Abstract
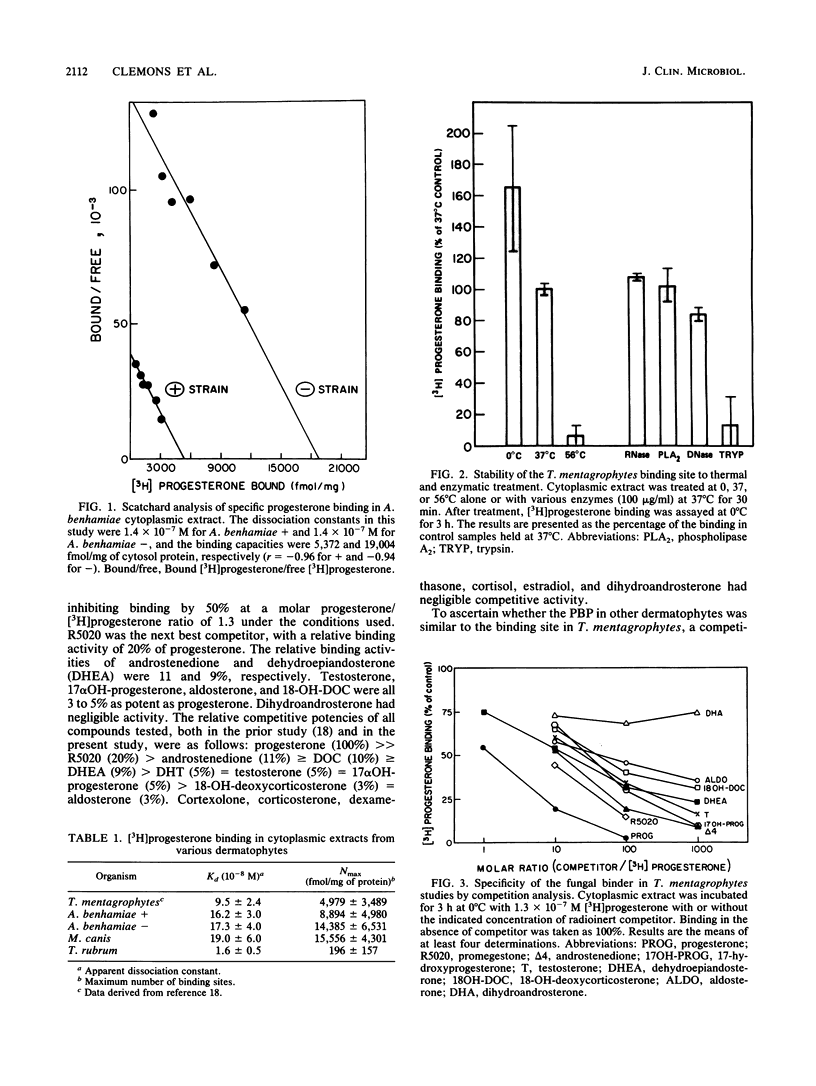
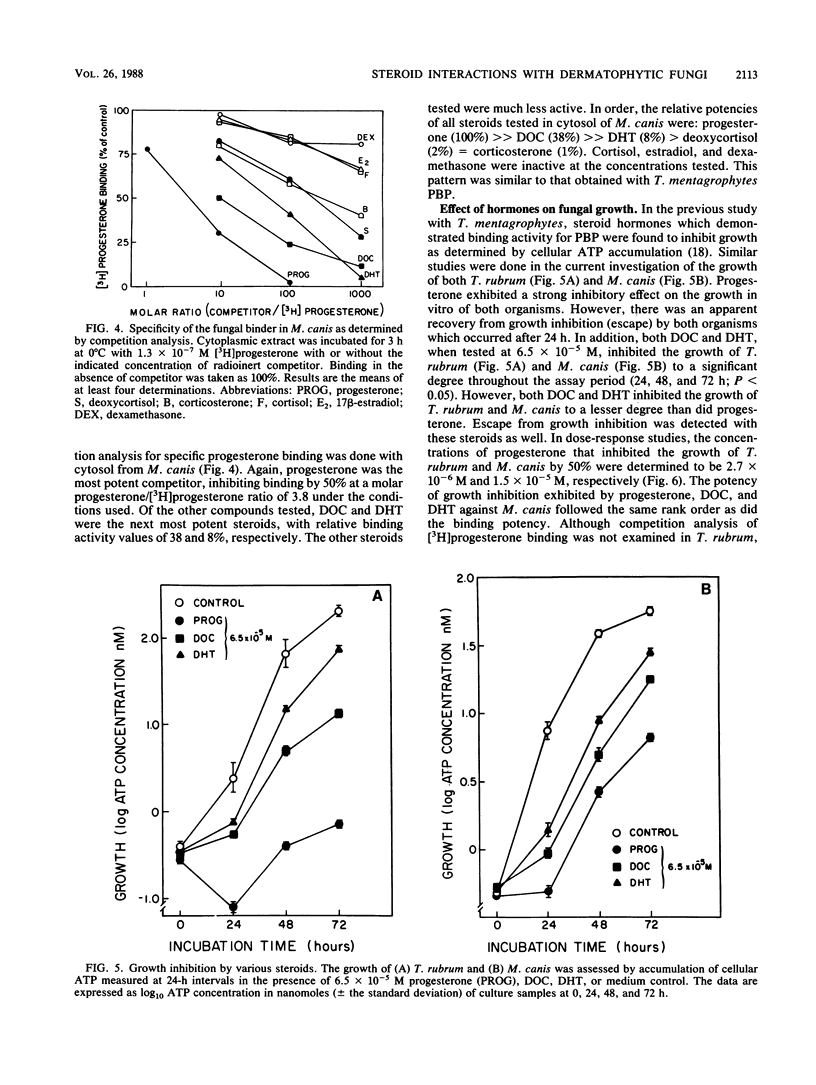
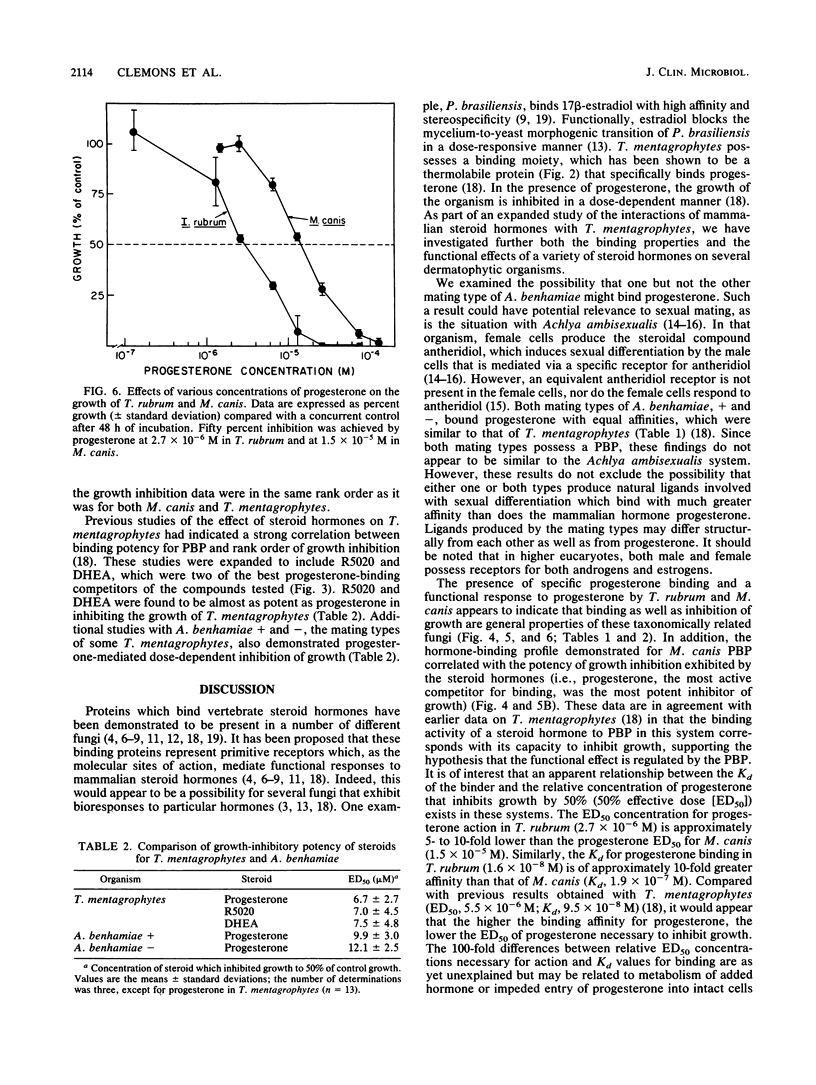
We reported previously that Trichophyton mentagrophytes contains a cytoplasmic macromolecule which specifically binds progesterone. Progesterone is also an effective inhibitor of growth of the fungus. We report here studies which characterize more fully the specific binding properties and the functional responses of T. mentagrophytes and taxonomically related fungi to a series of mammalian steroid hormones. Scatchard analysis of [3H]progesterone binding in both the + and - mating types of Arthroderma benhamiae and in Microsporum canis revealed a single class of binding sites with approximately the same affinity as that in T. mentagrophytes (Kd, 1 X 10(-7) to 2 X 10(-7) M). Trichophyton rubrum had a protein with a higher binding affinity (Kd, 1.6 X 10(-8) M). Characterization of the [3H]progesterone-binding sites in T. mentagrophytes showed the binder to be a protein which was destroyed by trypsin and heating to 56 degrees C. Previous examination of the steroid-binding specificity in T. mentagrophytes had demonstrated that deoxycorticosterone (DOC) and dihydrotestosterone (DHT) were effective competitors for [3H]progesterone binding. Expansion of this study to include other competitors revealed that R5020 (a synthetic progestin), androstenedione, and dehydroepiandosterone possessed relative binding affinities which were 20, 11, and 9% of that of progesterone, respectively. Other ligands tested were less effective. Competition studies for the binder in M. canis resulted in similar findings: DOC and DHT were effective competitors for [3H]progesterone binding. The growth of A. benhamiae + and -, M. canis, and T. rubrum were all inhibited by progesterone in a dose-responsive manner, with 50% inhibition achieved at concentrations of 9.8 x 10(-6), 1.2 x 10(-5), 1.5 x 10(-5), and 2.7 x 10(-6) M. respectively,.