Abstract
The constitutive expression of angiogenic and tumorigenic chemokines by tumour cells facilitates the growth of tumours. The transcription of these angiogenic and tumorigenic chemokine genes is modulated, in part, by the nuclear factor-κB (NF-κB) family of transcription factors. In some tumours, there is constitutive activation of the kinases that modulate the activity of inhibitor of NF-κB (IκB) kinase (IKK), which leads to the constitutive activation of members of the NF-κB family. This activation of NF-κB is associated with the dysregulation of transcription of genes that encode cytokines, chemokines, adhesion factors and inhibitors of apoptosis. In this review, I discuss the factors that lie upstream of the NF-κB cascade that are activated during tumorigenesis and the role of the putative NF-κB enhanceosome in constitutive chemokine gene transcription during tumorigenesis.
Chemokines are small, pro-inflammatory peptides; they are often expressed in response to the induction of expression of nuclear factor-κB (NF-κB) by cytokines or other stimuli. Chemokines regulate the transport, activation and, sometimes, proliferation of several cell types, including myeloid, lymphoid, endothelial and epithelial cells1,2. There are four chemokine subfamilies — CXC, C, CX3C and CC — based on the positions of conserved cysteine residues near the amino terminus of the proteins1 (TABLE 1). Melanoma growth-stimulatory activity (CXCL1) and interleukin-8 (IL-8, CXCL8) are members of the CXC-chemokine family, which also includes interferon-γ (IFN-γ)-inducible gene 10 (CXCL10), IFN-inducible T-cell α-chemoattractant (CXCL11), monocyte induced by IFN-γ (CXCL9), epithelial-derived neutrophil-activating peptide 78 (ENA-78, CXCL5), granulocyte chemotactic protein 2 (CXCL6), neutrophil activating peptide 2 (CXCL7), a mitogen for B-cell progenitors known as stromal-derived factor (CXCL12) and B-cell-activating factor 1/B-lymphocyte chemoattractant (BCA1/BLC, CXCL13)1. The expression of various members of the chemokine superfamily is induced by several cytokines, such as IL-1 and tumour-necrosis factor (TNF), through an NF-κB-mediated event; by IFN-γ through the Janus-activated kinase (JAK)–signal transducer and activator of transcription (STAT) pathway; or by activating protein 1 (AP1)-mediated transcription. The transcription of chemokine genes is often inhibited by transforming growth factor-β (TGF-β) and glucocorticoids1.
Table 1.
CXC-, C-, CX3C- and CC-chemokine and receptor families
| Systematic name | Chromosome | Human ligand | Mouse ligand | Chemokine receptor(s) |
|---|---|---|---|---|
| CXC-chemokine/receptor family | ||||
| CXCL1 | 4q21.1 | GROα/MGSAα | GRO/MIP2/KC? | CXCR2>CXCR1 |
| CXCL2 | 4q21.1 | GROβ/MGSAβ | GRO/MIP2/KC? | CXCR2 |
| CXCL3 | 4q21.1 | GROγ/MGSAγ | GRO/MIP2/KC? | CXCR2 |
| CXCL4 | 4q21.1 | PF4 | PF4 | Unknown |
| CXCL5 | 4q21.1 | ENA78 | GCP2/LIX? | CXCR2 |
| CXCL6 | 4q21.1 | GCP2 | GCP2/LIX? | CXCR1, CXCR2 |
| CXCL7 | 4q21.1 | NAP2 | Unknown | CXCR2 |
| CXCL8 | 4q21.1 | IL-8 | Unknown | CXCR1, CXCR2 |
| CXCL9 | 4q21.1 | MIG | MIG | CXCR3 (CD183) |
| CXCL10 | 4q21.1 | IP10 | IP10/CRG2 | CXCR3 (CD183) |
| CXCL11 | 4q21.1 | ITAC | ITAC | CXCR3 (CD183) |
| CXCL12 | 10q11.21 | SDF1α/β | SDF1/PBSF | CXCR4 (CD184) |
| CXCL13 | 4q21.1 | BCA1 | BLC | CXCR5 |
| CXCL14 | 5q31.1 | BRAK/Bolckine | BRAK | Unknown |
| (CXCL15) | Unknown | Unknown | Lungkine/WECHE | Unknown |
| CXCL16 | 17p13 | Unknown | Unknown | CXCR6 |
| C-chemokine/receptor family | ||||
| XCL1 | 1q24.2 | Lymphotactin/SCM1α/ATAC | Lymphotactin | XCR1 |
| CX3C-chemokine/receptor family | ||||
| CX3CL1 | 16q13 | Fractalkine | Neurotactin/ABCD3 | CX3CR1 |
| CC-chemokine/receptor family | ||||
| CCL1 | 17q11.2 | I309 | TCA3/P500 | CCR3 |
| CCL2 | 17q11.2 | MCP1/MCAF/TDCF | JE? | CCR2 |
| CCL3 | 17q12 | MIP1α/LD78α | MIP1α | CCR1, CCR5 |
| CCL3L1 | 17q12 | LD78β | Unknown | CCR1, CCR5 |
| CCL4 | 17q12 | MIP1β | MIP1β | CCR5 (CD195) |
| CCL5 | 17q12 | RANTES | RANTES | CCR1, CCR3, CCR5 (CD195) |
| (CCL6) | Unknown | C10/MRP1 | Unknown | |
| CCL7 | 17q11.2 | MCP3 | MARC? | CCR1, CCR2, CCR3 |
| CCL8 | 17q11.2 | MCP2 | MCP2? | CCR3, CCR5 (CD195) |
| (CCL9/10) | Unknown | MRP2/CCF18/MIP1γ | CCR1 | |
| CCL11 | 17q11.2 | Eotaxin | Eotaxin | CCR3 |
| (CCL12) | Unknown | MCP5 | CCR3 | |
| CCL13 | 17q11.2 | MCP4 | Unknown | CCR2, CCR3 |
| CCL14 | 17q12 | HCC1 | Unknown | CCR1, CCR5 |
| CCL15 | 17q12 | HCC/LKN1/MIP1δ | Unknown | CCR1, CCR3 |
| CCL16 | 17q12 | HCC4/LEC/LCC1 | Unknown | CCR1, CCR2 |
| CCL17 | 16q13 | TARC | TARC/ABCD2 | CCR4 |
| CCL18 | 17q12 | DC-CK1/PARC/AMAC1 | Unknown | Unknown |
| CCL19 | 9p13.3 | MIP3β/ELC/exodus-3 | MIP3β/ELC/exodus-3 | CCR7 (CD197) |
| CCL20 | 2q36.3 | MIP3α/LARC/exodus-1 | MIP3α/LARC/exodus-1 | CCR6 |
| CCL21 | 9p13.3 | 6Ckine/SLC/exodus-2 | 6Ckine/SLC/exodus-2/TCA4 | CCR7 (CD197) |
| CCL22 | 16q13 | MDC/STCP1 | ABCD1 | CCR4 |
| CCL23 | 17q12 | MPIF1/CKβ8/CKβ8-1 | Unknown | CCR1 |
| CCL24 | 7q11.23 | Eotaxin-2/MPIF2 | MPIF2 | CCR3 |
| CCL25 | 19p13.3 | TECK | TECK | CCR9 |
| CCL26 | 7q11.23 | Eotaxin-3 | Unknown | CCR3 |
| CCL27 | 9p13.3 | CTACK/ILC | ALP/CTACK/ILC/ESkine | CCR10 |
| CCL28 | 5p12 | MEC | CCR3/CCR10 | |
Reproduced, with permission, from REF. 123 (© 2001 The Society for Leukocyte Biology).
As the CXC-chemokines CXCL1 and CXCL8 have been associated with tumour growth, metastasis and angiogenesis, I concentrate on these important chemokines. The biological functions of chemokines are mediated through seven-transmembrane G-protein-coupled receptors. CXCL8 and CXCL6 bind to the chemokine receptors CXCR1 and CXCR2, whereas CXCL1 and other CXC-chemokines that have a Glu-Leu-Arg (ELR) motif at their amino terminus (CXCL2, -3 and-5) bind to and activate CXCR2 only2. There are also viral receptors and binding proteins for these chemokines3. Receptor binding initiates a series of downstream signals, including calcium mobilization and the activation of phospholipase-Cβ (PLCβ), phosphatidylinositol 3-kinase (PI3K), RAS, the RHO family of GTPases, p21-activated kinase (PAK), extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2), p38 mitogen-activated protein kinase (p38 MAPK) and NF-κB1,4,5.
