged GTPase to visualize the distribution of signal molecules is not applicable in observing Rho GTPase localization because even in the stimulated cells, only a small part of Rho GTPases are activated and this small amount of activated GTPases is masked by the large amount of non-activated forms. Fluorescence resonance energy transfer (FRET) is a distance-dependent interaction between the electronically excited states of two dye molecules, in which excitation is transferred from a donor molecule to an acceptor molecule without the emission of a photon. FRET is an important technique for investigating a variety of biological phenomena that produce changes in molecular proximity. Thus, it can be used to study the colocalization and/or the interaction of two molecules. A single-molecule FRET probe was first developed to test Ca2+ oscillation (Miyawaki et al., 1997) and later used to monitor the localized activation of Ras and Rho GTPases (Mochizuki et al., 2001; Itoh et al., 2002).
In this manuscript, we used a PH-Akt-GFP readout to monitor the generation and localization of PtdIns(3,4,5)P3 in cells expressing either wild-type (WT) or a poorly internalized mutant of CXCR2 (I323 to A and L324 to A in the LLKIL motif) (LLKIL mutant CXCR2), after cells were exposed to a chemokine gradient. We show that PH-Akt-GFP is recruited to the leading edge of human embryonic kidney 293 (HEK293) cells expressing WT CXCR2, but not in cells expressing LLKIL mutant CXCR2. Furthermore, the Rho GTPases, Rac1 and Cdc42, were also found to accumulate in the leading edge of HEK 293 cells expressing WT CXCR2 in response to a chemokine gradient, based on FRET analysis. This localized activation of Rac1 and Cdc42 was abolished in cells expressing ILKLL mutant CXCR2, which showed defective AP-2 and HIP binding and defective receptor internalization. These results suggest that either AP-2/HIP binding or internalization of CXCR2, or both, play roles in the localized activation or recruitment of intracellular molecules and thus affect the chemotactic responses to the chemokine gradient.
Materials and Methods
Materials
The HEK 293 cell line was purchased from ATCC (Manassas, VA). Chemokine CXCL1 (MGSA/GRO) was purchased from R&D systems (Minneapolis, MN) and CXCL8 (IL-8) was from PeproTech (Rocky Hill, NJ). The CXCL1 murine homologue macrophage inflammatory protein-2 (MIP-2) was kindly provided by E. Lolis (Yale University School of Medicine, New Haven, CT). Cdc42, Rac1 and Erk1/2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Erk (p-Erk) antibody was purchased from Santa Cruz and Phospho-Akt (Ser 473) antibody was purchased from Cell Signaling Technology (Beverly, MA). FITC-conjugated goat-anti-rabbit antibody was purchased from Chemocon (Temecula, CA). H. Bourne (University of California San Francisco, San Francisco, CA) kindly provided the PH-Akt-GFP DNA plasmid. FRET probes for Rac1 (Raichu Rac1), Cdc42 (Raichu Cdc42) were kind gifts from M. Matsuda (Osaka University, Osaka, Japan). FRET constructs containing constitutively active Rac1 (V12), containing a mutation from G to V at amino acid residue 12, and dominant negative Rac1 (N17), containing a mutation from S to N at amino acid residue 17, were also provided by M. Matsuda in Osaka University.
Cell culture and transfection
HEK293 cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin, 3 mM glutamine, 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) at 37°C, 5% CO2. Transfection was performed with Lipofectamine (Invitrogen Life Technology) based on the manufacturer’s protocol. Transiently transfected cells were assayed 48 hours after transfection. For selection of stable polyclonal cell lines, 800 μg/ml G418 (Sigma, St Louis, MO) was added 24 hours after transfection and cells were maintained in DMEM medium containing 800 μg/ml G418 through subculture procedures until a pooled, stable cell line was established.
Time-lapse video microscopy
Transfected cells were trypsinized, allowed to recover in 37°C for 1.5 hour and replated at low density on collagen IV (Sigma, 20 μg/ml) coated glass-bottom culture dish in DMEM medium with 10% serum. The medium was replaced with serum-free DMEM before microscopic examination. A Zeiss 200M inverted fluorescence microscope (Carl Zeiss Microimage, Germany) with temperature- and CO2-controlled chamber was used for all time-lapse video microscopy with the software Openlab (Improvision, Lexington, MA). Chemokine was delivered from a point source in a small vessel that is separated from the assay medium with an 8 μm-pore membrane. Two-hundred microliters of a solution of MIP-2 chemokine (1 μg/ml in serum-free DMEM) were loaded into the vessel immediately before the time-lapse video was taken. This way, chemokine was slowly released into the assay medium through the membrane by gravity and thus generated a concentration gradient. The time-lapse videos were taken at 20-second intervals. For GFP analysis of PH-Akt polarization, approximately 20 cells of three independent experiments were analyzed in detail. For FRET analysis, pairs of cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) images were taken sequentially and ratio images of YFP:CFP were generated with the program Openlab. Four independent experiments were performed and triplicate detailed cellular analyses were studied.