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capek A., Simek A. Antimicrobial agents. IX. Effect of steroids on dermatophytes. Folia Microbiol (Praha) 1971;16(4):299–302. doi: 10.1007/BF02872811. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Huppert M., Sun S. H., McGuire W. L. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect Immun. 1981 May;32(2):897–907. doi: 10.1128/iai.32.2.897-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Do Y., Burshell A., Stathis P., Loose D. S. An estrogen-binding protein and endogenous ligand in Saccharomyces cerevisiae: possible hormone receptor system. Science. 1982 Oct 15;218(4569):297–298. doi: 10.1126/science.6289434. [DOI] [PubMed] [Google Scholar]
- Hoeprich P. D., Finn P. D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972 Oct;126(4):353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Feldman D. Characterization of a unique corticosterone-binding protein in Candida albicans. J Biol Chem. 1982 May 10;257(9):4925–4930. [PubMed] [Google Scholar]
- Loose D. S., Schurman D. J., Feldman D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature. 1981 Oct 8;293(5832):477–479. doi: 10.1038/293477a0. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stevens D. A., Schurman D. J., Feldman D. Distribution of a corticosteroid-binding protein in Candida and other fungal genera. J Gen Microbiol. 1983 Aug;129(8):2379–2385. doi: 10.1099/00221287-129-8-2379. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stover E. P., Restrepo A., Stevens D. A., Feldman D. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J Antimicrob Chemother. 1980 Nov;6(6):749–761. doi: 10.1093/jac/6.6.749. [DOI] [PubMed] [Google Scholar]
