Abstract
Constitutive activation of NF-κB is an emerging hallmark of various types of tumors including breast, colon, pancreatic, ovarian, and melanoma. In melanoma cells, the basal expression of the CXC chemokine, CXCL1, is constitutively up-regulated. This up-regulation can be attributed in part to constitutive activation of NF-κB. Previous studies have shown an elevated basal IκB kinase (IKK) activity in Hs294T melanoma cells, which leads to an increased rate of IκB phosphorylation and degradation. This increase in IκB-α phosphorylation and degradation leads to an ~19-fold higher nuclear localization of NF-κB. However, the upstream IKK kinase activity is up-regulated by only about 2-fold and cannot account for the observed increase in NF-κB activity. We now demonstrate that NF-κB-inducing kinase (NIK) is highly expressed in melanoma cells, and IKK-associated NIK activity is enhanced in these cells compared with the normal cells. Kinase-dead NIK blocked constitutive NF-κB or CXCL1 promoter activity in Hs294T melanoma cells, but not in control normal human epidermal melanocytes. Transient overexpression of wild type NIK results in increased phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), which is inhibited in a concentration-dependent manner by PD98059, an inhibitor of p42/44 MAPK. Moreover, the NF-κB promoter activity decreased with overexpression of dominant negative ERK expression constructs, and EMSA analyses further support the hypothesis that ERK acts upstream of NF-κB and regulates the NF-κB DNA binding activity. Taken together, our data implicate involvement of IκB kinase and MAPK signaling cascades in NIK-induced constitutive activation of NF-κB.
The CXC chemokine CXCL1, previously designated as melanoma growth-stimulatory activity was originally purified from conditioned medium from the human malignant melanoma cell line Hs294T and was later identified to be homologous to the growth-regulated gene, GRO, a potent chemoattractant for neutrophils, lymphocytes, and monocytes (1–3). In recent years, CXCL1 has been shown to play an important role in tumorigenesis and angiogenesis. In human malignant melanoma cells, CXCL1 basal expression is constitutively up-regulated (4). We have previously shown that CXCL1 transcription is constitutively activated in the human malignant melanoma cell line, Hs294T, through interaction of the HMG1 (Y), Sp1, NF-κB, and IUR elements in the promoter/enhancer of the CXCL1 gene (5, 6). Melanoma cells exhibit more rapid decay of IκB due to endogenous activation of IKK1 (7–9).
Subsequent research has shown that disregulation of NF-κB transcription machinery and constitutive expression of chemotactic cytokines are factors thought to be common early events in malignant tumor progression (for a review, see Ref. 10). Rel/NF-κB (nuclear factor-κB), a family of structurally related DNA-binding proteins, has been implicated in the regulation of cell growth and oncogenesis by inducing proliferative and antiapoptotic gene products (11, 12). In nonstimulated cells, NF-κB is sequestered in the cytoplasm and is complexed with IκB, a family of inhibitory proteins, which bind to NF-κB and mask its nuclear localization signal, thereby preventing nuclear transport (for a review, see Ref. 10). NF-κB translocation to the nucleus requires IκB phosphorylation (Ser32 and Ser36), ubiquitination, and ultimately proteolytic degradation. The cytokine-induced IκB phosphorylation and subsequent degradation is regulated by activation of a recently described macro-molecular complex, the “signalosome” called IκB kinase or IKK (700–900 kDa) (13–19). The IKK complex consists of two catalytic units IKKα and IKKβ (also referred as IKK1 and IKK2), which can directly phosphorylate IκB and a regulatory subunit IKKγ or NEMO (20). Both of these kinases IKK1 and -2 can phosphorylate Iκ-α at serine 32 and 36 in vitro. Furthermore, a number of recent studies of transient overexpression have suggested that some mitogen-activated protein kinase kinase kinases, including NF-κB-inducing enzyme (NIK) and MEKK1–3, are involved in the activation of the IKK complex (16, 21–23).
