Summary
Metastatic melanoma is an aggressive skin cancer that is notoriously resistant to current cancer therapies. In human melanoma, nuclear factor-kappa B (NF-κB) is upregulated, leading to the deregulation of gene transcription. In this review, we discuss (i) the relationship between gene alteration in melanoma and upregulation of NF-κB, (ii) mechanisms by which activated NF-κB switch from pro-apoptotic to anti-apoptotic functions in melanoma and (iii) autocrine mechanisms that promote constitutive activation of NF-κB in metastatic melanoma.
Keywords: NF-κB, IKK, melanoma, MAP3K, cytokines, apoptosis
Introduction
Melanoma is a skin cancer that originates in melanocytes, specialized pigment-producing cells found in both the basal layer of the epidermis and in the eye (Hurst et al., 2003). The known environmental risk factor is exposure to ultraviolet light, and the risk is greatly increased in people with fair skin (Bauer and Garbe, 2003; Bliss et al., 1995). Normally, melanocytes synthesize melanin pigments and transfer them to surrounding keratinocytes. The resulting skin pigmentation protects against damage caused by solar ultraviolet radiation (Gilchrest et al., 1999). Melanoma progresses from pigmented lesions called benign nevi, which are then converted to dysplastic nevi (Clark, 1991; Clark et al., 1989; Koh, 1991; Mooi, 1997; Parmiter and Nowell, 1993). Further progression leads to an in situ melanoma, which grows laterally and is mostly confined to the epidermis. This stage is known as radial growth phase (RGP) melanoma (Clark, 1991). RGP melanoma can be treated efficiently by surgical dissection, with low risk of relapse or metastasis. However, if left untreated, the melanoma can progress to the vertical growth phase (VGP), which is associated with invasion of the dermis by melanoma cells and the acquisition of metastatic potential (Clark, 1991). Due to the complex nature of the disease, melanoma has proven to be highly resistant to conventional chemotherapy treatment with dacarbazine (DTIC) or its derivative temozolomide (TMZ), which exhibits the best single agent activity with a response rate of only 15–20% (Balch and Cascinelli, 2001; Sondak et al., 2001). Patients at high risk for recurrence are frequently given interferon-α (IFN-α) and/or interleukin-2 (IL-2) as an adjuvant. The effectiveness of this treatment is widely debated, and even its supporters acknowledge that benefits are small and offset by a high level of toxicity (de Gast et al., 2003; Meric et al., 2003; Punt and Eggermont, 2001).
The activation of nuclear factor-kappa B (NF-κB) has been proposed as an event that promotes melanoma tumor progression (Huang et al., 2000a; Payne and Cornelius, 2002; Richmond, 2002). In human melanoma, a number of NF-κB-regulated chemokines are constitutively expressed at high levels: CXC ligand 8 (CXCL8 or IL8, interleukin-8; Singh et al., 2005), CXCL1 (Melanoma growth stimulatory activity or MGSA; Richmond et al., 1985), CCL5 (regulated on activation, normal T expressed and secreted, or RANTES; Mrowietz et al., 1999) and CCL2 (monocyte chemotactic protein-1, or MCP1; Bottazzi et al., 1992). These NF-κB-regulated chemokines, when transcriptionally activated, are thought to enhance melanoma progression through autocrine and paracrine loops (Payne and Cornelius, 2002; Richmond, 2002; Strieter, 2001). Indeed, overexpression of CXCL8 causes metastatic tumor growth in normal primary melanoma cells (Schaider et al., 2003; Singh et al., 2005), and is associated with the transition from RGP to VGP in melanoma (Leslie and Bar-Eli, 2005). Antibodies which neutralize CXCL8 inhibit tumor angiogenesis in human melanoma (Huang et al., 2000a, 2002), and melanoma patients responding to chemotherapy exhibited a significant decrease in CXCL8 serum levels (Brennecke et al., 2005). Overexpression of the murine homologue of CXCL1 in INK4a/ARF−/− immortalized melanocytes increases melanoma tumor incidence (Yang et al., 2001b) and induces malignancy in squamous cell carcinoma in nude mice (Dong et al., 2001). Furthermore, antibodies that are specific for these ligands or their receptors slow the growth of melanoma tumors in mice (Payne and Cornelius, 2002).
All members of the NF-κB family [Rel A (p65), Rel B, C-Rel, NF-κB1 (p50), and NF-κB2 (p52)] contain a Rel homology domain (RHD) in the N-terminal region that mediates dimerization and DNA binding (Dixit and Mak, 2002; Ghosh and Karin, 2002; Hayden and Ghosh, 2004; Richmond, 2002). Inactivated forms of p65, Rel B, and C-Rel are associated with cytoplasmic IkB (inhibitor protein of NF-κB), while p100 (precursor of p52) and p105 (precursor of p50) contain intrinsic inhibitory domains. IkB proteins are regulated through a mechanism in which they are phosphorylated by the IkB kinase complex (IKK) and subsequently degraded by the 26S proteasome (Dixit and Mak, 2002; Ghosh and Karin, 2002). p65 is phosphorylated by a number of kinases during the phosphorylation and degradation of IkBs, and these events enhance the nuclear translocation of p65 (Naumann and Scheidereit, 1994; Sakurai et al., 1999). Upon stimulation, p100 and p105 are cleaved into active forms p52 and p50 respectively. These post-translational modifications generate active NF-κB complexes, most importantly the p65/p50 heterodimer, which represents the major activated form of NF-κB in many cell types (Dixit and Mak, 2002; Ghosh and Karin, 2002; Richmond, 2002).
Although causality is often difficult to determine in melanomas, sun exposure and genetic susceptibility are considered important predisposing factors. On the contrary, in vitro and in vivo studies have shown that NF-κB activity is upregulated in dysplastic nevi and lesions of human melanoma when compared with human nevi or melanocytes in normal skin (Dhawan and Richmond, 2002; McNulty et al., 2001, 2004). Inhibition of NF-κB in highly metastatic melanoma xenografts in nude mice resulted in a decrease in angiogenesis as measured by microvessel density, which correlated with a decrease in the level of CXCL8 expression (Huang et al., 2000b). In this review, we discuss (i) relationships between gene alteration in melanoma and the upregulation of NF-κB, (ii) mechanisms by which activation of NF-κB results in a switch from a pro-apoptotic to anti-apoptotic function in melanoma, and (iii) autocrine mechanisms that sustain the constitutive activation of NF-κB in metastatic melanoma.
Gene mutation in sporadic melanoma and NF-κB upregulation
Exposure to UV light is known as an inducer of gene mutation in sporadic melanoma (Bauer and Garbe, 2003; Bliss et al., 1995; Clark et al., 1989; Gilchrest et al., 1999). Specific gene mutations reported with high frequency include the 16 kDa cyclin-dependent kinase 4 (CDK4) inhibitor (p16 INK4a), the 14 kDa protein derived from the alternative reading frame of INK4 (p14 INK4/ARF), p53, human neuroblastoma retrovirus-associated sequences (N-Ras), v-Raf murine sarcoma viral oncogene homolog B1 (B-Raf), and a lipid phosphatase known as phosphatase and tensin homolog (PTEN; Table 1).
Table 1.