Chemotaxis assay
The modified Boyden chamber (96-well) (Neuroprobe, Gaithersburg, MI) was used for chemotaxis assay. HEK293 cells stably expressing WT CXCR2 were transfected with dominant Akt (kinase defective) or vector as control. Forty-eight hours after transfection, cells were trypsinized, then incubated in serum-containing medium for 2 hours at 37°C with rotation to allow restoration of receptor expression at the membrane. Lower compartments of the Boyden chamber were filled with the indicated concentrations of chemokine (ranging from 2.5 ng/ml to 250 ng/ml) in DMEM containing 1 mg/ml BSA, and then covered very carefully with a 10-μm-pore polycarbonate membrane without trapping any air. The membrane was pre-coated with human collagen IV (Sigma, MO) (20 μg/ml in DMEM) for 2 hours at 37°C. After the 2-hour recovery in serum-containing medium, cells were washed and resuspended in DMEM containing 1 mg/ml BSA. Two-hundred microliters of cells at a density of 106 cells/ml were loaded into the upper compartments and the chamber was incubated at 37°C, 5% CO2 for 4.5 hours. The membrane was then removed from the chamber and the loosely attached cells were scraped from the top surface. The cells on the bottom face of the membrane were fixed in Diff-quick fixative (Dade Diagnostics, Aguada, PR), stained in 1% crystal violet and washed extensively with tap water. Cells that had migrated across the membrane were counted under microscope using a 20× objective. Five fields were counted for each sample in duplicate or triplicate. The chemotaxis index was calculated as the number of cells that had migrated into the wells with chemokine versus the number of cells that had migrated into the wells without chemokine (control).
Affinity precipitation assay for Rac 1 and Cdc42 activity
PBD (p21 binding domain)-based assays of Cdc42 and Rac1 were performed as described by Benard et al., (Benard et al., 1999). Briefly, CXCR2-expressing HEK293 cells were stimulated with 50 ng/mL MIP-2 for indicated times, and immediately lysed by sonication in RIPA buffer containing cocktail protease inhibitor. Four-hundred micrograms of protein of each whole cell extract was incubated with purified glutathione S-transferase (GST)-conjugated PBD (GST-PBD) beads for 30 min at 4°C. The GTP bound and total levels of Cdc42 and Rac1 were detected by western blotting using Cdc42 and Rac1 antibodies.
Statistical analysis
Student’s t tests were performed to test statistical significance for the paired data comparisons. ANOVA (analysis of variance) tests were used to test the significant differences for the group data comparisons.
Results
Recruitment of PH-Akt-GFP in chemotaxing cells mediated by WT CXCR2, not LLKIL mutant CXCR2
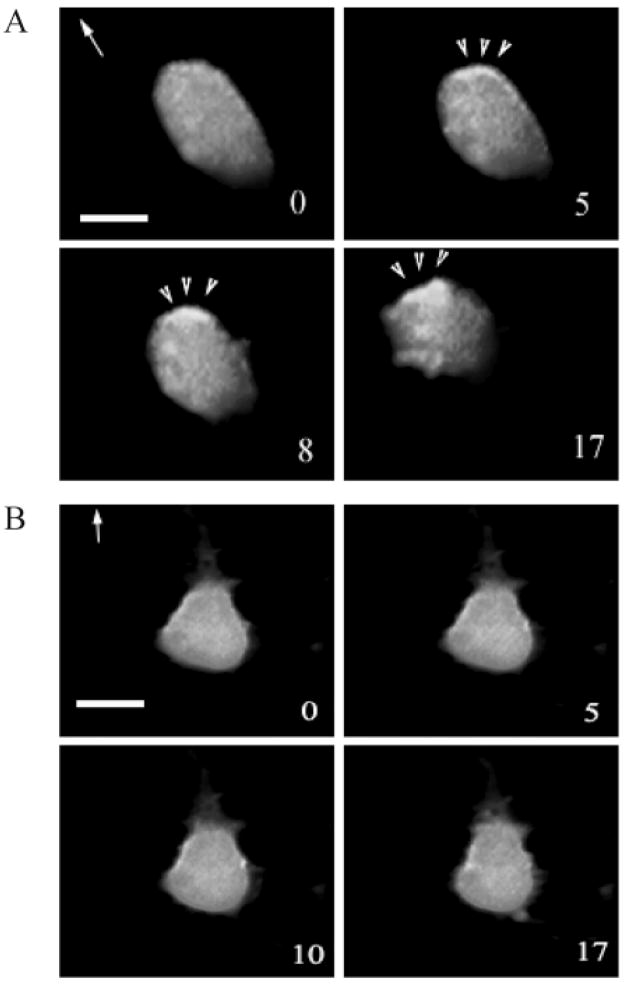
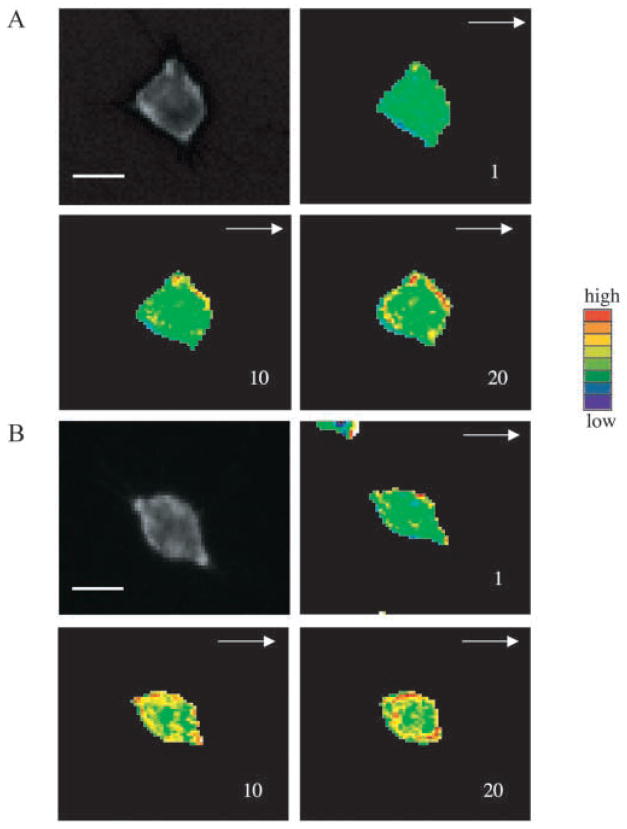