NIK was identified by means of its association with TNF receptor-associated factor 2 (TRAF2) and has been shown to potently activate NF-κB when overexpressed (21). Expression of kinase-defective forms of NIK blocks TNF-α- and IL-1-induced NF-κB activation (24, 25). NIK also interacts with other TRAF proteins, including TRAF3, which are not involved in NF-κB activation (26). Furthermore, the region of TRAF2 to which NIK binds can be replaced with heterologous oligomerization domains, and resulting chimeric proteins, which no longer bind NIK, but can still activate IKK (27). The fact that NIK strongly and preferentially interacts with both IKKκ and -β and activates their phosphorylation has been confirmed using the yeast two-hybrid system as well as protein interaction studies (28, 29). However, recent results from IKK and NIK knockout studies demonstrated that IKKα and NIK are not required for IKK activation by TNF-α, but are required for IKK activation by LT-β (30, 31).
A recent report has shown that kinase-proficient NIK promotes neurite formation, a process involving activation of both the IKK and MAPK pathways (32). This indicates the possibility of NIK exerting a broader range of effects than was previously suspected. Several recent studies have suggested that mitogen-activated kinases (MAPKs) can participate in the activation of NF-κB in the cytoplasm as well as in the modulation of its transactivation potential in the nucleus. The MEK-ERK pathway has been shown to be required for activation of AP1 and C/EBP transcription factors (33, 34). Recently, another report demonstrated that overexpression of the MEK-ERK pathway negatively regulates NF-κB transcriptional activity (35). Moreover, it has been shown that p38 MAPK regulates NF-κB gene expression by modulating the phosphorylation and activation of TATA-binding protein (36). The p38 MAPK inhibitor, SB203580, enhances nuclear factor-κB transcriptional activity by a nonspecific effect upon the ERK pathway (37).
Although the kinases responsible for phosphorylation of IκB and activation of IKK have been recently identified, the pathway(s) responsible for elevated constitutive activation of NF-κB in tumor cells is not clearly understood. We recently reported increased degradation of IκBα and an elevated constitutive IκB kinase activity in Hs294T melanoma cells compared with normal melanocytes (7, 8). This results in a higher level of IκB-α phosphorylation, a 19-fold higher nuclear localization of NF-κB, and higher NF-κB activity, leading to higher CXCL1 transcription. However, the upstream IKK kinase activity was up-regulated only about 2-fold and could not account for the larger increase in NF-κB activity. This finding suggested the possibility of additional pathway(s) responsible for NF-κB regulation. In this study, using various melanoma cell lines, we demonstrate that NIK basal expression as well as IKK-associated NIK activity is higher in melanoma cells compared with normal melanocytes. Furthermore, using wild type and kinase-dead mutant constructs of NIK, we have shown that NIK activity is required for the induction of NF-κβ promoter activity in melanoma cells. We also describe a novel pathway by which MAPK activation via NIK regulates NF-κB activation in human melanoma cells.
MATERIALS AND METHODS
Plasmids and Reagents
Eukaryotic expression vectors for wild type NIK and kinase-inactive NIK were used as described previously (22). Wild type MEKK1 and MEKK1 dominant negative expression constructs were the kind gift of Melanie Cobb and have been described elsewhere (38). The ERK2 dominant negative and ERK2 constitutively active (ERK2-MEK1 fusion protein) expression constructs were also obtained from Melanie Cobb and have been described previously (39). The CXCL1/GROα-LUC 350-bp promoter was constructed by inserting the minimal promoter (−306 to +45) of CXCL1 into a pGL2 Basic vector (7, 8). The NF-κB luciferase reporter vector contains five tandem repeats of the NF-κB element 5′ to the transcription initiation site and is contained in pLUC-MCS reporter vector (Stratagene; La Jolla, CA). Rabbit anti-NIK (sc 7211), rabbit anti-IKKα (sc 7218), rabbit anti-IKKβ (sc 7607), mouse anti-MEKK1 (sc 448), mouse anti-p-ERK1/2 (sc 7383), and mouse anti-ERK2 (sc 1647) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and anti-p-IκB was obtained from Cell Signaling Technology (Beverly, MA). Glutathione S-transferase-IκBα purified protein was also obtained from Santa Cruz Biotechnology. PD98059 was obtained from Calbiochem.