Genetic mutations recorded in sporadic melanoma gene mutations including loss or alteration of nucleotides with high frequency in melanoma are listed
| Pathway | Gene mutation (frequency %) | Reference |
|---|---|---|
| p16 INK | p16INK loss/mutation (8–35) | Begg et al. (2005); Berwick et al. (2004); Puig et al. (2005) |
| p14 INK/ARFloss/mutation (20–40) | Ghiorzo et al. (2004); Rizos et al. (2001) | |
| p53 | p53 loss/mutation (10) | Albino et al. (1994); Papp et al. (2003); Sherr (2001); Straume et al. (2000) |
| MAPK | N-Ras mutation (15–30) | Borner et al. (1999) |
| B-Raf mutation (26–70) | Davies et al. (2002); Kumar et al. (2003); Yazdi et al. (2003) | |
| PI3K | PTEN deletion (10–15) | Celebi et al. (2000); Tsao et al. (2003); Wu et al. (2003); Zhou et al. (2000) |
The mutation frequencies were documented by Gray-Schopfer et al. (2005).
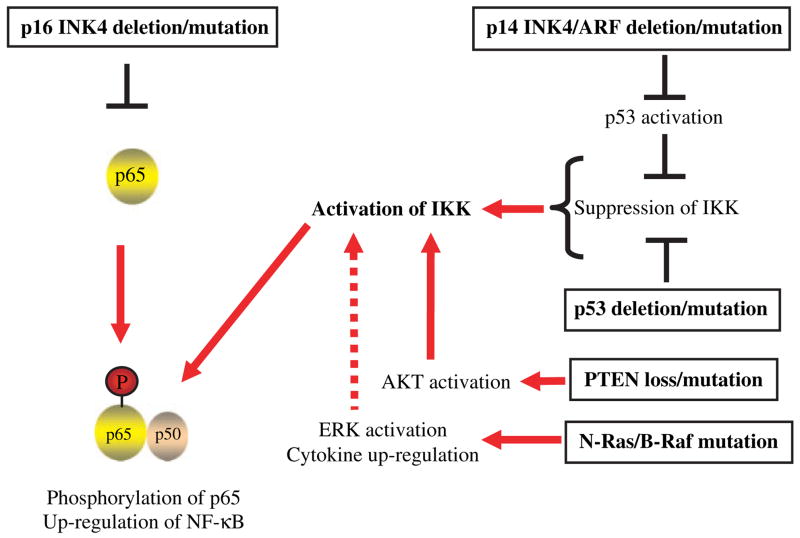
There is increasing evidence that melanoma progression is mediated through multiple genetic routes. For example, an analysis of 41 melanoma samples revealed an uneven distribution of B-Raf, N-Ras, PTEN, P53 and p16 INK4a mutations, and among the samples, 12 distinct mutational profiles were identified (Daniotti et al., 2004). These gene mutations are thought to coordinately promote the dermatological transitions from normal melanocyte to malignant melanoma as shown in Figures 1 and 2A. In the first section of this review, these gene mutations are reviewed in terms of the upregulation of NF-κB, as shown in Figure 1.
Figure 1.
Gene mutations in sporadic melanoma and NF-κB upregulation. Gene mutations with high frequency in melanoma are listed in Table 1. These mutations directly/indirectly induce NF-κB upregulation. Red arrow (→) indicates direct positive regulation and dotted red arrow indicates indirect positive regulation. The black colored symbol (⊦) indicates negative regulation. This diagram is based on the following articles: Becker et al. (2005); Castelli et al. (1994); Celebi et al. (2000); Cooray (2004); Dhawan et al. (2002); Gu et al. (2004); Ikenoue et al. (2003, 2004); Karasarides et al. (2004); Kim and Lee (2005); Koul et al. (2001); Li and Sarkar (2002); Tsao et al. (2003); Wang et al. (2005); Wolff and Naumann (1999); Zhou et al. (2000).
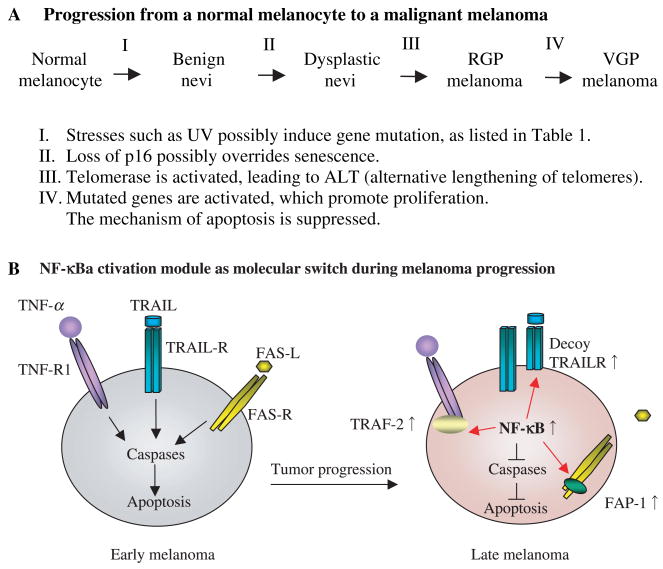
Figure 2.
NF-κB as a molecular switch in melanoma. (A) Model of progression from a normal melanocyte to a malignant melanoma. Adapted from Clark’s model (Clark, 1991; Clark et al., 1989) with other reviews (Bennett, 2003; Gray-Schopfer et al., 2005). Oncogene-induced senescence (OIS) and/or ALT-induced senescence are overridden by various gene mutations such as those in p16 and p53. (B) NF-κB activation module as molecular switch during melanoma progression. In late melanoma stage, NF-κB is activated and inhibits cell apoptosis (see detail in text). This model is modified form Ivanov et al. (2003) with other articles (Baldwin, 1996; Chan et al., 1999; Ivanov et al., 2003, 2006; Oya et al., 2001; Ravi et al., 2001; Sato et al., 1995; Zhang et al., 2000).
NF-κB upregulation and p16 INK4a mutation
p16 INK4a regulates not only cell proliferation (Hayward, 2000; Rocco and Sidransky, 2001; Ruas and Peters, 1998; Sharpless and DePinho, 1999), but also the activity of the retinoblastoma (RB) protein family members, which are tumor suppressors and inhibitors of cell proliferation (Castellano and Parmiani, 1999; Soufir et al., 1999). The p16 INK4a/RB pathway is critical to the prevention of melanoma, as p16 INK4a or RB deficiency leads to the overexpression of cyclin D1, enhancement of proliferation, and/or immortalization (Bartkova et al., 1996; Sauter et al., 2002; Utikal et al., 2005).
Wild-type p16 INK4a has been shown to bind to the NF-κB subunit p65, whereas mutated p16 INK4a exhibits reduced binding (Becker et al., 2005; Wolff and Naumann, 1999). Expression of wild-type p16 INK4a strongly inhibits NF-κB transcriptional activity (Becker et al., 2005), suggesting that loss of p16 INK4a directly leads to the upregulation of NF-κB activation (Figure 1).
NF-κB upregulation and p14 INK/ARF-P53 mutation
p14 INK4/ARF activates a key tumor suppressor, p53 (Albino et al., 1994; Papp et al., 2003; Sherr, 2001; Straume et al., 2000). Thus, p14 INK/ARF loss/inactivating mutation is associated with the reduction or loss of p53 activation (Ghiorzo et al., 2004; Rizos et al., 2001).