The recruitment of PH-domain containing proteins by PtdIns(3,4,5)P3 to the leading edge of chemotaxing cells has been shown both in Dictoystelium and differentiated mammalian HL-60 cells (Meili et al., 1999; Servant et al., 2000). We examined the ligand induced intracellular relocalization of the PH-domain from Akt/PKB in HEK293 cells that stably expressed the CXC chemokine receptor CXCR2. A DNA vector expressing PH-Akt-GFP was transiently transfected into these cells which were then used to monitor the PH-domain movement in response to the chemokines IL-8, MGSA/GRO or its murine homologue MIP-2. Three experiments were performed and cells expressing PH-Akt were analyzed by time-lapse video fluorescence-microscopy after treatment with MIP-2. Fig. 1A shows that the PH-Akt domain was recruited to the leading edge of cells during chemotaxis in response to ligand stimulation (arrow heads). Of the cells selected randomly for analysis, approximately 50% exhibited the response observed in Fig. 1. We previously reported that the LLKIL mutant CXCR2 exhibits impaired ligand-induced receptor internalization and impaired ability to mediate chemotaxis in response to ligand (Fan et al., 2001b). Here, we also show that ligand-induced recruitment of PH-Akt-GFP to the leading edge was diminished in cells expressing this mutant receptor (Fig. 1B). In all three experiments performed, none of the cells expressing LLKIL mutant CXCR2 exhibited polarized localization of PH-Akt-GFP after ligand stimulation. Video clips for Fig. 1A and B are available online (see Movies 1 and 2 in supplementary material).
Fig. 1.
Recruitment of PH-Akt-GFP to the leading edge of the cell during chemotaxis. A DNA vector expressing PH-Akt-GFP was transiently transfected into HEK293 cells stably expressing either (A) WT CXCR2 or (B) LLKIL mutant CXCR2. Time-lapse fluorescence microscopic images were taken every minute immediately after a point source of ligand (1 μg/ml MIP-2) was applied. The number in each picture shows the time-lapse in minutes and the arrow indicates the direction of ligand application. The arrowheads point towards the accumulation of PH-Akt-GFP. Scale bars, 20 μm. Each experiment was repeated at least three times and representative images are shown. The time-lapse video clips are available online (Movies 1 and 2 in supplementary material).
Role of Akt activity in CXCR2-mediated chemotaxis
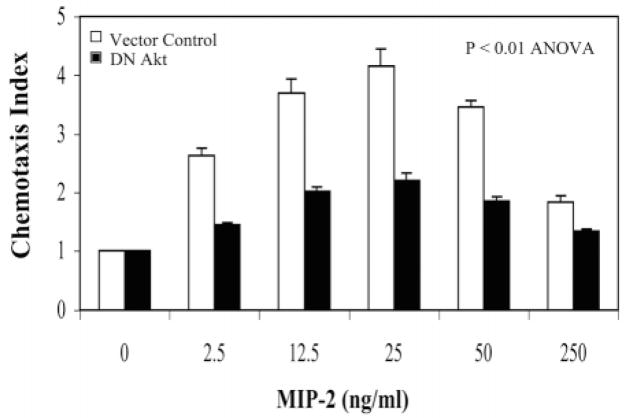
It is well known that the PH-domain containing protein Akt/PKB is activated when it is recruited to the membrane by PtdIns(3,4,5)P3, a product of phosphorylation by PI 3 kinase. It has been reported that Akt is phosphorylated and activated quickly in response to chemoattractant stimulation. However, to follow this phosphorylation by western blot usually requires a much higher concentration of chemoattractant than is required for the chemotaxis assay (Heit et al., 2002). We examined the effect of MIP-2 on CXCR2-induced Akt phosphorylation in HEK 293 cells expressing WT CXCR2. A western blot probed with a phospho-specific Akt antibody revealed that HEK 293 cells exhibit high basal Akt phosphorylation, and thus do not show a dramatic increase in Akt phosphorylation in response to 100 ng/ml MIP-2 (data not shown). However, Akt activity is required for chemotaxis in response to CXCR2 activation, since a Boyden chamber assay showed that transfection of cells with dominant negative (kinase defective) Akt significantly attenuated the cell migration in response to MIP-2 (P<0.01, ANOVA) (Fig. 2). By contrast, the transfection of CXCR2-expressing HEK293 cells with wild-type Akt did not significantly change the chemotactic response compared to the vector control (data not shown).