Cell Culture and Transfection
The human melanoma cell lines Hs294T, SKMel 5, SKMel 28, WM115, WM852, normal lung cell line BEAS2B, and lung cancer cell lines H157 and H358 were obtained from American Type Culture Collection (Manassas, VA). Retinal pigment epithelial (RPE) cell cultures established from the North Carolina Organ Donor and Eye Bank within 24 h of death were kindly provided by Glenn Jaffe (Duke University). Normal human epidermal keratinocytes (NHEM) established from foreskin were obtained from the Skin Disease Research Center core facility at Vanderbilt University Medical Center. The normal immortalized breast cell line MCF10A and cancer cell lines MCF7 and MDA468 were kindly provided by Lynn Matrisian (Vanderbilt University School of Medicine). RPE and melanoma cells were grown in Dulbecco’s modified Eagle’s medium and F-12 medium (1:1) supplemented with 10% fetal calf serum, 100 units/ml each of penicillin and streptomycin, and 2 mM glutamine in humidified 5% CO2 at 37 °C. NHEM cells were cultured in medium 154 supplemented with human melanocyte growth supplement (Cascade Biologics, Portland, OR). Breast and lung cancer cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 units/ml each of penicillin and streptomycin, and 2 mM glutamine in humidified 5% CO2 at 37 °C. One day prior to transfection, the cells were seeded in six-well cell culture plates to provide a final density of 40 –60% confluence (~3 × 105 cells/well). Cells were transfected with the β-galactosidase and luciferase reporter expression constructs using Effectene transfection reagent (Qiagen, Valencia, CA). Details of transfection of other expression constructs are given throughout. When necessary, additional DNA (pCMV) was added to make the total amount of transfected DNA 1 μg/well. Culture medium was changed 20 –24 h after transfection. Thereafter, cells were starved in serum-free medium for 16 h, and 42–48 h after transfection cells were washed with phosphate-buffered saline and lysed in 200 μl of reporter lysis buffer (Tropix, Bedford, MA) on ice for 10 min. Cell debris was removed by centrifugation at maximum speed at 4 °C for 5 min. A 14-μl aliquot of extract was used to measure the expression of firefly luciferase using assay reagents from Tropix, and the resultant luciferase activities were measured with a monolight 2010 luminometer. Relative transfection efficiency in each sample was determined by the normalization to the activity of co-transfected β-galactosidase. Unless otherwise indicated in the figure legends, all data were collected from three independent experiments, each in duplicate. Normalized data were plotted as a histogram. -Fold stimulation was calculated for each sample by dividing the normalized luciferase activity by the value obtained from the control transfection containing empty parental expression vectors (pCMV).
Immunoblot Analysis
Whole cell extracts were obtained according to our standard protocol using radioimmune precipitation buffer (phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) with complete protease inhibitors (Roche Molecular Biochemicals), phosphatase inhibitors (1 mM sodium orthovanadate, 50 mM sodium fluoride), and 100 μg/ml phenylmethylsulfonyl fluoride. Whole cell lysates (50 μg) were resolved on 10% SDS-PAGE, transferred to the nitrocellulose membrane, blocked with 5% milk in TBS-T, and probed with the appropriate antibodies. The antibodies were visualized with either horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Roche Molecular Biochemicals) using enhanced chemiluminescence (Pierce).
Immunoprecipitation and Kinase Assays
Normal and melanoma cells were grown in 100-mm cell culture dishes. Cells were placed in serum-free medium for 18 –24 h. Thereafter, cells were rinsed with ice-cold phosphate-buffered saline and incubated for 20 min at 4 °C in 1 ml of lysis buffer containing 1% Nonidet P-40, 50 mM HEPES (pH 7.6), 100 mM NaCl, 10% glycerol, 1 mM EDTA, 20 mM glycerophosphate, 20 mM p-nitrophenyl phosphate, 1 mM sodium orthovanadate, 1 mM NaF, and 1× Protease Inhibitor Mixture Set I. Cellular debris was removed by centrifugation, protein concentration was measured with a Bio-Rad protein estimation kit. For immunoprecipitation, 1 mg of total protein was incubated with 2 μg of the respective antibody (Santa Cruz Biotechnology) for 3 h at 4 °C followed by overnight incubation with 20 μl of protein A/G-Sepharose. The immunoprecipitates were collected by centrifugation at 2500 r.p.m for 5 min and washed three times with 0.5 ml of immunoprecipitation buffer, and loading buffer was added. Samples were resolved on a reducing 10% SDS-PAGE gel and probed with NIK and MEKK1 antibodies if immunoprecipitation was performed with IKKαβ. Alternatively, if immunoprecipitation was performed with NIK and MEKK1 antibodies, a kinase assay was performed using full-length glutathione S-transferase-IκBα as a substrate for co-immunoprecipitated IKK. The same blot was normalized with the antibody used for immunoprecipitation.