Both the CXC-chemokines and their receptors, and the CC-chemokines and their receptors, are involved in tumorigenesis and metastasis6. Expression of CXC-chemokines that have an ELR motif at the amino terminus is associated with enhanced tumorigenic capacity and metastasis, whereas expression of ELR-negative CXC-chemokines, C-chemokines and CC-chemokines by tumours is often associated with the inhibition of tumour growth or regression3,7. Much research into the dysregulated expression of chemokines during tumour formation has used the melanoma model, in which CXCL1, CXCL8 and regulated on activation, normal T-cell expressed and secreted (RANTES, CCL5) are expressed frequently at high levels in metastatic lesions8. This constitutive expression of chemokines occurs due to transcriptional activation of the encoding genes. In part, the endogenous transcription of chemokine genes in melanoma cells is due to altered NF-κB activation. Tumour angiogenesis, growth and metastasis are facilitated by the NF-κB-modulated transcription of chemokine genes. In this review, I explore the mechanisms by which constitutively activated kinases that function upstream of the NF-κB cascade facilitate chemokine-mediated tumorigenesis. I also examine the role of NF-κB-interacting factors — which are thought to form an ENHANCEOSOME-like structure — in the dysregulation of transcription of chemokine genes during tumour development.
Chemokines and cancer
Constitutive secretion of chemokines in melanoma
CXCL1, CXCL8 and CCL5 have been shown to be expressed constitutively in human melanoma cell lines and tumours (see REF. 8 for a full review). The over-expression of these chemokines in melanocytes and melanoma tumour cells is associated with enhanced tumour-forming capacity and metastatic potential. Antibodies that are specific for these ligands or their receptors slow the growth of melanoma tumours in mice8. Human melanoma lesions are reported to express several chemokine receptors6,8–11.
A role for CXCL1 and CXCL8 in tumorigenesis is supported further by data showing that the overexpression of one of the mouse homologues of CXCL1 — known as KC (on the basis of the ordinate and abscissa of the cloning position) — enhanced the growth and metastasis of KC-deficient PAM212 squamous-cell carcinoma cells12. KC-expressing PAM212 cells injected into BALB/c mice formed tumours that grew more rapidly than PAM212 parental cells, but this growth advantage associated with the expression of KC was not observed in BALB/c Cxcr2 −/− mice. When BALB/c mice were injected with PAM–Ly2 cells — a metastatic variant of PAM212 that expresses KC — and the tumour-bearing mice were treated with the NF-κB inhibitor PS-341, which blocks the proteasomal degradation of inhibitor of NF-κB (IκB), the growth of the tumours was inhibited by 50%. The mice that were treated with PS-341 had blocked expression of KC and vascular endothelial growth factor (VEGF), which inhibited tumour growth and angiogenesis12. Similar experiments carried out with xenografts of human squamous-cell carcinoma cell lines into severe combined immunodeficient (SCID) mice gave similar results. In general, the greater the constitutive activation of NF-κB, the greater the sensitivity to PS-341-induced apoptosis. Using DIFFERENTIAL DISPLAY and complementary DNA MICROARRAYS to investigate differences in gene expression between PAM212 cells that metastasize and those that do not, Dong et al.13 found that of the genes that are differentially expressed in the metastatic cells, 10 out of 22 are NF-κB dependent in their expression. CXCL1, certain signalling molecules (c-Met, Syk and Yes-associated protein), proteins involved in cell growth (p21, p27 and cyclin D) and proteins involved in apoptosis (cellular inhibitor of apoptosis 1, Iap1; the phosphoprotein enriched in astrocytes of 15 kDa, Pea15; and Fas ligand, FasL) were among the proteins encoded by these NF-κB-inducible genes. So, genes that are regulated by NF-κB, including chemokine genes, modulate squamous-cell carcinoma and melanoma tumorigenesis.
Regulation of expression of chemokine receptors
The expression of chemokine receptors is also modulated by NF-κB. The expression of CXCR2 is regulated through an NF-κB element, and the expression of this receptor is induced in response to granulocyte–macrophage colony-stimulatory factor (GM-CSF), IL-4 and IL-13, whereas lipopolysaccharide (LPS) induces the loss of expression of CXCR2 (REFS 14–17). The expression of CXCR1 and CXCR2 has been detected in melanoma tumours, which indicates that melanoma cells might be targets for autocrine stimulation by CXCL8 (REF. 9). However, some melanoma tumour cell lines express CXCR4, CCR7 and CCR10, but no detectable levels of other chemokine receptors6. The expression of CXCR3 and CXCR4 has been noted in BLM and Sk-Mel37 human melanoma cells10. The ligand for CXCR4 (CXCL12) is produced by lymph node, liver, lung and bone marrow. Ligands for these receptors6 stimulate small GTPases and induce reorganization of the actin cytoskeleton, which indicates that these receptors might be involved in the migration and, perhaps, metastasis of these tumour cells. The activation of NF-κB also occurs as a consequence of activation of chemokine receptors. The melanoma cell lines that express moderate levels of CXCR3 and CXCR4 (BLM and SK-Mel37) have barely detectable levels of immunoreactive CXCR1 and CXCR2. When B16 melanoma cells were transduced with a retroviral expression vector for CCR7, enhanced lymph-node metastasis was observed, which could be blocked by antibodies specific for secondary lymphoid tissue chemokine (CCL21), the ligand for CCR7 (REF. 11). Together, these studies indicate that there is differential expression of chemokine receptors between melanoma cell lines and melanoma tumours. The expression of these receptors can often be correlated with the metastatic capacity of the tumour cells and their organ-specific metastasis.
Box 1 | Members of the REL family and their regulators
Members that activate transcription
v-Rel — an avian retroviral protein
c-REL — the cellular homologue of v-Rel
RELA/p65 — the main REL protein
RELB — a less commonly expressed REL protein
Dorsal — a Drosophila Rel-like protein
Dorsal-related immunity factor (Dif) — a Drosophila Rel-like protein
p50 subunit of nuclear factor-κB (NF-κB) — derived from processing of p105
p52 subunit of NF-κB — derived from processing of p100
Members that sequester NF-κB in the cytoplasm
Inhibitor of NF-κB, α (IκBα)
IκBβ
IκBε— lacks the PEST domain (a sequence that is rich in proline, glutamate, serine and threonine residues)
IκBγ
Drosophila Relish
Members that enhance transcription or restrain NF-κB nuclear localization
B-cell leukaemia/lymphoma 3 (BCL3)
Kinases that phosphorylate IκB
IκB kinase-α (IKKα) — phosphorylates IκB and facilitates the processing of p105
IKKβ— phosphorylates IκBα at Ser32 and Ser36, and IκBβ at Ser19 and Ser23
Adaptor molecules for the IKKs
IKKγ (NF-κB essential modulator, NEMO) — a regulatory factor for IKKα and IKKβ
CXCL8 and its receptors, CXCR1 and CXCR2, are expressed in ovarian-cancer cells18. CXCR1 is expressed largely on the luminal side of the epithelial tumour cells, but there is no such polar distribution of CXCR2. CXCL8 and both CXCR1 and CXCR2 are expressed in human colon-carcinoma cells, and antibodies specific for CXCR1, CXCR2 or CXCL8 inhibited the invasive capacity of colon-carcinoma cells19. Antibodies specific for CXCR1 or CXCR2 also produced a moderate inhibition of the proliferation of the colon-cancer cells and of the adhesion of colon-carcinoma cells to endothelial cells.
NF-κB activation and cancer
Several tumour systems have been shown to have constitutive activation of NF-κB. This enhanced NF-κB activity is thought to allow tumour cells to constitutively express both angiogenic and angiostatic chemokines, cytokines such as VEGF, IL-1 and IL-6, and other factors that are reported to affect melanoma growth and escape from apoptosis. The expression of CCL5 in melanoma and breast cancer has been shown to correlate with the metastatic capacity of the tumour20,21. Because the expression of CCL5 is induced by NF-κB22, down-modulation of CCL5 through the inhibition of NF-κB offers promise for therapeutic intervention. We have observed that once the expression of CXCL1 and CXCL8 has been induced, these secreted chemokines feed back on the activation of NF-κB to keep the cycle going. The endogenous activation of NF-κB in melanoma tumour cells can be inhibited by ~50% with an antibody specific for CXCL1, which indicates that chemokine autocrine loops might be important for perpetuating the constitutive activation of NF-κB in melanoma, but that additional cytokines or alterations in the signal-transduction pathway also contribute to the dysregulation of NF-κB in melanoma23.