Fig. 2.
Akt activity is required for CXCR2-mediated chemotaxis. HEK293 cells stably expressing WT CXCR2 were transiently transfected with either dominant negative Akt (black bars) or vector control (open bars). The chemotaxis assays were performed in a modified Boyden chamber. Duplicates were used for each sample and the number of cells in ten fields was counted for each well. The chemotaxis index was calculated as described in Materials and Methods. Error bars indicate the s.e.m. ANOVA analysis was performed to evaluate the statistical difference between vector control and dominant negative Akt (P<0.01).
Inhibition of PH-Akt accumulation by the inhibition of dynamin
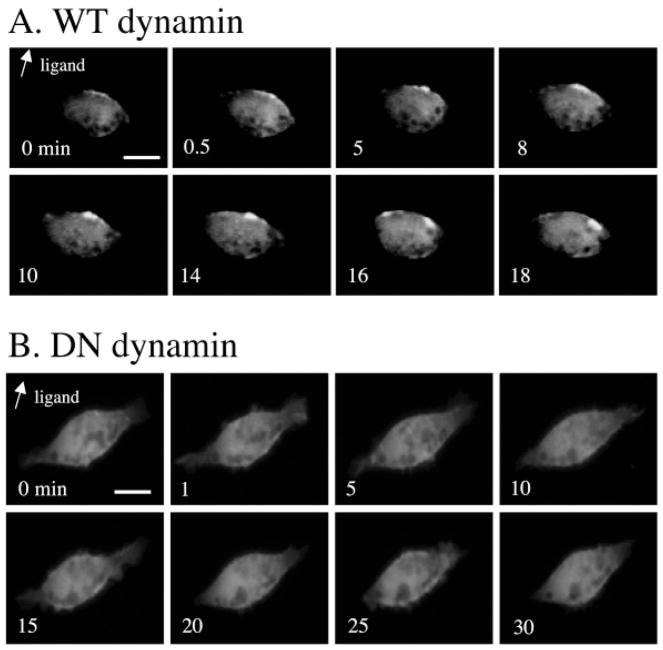
Dynamin is a large GTPase that plays a crucial role in the formation of the endocytic vesicles from clathrin-coated pits. The inhibition of dynamin GTPase activity blocks CXCR2 internalization into endocytic vesicles in response to ligand (Yang et al., 1999). Here, HEK293 cells stably expressing WT CXCR2 were transiently transfected with expression vector for PH-Akt-GFP and either wild-type dynamin or dominant negative dynamin (K44A). Time-lapse microscopic images were taken every 30 seconds after the chemokine was delivered in a directional manner. The cells expressing wild-type dynamin still showed recruitment of PH-Akt-GFP to the leading edge of the migrating cell, in a manner comparable to that observed in non-dynamin transfected cells (Fig. 3A). By contrast, expression of the GTPase-deficient dominant negative dynamin inhibited recruitment of PH-Akt in a polarized manner toward the leading edge of the cell in all the cells studied in detail (Fig. 3B). Altogether, these data support the model that the internalization of CXCR2 is required for normal MIP-2 chemotactic responses.
Fig. 3.
Inhibition of CXCR2 internalization by dominant negative dynamin results in inhibition of PH-Akt recruitment. HEK 293 cells stably expressing WT CXCR2 were co-transfected with PH-Akt-GFP, and either (A) wild-type dynamin or (B) dominant negative dynamin (K44A). Time-lapsed fluorescence microscopy was carried out for each group of transfected cells. Point source of ligand (MIP-2) was given as described in Materials and Methods and the arrow indicates the direction of ligand application. Scale bars, 20 μm. The number inside each picture gives the time-lapse in minutes after initiation of ligand stimulation.
Detection of the distribution of Rac1 and Cdc42 activities in live cells by FRET
The Rho GTPase family members play a very important role in cell migration (Ridley, 2001). Itoh et al., used single-molecule FRET probes to test the spatiotemporal regulation of Rac1 and Cdc42 activities in live HT1080 cells (Itoh et al., 2002). These single-molecule probes are fusion proteins consisting of a sensor (Rac1 or Cdc42) and an effector (Cdc42/Rac interactive binding (CRIB) domain), and the sensor and effector are flanked by either YFP or CFP at the end. Once Rac1 and Cdc42 are activated, they bind to the CRIB domain of the fusion protein, fold the fusion protein and thus bring YFP and CFP close to each other to generate FRET. The FRET activity generated by activation of both Rac1 and Cdc42 gradually increased toward the leading edge of moving cells (Itoh et al., 2002). We used the same probes to test the redistribution of activated Rac1 and Cdc42 in CXCR2-expressing HEK293 cells in response to the CXCR2 ligand. To be certain that these FRET probes would work in our system, we first tested the fluorescence spectrum of the cell lysates after they were transfected with FRET probes and the receptor. The cell lysates were excited with light of the wavelength of 433 nm. The 475 nm emission peak is the primary peak of CFP, the 525 nm emission of YFP is the derivative peak owing to the energy transfer from the primary CFP peak. The ratio value of 525:475 indicated FRET intensity. The profiles of emission spectrum for different FRET probes showed that the construct containing constitutively active Rac1 (V12) had the highest FRET signal because Rac1 (V12) binds to the CRIB domain all the time. Dominant negative Rac1 (N17) loses affinity of G proteins for guanine nucleotides and had the lowest FRET signal, while wild-type Rac1 FRET signal strength was between that of Rac1 (V12) and Rac1 (N17). Our data were equivalent to those previously reported by Itoh et al. (Itoh et al., 2002) and therefore are not shown here.