The IKKα promoter contains a p53 binding site, which inhibits gene transcription (Gu et al., 2004). Therefore, loss of p53 leads to the upregulation of IKKα and the activation of NF-κB. An inhibitor of p53-dependent transcription leads to an increase in UV-induced activation of NF-κB (Wang et al., 2005). These experimental data suggest that p53 loss/mutation directly leads to the upregulation of NF-κB (Figure 1).
NF-κB upregulation and N-Ras/B-Raf mutation
The responses of cells to their environment are controlled by conserved signaling mechanisms that transmit signals from the cell surface to the nucleus. The Ras/Raf/mitogen-activating-kinase (MAPK) cascade leads to cell proliferation and the inhibition of cell apoptosis. Ras proteins, small guanine–nucleotide binding proteins that are embedded in the inner surface of the plasma membrane, are inactive in their GDP-bound state and are active in GTP-bound state. Ras proteins are activated by receptor tyrosine kinases or G-protein coupled receptors (Marais and Marshall, 1996 Robinson and Cobb, 1997). Ras-GTP can bind to several effector proteins, including Raf serine/threonine-specific kinases (Marais and Marshall, 1996; Robinson and Cobb, 1997). The B-Raf gene undergoes point transversion mutations in the kinase domain (predominantly V600E, where valine is substituted for glutamic acid) at high frequency (Davies et al., 2002), as shown in Table 1. Most cases of B-Raf mutations are associated with melanoma and are not commonly associated with other cancers (Brose et al., 2002; Davies et al., 2002). The B-Raf mutation is found in early stages of benign nevi (Jackson et al., 2005), and even causes oncogene-induced cell senescence (OIS) through the induction of p16 in human nevi (Michaloglou et al., 2005). However, as shown in Figure 3A, OIS is overridden when p16 is deleted or mutated in melanoma. When OIS is overridden, the kinase activity of mutated B-Raf is 500-fold greater than that of wild type B-Raf (Wan et al., 2004). This kinase activation leads to downstream effects such as the constitutive activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) in various melanoma cell lines and melanoma tumors (Bloethner et al., 2005; Karasarides et al., 2004; Zuidervaart et al., 2005).
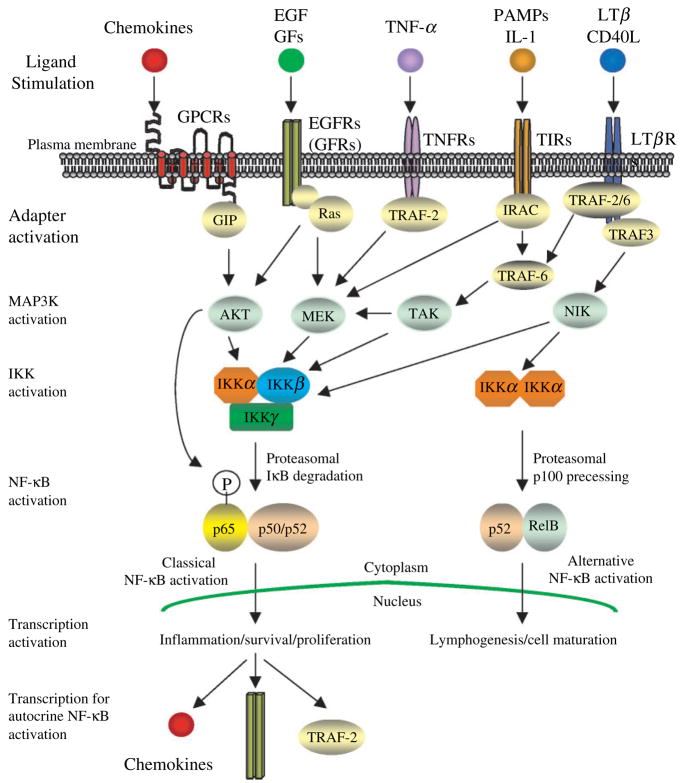
Figure 3.
Model for autocrine system by upregulated NF-κB in melanoma. This figure illustrates the autocrine system for constitutive activation of NF-κB in melanoma as detailed in Autocrine system for constitutive activation of NF-κB in melanoma of this review.
So far, it is not clear how mutant N-Ras and/or B-Raf activates NF-κB, although these mutated genes are known to activate ERK 1/2. In human melanoma cell lines with B-Raf mutations and constitutively active NF-κB and ERK, the anti-proliferative and pro-apoptotic reagent curcumin suppresses NF-κB/IKK activation, but not ERK activation (Siwak et al., 2005). These data suggest that ERK activation is not directly linked to the activation of NF-κB. However, constitutively active ERK also activates expression of various cytokines such as TNF-α and IL-1α/β, as well as chemokines, all of which are known activators of NF-κB (Castelli et al., 1994; Gaggioli et al., 2005). Therefore, mutant N-Ras and/or B-Raf may indirectly activate NF-κB through constitutive activation of ERK and the upregulation of inflammatory cytokines (Norris and Baldwin, 1999).
NF-κB upregulation and PTEN loss/mutation
The responses of cells to their environment are also controlled by another conserved signaling module, the phosphatidyl inositol (3, 4, 5) kinase (PI3K) cascade. In the PI3K cascade, PI3K binds to an activated tyrosine kinase receptor or G-protein coupled receptor and transfers the gamma phosphate group from ATP to the 3′-OH of phosphatidyl inositol (PI) substrates (Vanhaesebroeck et al., 1999). The phosphorylated PI, such as PIP2 or PIP3, in turn recruits adapter kinases to the cell membrane, which subsequently phosphorylates AKR thymoma (AKT; Cooray, 2004). AKT is a serine/threonine kinase that phosphorylates many target proteins (Cooray, 2004; Sliva, 2004). AKT also phosphorylates IKKα at the consensus sequence RXRXXS/T, leading to the p65 phosphorylation (Li and Stark, 2002; Sizemore et al., 2002). In addition, AKT has been reported to phosphorylate the NF-κB subunit p65, increasing the binding of the NF-κB complex to DNA (Koul et al., 2001). Inhibitors of PI3K block endogenous NF-κB activity in malignant melanoma cells (Dhawan et al., 2002). These results indicate that AKT directly mediates NF-κB activation.
PTEN is the regulatory molecule in the PI3K/AKT pathway and is known to shut off AKT activation by dephosphorylating phosphatidylinositol-3, 4, 5-triphosphate (PIP3) and blocking AKT membrane localization (Koul et al., 2001). PTEN is often deleted or mutated in melanoma tumors (Table 1), leading to the constitutive activation of AKT as well as NF-κB in human melanoma (Celebi et al., 2000; Dhawan et al., 2002; Tsao et al., 2003; Zhou et al., 2000).
NF-κB: molecular switch from pro-apoptotic to anti-apoptotic melanoma
Gene mutations lead to dermatological melanoma progression (Figure 2A), during which NF-κB is upregulated. NF-κB in known to coordinate the expression of over 150 genes and contribute to the balance between cell survival and apoptosis (Ivanov et al., 2003; Richmond, 2002). This section discusses how upregulated NF-κB coordinately regulates these genes and how NF-κB switches from pro-apoptotic to anti-apoptotic cell stages.