CXCL8, anoxia and tumour aggressiveness
Treatment of human malignant melanoma cells with ionizing radiation induces the coordinated activation of NF-κB and other regulators of transcription24. The activation of NF-κB correlates with CXCL8 expression, growth, angiogenesis and metastasis of human melanoma cells25,26. The induction of transcription of CXCL8 in necrotic/ANOXIC areas of melanoma tumours is dependent on NF-κB and AP1 activity27. Transfection of melanoma cells with a dominant-negative inhibitor of NF-κB reduced tumour growth, metastatic potential, CXCL8 production and growth of blood vessels into the tumour28. These data support an association between the endogenous activation of NF-κB and the enhanced metastatic potential of malignant melanoma cells, and indicate that targeting NF-κB might have some potential therapeutic effects in clinical trials. In support of this concept, the adenoviral-mediated transfer of IκB to melanoma cells renders them sensitive to TNF-induced cytotoxicity29. Using a cell-culture system, we have shown that delivery of the super-repressor form of IκB — which cannot be phosphorylated on Ser32 or Ser36 and, therefore, is not targeted for ubiquitylation and degradation by the proteasome — to melanoma cells blocks FOCUS FORMATION and targets tumour cells to undergo apoptosis30.
Mechanism of dysregulation of NF-κB
The NF-κB proteins constitute a family of proteins that are homologous to the chicken oncogene rel. These proteins are regulated at several levels — transcription, translation and post-translational processing (BOX 1). Normally, NF-κB (a p65–p50 or p65–p52 heterodimer) is sequestered in the cytoplasm, owing to the binding of IκB to the nuclear-localization sequence of the p65/RELA NF-κB protein. Activating signals lead to the phosphorylation and ubiquitylation of IκB and its degradation by the 26S proteasome, thereby freeing the NF-κB complex to move to the nucleus and bind specific DNA promoter sequences31. Much work has been done to clarify the role of IκB phosphorylation and ubiquitylation in its targeting to the proteasome32–34. Inducible pathways for the regulation of these events involve activation of the IκB kinase (IKK) complex — comprising IKKα, IKKβ and the adaptor molecule IKKγ (NEMO) — which leads to the phosphorylation of specific serine residues of IκB (Ser32 and Ser36 of IκBα, or Ser19 and Ser23 of IκBβ). In addition, there are non-inducible pathways for the ubiquitylation and degradation of IκB, which result in the targeting of this protein for degradation by the proteasome35.
Several members of the mitogen-activated protein kinase kinase kinase (MAP3K) family, such as NF-κB-inducing kinase (NIK) and MEKK1, affect the activation of NF-κB (FIG. 1). NIK was thought initially to phosphorylate and activate IKKα preferentially, whereas MEKK1 was proposed to phosphorylate and activate IKKβ preferentially36,37. However, recent work with Nik−/− and Mekk1−/− mice indicates that NIK and MEKK1 are not essential for TNF- and IL-1-mediated induction of NF-κB, but are essential for the activation of NF-κB by other factors. For example, NIK is required for the lymphotoxin-β (LTβ)-mediated activation of NF-κB38. Once in the nucleus, NF-κB binds DNA; then, additional proteins, such as CREB-binding protein (CBP)/p300, bind to NF-κB39 and stabilize transactivation through the formation of an enhanceosome-like structure.
Figure 1. NF-κB is activated by plasma-membrane receptors that transduce signals to kinases such as PI3K.
Phosphatidylinositol 3-kinase (PI3K) can activate AKT, which potentially affects the phosphorylation of both inhibitor of NF-κB (IκB) kinase (IKK; a complex of IKKα, IKKβ and NEMO) and p65 (RELA). In addition, linker proteins, such as tumour-necrosis factor (TNF)-receptor-associated factors (TRAFs), participate in the activation of NF-κB-inducing kinase (NIK), as well as transforming-growth-factor-β-activated kinase 1 (TAK1). NIK-mediated enhancement of NF-κB activity might be facilitated by its activation of mitogen-activated protein (MAP) kinases, such as extracellular-signal-regulated kinase 1 (ERK1) and ERK2. The activation of IKK leads to enhanced phosphorylation of IκB, followed by its ubiquitylation then degradation by the 26S proteasome. This frees the NF-κB p50 and p65 subunits to be transported to the nucleus. Nuclear NF-κB p50–p65 heterodimers that are not phosphorylated on the p65 subunit can only partially activate gene transcription, whereas if p65 is phosphorylated, the enhancement of transcription is greatly magnified. LTβ, lymphotoxin-β; LTβR, lymphotoxin-β receptor; NEMO, NF-κB essential modulator; PtdInsP3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, phosphatase and tensin homologue.
The dysregulation of NF-κB in haematopoietic tumours, as well as solid tumours, has gradually become established as a paradigm31 (BOX 2). This alteration in NF-κB activity is often accompanied by altered expression of chemokines, growth factors and cytokines, oncogenes and tumour suppressors30,40–46, adhesion proteins and proteases47, and inhibitors of apoptosis48. Increased expression of p50 or p100 (the precursor of p52) has been reported in biopsies of non-small-cell lung cancer and human breast cancer, as well as head and neck cancer cells49–52. During TPA/DMBA (tetradecanoylphorbol 13-acetate/7,12-dimethylbenz(a)anthracene)-mediated induction of mouse skin cancer, there is an increase in the nuclear localization of p50 and B-cell leukaemia/lymphoma 3 (Bcl3) in the developing tumours53. The transformation of human thyroid cancer cell lines is an NF-κB-related event54. Nuclear localization of NF-κB is associated with breast cancer and might be related to the escape of these tumour cells from apoptosis55. Malignant melanoma cells have elevated constitutive activation of IKK compared with normal human epidermal melanocytes, which results in the more rapid degradation of IκB, nuclear localization of p50–p65 NF-κB and enhanced transactivating capacity of the NF-κB complex23,56,57. As the activation of NF-κB induces the transcription of inhibitors of apoptosis, as well as factors that are associated with tumour angiogenesis, metastasis and growth, we have come to understand that NF-κB could be an important potential therapeutic target in cancer, particularly if we can identify which of the upstream kinases that affect NF-κB become dysregulated during tumorigenesis. Moreover, it has been shown clearly that blocking the activity of NF-κB targets tumour cells for apoptosis12,58. In cell models in which the activation of NF-κB facilitates the escape of tumour cells from apoptosis59,60, the co-administration of an inhibitor of NF-κB, such as the proteasome inhibitor PS-341, would be expected to increase tumour kill after irradiation14.
NIK-mediated modulation of NF-κB
NIK was first identified on the basis of its association with TNF-RECEPTOR-ASSOCIATED FACTOR 2 (TRAF2)61. NIK associates with, and is reported to activate the phosphorylation of, IKKα and IKKβ. When overexpressed, NIK enhances NF-κB activity, and the expression of kinase-defective NIK blocks NF-κB activation in response to most inducers. However, NIK-deficient mice still respond to TNF and IL-1 with enhanced NF-κB activity, which indicates that NIK is not required for the activation of NF-κB by these cytokines. NIK is involved in the processing of p100 to the NF-κB p52 subunit62. This p52 subunit either homodimerizes or interacts with p65 to form the NF-κB p65–p52 heterodimer. So, the enhanced NIK activity in tumours57 could be associated with the enhanced level of nuclear p52 that is reported to be present in breast cancers63. NIK is associated also with the phosphorylation and activation of IKKα, which can affect the transactivation potential of p65 (REF. 64). Mice that have a mutation in NIK (aly/aly mice) have a loss of signalling in response to the chemokines CCL21 and CXCL13 (REF. 64). The chemotactic response is lost, as is the activation of NF-κB in response to CCL21-mediated activation of CCR7 (REF. 65).