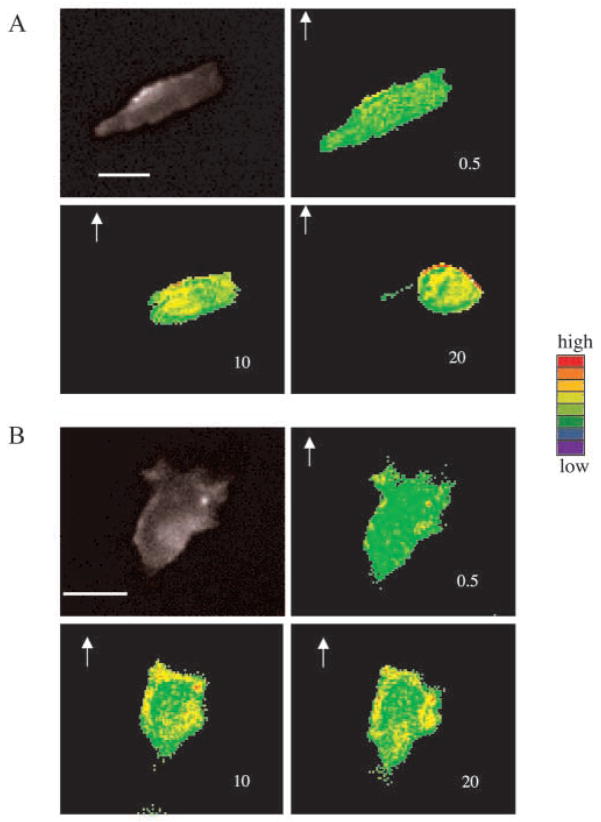
To trace the activities of Rac1 and Cdc42 in live cells, FRET probes were transiently transfected in the cells stably expressing either WT or LLKIL mutant CXCR2. The FRET signal was analyzed by taking the ratio of YFP and CFP images. Fig. 4A shows that the Rac1 FRET signal increased after ligand was applied, indicating Rac1 activities increased with the ligand stimulation. A stronger FRET signal (indicating Rac1 activation) was observed at the leading edge of the cell in more than 80% of the cells expressing WT CXCR2 of four independent experiments. In cells expressing the LLKIL mutant CXCR2, we did not observe a polarization of the FRET signal, indicating no localized activation of Rac at the leading edge of the cells (Fig. 4B). Also, Rac1 activity still increased after the ligand stimulation, but there was no polarized distribution of Rac1 as in cells expressing the WT receptor. A very similar pattern was seen in the Cdc42 responses after ligand stimulation in the cells expressing WT and mutant CXCR2 (Fig. 5A, B). Video clips for Figs 4 and 5 are available online (see Movies 3–6 in supplementary material). If cells stably expressing WT CXCR2 were co-transfected with the Cdc42 FRET construct and either dominant negative or constitutively active Cdc42, increases of FRET signals were inhibited. This is probably because the overexpressed dominant negative Cdc42 or the constitutively active Cdc42 compete with the Cdc42 component within the FRET probe for Cdc42 activators (such as GEFs). See online for more information on video (see Movies 7 and 8 in supplementary material).
Fig. 4.
FRET images of Rac1 in chemotaxing cells. Membrane-bound Rac1 activities were tested by FRET assay in HEK 293 cells expressing (A) WT or (B) LLKIL mutant CXCR2. FRET images were made as ratio images from two different fluorescent images and plotted with pseudocolor (red, highest activity; blue, lowest activity. Although the overall activities were increased in both types of cells expressing either receptor, the distribution of Rac1 activities was only polarized to the leading pseudopod in WT CXCR2-expressing cell with a higher level in the leading edge. The number in each image indicates the time-lapse in minutes after ligand application, the arrow indicates the direction of ligand application. Scale bars, 20 μm. Each experiment was repeated at least three times and representative images are shown. The time-lapse video clips are available in online (Movies 3 and 4 in supplementary material).
Fig. 5.
FRET images of Cdc42 in chemotaxing cells. Membrane-bound Cdc42 activities were tested by FRET assay in HEK 293 cells expressing (A) WT or (B) LLKIL mutant CXCR2. FRET images were made as ratio images from two different fluorescent images and plotted with pseudocolor, in which, red represents the highest activity and blue is lowest. Although the overall activities were increased in both types of cells expressing either receptor, the distribution of Cdc42 activities was only polarized in WT CXCR2-expressing cell with a higher level in the leading edge. The number showing in each image indicates the time-lapse in minutes after ligand application, the arrow indicates the direction of ligand application. Scale bars, 20 μm. Each experiment was repeated at least three times and representative images are shown. The time-lapse video clips are available online (Movies 5 and 6 in supplementary material).