In melanocytes or early stages of melanoma, NF-κB upregulates three major pro-apoptotic pathways, which leads to caspase activation through (i) tumor necrosis factor receptor-1 (TNFR-1; Baldwin, 1996), (ii) TNF-related apoptosis-inducing ligand receptor 1/2 (TRAILR-1/2; Ravi et al., 2001), and (iii) Fas receptor (FAS-R; Chan et al., 1999; Ivanov and Ronai, 2000; Figure 2B). On the contrary, in late stages of metastatic melanoma, NF-κB upregulation inhibits these three pro-apoptotic pathways through the upregulation of (i) tumor necrosis factor receptor-associated factor-1 (TRAF-1) and TRAF-2 (Baldwin, 1996) to inhibit the TNF-R1/caspase-8-mediated pro-apoptotic pathway (Wang et al., 1998), (ii) TRAIL decoy receptor, inhibiting the TRAIL-mediated cell-death pathway (Bernard et al., 2001; Oya et al., 2001; Zhang et al., 2000), and (iii) Fas-associated phosphatase-1 (FAP-1; Sato et al., 1995), which inhibits FAS-R trafficking from cytoplasm to membrane (Ivanov et al., 2006; Figure 2B).
In late-stage melanoma, upregulated NF-κB also enhances several anti-apoptotic molecules such as inhibitor of apoptosis (IAP; Deveraux et al., 1998), caspase-8 (FLICE) inhibitory protein (FLIP; Micheau et al., 2001) and BclxL genes (Ravi et al., 2001). In addition, NF-κB upregulates Myc (Kraehn et al., 2001) and the cell cycle regulatory proteins, cyclin D1 and cyclin dependent kinase 2 (CDK2; Guttridge et al., 1999; Hinz et al., 1999), which further contribute to melanoma tumor growth.
Autocrine system for constitutive activation of NF-κB in melanoma
As discussed above, in malignant melanoma NF-κB is activated because of the activity of IKK complex-mediated degradation of IkB family members. IkB family members include IkBα IkBβ, BCl-3, IkBε, IkBγ, and the domains inside NF-κB precursors p100 and p105 (Hatada et al., 1993).
As shown in Figure 3, the classical NF-κB activation pathway (Ghosh and Karin, 2002) includes an activated IKK complex composed of two kinase subunits, IKKα and IKKβ, and a regulatory subunit IKKγ. In this pathway, IKKβ is necessary and sufficient to phosphorylate IkB molecules, which are bound to p65-containing homo- and heterodimers (Figure 3). Mice with p65-null mutation are lethal in the embryonic developmental stage, causing liver degeneration via TNF-α signaling (Beg et al., 1995). The lethality caused by p65-null mutation was suppressed by crossing the p65 null mice onto a TNFα-null background (Alcamo et al., 2001; Beg and Baltimore, 1996; Doi et al., 1999). p50 null mice are not embryonic lethal, but exhibit decreased immunoglobulin production and abnormal immunoglobulin responses (Campbell et al., 2000; Sha et al., 1995).
There is an alternative NF-κB activation pathway (Ghosh and Karin, 2002) that contains the activated IKKα homodimer complex (Figure 3). In this pathway, IKKα binds to the p100/RelB complex, then phosphorylates and processes p100, producing the active p52/RelB heterodimer (Figure 3). Rel B binds to p100, but does not homodimerize or heterodimerize with p65 (Ryseck et al., 1992). p52− null mice exhibit defects in their peripheral B-cell population, humoral response, as well as spleenic architecture (Caamano et al., 1998; Franzoso et al., 1998). Rel B-null mice exhibit decreased NF-κB activity in the thymus and spleen, and increased inflammatory infiltration into multiple organs. Rel B is critical to the coordinated activation of genes, which determine lineage commitment in the immune system (Burkly et al., 1995; Weih et al., 1995). This alternative pathway is activated by a LTβR family-mediated cascade, as shown in Figure 3.
In general, these previous data indicate that p65-containing NF-κB complexes in the classical pathway are crucial to protection from apoptosis, whereas RelB/p52 dimerization in the alternative pathway is responsible for lymphoid organogenesis (Figure 3). Following the degradation of IkB molecules or domains, the released and activated NF-κB complexes are free to translocate into the nucleus and bind to the consensus enhancer sequence (Parry and Mackman, 1994) on the promoters of various target genes. We also observed this sequence in the promoter enhancer region of NF-κB-regulated chemokine genes CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8. In the following five cascades, we discuss the mechanism of IKK activation with the NF-κB autocrine activation loop in melanoma.
Chemokines/GPCR/GIP cascade for autocrine NF-κB activation
As reviewed above, a number of NF-κB-regulated chemokines such as CXCL8 (Singh et al., 2005), CXCL1 (Richmond et al., 1985), CCL5 (Mrowietz et al., 1999) and CCL2 are overexpressed in melanoma (Bottazzi et al., 1992). Chemokines and their receptors direct cell movement and gene expression signaling in melanoma. These chemokines are divided into the α (CXC), β(CC), γ(C), and δ(CXXXC) subclasses according to the configuration of the first two cysteine residues on their N-termini (Premack and Schall, 1996), which bind to G-protein coupled receptors (GPCR). The GPCRs then activate heterotrimeric G-proteins, which dissociate from the receptors and initiate second messenger signaling (Ganju et al., 1998; Hamm, 1998). GPCRs also interact with many other proteins, designated GPCR interacting proteins (GIP), most commonly through consensus domains located on their intracellular carboxyl terminal domains (Bockaert et al., 2004). For example, β-arrestin, a common GIP, binds to most GPCRs, regulating the receptor function (Sun et al., 2002). The activation of some GPCR/β-arrestin complexes mediates new signals via activation of MAP kinases such as c-Jun N-terminal kinase 3 (JNK3), extracellular-signal-regulated kinase 1/2 (ERK1/2), and p38 MAP kinase (McDonald and Lefkowitz, 2001; Shenoy and Lefkowitz, 2003; Tilton et al., 2000).
Human melanoma lesions are known to overexpress GPCRs that play a role in these chemokine-mediated signaling pathways (Luan et al., 1997; Muller et al., 2001; Payne and Cornelius, 2002; Robledo et al., 2001; Wiley et al., 2001). The constitutive expression of CXCL-8 receptors (CXCR1 and CXCR2) leads to a IL-8-mediated metastatic phenotype in human malignant melanoma cells (Varney et al., 2003). Synthesis of the autocrine CXCL-8 also leads to a CXCL-8-dependent proliferation and angiogenesis in a subgroup of human melanomas (Bobrovnikova-Marjon et al., 2004; Leslie and Bar-Eli, 2005; Singh et al., 1999). The expression of CXCL-1 also further activates NF-κB through the sensitization of GPCRs (Richmond, 2002; Yang et al., 2001b). NF-κB is also activated by CXCL1 through the MEKK1 and p38 MAPK pathway (Wang and Richmond, 2001). Melanoma cells exposed to CCL27 undergo rapid activation of AKT and exhibit resistance to cell death induced by melanoma antigen-specific cytotoxic T cells, or by Fas-mediated apoptosis (Murakami et al., 2003, 2004).
These data suggest that GPCR signaling mediates melanoma tumor progression through an autocrine system, which is mediated by the activation of PI3K/AKT as well as MAPK cascades (Figure 3).