Box 2 | Tumours that have elevated NF-κB
Dysregulation of NIK NF-κB activity in melanoma
IKK-associated NIK activity is enhanced in melanoma cell lines compared with normal human epidermal melanocytes (NHEMs)57. A catalytically inactive form of NIK blocks constitutive NF-κB or CXCL1 promoter activity in melanoma cells, but not in control NHEMs. Transient overexpression of wild-type NIK results in increased phosphorylation of ERK1 and ERK2. This phosphorylation is inhibited in a concentration-dependent manner by PD98059, an inhibitor of p42/p44 MAPK. Moreover, the NF-κB promoter activity in melanoma cells decreases with the over-expression of dominant-negative ERK57. Although these data support the hypothesis that NIK-mediated ERK activation contributes to NF-κB activity, they should not be over-interpreted because transfection/overexpression models were used.
AKT modulates NF-κB transactivation
Once IκB is degraded by the proteasome, the nuclear-localization sequence of the p50–p65 or p52–p65 NF-κB complex is exposed and, consequently, this complex can move to the nucleus and bind to the NF-κB element of the promoter of responsive genes. However, simply binding to the DNA is not sufficient. The p65 component needs to be phosphorylated in its transactivation domain to be fully active. Zhong et al.66 showed that phosphorylation of p65 on Ser276 by protein kinase A (PKA) is required for its transactivation functions. In addition, other kinases, including casein kinase II (CKII), phosphorylate p65 at serine residues that differ from those that are phosphorylated by PKA, and this enhances the transcription activity of NF-κB67. The p65 phosphorylation event might also be mediated by IKKβ68 or, possibly, IKKα69. In addition, it has been proposed recently that the serine/threonine protein kinase AKT, also known as PKB — which was isolated from the AKR mouse thymoma — might also facilitate the phosphorylation of p65 on Ser529 and Ser536 (REFS 69–73). In response to the activation of PI3K, AKT is recruited to the cell membrane by phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) or phosphatidylinositol-3,4-bisphosphate, where it becomes phosphorylated by 3′-phosphoinositide-dependent kinase 1 (PDK1) on Thr308 in the kinase domain and by PDK2 on Ser473. AKT phosphorylates several substrates at an Arg-Xaa-Arg-Xaa-Xaa-Ser/Thr motif, some of which mediate escape from apoptosis and contribute to the activation of nuclear NF-κB. Indeed, IKKα has a perfect consensus sequence for AKT-mediated phosphorylation, and Li and Stark have suggested that AKT-mediated activation of IKKα could occur upstream of the phosphorylation of p65 by IKK70. However, work from Delhase et al. does not support this possibility71. Moreover, phosphorylation of the p50 NF-κB subunit by the PI3K–AKT pathway is also reported to increase the binding of the NF-κB complex to DNA72.
The lipid phosphatase known as phosphatase and tensin homologue (PTEN) is known to switch off the activation of AKT by dephosphorylating PtdInsP3, which blocks the membrane localization of AKT. PTEN is also reported to block NF-κB activation without affecting the IκB degradation pathway72. PTEN is often deleted or mutated in melanoma and other types of tumour74,75. The activation of AKT that we observe in some human melanoma cells in vitro is accompanied by the loss or reduction of expression of PTEN (P. Dhawan, D. Ellis and R. A., unpublished observations). The loss of PTEN with consequent enhanced activation of AKT has also been reported for several other types of tumour, which indicates that this is a frequent step associated with transformation.
RAS-mediated activation of NF-κB
The activation of NF-κB can suppress apoptosis induced by oncogenic RAS76. The RAS superfamily is comprised of small GTPase proteins. One family member, HRAS (named after the Harvey-Ras oncogene), is associated with transformation. Paradoxically, when cells are transformed with HRAS, transcriptional activity is mediated through an NF-κB element, although this does not result in an increase in the nuclear level of p50–p65 (REF. 77). Oncogenic RAS activates NF-κB transcriptional activity77. Moreover, inhibitors of PI3K block the endogenous NF-κB activity of malignant melanoma cells, although these inhibitors will not block IKK-mediated phosphorylation of IκBα (P. Dhawan, D. Ellis and R.A., unpublished observations). Embryonic fibroblasts from both IKKβ- and IKKα-null mice cannot induce NF-κB transactivation. However, the restoration of IKKβ in IKKαβ-null mice will not restore cytokine-stimulated activation of RELA/p65 (REF. 69). Both IKKα and IKKβ are required for the activation of the transactivating activity of the RELA/p65 subunit of NF-κB, presumably because IKKα is required for the phosphorylation and transactivation of p65 (REF. 69). So, one can envision a pathway whereby RAS activates PI3K, which activates AKT, which activates IKKα-mediated phosphorylation of p65.
Other kinases can modulate NF-κB
Although many kinases have been shown to contribute to the activation of NF-κB, it has been difficult to show clearly the requirement for an activating kinase upstream of the IKK complex. NIK is a MAP3K, as are TGF-β-activated kinase 1 (TAK1), MEKK1 and MEKK3 (see REF. 70 for a review). Deletion of NIK and MEKK1 does not block TNF- or IL-1-mediated induction of IKK activity, but the possibility that TAK1 and MEKK3 are potential modulators of IL-1- and TNF-mediated induction of IKK remains open. NIK is required for LTβ-receptor-mediated activation of IKK38. In addition to the MAP3Ks and AKT, several other indirect modulators of NF-κB activation have been described, including RAS, RAF and TAX78–83. Protein kinase C (PKC) has also been noted as a potential modulator of IKK activity, and PKC-mediated activation of IKK is linked to tumorigenesis84–88. So, when trying to discern why there is dysregulation of NF-κB in tumour cells, it is important to look at several upstream signal-transduction pathways.
Chemokines and NF-κB
NF-κB has been shown to be an essential modulator of transcription of the following chemokines: CXCL1, -2, -3, -5, -8, -9, -10 and -12, and CCL2, -3, -4, -5, -11 and -17. However, the context of the NF-κB element in the promoter of the 5′ regulatory regions of these genes varies considerably. This finding probably indicates that there are more-subtle mechanisms for the modulation of transcription that involve other co-regulatory factors. The constitutive expression of chemokines in tumour cells has been documented extensively. However, even though the expression of many of the chemokines is regulated by NF-κB and tumours have constitutive activation of NF-κB, there is variability in the expression of specific chemokines between tumours. As the ELR-motif-containing CXC-chemokines CXCL1 and CXCL8 are known for their modulation of tumour angiogenesis and tumour growth, I concentrate here on the modulation of the transcription of these chemokines by NF-κB.
Activation of NF-κB enhances chemokine transcription
The constitutive activation of NF-κB facilitates the immortalization of melanoma and mammary tumour cells, together with their escape from apoptosis89. One consequence of this dysregulated NF-κB activity23,55–58,61–68 is that there is constitutive transcription of the NF-κB-dependent chemokines CXCL1 and CXCL8 in human tumours90–96. Constitutive expression of these chemokines can transform immortalized melanocytes and is associated with increased metastasis in colon carcinoma, squamous-cell carcinoma and melanoma19,28,91,97.
Transcriptional regulation of CXCL1 and CXCL8
Although cytokine-mediated activation of NF-κB is the main mechanism for the transcriptional induction of chemokines, NF-κB does not act alone in this induction. Moreover, the factors that interact with NF-κB to modulate transcription differ between CXCL1 and CXCL8 (FIG. 2). Whereas cooperation between NF-κB and either AP1 or the CCAAT/enhancer-binding protein (C/EBP) element is required for the transcriptional modulation of CXCL8 (REF. 96), for CXCL1, NF-κB cooperates with a transcription factor that binds to GC-rich complexes (SP1), the high-mobility group protein HMGIY (as determined by an ELECTROPHORETIC MOBILITY-SHIFT ASSAY and immediate upstream region (IUR) elements to modulate the transcription of this chemokine42. SP1 and IUR elements are involved in basal and cytokine-induced transcription of the CXCL1 gene. The factors that bind to these elements might form an enhanceosome-like transcriptional response element. The IUR binds both positive and negative regulatory factors. The 115-kDa poly-ADP ribose polymerase (PARP) binds to the TCGATC sequence of the IUR98, and thereby positively modulates CXCL1 transcription, perhaps by displacing the binding of the negative modulatory factor CCAAT-displacement protein (CDP)99. Inhibitors of the ADP-ribose-polymerase function of PARP reduce CXCL1 promoter activity, which indicates that not only DNA binding, but also the enzymatic activity of PARP, might be required for the co-activating function of PARP in CXCL1 transcription98,99.