The up-regulation of Erk1/2 and Cdc42 activities were not affected by LLKIL mutant receptors
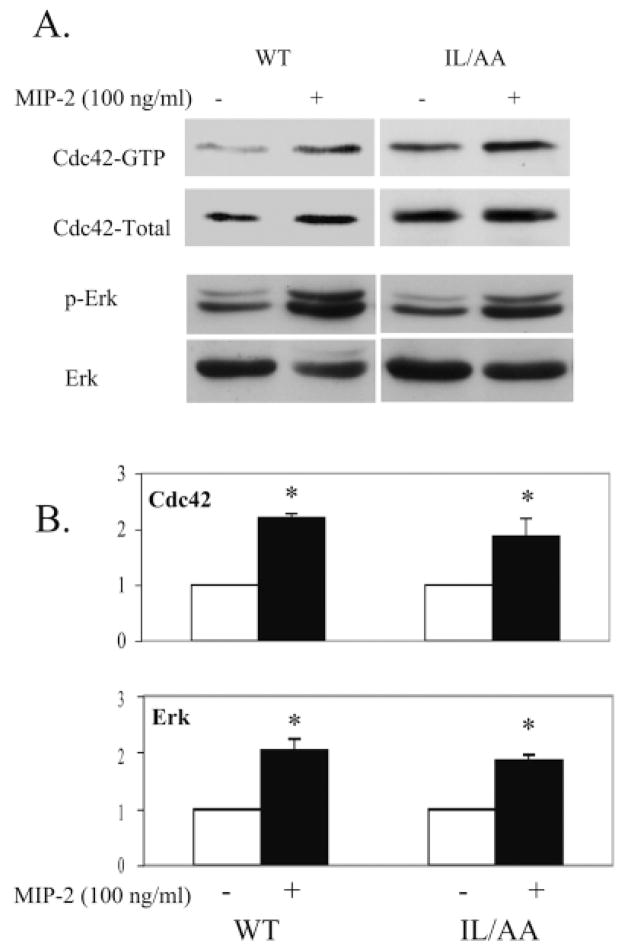
The MAP kinase pathway can be upregulated in response to many different kinds of stimulation, although data, regarding whether this kinase activity is required for chemotaxis, are contradictory from cell to cell or from receptor to receptor (Heit et al., 2002). We reported previously that Erk1/2 activity was stimulated in HEK293 cells expressing WT CXCR2 in response to ligand treatment and this activation was not impaired by inhibiting CXCR2 internalization with dominant negative dynamin (Yang et al., 1997; Yang et al., 1999). In this study, the Erk1/2 activation was also tested and compared in HEK 293 cells expressing either WT or LLKIL mutant CXCR2. Erk1/2 activity increased in response to ligand in cells expressing LLKIL mutant CXCR2, equivalent to that observed in cells expressing WT CXCR2 (Fig. 6). Student’s t-test indicated significant increases in Erk activation (P=0.025 for cells expressing WT receptors and P=0.05 for mutant receptors). However, there is no significant difference (P=0.45) in the intensity of Erk activation in the two groups. Furthermore, the activities of Cdc42 were also tested in a GST-PBD/Pak pull-down assay (see Materials and Methods for details) in cells expressing either WT or LLKIL mutant CXCR2. We found that the activities of Cdc42 increased about twofold in both types of cells upon agonist stimulation (100 ng/ml MIP-2) (P<0.05, Student’s t-test) (Fig. 6). The upregulation of Rac1 and Cdc42 activities in response to agonist stimulation are also observed in FRET image analyses of both WT and mutant receptor experiments (Figs 4 and 5). These data are consistent with earlier reports in that, cells expressing non-internalizing CXCR2 maintain or even increase chemokine signals in length and strength (Richardson et al., 1998).
Fig. 6.
Activation of Cdc42 and Erk1/2 in HEK293 cells expressing either WT or LLKIL mutant receptors upon agonist stimulation. HEK 293 cells stably expressing either WT CXCR2 or LLKIL mutant receptor were treated without or with MIP-2 (100 ng/ml) for 5 minutes. The affinity precipitation assay (see Materials and Methods for details) was performed for Cdc42 activity and western blots with an p-Erk1/2-specific antibody was performed for the Erk1/2 activation test. Experiments were repeated at least three times with similar results. (A) A representative western blot from three experiments. (B) Quantification of the western blots with error bars for s.e.m. Significant differences of ligand treatment versus non-treatment are indicated by asterisks (P<0.05, Student’s t-test).
Chemotactic responses are inhibited by either increasing or decreasing the activities of Rac1 and Cdc42
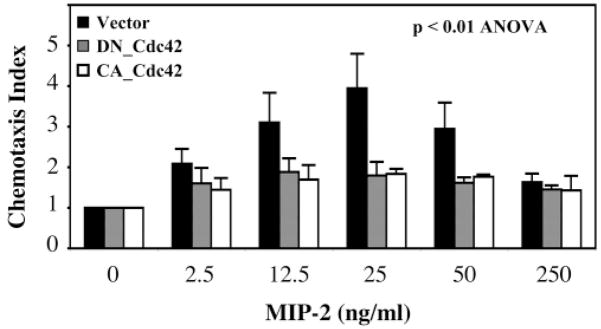
As shown above, the failure of cells expressing LLKIL mutant CXCR2 to exhibit a normal chemotactic response to the CXCR2 ligand was not because Rac1 or Cdc42 signal molecules were not activated, but rather because these activated molecules were not concentrated in a polarized manner at the leading edge of the cell. Moreover, we show that expression of either constitutively active or dominant negative Cdc42 inhibited the WT CXCR2-mediated chemotaxis when compared to vector control (P<0.01, ANOVA) (Fig. 7). As expected, there is no significant difference between dominant negative and constitutive active groups (P=0.89). These data suggest that the oscillation of the activation of these Rho GTPases (on-off cycling) is important in the mediation of chemotaxis.