EGF/EGFR/Ras cascade for autocrine NF-κB activation
The binding of epidermal growth factor (EGF) to its receptor leads to the activation of NF-κBs, DNA binding to the consensus sequences, and an increase in NF-κB-dependent gene transcription (Haussler et al., 2005). The inhibition of NF-κB causes a reduction in EGF-induced cyclin D1 promoter activity (Haussler et al., 2005). These recent experimental data indicate that, in melanoma, EGF receptor (EGFR) mediates Ras/AKT/MEKK3 and NF-κB activation through autocrine loops. Indeed, a combination of specific inhibitors for PI3K/AKT and MAPK kinase kinase (MAPK3)/ERK induces high levels of apoptosis in melanomas (Ivanov and Hei, 2005).
Members of the human EGFR family are expressed in cultured human melanocytes, and several combinations of heterodimers exhibit the differential responses to the ligand TGF-α in migration and proliferation (Gordon-Thomson et al., 2005). This indicates a molecular switch function for the EGFR family in melanoma growth (Gordon-Thomson et al., 2005). In this subsection, EGFR family-mediated signaling is specifically discussed, because among the large number of the growth factor receptors, EGFR upregulation is commonly reported in relation to melanoma progression.
The human EGFR family contains HER1 (receptor for EGF), HER2 (orphan receptor), HER3, and HER4 (receptors for hereglin; Coussens et al., 1985; Plowman et al., 1993; Ullrich et al., 1984). The binding of ligands to the cysteine-rich ectodomains of receptors results in the formation of homodimeric and heterodimeric complexes, which is rapidly followed by the activation of the cytoplasmic receptor tyrosine kinase (RTK; Riese and Stern, 1998). The activation of the intrinsic RTK auto-phosphorylates C-terminal tyrosine residues, which in turn recruit signaling molecules (Alroy and Yarden, 1997). The activated RTK is known to bind SH2 domain-containing proteins such as Shc and Grb2, leading to the activation of Ras/MAPK (Segatto et al., 1993), PLCγ (phospholipase Cγ; Fazioli et al., 1991), and PI3K (Medzhitov et al., 1998) pathways.
So far, we have discussed NF-κB as a downstream target of signaling pathways initiated via EGFR in melanoma. In Ras inducible transgenic mice, the activation of Ras leads to the upregulation of the EGF family, and the mice exhibited enhanced melanoma progression (Bardeesy et al., 2005). This indicates that an autocrine loop of EGFR signaling cascade contributes to melanoma progression (Bardeesy et al., 2005). However, the EGF promoter does not have an NF-κB binding element within − 300 and +1 of the promoter. On the other hand, EGFR (HER1) (reference accesssion no. NM_005228) does contain the NF-κB-binding consensus sequence GGGAACGCCC at position − 275 (TFSEARCH http://www.cbrc.jp), similarly HER2 (NM_004448) contains GGGAGTTGCC at position − 79, and HER4 (NM_005235) contains GGGATCTCTG at position − 51. These sequences exceed the 85% threshold in relation to the NF-κB consensus sequence in M00054. In contrast, the HER3 (NM_004448) promoter does not contain an NFκB binding site. Though the NF-κB binding site in the EGFR (HER1) promoter at position − 275 is distant from the TATA box, the proximal location of the NF-κB binding sites in HER2 and HER4 promoters suggest possible functions as enhancer regions for core transcription factor binding sites. HER2 is an orphan receptor which homo- or heterodimerizes with other EGF receptors and transduces signaling to downstream cascades. Therefore, the upregulation of NF-κB possibly upregulates EGFRs, especially HER2 and HER4, to mediate an EGFR autocrine activation system (Figure 3). Indeed, constitutively active HER2 can activate NF-κB and induce resistance to apoptotic stimuli by TNF-α in NIH3T3 cells (Makino et al., 2004).
IL-1/IL-1R/IRAC cascade for constitutive NF-κB activation
Toll receptors are known to sense the invasion of microorganisms with pathogen-associated microbial patterns (PAMPs) among many species (Belvin and Anderson, 1996). Toll-like receptor (TLR) in human also recognize many PAMPs including LPS, double stranded RNA (dsRNA), non-methylated CpG DNA, and flagellum in innate immune system (Liew et al., 2005). Both TLR and interleukin-1 receptor (IL-1R), designated TIR for these two receptors, contain a highly homologous intracellular domain (TIR domain; Suzuki et al., 2002). Upon the activation of PAMS or IL-1 family, the TIR domain recruits MyD88 protein, followed by serine/threonine kinase, interleukin-1 receptor associated kinase (IRAK; O’Neill et al., 2003). The activated IRAK binds to TNF-receptor-associated factor 6 (TRAF-6; Jiang et al., 2002). TRAF-6 deficient cells exhibit a complete loss of NF-κB DNA binding induced by IL-1R, which indicates that the activation of TRAF-6 upregulates NF-κB (Lomaga et al., 1999). TRAF-6 possesses an E3 ubiquitin ligase activity, which modifies protein function in K63-linked ubiquitination without leading to the degradation, and this modification activates a member of the MAP3K including TGF-beta-activated kinase 1 (TAK1; Jiang et al., 2002). The activated TAK-1 phosphorylates and activates both IKKα and IKKβ, leading to NF-κB activation (Jiang et al., 2002). When melanoma cells constitutively expressing TLR-4 are treated with LPS, CXCL8 is overexpressed, suggesting that NF-κB is activated through the TIR domain-mediated signaling in melanoma to stimulate CXCL8 transcription (Molteni et al., in press).
Hiscott et al. reported that the promoter of the IL-1β gene contains an NF-κB consensus sequence, GGGAAAATCC, at the − 300-nt position (Hiscott et al., 1993). However, the other TIR-mediated signaling molecules listed in this review do not have the NF-κB binding consensus sequence within − 300 and +1. We therefore deduced that the autocrine NF-κB activation loop does not function in this pathway. The TIR-mediated NF-κB activation pathway can respond with varied and unexpected PAMP stimulation, including processed dsDNAs accompanied by sudden infection with viral or bacterial pathogens. In this pathway, NF-κB activation is transient, allowing return to the homeostatic regulated state.
TNFR cascade for constitutive NF-κB activation
As discussed previously in NF-κB: molecular switch from pro-apoptotic to anti-apoptotic melanoma, TNFR-mediated signaling regulates both cell death and survival. Upon TNF-α stimulation, the TNFR is known to recruit two proteins which have opposite biological effects, TNFR-associated death domain associated protein (TRADD) and TRAF-2 (Hsu et al., 1996; Rothe et al., 1995). TRADD binds to and activates caspase-8, leading to cell apoptosis (Bender et al., 2005; Hsu et al., 1996). On the contrary, TRADD also binds to TRAF-2, which leads to NF-κB activation and inhibition of TRADD-induced cell apoptosis (Rothe et al., 1995). TRAF2 is subsequently modified by ubiquitination because of the release of a ubiquitination inhibitor, which directly leads to IKK activation (Reiley et al., 2005). Activated TRAF-2 also interacts with MAPK3, which leads to the activation of the IKK complex (Yang et al., 2001a). These IKK activation pathways, mediated by TRAF-2, are illustrated in Figure 3.
These data indicate that homeostasis between proapoptotic and anti-apoptotic pathways are maintained via TNFR-mediated signaling. However, upregulation of TRAF-2 and NF-κB emphasize the pro-apoptotic pathway. Elevated TRAF-2 expression has been observed in various human tumors, including melanomas (Devergne et al., 1996; Murray et al., 2001; Zapata et al., 2000). The promoter of TRAF2 contains an NF-κB binding site GGAATTTCC at the − 63 position (TFSEARCH http://www.cbrc.jp). The upregulation of NF-κB and TRAF-2 seems to mediate an autocrine activation loop that promotes the progression of melanoma.