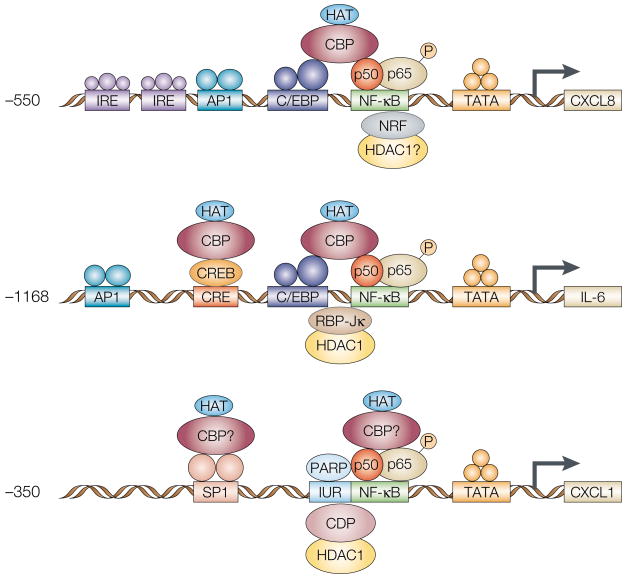
Figure 2. Similarities between the proposed enhanceosomes for IL-6, CXCL8 and CXCL1.
Similar key regulatory elements participate in the modulation of transcription of CXCL8, interleukin-6 (IL-6) and CXCL1. The transcription of CXCL8 is largely modulated through a nuclear factor-κB (NF-κB) element, which works in concert with adjacent activating protein 1 (AP1) and/or C/EBP elements to modulate the expression of this chemokine. The transcription of CXCL8 is also modulated by an NF-κB-repressing factor (NRF)120. This negative regulatory element has a dual role; in the absence of stimulation, NRF inhibits the transcription of CXCL8, but after an IL-1 signal, the NRF is required for the full induction of CXCL8 transcription. An enhanceosome model of cytokine gene expression, analogous to the CXCL8 paradigm, has been proposed for the regulation of the IL-6 promoter. Both CXCL8 and IL-6 promoters have binding sequences for NF-κB, C/EBP and the TATA BOX121. There is competition between NF-κB and an inhibitory factor, RBP-Jκ for the NF-κB promotor element. Promoter activation relies on the p65 NF-κB subunit, which recruits CREB-binding protein (CBP/p300) to the site122. The binding of CBP/p300, which has histone acetylase (HAT) activity, will stabilize transcription from these promoters. In an independent study, CCAAT displacement protein (CDP) has been shown to interact physically with CBP/p300 and is a target for acetylation at specific residues near the homeodomain. So, CDP and CBP seem to have antagonistic roles in the regulation of IL-6 and CXCL8 transcription. The transcriptional repression of CDP is probably associated with its ability to recruit the histone-deacetylase activity of HDAC1. HDAC1 deacetylates the histones and, as a result, the chromatin structure is closed and unavailable for transcription, with the end result being the consequent silencing of the chromatin. We propose that similar interactions between NF-κB, CBP and CDP might be involved in the transcription of CXCL1. c/EBP, CCAAT, enhancer-binding protein; CRE, cyclic AMP response element; IRE, interferon response element; IUR, immediate upstream region; PARP, poly-ADP ribose polymerase; RBP-Jκ, recombination signal binding protein-Jκ.
Within the IUR element, there is an overlapping consensus sequence (GGGATCGATC) that binds the 170–180-kDa CDP protein. Mutations in the IUR element that map to the putative CDP-binding site inhibit the binding of CDP to the IUR element and favour increased transcription from the CXCL1 promoter. CDP can negatively regulate the expression of CXCL1, whereas PARP can potentiate the transcription of this gene99–101. CDP has been shown previously to repress actively the transcription of several other genes101–105 either by displacing positive transactivating factors or through the recruitment of histone DEACETYLASE (HDAC), which would affect the open/closed state of the chromatin and thereby affect transcription. The action of CDP might be antagonized by the binding of CBP/p300, which has been shown to bind NF-κB and SP1, and —through its histone-acetylase activity — to stabilize the enhanceosome and enhance transcription25,106,107.
The transcription of CXCL8 is modulated mainly through an NF-κB element that works in concert with adjacent AP1 and nuclear factor induced by IL-6 (NF-IL-6) elements. There is some cell-type specificity with regard to the requirement for transactivation through either the AP1 and NF-κB elements or the NF-IL-6 and NF-κB elements97. The transcription of CXCL8 is also modulated by an NF-κB-repressing factor (NRF).
Inhibitors of NF-κB for tumour therapy
It is now clear that many types of tumour have constitutive activation of NF-κB (BOX 2). This activation of NF-κB leads to the endogenous expression of factors that are associated with tumorigenesis (VEGF, CXCL1 and CXCL8), escape from apoptosis (inhibitor of apoptosis proteins, IAPs) and metastasis (metalloproteinases). However, whereas the expression of CXCL1, CXCL8 and CCL2 is associated with increased tumorigenicity and metastasis — CCL1, CXCL1 and CXCL8 in melanoma and colon adenocarcinoma19,108,109 — the treatment of various types of tumour with CXCL9, CXCL10, CCL1, CCL3, CCL5, CCL16, CCL19, CCL20, CCL21 and XCL1 is associated with tumour regression and increased survival, possibly due to the recruitment of infiltrating leukocytes and dendritic cells (DCs). As the expression of many of these chemokines is modulated by NF-κB also, the inhibition of NF-κB presents the risk of inhibiting the migration of natural killer (NK) cells, tumour-infiltrating lymphocytes and DCs into the developing tumour. However, this negative effect is, apparently, offset by the enhanced apoptosis of the tumour cells due to the inhibition of NF-κB. Indeed, treatment with NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) has been shown to inhibit both NF-κB and tumour growth, without detrimental effects on the host31. So, reagents such as sulindac or other NSAIDs, the proteasome inhibitor PS-341 and the IKK adaptor protein NEMO-binding peptide are potential therapeutic reagents for tumours60,110–112 (FIG. 3). Indeed, initial preclinical and clinical data with PS-341 indicate that it will provide a powerful means of killing tumour cells by targeting them for apoptosis113–115.
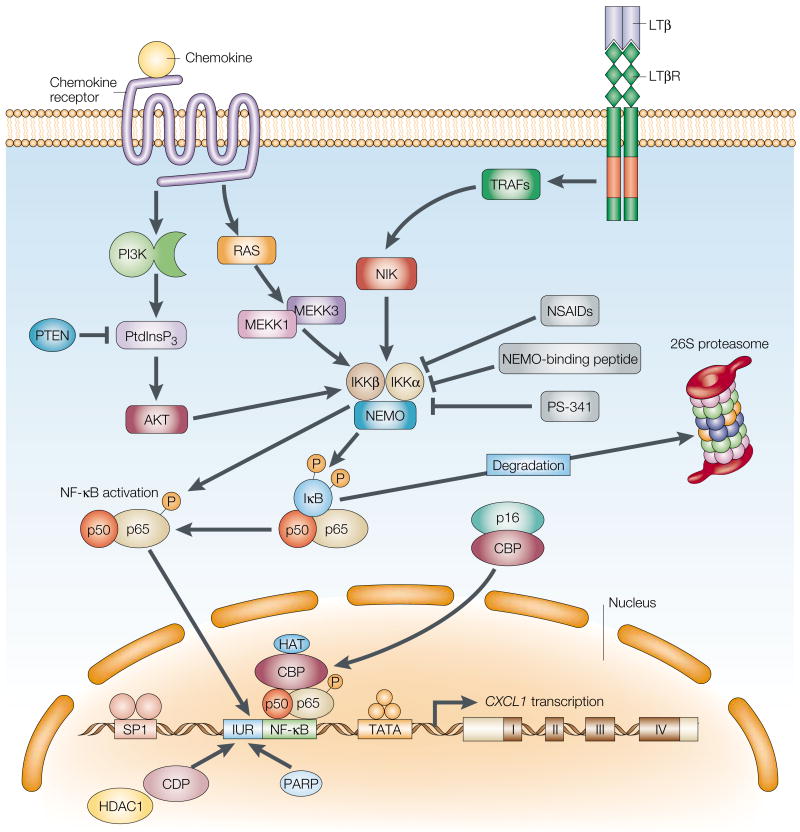
Figure 3. Model of potential components involved in the constitutive activation of NF-κB and enhanced expression of CXCL1 and CXCL8 in melanoma.