Kinase assays were performed in 20 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM MnCl2, 100 mM NaCl, 100 μM Na3VO4, 20 mM glycerophosphate, and 1 mM dithiothreitol. The amount of the substrate ATP, [γ-32P]ATP (2000 Ci/mmol; PerkinElmer Life Sciences), or IκB is specified for each individual experiment. Reactions were performed at 30 °C for 30 min. Samples were analyzed by 10% SDS-polyacrylamide gel electrophoresis under reducing conditions.
Electrophoretic Mobility Shift Assays (EMSAs)
Preparation of nuclear and cytoplasmic extracts for EMSAs were performed as described previously (40). All extracts contained a 1× concentration of complete protease inhibitor mixture (Roche Molecular Biochemicals). Briefly, nuclear extracts were prepared 48 h post-transfection. The double-stranded probe containing an NF-κB consensus site 5′-agttgaggggactttcccaggc-3′ from Promega (Madison, WI) was labeled. A typical binding reaction with 10 μg of nuclear extracts, 2 μg of nonspecific competitor poly(dI-dC), 200 ng of single-stranded oligonucleotide, 20 mM HEPES-NaOH (pH 7.6), 100 mM NaCl, 1 mM dithiothreitol, and 2% glycerol was performed for 15 min. Thereafter, the binding reaction mixture was incubated with 50,000 cpm (40 fmol) of radiolabeled probe for 20 min. Complexes were resolved on a 6% native polyacrylamide gel for 2 h at 170 V. After electrophoresis, the gel was dried and processed for autoradiography. In supershift analyses, the antibodies to p65 and p50 were preincubated with the nuclear extracts for 10 min at room temperature, and then the binding reaction was performed as above. The concentration of antibody in each EMSA reaction was 2 μg/10 μg of nuclear extracts.
Statistical Analysis
Student’s t test for paired samples was used to determine statistical significance of the transfection data. Differences were considered statistically significant at a value of p ≤ 0.05.
RESULTS
NF-κB DNA Binding and Nuclear Translocation Are Higher in Melanoma Cells
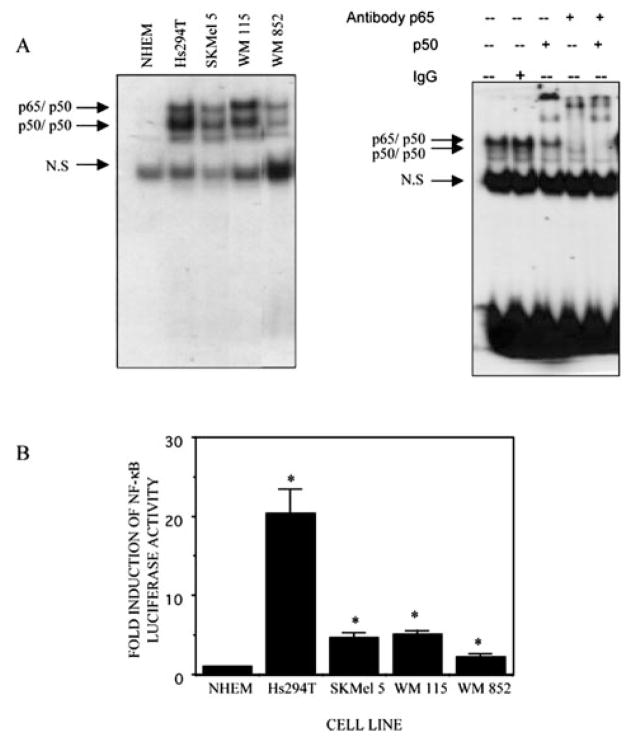
The basal DNA binding activity of NF-κB as analyzed by EMSA analysis is constitutively high in most of the melanoma cell lines tested in this study (Hs294T, SKMel 5, WM115, and WM852). However, this constitutive NF-κB activation was almost 19-fold elevated in Hs294T cells compared with NHEM cells (Fig. 1A, left panel). Supershift analyses of NF-κB complexes performed with anti-p65, anti-p50 antibodies further confirmed the specificity of the binding complexes (Fig. 1A, right panel).