Fig. 7.
The inhibition of dominant negative and constitutively active Cdc42 on chemotaxis in HEK 293 cells. HEK 293 cells stably expressing WT CXCR2 were transfected with pCDNA3 vector (control, black bars) or dominant negative Cdc42 (DN-Cdc42, gray bars) or constitutively active Cdc42 (CA-Cdc42, open bars) constructs. Chemotaxis assays were performed 48 hours after transfection. Each assay was repeated at least three times. Error bars give s.e.m. An ANOVA was performed for the comparison between vector control and either dominant negative or constitutive active Cdc42 (P<0.01).
Discussion
Desensitization and internalization of G-protein-coupled receptors after exposure to the ligand plays an important role in adjusting the responses of cells to the stimuli. However, the requirement of receptor internalization for chemotactic response has remained controversial. Some studies showed that the internalization and recycling of G-protein-coupled receptors is important for chemotaxis. This is the case for the fMLP receptor in neutrophils (Perez et al., 1989), CXCR1 in neutrophils (Samanta et al., 1990), protease-activated receptor-1 in murine hematopoietic progenitor cells (Sambrano and Coughlin, 1999) and CXCR4/CCR5 in HEK 293 cells (Fernandis et al., 2002; Roland et al., 2003). Some of these reports indicated that, while calcium mobilization and Erk1/2 phosphorylation were independent of receptor endocytosis, the receptor-mediated chemotaxis was inhibited in cells expressing C-terminal-truncated receptor (with receptor endocytosis being completely abolished) (Fernandis et al., 2002; Roland et al., 2003). By contrast, several other studies showed that the receptor internalization is not required for the receptor-mediated chemotaxis (Arai et al., 1997; Kim et al., 1997; Hsu et al., 1997; Kraft et al., 2001; Richardson et al., 2003). In those studies, the mutant receptors that exhibited compromised agonist-stimulated receptor phosphorylation and/or internalization were found to retain chemotactic responses. However, in each case, the mutations made by either replacing all the Ser or Thr residues with Ala in the C-terminal domain or simply truncating the C-terminal tail of the G-protein-coupled receptors, did not completely eliminate receptor internalization. Moreover, the truncated CXCR2 receptor, in which the C-terminal tail with Ser/Thr residues was deleted (CXCR2/331T), exhibited compromised internalization in 3ASubE and RBL cells (Richardson et al., 1998), whereas these same CXCR2/331T mutant receptors were internalized equivalently to WT receptor in HEK 293 cells (Fan et al., 2001b). Hence, the data obtained from different chemokine receptors or even the same receptors in different cell lines represent the complexity of chemotaxis in the mammalian system. This complexity and multiplicity of modulation of receptor trafficking provides a mechanism for even finer tuning of the regulation of chemotaxis.
Here, we show that the LLKIL motif of CXCR2 is crucial for recruitment of activated PH-Akt, Rac1 and Cdc42 to the leading edge of HEK293 cells during chemotaxis. The poorly internalizing LLKIL mutant CXCR2 (with a mutation in LLKIL motif, which is necessary for AP-2 and HIP binding) failed to show this polarization of activated intracellular signaling molecules. Moreover, blocking the cell endocytosis machinery with dominant negative dynamin also impaired polarization of these signals in response to the ligand. The Rho GTPases, Rac1 and Cdc42, were activated equally upon ligand stimulation in the cells expressing either WT receptor or LLKIL mutant receptors. However, only in the cells expressing WT CXCR2, did we observe polarized localization of these activated signals. The LLKIL mutant CXCR2-expressing cells displayed strong activation of Rac1 and Cdc42 but there was no polarized localization of these activated molecules toward the chemokine gradient. Biochemical assays of MIP-2-stimulated Cdc42 activity confirmed that these GTPases are activated in response to ligand independent of receptor internalization. Moreover, data showing expression of dominant negative or constitutively active Cdc42 attenuated CXCR2-mediated chemotaxis support the model in which polarization of the activated signals are required for chemotaxis.
There is some evidence that feedback loops are important for chemotaxis, i.e. the PI 3 kinase-Rac-actin feedback loop (Weiner et al., 2002) and Gβγ-PAK1-PIXα-Cdc42 signaling complex (Li et al., 2003). A model postulated by Parent and Devreotes, suggests that the net effect of a localized activation opposed by a global inhibition determines the intracellular polarization in chemotaxing cells (Parent and Devreotes, 1999). This localized activation is achieved through mutual negative feedback pathways. Our data with dominant negative and constitutive active Cdc42 also imply that oscillation of activation of Rho GTPases is an important mediation of chemotaxis.