We postulate that the constitutive activation of nuclear factor-κB (NF-κB) is modulated by receptor-mediated signals, such as those mediated through chemokine receptors and the LTβ receptor, which result in the persistent activation of NF-κB-inducing kinase (NIK), AKT and, potentially, mitogen-activated protein (MAP) kinase kinase kinase 1 (MEKK1). This activation of the inhibitor of NF-κB (IκB) kinase (IKK; a complex of IKKα, IKKβ and NEMO) leads to the phosphorylation and degradation of IκB and the phosphorylation of RELA/p65, which can, in turn, lead to constitutive expression of chemokines. We postulate that chemokine expression can be blocked by treatment with non-steroidal anti-inflammatory drugs (NSAIDs), NEMO-binding peptide and PS-341, which would target tumour cells for apoptosis. CBP, CREB-binding protein; CDP, CCAAT displacement protein; HAT, histone acetyltransferase; HDAC1, histone deacetylase 1; IUR, immediate upstream region; LTβ, lymphotoxin-β; LTβR, lymphotoxin-β receptor; NEMO, NF-κB essential modulator; PARP, poly-ADP ribose polymerase; PI3K, phosphatidylinositol 3-kinase; PtdInsP3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, phosphatase and tensin homologue; TRAF, tumour-necrosis-factor-receptor-associated factor.
Conclusions
It has become clear that the dysregulation of NF-κB is a potential target for therapeutic intervention for cancers. This is due, in part, to the role of NF-κB in the modulation of expression of chemokines and inflammatory cytokines, and also to the role of NF-κB in the regulation of expression of inhibitors of apoptosis. An important point for consideration, however, is that NF-κB also modulates the expression of the angiostatic chemokines that participate in the recruitment of tumour-infiltrating lymphocytes and NK cells into the developing tumour. So, blocking NF-κB could be a double-edged sword, were it not for the fact that NF-κB does not act alone, but cooperates with several other transcription factors to modulate gene expression. The angiostatic chemokines are transcriptionally activated by IFN-γ, in addition to NF-κB. Clearly, characterizing the enhanceosome for the angiostatic chemokines, the chemokines that suppress tumorigenesis, and those that stimulate tumour growth and angiogenesis will allow investigators to develop more-precise ways to block the growth of tumours while maintaining full participation of the immune system in this blocking process.
Acknowledgments
The work described in this review was facilitated by funding from the Department of Veterans Affairs (Senior Career Scientist Award and Merit Award) and the National Cancer Institute. The figures were contributed by C. S. Nirodi and P. Dhawan. I am also endebted to M. Boothby (Vanderbilt University School of Medicine) for critical review of this manuscript.
- ENHANCEOSOME
Gene transcription is achieved by the assembly of higher-order, three-dimensional transcription factor/enhancer DNA complexes, termed enhanceosomes. Enhanceosomes activate transcription by recruiting chromatin-modifying activities and basal transcription factors to the nearby promoters
- SCID
(Severe combined immunodeficiency). Mice with this defect in their immune system do not have B or T cells and can, therefore, accept tumour cells from another species without rejection
- DIFFERENTIAL DISPLAY
This is a powerful tool for the comparison of gene expression between two or more messenger RNA populations
- MICROARRAYS
This technique allows the screening of messenger RNA extracted from cells against DNA from many thousands of genes. The DNA from each gene is positioned on a solid support in a highly ordered array
- ANOXIA
Limited oxygen supply
- FOCUS FORMATION
The ability of tumour cells to grow in an anchorage-independent manner by adhering to one another, thereby forming ‘foci’ of tumour cells
- NEMO
(NF-κB essential modulator). This is the regulatory component of the inhibitor of NF-κB (IκB) kinase (IKK) complex, also known as IKKγ
- TNF-RECEPTOR-ASSOCIATED FACTORS
(TRAFs). A term that originated with proteins that were found to bind to the cytoplasmic domain of the tumour-necrosis factor (TNF) receptor in a yeast two-hybrid screen
- TATA BOX
A DNA motif that binds several factors (TATA-binding proteins, TBPs and TBP-associated factors TAFs) that facilitate the initiation of transcription
- ELECTROPHORETIC MOBILITY-SHIFT ASSAY
A technique for detecting DNA–protein complex formation. It involves the incubation of nuclear extracts with a radiolabelled oligonucleotide probe, then separating the probe that has bound to nuclear proteins from the free radiolabelled probe by gel electrophoresis, followed by autoradiography
- DEACETYLATION
Acetylation is a post-translational modification of chromatin components, particularly histones. Histone deacetylases have been identified as components of nuclear co-repressor complexes
- NON-STEROIDAL ANTI-INFLAMMATORY DRUGS
(NSAIDs). Drugs, such as aspirin, that are used to ablate the inflammatory response. These drugs can stimulate apoptosis and inhibit angiogenesis, thereby suppressing malignant transformation and tumour growth. They work, in part, by suppressing NF-κB activation
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 3.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer. Nature Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 4.Chensue SW. Molecular machinations, chemokine signals in host–pathogen interactions. Clin Microbiol Rev. 2001;14:821–835. doi: 10.1128/CMR.14.4.821-835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devalaraja MN, Richmond A. Multiple chemotactic factors, fine control or redundancy? Trends Pharmacol Sci. 1999;20:151–156. doi: 10.1016/s0165-6147(99)01342-5. [DOI] [PubMed] [Google Scholar]
- 6.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. This article reviews the role of chemokines as regulators of tumour growth and metastasis, or the inhibition of tumour growth. [DOI] [PubMed] [Google Scholar]
- 7.Strieter RM. Chemokines: not just leukocyte chemoattractants in the promotion of cancer. Nature Immunol. 2001;2:285–286. doi: 10.1038/86286. A review of opposing roles of angiogenic and angiostatic CXC-chemokines in tumour growth. [DOI] [PubMed] [Google Scholar]
- 8.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. This article reviews the roles of CXCL1, CXCL8 and CCL5 in melanoma. [DOI] [PubMed] [Google Scholar]
- 9.Luan J, et al. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukocyte Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 10.Robledo MM, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 11.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC-chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 12.Sunwoo JB, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κB, cell survival, tumor growth, and angiogenesis in squamous-cell carcinoma. Clin Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 13.Dong G, et al. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis and the NF-κB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- 14.Cusack JC, Jr, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341, implications for systemic nuclear factor-κB inhibition. Cancer Res. 2001;61:3535–3540. This article shows that use of the proteasome inhibitor PS-341 to block the activation of NF-κB results in the inhibition of tumour growth when it is used in combination with the chemotherapeutic agent CPT-11. [PubMed] [Google Scholar]
- 15.Sprenger H, Lloyd AR, Meyer RG, Johnston JA, Kelvin DJ. Genomic structure, characterization, and identification of the promoter of the human IL-8 receptor A gene. J Immunol. 1994;153:2524–2532. [PubMed] [Google Scholar]
- 16.Bonecchi R, et al. Induction of functional IL-8 receptors by IL-4 and IL-13 in human monocytes. J Immunol. 2000;164:3862–3869. doi: 10.4049/jimmunol.164.7.3862. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd AR, et al. Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin-8 receptors on polymorphonuclear leukocytes. J Biol Chem. 1995;270:28188–28192. doi: 10.1074/jbc.270.47.28188. [DOI] [PubMed] [Google Scholar]
- 18.Ivarsson K, Ekerydh A, Fyhr IM, Janson PO, Brannstrom M. Upregulation of interleukin-8 and polarized epithelial expression of interleukin-8 receptor A in ovarian carcinomas. Acta Obstet Gynecol Scand. 2000;79:777–784. [PubMed] [Google Scholar]
- 19.Li A, Varney ML, Singh RK. Expression of interleukin-8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 20.Azenshtein E, et al. The CC-chemokine RANTES in breast carcinoma progression, regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- 21.Mrowietz U, et al. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genin P, Algarte M, Roof P, Lin R, Hiscott J. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription. J Immunol. 2000;164:5352–5361. doi: 10.4049/jimmunol.164.10.5352. [DOI] [PubMed] [Google Scholar]
- 23.Yang JM, Richmond A. Constitutive IKK activity correlates with NF-κB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. This article shows that melanoma cell lines have constitutive IKK activity, and also constitutive phosphorylation of p65, which results in constitutive NF-κB transcriptional activity and enhanced expression of chemokines. The article also shows that the chemokine CXCL1 contributes to the constitutive activation of IKK through an autocrine loop. [PubMed] [Google Scholar]
- 24.Yang CR, et al. Coordinate modulation of Sp1, NF-κB and p53 in confluent human malignant melanoma cells after ionizing radiation. FASEB J. 2000;14:379–392. doi: 10.1096/fasebj.14.2.379. [DOI] [PubMed] [Google Scholar]