LTβ family/LTβ R family/TRAFs cascade for constitutive NF-κB activation
Lymphotoxin-β receptor (LTβR) and CD40, members of the tumor necrosis factor receptor family, play essential roles in the embryonic development and organization of secondary lymphoid tissues (Caamano et al., 1998; Davies et al., 2005; Franzoso et al., 1998; Kuai et al., 2003). Upon ligand activation by lymphotoxin β(LTβ) or LIGHT, LTβR recruits TRAF2, TRAF3 and cIAP1 (Kuai et al., 2003). This action leads to the activation of NF-κB and c-Jun N-terminal kinase MAP kinase (JNK), and eventually to cell death (Kuai et al., 2003). The activation of CD40 with its ligand CD40L leads to the receptor interaction with TRAF2 and TRAF6, and exhibits the upregulation of NF-κB, JNK, and p38 MAPK (Davies et al., 2005). These data suggest that the LTβR family activates the classical NF-κB activation pathway (Figure 3). Conversely, the activation of the LTβR family also activates the NF-κB inducing kinase (NIK), a member of the MAP3K family (Malinin et al., 1997; Song et al., 1997) via a specific sequence motif located in the N-terminal region of NIK, which directly phosphorylates and activates IKKα homodimers, leading to the activation of the p52/RelB complex (Senftleben et al., 2001; Xiao et al., 2001). There is increasing evidence that the upstream activator of NIK is receptor-bound TRAF3, not TRAF-2/6 (Takaori-Kondo et al., 2000; Wu et al., 2006), indicating that the alternative NF-κB activation pathway is activated by the TRAF-3-mediated pathway (Figure 3). There is also evidence that NIK is participating in the classical pathway as well as an alternative pathway for NF-κB activation. CHUK, a NIK associating protein, specifically activates NF-κB in IL-1- or TNF-α-mediated pathways through its association with IkB, leading to the ubiquitination and proteasome-mediated degradation of IkB (Regnier et al., 1997). NIK binds to and activates IKKα with a specific sequence motif in the alternative pathway (Senftleben et al., 2001; Xiao et al., 2001). NIK is also proposed to participate in the classical pathway by binding to IKK complex, where IKKα oligomerizes with IKKβ and IKKγ (Woronicz et al., 1997), which suggests that NIK possibly activates the IKK α/β complex in the classical pathway. NIK activity, therefore, is indicated as playing a pivotal role in translating the signal from extracellular stimuli to the classical or alternative NF-κB activation pathways (Figure 3).
Conclusion
It has become clear that the upregulation of NF-κB is associated with melanoma tumor progression. During melanoma progression, the upregulation of NF-κB further amplifies in the autocrine loop through the EGFR-mediated pathway as well as through GPCR-, TIR-, TNFR-, LTβR-mediated pathways. The autocrine loops deregulate the balance between cell death and survival. In this review, the possible mechanism of cross-talk between classical and alternative NF-κB activation pathways also is discussed. The regulatory molecules NIK and TRAF2/6 are pivotal in connecting these NF-κB activation pathways. In melanoma, it is important to examine NF-κB activation as a potential therapeutic target in the future. NF-κB activity may provide a double-edged sword for the modulation of cell proliferation and cell death in melanoma.
Acknowledgments
This work was supported by grants to AR from the NCI (CA 116021 and CA 098807) and from the Department of Veterans Affairs (Career Scientist Award to AR). We thank Michael Dohn, for his careful reading of the manuscript and editorial advice.
References
- Albino AP, Vidal MJ, McNutt NS, Shea CR, Prieto VG, Nanus DM, Palmer JM, Hayward NK. Mutation and expression of the p53 gene in human malignant melanoma. Melanoma Res. 1994;4:35–45. doi: 10.1097/00008390-199402000-00006. [DOI] [PubMed] [Google Scholar]
- Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, Scott M, Doerschuk CM, Hynes RO, Baltimore D. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-kappa B in leukocyte recruitment. J Immunol. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand–receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Balch CM, Cascinelli N. The new melanoma staging system. Tumori. 2001;87:S64–S68. [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Kim M, Xu J, Kim RS, Shen Q, Bosenberg MW, Wong WH, Chin L. Role of epidermal growth factor receptor signaling in RAS-driven melanoma. Mol Cell Biol. 2005;25:4176–4188. doi: 10.1128/MCB.25.10.4176-4188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Guldberg P, Alsner J, Kirkin AF, Zeuthen J, Bartek J. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56:5475–5483. [PubMed] [Google Scholar]
- Bauer J, Garbe C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297–306. doi: 10.1034/j.1600-0749.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Becker TM, Rizos H, de la Pena A, Leclercq IA, Woodruff S, Kefford RF, Mann GJ. Impaired inhibition of NF-kappaB activity by melanoma-associated p16INK4a mutations. Biochem Biophys Res Commun. 2005;332:873–879. doi: 10.1016/j.bbrc.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Begg CB, Orlow I, Hummer AJ, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97:1507–1515. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- Bennett DC. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22:3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- Bernard D, Quatannens B, Vandenbunder B, Abbadie C. Rel/NF-kappaB transcription factors protect against tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J Biol Chem. 2001;276:27322–27328. doi: 10.1074/jbc.M011183200. [DOI] [PubMed] [Google Scholar]
- Berwick M, Orlow I, Mahabir S, et al. Estimating the relative risk of developing melanoma in INK4A carriers. Eur J Cancer Prev. 2004;13:65–70. doi: 10.1097/00008469-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Bliss JM, Ford D, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case–control studies. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:367–376. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- Bloethner S, Chen B, Hemminki K, Muller-Berghaus J, Ugurel S, Schadendorf D, Kumar R. Effect of common BRAF and N-Ras mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–1232. doi: 10.1093/carcin/bgi066. [DOI] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating protein-1. Cancer Res. 2004;64:4858–4869. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Borner C, Schlagbauer Wadl H, Fellay I, Selzer E, Polterauer P, Jansen B. Mutated N-ras upregulates Bcl-2 in human melanoma in vitro and in SCID mice. Melanoma Res. 1999;9:347–350. doi: 10.1097/00008390-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol. 1992;148:1280–1285. [PubMed] [Google Scholar]
- Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–522. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Caamano JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Gerondakis S, O’Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M, Parmiani G. Genes involved in melanoma: an overview of INK4a and other loci. Melanoma Res. 1999;9:421–432. [PubMed] [Google Scholar]
- Castelli C, Sensi M, Lupetti R, Mortarini R, Panceri P, Anichini A, Parmiani G. Expression of interleukin 1 alpha, interleukin 6, and tumor necrosis factor alpha genes in human melanoma clones is associated with that of mutated N-RAS oncogene. Cancer Res. 1994;54:4785–4790. [PubMed] [Google Scholar]