Clearly, the modulation of chemotaxis is a complex issue. The data presented here provide additional insight into the question of how a cell interprets an external chemokine gradient, translates and amplifies that gradient into an intracellular gradient of signaling molecules. We show that HEK 293 cells expressing mutant receptors that internalize poorly because their AP-2/HIP binding motif is mutated, do not exhibit a focal distribution of PH-Akt, Rac1 or Cdc42 in response to ligand. Cells expressing these mutant receptors also do not undergo normal chemotaxis in response to CXCR2 ligands. Therefore, our data indicate that in the case of CXCR2-mediated chemotaxis, receptor internalization and recycling could be key events in the continuous directional sensing of a chemokine gradient.
Acknowledgments
We thank Tamás Balla for permission to use his construct PH-Akt-GFP. We thank Henry Bourne, Gary Bokoch, and Michiyuki Matsuda for kindly providing the DNA constructs used in this study. Thanks also to the scientists providing advice in the following aspects: David Piston for his advice on FRET analysis using the fluorescence spectrophotometer, Sam Wells for advice in using confocal and time-lapse video microscopy, Linda Horton and Yingchun Yu for technical assistance. We thank Chang Chung, Alisa Weaver and Evemie Schutyser for their thoughtful comments and the critical reading of the manuscript. This study was supported by grants from the NIH, CA-34590 (A.R.), the Vanderbilt-Ingram Cancer Center grant CA-68485-(Harold Moses) and the Department of Veterans Affairs for a Career Scientist Award (A.R.).
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/117/23/5489/DC1
References
- Arai H, Monteclaro FS, Tsou CL, Franci C, Charo IF. Dissociation of chemotaxis from agonist-induced receptor internalization in a lymphocyte cell line transfected with CCR2B. Evidence that directed migration does not require rapid modulation of signaling at the receptor level. J Biol Chem. 1997;272:25037–25042. doi: 10.1074/jbc.272.40.25037. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Yang W, Sai J, Richmond A. Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J Biol Chem. 2001a;276:16960–16968. doi: 10.1074/jbc.M009292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001b;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Yang W, Sai J, Richmond A. Hsc/Hsp70 interacting protein (hip) associates with CXCR2 and regulates the receptor signaling and trafficking. J Biol Chem. 2002;277:6590–6597. doi: 10.1074/jbc.M110588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Fernandis AZ, Cherla RP, Chernock RD, Ganju RK. CXCR4/CCR5 down-modulation and chemotaxis are regulated by the proteasome pathway. J Biol Chem. 2002;277:18111–18117. doi: 10.1074/jbc.M200750200. [DOI] [PubMed] [Google Scholar]
- Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MH, Chiang SC, Ye RD, Prossnitz ER. Phosphorylation of the N-formyl peptide receptor is required for receptor internalization but not chemotaxis. J Biol Chem. 1997;272:29426–29429. doi: 10.1074/jbc.272.47.29426. [DOI] [PubMed] [Google Scholar]
- Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- Kim JY, Soede RD, Schaap P, Valkema R, Borleis JA, van Haastert PJ, Devreotes PN, Hereld D. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J Biol Chem. 1997;272:27313–27318. doi: 10.1074/jbc.272.43.27313. [DOI] [PubMed] [Google Scholar]
- Kraft K, Olbrich H, Majoul I, Mack M, Proudfoot A, Oppermann M. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J Biol Chem. 2001;276:34408–34418. doi: 10.1074/jbc.M102782200. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- Nieto M, Frade JM, Sancho D, Mellado M, Martinez AC, Sanchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med. 1997;186:153–158. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. A cell’s sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- Perez HD, Elfman F, Marder S, Lobo E, Ives HE. Formyl peptide-induced chemotaxis of human polymorphonuclear leukocytes does not require either marked changes in cytosolic calcium or specific granule discharge. Role of formyl peptide receptor reexpression (or recycling) J Clin Invest. 1989;83:1963–1970. doi: 10.1172/JCI114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–23836. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE, Devaux C, Biard-Piechaczyk M. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- Samanta AK, Oppenheim JJ, Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990;265:183–189. [PubMed] [Google Scholar]
- Sambrano GR, Coughlin SR. The carboxyl tail of protease-activated receptor-1 is required for chemotaxis. Correlation of signal termination and directional migration. J Biol Chem. 1999;274:20178–20184. doi: 10.1074/jbc.274.29.20178. [DOI] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Neptune ER, Sedat JW, Bourne HR. Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol Biol Cell. 1999;10:1163–1178. doi: 10.1091/mbc.10.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul JD, Voermans C, van Gelderen J, Anthony EC, van der Schoot CE, Hordijk PL. Leukocyte-endothelium interaction promotes SDF-1-dependent polarization of CXCR4. J Biol Chem. 2003;278:30302–30310. doi: 10.1074/jbc.M304764200. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Schraw WP, Mueller SG, Richmond A. Interruption of G protein-coupling in CXCR2 does not alter ligand binding, but eliminates ligand-activation of GTPgamma35S binding, calcium mobilization, and chemotaxis. Biochemistry. 1997;36:15193–15200. doi: 10.1021/bi971594u. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang D, Richmond A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, re-sensitization, and signal transduction. J Biol Chem. 1999;274:11328–11333. doi: 10.1074/jbc.274.16.11328. [DOI] [PubMed] [Google Scholar]