- 25.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, De Guzman A, Bucana CD, Fidler IJ. Level of interleukin-8 expression by metastatic human melanoma cells directly correlates with constitutive NF-κB activity. Cytokines Cell Mol Ther. 2000;6:9–17. doi: 10.1080/13684730050515868. [DOI] [PubMed] [Google Scholar]
- 27.Kunz M, et al. Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma. A potential mechanism for high tumor aggressiveness. Am J Pathol. 1999;155:753–763. doi: 10.1016/S0002-9440(10)65174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-κB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- 29.Bakker TR, Reed D, Renno T, Jongeneel CV. Efficient adenoviral transfer of NF-κB inhibitor sensitizes melanoma to tumor necrosis factor-mediated apoptosis. Int J Cancer. 1999;80:320–323. doi: 10.1002/(sici)1097-0215(19990118)80:2<320::aid-ijc24>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, et al. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin AS. Control of oncogenesis and cancer-therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. An excellent review of NF-κB, its role in cancer and the potential for therapeutic intervention using inhibitors of NF-κB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beg AA, et al. IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 1992;10:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 33.Didonato J, Mercurio F, Karin M. Phosphorylation of IκB-α precedes but is not sufficient for its dissociation from NF-κB. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiDonato J, et al. Mapping of the inducible I-κB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pando MP, Verma IM. Signal-dependent and -independent degradation of free and NF-κB-bound IκB-α. J Biol Chem. 2000;275:21278–21286. doi: 10.1074/jbc.M002532200. [DOI] [PubMed] [Google Scholar]
- 36.Ling L, Cao ZD, Goeddel DV. NF-κB-inducing kinase activates IKK-α by phosphorylation of ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. An introduction to the biological role of NIK as an activator of IKKα. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano H, et al. Differential regulation of I-κB kinase-α and -β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase ERK kinase kinase. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C, et al. NF-κB-inducing kinase is dispensable for activation of NF-κB in inflammatory settings but essential for lymphotoxin-β receptor activation of NF-κB in primary human fibroblasts. J Immunol. 2001;167:5895–5903. doi: 10.4049/jimmunol.167.10.5895. [DOI] [PubMed] [Google Scholar]
- 39.Perkins ND, et al. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 40.Shattuck-Brandt RL, Richmond A. Enhanced degradation of I-κB-α contributes to endogenous activation of NF-κB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 41.Shattuck RL, Wood LD, Jaffe GJ, Richmond A. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood LD, Farmer AA, Richmond A. HMGI (Y), and SP1 in addition to NF-κB regulate transcription of the MGSA/GROα gene. Nucleic Acids Res. 1995;23:4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GROα gene requires both NF-κB and novel constitutive factors. J Biol Chem. 1995;270:30619–30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 44.Kondo S, Kono T, Sauder DN, McKenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol. 1993;101:690–694. doi: 10.1111/1523-1747.ep12371677. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Becker D. Antisense targeting of bFGF and FGF receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nature Med. 1997;8:887–893. doi: 10.1038/nm0897-887. [DOI] [PubMed] [Google Scholar]
- 46.Shih IM, Herlyn M. Autocrine and paracrine roles for growth factors in melanoma. In Vitro. 1994;8:113–124. [PubMed] [Google Scholar]
- 47.Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-κB/IκB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 48.Karin M, Lin A. NF-κB at the crossroads of life and death. Nature Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. This article provides an up-to-date view of the role of NF-κB activation in the modulation of factors that are involved in apoptosis and cell survival. [DOI] [PubMed] [Google Scholar]
- 49.Dejardin E, et al. Highly expressed p100/p52 (NF-κB2) sequesters other NF-κB-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–1841. [PubMed] [Google Scholar]
- 50.Tamatani T, et al. Enhanced IκB kinase activity is responsible for the augmented activity of NF-κB in human head and neck carcinoma cells. Cancer Lett. 2001;171:165–172. doi: 10.1016/s0304-3835(01)00611-5. [DOI] [PubMed] [Google Scholar]
- 51.Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-κB transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- 52.Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronov V. The NF-κB transcription factor and cancer: high expression of NF-κB and IκB-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47:145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 53.Budunova IV, et al. Increased expression of p50–NF-κB and constitutive activation of NF-κB transcription factors during mouse skin carcinogenesis. Oncogene. 1999;18:7423–7431. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]
- 54.Visconti R, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NF-κB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 55.Sovak MA, et al. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devalaraja M, Wang DZ, Ballard DW, Richmond A. Elevated constitutive IKK activity and IκB-α phosphorylation in Hs294T melanoma cells lead to increased basal MGSA/GROα transcription. Cancer Res. 1999;59:1372–1377. [PubMed] [Google Scholar]
- 57.Dhawan P, Richmond A. The role of endogenous NIK and MEKK1 in the constitutive activation of NF-κB in human melanomas. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. This study shows that, in human melanoma, there is constitutive activation of NIK, and that blocking NIK blocks the constitutive activation of NF-κB in melanoma cells. Moreover, this NIK-mediated effect on NF-κB activity requires the activation of MEKK1 and ERK1/ERK2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cusack J, Liu R, Baldwin A. Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-κB activation. Cancer Res. 2000;60:2323–2330. [PubMed] [Google Scholar]
- 59.Budunova IV, et al. Increased expression of p50–NF-κB and constitutive activation of NF-κB transcription factors during mouse skin carcinogenesis. Oncogene. 2000;19:3003–3012. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]
- 60.Mayo MW, Baldwin AS. The transcription factor NF-κB, control of oncogenesis and cancer-therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 61.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 62.Xiao G, Harhaj EW, Sun SC. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 63.Cogswell PC, Guttridge DC, Funkhouse WK, Baldwin AS., Jr Selective activation of NF-κB subunits in human breast cancer: potential roles for NF-κB2/p52 and for Bcl-3 . Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 64.Senftleben U, et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 65.Fagarasan S, et al. Alymphophasia (aly)-type nuclear factor-κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J Exp Med. 2000;191:1477–1486. doi: 10.1084/jem.191.9.1477. This article shows that loss of NIK not only affects signalling through the lymphotoxin-β receptor, but also alters the response to chemokines, which indicates that chemokine receptors might also activate NIK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong H, Voll RE, Ghosh S. Rearranged NF-κB2 gene in the HUT78 T-lymphoma cell line codes for a constitutively nuclear factor lacking transcriptional repressor functions. Mol Cell. 1998;1:661–671. [Google Scholar]
- 67.Bird TA, Schooley K, Dower SK, Hagen H, Virca GD. Activation of nuclear transcription factor NF-κB by interleukin-1 is accompanied by casein-kinase-II-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606–32612. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- 68.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 69.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the IκB kinase α- and β-subunits in liberating nuclear factor-κB (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-κB. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. This article shows the role of AKT in the activation of IKKα, which potentially phosphorylates the p65 subunit of NF-κB to potentiate its transactivating capacity. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Stark GR. NF-κB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 71.Delhase M, Li N, Karin M. Kinase regulation in inflammatory response. Nature. 2000;406:367–368. doi: 10.1038/35019154. [DOI] [PubMed] [Google Scholar]
- 72.Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine induced NF-κB activation without interfering with the IκB degradation pathway. J Biol Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- 73.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 74.Celebi JT, Shendrik I, Silvers DN, Peacocke M. Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet. 2000;37:653–657. doi: 10.1136/jmg.37.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou XP, et al. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayo MW, et al. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 77.Finco TS, et al. Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 78.Norris JL, Baldwin AS. Oncogenic Ras enhances NF-κB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 79.Troppmair J, Hartkamp J, Rapp UR. Activation of NF-κB by oncogenic Raf in HEK293 cells occurs through autocrine recruitment of the stress kinase cascade. Oncogene. 1998;17:685–690. doi: 10.1038/sj.onc.1201981. [DOI] [PubMed] [Google Scholar]