- Celebi JT, Shendrik I, Silvers DN, Peacocke M. Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet. 2000;37:653–657. doi: 10.1136/jmg.37.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H, Bartos DP, Owen-Schaub LB. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50-p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WH. Tumour progression and the nature of cancer. Br J Cancer. 1991;64:631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Daniotti M, Oggionni M, Ranzani T, et al. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23:5968–5977. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Davies CC, Mak TW, Young LS, Eliopoulos AG. TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells. Mol Cell Biol. 2005;25:9806–9819. doi: 10.1128/MCB.25.22.9806-9819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Hatzivassiliou E, Izumi KM, Kaye KM, Kleijnen MF, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein–Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- Dixit V, Mak TW. NF-kappaB signaling. Many roads lead to Madrid Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- Doi TS, Marino MW, Takahashi T, Yoshida T, Sakakura T, Old LJ, Obata Y. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- Fazioli F, Kim UH, Rhee SG, Molloy CJ, Segatto O, Di Fiore PP. The erbB-2 mitogenic signaling pathway: tyrosine phosphorylation of phospholipase C-gamma and GTPase-activating protein does not correlate with erbB-2 mitogenic potency. Mol Cell Biol. 1991;11:2040–2048. doi: 10.1128/mcb.11.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Poljak L, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Deckert M, Robert G, Abbe P, Batoz M, Ehrengruber MU, Ortonne JP, Ballotti R, Tartare-Deckert S. HGF induces fibronectin matrix synthesis in melanoma cells through MAP kinase-dependent signaling pathway and induction of Egr-1. Oncogene. 2005;24:1423–1433. doi: 10.1038/sj.onc.1208318. [DOI] [PubMed] [Google Scholar]
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1 alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- de Gast GC, Batchelor D, Kersten MJ, Vyth-Dreese FA, Sein J, van de Kasteele WF, Nooijen WJ, Nieweg OE, de Waal MA, Boogerd W. Temozolomide followed by combined immunotherapy with GM-CSF, low-dose IL2 and IFN alpha in patients with metastatic melanoma. Br J Cancer. 2003;88:175–180. doi: 10.1038/sj.bjc.6600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorzo P, Pastorino L, Bonelli L, Cusano R, Nicora A, Zupo S, Queirolo P, Sertoli M, Pugliese V, Bianchi-Scarra G. INK4/ARF germline alterations in pancreatic cancer patients. Ann Oncol. 2004;15:70–78. doi: 10.1093/annonc/mdg498. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C, Jones J, Mason RS, Moore GP. ErbB receptors mediate both migratory and proliferative activities in human melanocytes and melanoma cells. Melanoma Res. 2005;15:21–28. doi: 10.1097/00008390-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer VC, da Rocha Dias S, Marais R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 2005;24:165–183. doi: 10.1007/s10555-005-5865-1. [DOI] [PubMed] [Google Scholar]
- Gu L, Zhu N, Findley HW, Woods WG, Zhou M. Identification and characterization of the IKKalpha promoter: positive and negative regulation by ETS-1 and p53, respectively. J Biol Chem. 2004;279:52141–52149. doi: 10.1074/jbc.M407915200. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hatada EN, Naumann M, Scheidereit C. Common structural constituents confer I kappa B activity to NF-kappa B p105 and I kappa B/MAD-3. EMBO J. 1993;12:2781–2788. doi: 10.1002/j.1460-2075.1993.tb05939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler U, von Wichert G, Schmid RM, Keller F, Schneider G. Epidermal growth factor activates nuclear factor-kappaB in human proximal tubule cells. Am J Physiol Renal Physiol. 2005;289:F808–F815. doi: 10.1152/ajprenal.00434.2003. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hayward N. New developments in melanoma genetics. Curr Oncol Rep. 2000;2:300–306. doi: 10.1007/s11912-000-0022-z. [DOI] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Marois J, Garoufalis J, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Huang S, DeGuzman A, Bucana CD, Fidler IJ. Level of interleukin-8 expression by metastatic human melanoma cells directly correlates with constitutive NF-kappaB activity. Cytokines Cell Mol Ther. 2000a;6:9–17. doi: 10.1080/13684730050515868. [DOI] [PubMed] [Google Scholar]
- Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000b;6:2573–2581. [PubMed] [Google Scholar]
- Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch Dermatol. 2003;139:1067–1073. doi: 10.1001/archderm.139.8.1067. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Hikiba Y, Kanai F, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132–8137. [PubMed] [Google Scholar]
- Ikenoue T, Hikiba Y, Kanai F, et al. Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor kappaB pathway and cellular transformation. Cancer Res. 2004;64:3428–3435. doi: 10.1158/0008-5472.CAN-03-3591. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Hei TK. Combined treatment with EGFR inhibitors and arsenite upregulated apoptosis in human EGFR-positive melanomas: a role of suppression of the PI3K-AKT pathway. Oncogene. 2005;24:616–626. doi: 10.1038/sj.onc.1208125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Ronai Z. p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene. 2000;19:3003–3012. doi: 10.1038/sj.onc.1203602. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–1852. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Harland M, Turner F, et al. No Evidence for BRAF as a melanoma/nevus susceptibility gene. Cancer Epidemiol Biomarkers Prev. 2005;14:913–918. doi: 10.1158/1055-9965.EPI-04-0568. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee CH. MAP kinase activation is required for the MMP-9 induction by TNF-stimulation. Arch Pharm Res. 2005;28:1257–1262. doi: 10.1007/BF02978209. [DOI] [PubMed] [Google Scholar]
- Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFkappaB activation without interfering with the IkappaB degradation pathway. J Biol Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- Kraehn GM, Utikal J, Udart M, Greulich KM, Bezold G, Kaskel P, Leiter U, Peter RU. Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br J Cancer. 2001;84:72–79. doi: 10.1054/bjoc.2000.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai J, Nickbarg E, Wooters J, Qiu Y, Wang J, Lin LL. Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J Biol Chem. 2003;278:14363–14369. doi: 10.1074/jbc.M208672200. [DOI] [PubMed] [Google Scholar]
- Kumar R, Angelini S, Hemminki K. Activating BRAF and N-Ras mutations in sporadic primary melanomas: an inverse association with allelic loss on chromosome 9. Oncogene. 2003;22:9217–9224. doi: 10.1038/sj.onc.1206909. [DOI] [PubMed] [Google Scholar]
- Leslie MC, Bar-Eli M. Regulation of gene expression in melanoma: new approaches for treatment. J Cell Biochem. 2005;94:25–38. doi: 10.1002/jcb.20296. [DOI] [PubMed] [Google Scholar]
- Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Makino K, Day CP, Wang SC, Li YM, Hung MC. Upregulation of IKKalpha/IKKbeta by integrin-linked kinase is required for HER2/neu-induced NF-kappaB antiapoptotic pathway. Oncogene. 2004;23:3883–3887. doi: 10.1038/sj.onc.1207485. [DOI] [PubMed] [Google Scholar]
- Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- McDonald PH, Lefkowitz RJ. Beta-arrestins: new roles in regulating heptahelical receptors’ functions. Cell Signal. 2001;13:683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- McNulty SE, Tohidian NB, Meyskens FL. RelA, p50 and inhibitor of kappa B alpha are elevated in human metastatic melanoma cells and respond aberrantly to ultraviolet light B. Pigment Cell Res. 2001;14:456–465. doi: 10.1034/j.1600-0749.2001.140606.x. [DOI] [PubMed] [Google Scholar]
- McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Meric JB, Rixe O, Khayat D. Metastatic malignant melanoma. Drugs Today (Barc) 2003;39(Suppl C):17–38. [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-κB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni M, Marabella D, Orlandi C, Rossetti C. Melanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4. Cancer Lett. doi: 10.1016/j.canlet.2005.04.006. in press. [DOI] [PubMed] [Google Scholar]