- Powell B. L., Drutz D. J., Huppert M., Sun S. H. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect Immun. 1983 May;40(2):478–485. doi: 10.1128/iai.40.2.478-485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Salazar M. E., Cano L. E., Stover E. P., Feldman D., Stevens D. A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984 Nov;46(2):346–353. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl R. M., Toft D. O. Analysis of the steroid receptor of Achlya ambisexualis. J Biol Chem. 1984 Dec 25;259(24):15324–15330. doi: 10.1515/9783110885026.733. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Toft D. O. Effect of culture medium composition on pheromone receptor levels in Achlya ambisexualis. J Steroid Biochem. 1985 Oct;23(4):483–489. doi: 10.1016/0022-4731(85)90196-7. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Toft D. O., Meyer M. D., Carlson G. L., McMorris T. C. Detection of a pheromone-binding protein in the aquatic fungus Achlya ambisexualis. Exp Cell Res. 1984 Aug;153(2):544–549. doi: 10.1016/0014-4827(84)90623-2. [DOI] [PubMed] [Google Scholar]
- Schär G., Stover E. P., Clemons K. V., Feldman D., Stevens D. A. Progesterone binding and inhibition of growth in Trichophyton mentagrophytes. Infect Immun. 1986 Jun;52(3):763–767. doi: 10.1128/iai.52.3.763-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover E. P., Schär G., Clemons K. V., Stevens D. A., Feldman D. Estradiol-binding proteins from mycelial and yeast-form cultures of Paracoccidioides brasiliensis. Infect Immun. 1986 Jan;51(1):199–203. doi: 10.1128/iai.51.1.199-203.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