- 80.Yin MJ, et al. HTLV-1 Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 81.Chu Z, DiDonato J, Hawiger J, Ballard D. The tax oncogene of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 82.Geleziunas R, et al. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase-α (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mosialos G. The role of Rel/NF-κB proteins in viral oncogenesis and the regulation of viral transcription. Semin Cancer Biol. 1997;8:121–129. doi: 10.1006/scbi.1997.0063. [DOI] [PubMed] [Google Scholar]
- 84.LaPorta CA, Camolli R. PKC-dependent modulation of IκBα–NF-κB pathway in low metastatic B16F1 murine melanoma cells and in highly metastatic BL6 cells. Anticancer Res. 1998;18:2591–2597. [PubMed] [Google Scholar]
- 85.Biswas DK, et al. The nuclear factor-κB (NF-κB): a potential therapeutic target for estrogen receptor-negative breast cancers. Proc Natl Acad Sci USA. 2001;98:10386–10391. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson L, Szabo C, Salzman AL. Protein kinase-C-dependent activation of NF-κB in enterocytes is independent of IκB degradation. Gastroenterology. 1999;117:106–114. doi: 10.1016/s0016-5085(99)70556-1. [DOI] [PubMed] [Google Scholar]
- 87.Han Y, Meng T, Murray NR, Fields AP, Brasier AR. Interleukin-1-induced nuclear factor-κB–IκB-α autoregulatory feedback loop in hepatocytes. A role for protein kinase Cα in post-transcriptional regulation of IκB-α resynthesis. J Biol Chem. 1999;274:939–947. doi: 10.1074/jbc.274.2.939. [DOI] [PubMed] [Google Scholar]
- 88.Anrather J, Csizmadia V, Soares MP, Winkler H. Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Cζ in primary endothelial cells. J Biol Chem. 1999;274:13594–13603. doi: 10.1074/jbc.274.19.13594. [DOI] [PubMed] [Google Scholar]
- 89.Wang D, Richmond A. NF-κB activation by the CXC-chemokine MGSA/GROα involves the MEKK1/p38 MAP kinase pathway. J Biol Chem. 2001;276:3650–3659. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richmond A, Thomas HG. Melanoma growth stimulatory activity, a novel growth factor with a tissue distribution not restricted to melanoma tissue. J Cell Biochem. 1986;36:185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- 91.Singh RK, et al. Expression of interleukin-8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 92.Singh RK, et al. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin-8. Cancer Res. 1995;55:3669–3674. [PubMed] [Google Scholar]
- 93.Schadendorf D, et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2267–2675. [PubMed] [Google Scholar]
- 94.Schadendorf D, et al. Metastatic potential of human melanoma cells in nude mice — characterization of phenotype, cytokine secretion and tumor-associated antigens. Br J Cancer. 1996;74:194–199. doi: 10.1038/bjc.1996.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loukinova E, et al. Growth-regulated oncogene-α expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC-receptor-2-dependent mechanism. Oncogene. 2000;19:3477–3486. doi: 10.1038/sj.onc.1203687. [DOI] [PubMed] [Google Scholar]
- 96.Matsusaka T, et al. Transcription factors NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin-6 and interleukin-8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balentien E, et al. Effects of MGSA/GROα on melanocyte transformation. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- 98.Nirodi CS, Richmond A. Role of poly (ADP-ribose) polymerase (PARP) in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene. J Biol Chem. 2001;276:9366–9374. doi: 10.1074/jbc.M009897200. This article shows that PARP participates with NF-κB in the modulation of transcription of CXCL1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nirodi CS, et al. The 170-kDa CCAAT displacement protein (CDP/Cut) selectively binds the IUR cis-element in the CXCL1 promoter. The role of CDP in the negative regulation of CXCL1 gene expression. J Biol Chem. 2001;276:9366–9374. This study further defines the CXCL1 enhanceosome and shows that binding of the transcriptional repressor CDP to an element adjacent to the NF-κB-binding site represses the transcription of CXCL1. [Google Scholar]
- 100.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-κB transcriptional activation. J Biol Chem. 1999;380:953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 101.Ludlow C, Choy R, Blochlinger K. Functional analysis of Drosophila and mammalian Cut proteins in flies. Dev Biol. 1996;178:149–159. doi: 10.1006/dbio.1996.0205. [DOI] [PubMed] [Google Scholar]
- 102.Coqueret O, Berube G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 1998;17:4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mailly FG, et al. The human Cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding-site occupancy. Mol Cell Biol. 1996;16:5346–5357. doi: 10.1128/mcb.16.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li S, et al. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/Cut homolog, is associated with histone deacetylation. J Biol Chem. 1999;274:7803–7815. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- 105.Li S, Aufiero B, Schiltz RL, Walsh MJ. Regulation of the homeodomain CCAAT displacement/Cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP. Proc Natl Acad Sci USA. 2000;12:1627–1631. doi: 10.1073/pnas.130028697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao H, Hasegawa T, Isobe K. p300 collaborates with Sp1 and Sp3 in p21(Waf1/Cip1) promoter activation induced by histone deacetylase inhibitor. J Biol Chem. 2000;275:1371–1376. doi: 10.1074/jbc.275.2.1371. [DOI] [PubMed] [Google Scholar]
- 107.Kundu TK, et al. Activator-dependent transcription from chromatin in vitro involving targeted histone acetylation by p300. Mol Cell Biol. 2000;6:551–561. doi: 10.1016/s1097-2765(00)00054-x. This article shows the role of p300 in acetylation of histones and the stabilization of the transcriptional machinery. [DOI] [PubMed] [Google Scholar]
- 108.Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth and susceptibility to IL-2 therapy of murine melanoma. J Immunol. 1992;148:1280–1285. [PubMed] [Google Scholar]
- 109.Nakashima E, et al. Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm Res. 1995;12:1598–1604. doi: 10.1023/A:1016276613684. [DOI] [PubMed] [Google Scholar]
- 110.Berman KS, et al. Sulindac enhances tumor necrosis factor-α-mediated apoptosis of lung cancer cell lines by inhibition of nuclear factor-κB. Clin Cancer Res. 2002;8:354–360. [PubMed] [Google Scholar]
- 111.May MJ, et al. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 112.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. This article emphasizes the available data that indicate that, by developing methods of disrupting the NF-κB pathway, new advances can be made in the therapeutic intervention of acute and chronic inflammatory conditions, as well as malignancies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hideshima T, et al. NF-κB as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 114.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62:1083–1086. [PubMed] [Google Scholar]
- 115.Adams J. Proteasome inhibition, a novel approach to cancer therapy. Trends Mol Med. 2002;8:49–54. doi: 10.1016/s1471-4914(02)02315-8. This article reviews the current status of the use of proteasome inhibitors to block tumour growth. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Chang CC, Lombardi L, Dalla-Favera R. Rearranged NF-κB2 gene in the HUT78 T-lymphoma cell line codes for a constitutively nuclear factor lacking transcriptional repressor functions. Oncogene. 1994;9:1931–1937. [PubMed] [Google Scholar]
- 117.Higgins KA, et al. Antisense inhibition of the p65 subunit of NF-κB blocks tumorigenicity and causes tumor regression. Proc Natl Acad Sci USA. 1993;90:9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shah SA, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem. 2001;82:110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- 119.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell-survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 120.Nourbakhsh M, et al. The NF-κB repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-κB-flanking sequence element. J Biol Chem. 2001;276:4501–4508. doi: 10.1074/jbc.M007532200. This article models the components of the enhanceosome for CXCL8 and shows that a transcriptional repressor binds to an element flanking the NF-κB element. So, the models for the transcription of CXCL8, CXCL1 and IL-6 are similar. [DOI] [PubMed] [Google Scholar]
- 121.Stein B, Cogswell PS, Baldwin AS. Functional and physical associations between NF-κB and C/EBP family members: a Rel domain–bZIP interaction. Mol Cell Biol. 1993;13:3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li S, Aufiero B, Schiltz RL, Walsh MJ. Regulation of the homeodomain CCAAT displacement/Cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP. Proc Natl Acad Sci USA. 2000;97:7166–7171. doi: 10.1073/pnas.130028697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.International Union of Immunological Societies/World Health Organization Subcommittee on Chemokine Nomenclature. Chemokine/chemokine receptor nomenclature. J Leukocyte Biol. 2001;70:465–466. [Google Scholar]