- Mooi WJ. The dysplastic naevus. J Clin Pathol. 1997;50:711–715. doi: 10.1136/jcp.50.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U, Schwenk U, Maune S, Bartels J, Kupper M, Fichtner I, Schroder JM, Schadendorf D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA, Hwang ST. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003;198:1337–1347. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Murray PG, Flavell JR, Baumforth KR, Toomey SM, Lowe D, Crocker J, Ambinder RF, Young LS. Expression of the tumour necrosis factor receptor-associated factors 1 and 2 in Hodgkin’s disease. J Pathol. 2001;194:158–164. doi: 10.1002/path.873. [DOI] [PubMed] [Google Scholar]
- Naumann M, Scheidereit C. Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris JL, Baldwin AS. Oncogenic Ras enhances NF-kappaB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: adapter proteins involved in signal transduction by toll-like receptors. J Endotoxin Res. 2003;9:55–59. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene. 2001;20:3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- Papp T, Pemsel H, Rollwitz I, Schipper H, Weiss DG, Schiffmann D, Zimmermann R. Mutational analysis of N-ras, p53, CDKN2A (p16(INK4a)), p14(ARF), CDK4, and MC1R genes in human dysplastic melanocytic naevi. J Med Genet. 2003;40:E14. doi: 10.1136/jmg.40.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiter AH, Nowell PC. Cytogenetics of melanocytic tumors. J Invest Dermatol. 1993;100:254S–258S. [PubMed] [Google Scholar]
- Parry GC, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B-like sites that specifically bind c-Rel-p65 heterodimers. J Biol Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Lig-and-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- Puig S, Malvehy J, Badenas C, et al. Role of the CDKN2A locus in patients with multiple primary melanomas. J Clin Oncol. 2005;23:3043–3051. doi: 10.1200/JCO.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Punt CJ, Eggermont AM. Adjuvant interferon-alpha for melanoma revisited: news from old and new studies. Ann Oncol. 2001;12:1663–1666. doi: 10.1023/a:1013592219007. [DOI] [PubMed] [Google Scholar]
- Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- Reiley W, Zhang M, Wu X, Granger E, Sun SC. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–3895. doi: 10.1128/MCB.25.10.3886-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A, Lawson DH, Nixon DW, Chawla RK. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985;45:6390–6394. [PubMed] [Google Scholar]
- Riese DJ, II, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rizos H, Darmanian AP, Holland EA, Mann GJ, Kefford RF. Mutations in the INK4a/ARF melanoma susceptibility locus functionally impair p14ARF. J Biol Chem. 2001;276:41424–41434. doi: 10.1074/jbc.M105299200. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Robledo MM, Bartolome RA, Longo N, Rodriguez-Frade JM, Mellado M, Longo I, van Muijen GN, Sanchez-Mateos P, Teixido J. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:F115–F177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- Ryseck RP, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Yeo UC, von Stemm A, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–3206. [PubMed] [Google Scholar]
- Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, Berking C, Matsushima K, Herlyn M. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer. 2003;103:335–343. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- Segatto O, Pelicci G, Giuli S, Digiesi G, Di Fiore PP, McGlade J, Pawson T, Pelicci PG. Shc products are substrates of erbB-2 kinase. Oncogene. 1993;8:2105–2112. [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- Singh RK, Varney ML, Bucana CD, Johansson SL. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res. 1999;9:383–387. doi: 10.1097/00008390-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Singh SK, Morbach H, Nanki T, Girschick HJ. Differential expression of chemokines in synovial cells exposed to different Borrelia burgdorferi isolates. Clin Exp Rheumatol. 2005;23:311–322. [PubMed] [Google Scholar]
- Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- Sliva D. Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets. 2004;4:327–336. doi: 10.2174/1568009043332961. [DOI] [PubMed] [Google Scholar]
- Sondak VK, Ross MI, Schuchter LM. Early stage (I, II, III) melanoma. Curr Treat Options Oncol. 2001;2:183–191. doi: 10.1007/s11864-001-0032-6. [DOI] [PubMed] [Google Scholar]
- Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood. 1997;89:4461–4469. [PubMed] [Google Scholar]
- Soufir N, Moles JP, Vilmer C, Moch C, Verola O, Rivet J, Tesniere A, Dubertret L, Basset-Seguin N. P16 UV mutations in human skin epithelial tumors. Oncogene. 1999;18:5477–5481. doi: 10.1038/sj.onc.1202915. [DOI] [PubMed] [Google Scholar]
- Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res. 2000;6:1845–1853. [PubMed] [Google Scholar]
- Strieter RM. Chemokines: not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001;2:285–286. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, et al. Severe impairment of interleukin-1 and toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- Takaori-Kondo A, Hori T, Fukunaga K, Morita R, Kawamata S, Uchiyama T. Both amino- and carboxyl-terminal domains of TRAF3 negatively regulate NF-kappaB activation induced by OX40 signaling. Biochem Biophys Res Commun. 2000;272:856–863. doi: 10.1006/bbrc.2000.2860. [DOI] [PubMed] [Google Scholar]
- Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I, Thelen M. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313–324. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Mihm MC, Jr, Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol. 2003;49:865–872. doi: 10.1016/s0190-9622(03)02473-3. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Utikal J, Udart M, Leiter U, Peter RU, Krahn G. Additional Cyclin D(1) gene copies associated with chromosome 11 aberrations in cutaneous malignant melanoma. Int J Oncol. 2005;26:597–605. [PubMed] [Google Scholar]
- Vanhaesebroeck B, Jones GE, Allen WE, Zicha D, Hooshmand-Rad R, Sawyer C, Wells C, Waterfield MD, Ridley AJ. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999;1:69–71. doi: 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723–731. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Wang D, Richmond A. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogenactivated protein kinase pathway. J Biol Chem. 2001;276:3650–3659. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, Tong Q, He J, Huang C. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 2005;65:6601–6611. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- Wolff B, Naumann M. INK4 cell cycle inhibitors direct transcriptional inactivation of NF-kappaB. Oncogene. 1999;18:2663–2666. doi: 10.1038/sj.onc.1202617. [DOI] [PubMed] [Google Scholar]
- Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- Wu L, Nakano H, Wu Z. The C-terminal activating region 2 of the Epstein–Barr virus-encoded latent membrane protein 1 activates NF-κB through TRAF6 and TAK1. J Biol Chem. 2006;281:2162–2169. doi: 10.1074/jbc.M505903200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001a;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- Yang J, Luan J, Yu Y, Li C, DePinho RA, Chin L, Richmond A. Induction of melanoma in murine macrophage inflammatory protein 2 transgenic mice heterozygous for inhibitor of kinase/alternate reading frame. Cancer Res. 2001b;61:8150–8157. [PubMed] [Google Scholar]
- Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121:1160–1162. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- Zapata JM, Krajewska M, Krajewski S, et al. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J Immunol. 2000;165:5084–5096. doi: 10.4049/jimmunol.165.9.5084. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Nguyen T, Thomas WD, Sanders JE, Hersey P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482:193–199. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidervaart W, van Nieuwpoort F, Stark M, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer. 2005;92:2032–2038. doi: 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]