Fig. 1.
A, NF-κB translocation and DNA binding are constitutively activated in melanoma cell lines. The normal and melanoma cells were cultured and kept in serum-free medium for about 30 h before harvesting. Nuclear protein was isolated, and the binding reaction was performed with consensus NF-κB oligonucleotides (AGTTGAGGGGACTTTCCCAGGC) labeled with [γ-32P]ATP in the presence of 1–2 μg of poly(dI·dC). The reaction mixture was electrophoresed on a 6% native polyacrylamide gel that was then dried and processed for autoradiography. The arrow indicates the specific proteins associated with the NF-κB probe. The experiment shown here is representative of three independent experiments. For NF-κB supershift analysis, nuclear extracts (10 μg) from the Hs294T melanoma cell line were preincubated with 2 μg of anti-p65, anti-p50, anti-p65 and -p50 at room temperature for 10 min, then EMSA was performed as described above. B, basal NF-κB promoter activity in NHEM and melanoma cell lines (Hs294T, SKMel 5, WM115, and WM852). The NF-κB promoter activity was determined by luciferase assay from these cell lines grown in the absence of serum. Error bars represent S.E. The mean result from three individual assays performed in duplicate is shown as -fold induction as compared with NHEM. *, p ≤ 0.05.
Next, the transactivation of NF-κB promoter activity in melanoma cells was examined, as compared with normal melanocytes. The promoter activity was in agreement with the gel shift analyses and showed maximum promoter activity in Hs294T melanoma cells (Fig. 1B).
A Kinase-deficient Mutant of NIK Inhibits the NF-κB and CXCL1-dependent Gene Expression
Previously, we have shown that an elevated constitutive IκB kinase activity in Hs294T melanoma cells is responsible for higher NF-κB activity due to higher IκB-α phosphorylation and degradation. However, the kinase/s upstream of IKK have not been well studied in melanoma cells. Several kinases (e.g. NIK, MEKK1, TBK1/NAK, etc.) have been shown to be signaling intermediates that act as direct activators of the IKK complex (41). It is possible that cellular selection of kinase might be specific for cell type and/or dependent on distinct extracellular stimuli.
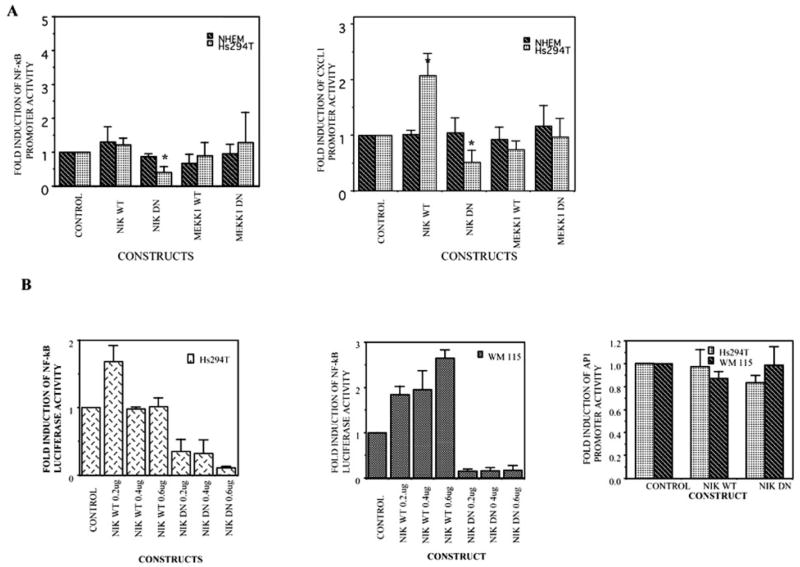
To identify the upstream kinase(s) responsible for IKK activation, we examined the effect of co-transfection of active or inactive forms of NIK and MEKK1 on NF-κB or CXCL1 promoter-luciferase activity in Hs294T melanoma cells (Fig. 2A). Overexpression of kinase-dead mutants of NIK (KK-AA) significantly suppressed the basal NF-κB/CXCL1-dependent luciferase activity (p ≤ 0.05), indicating the possibility that NIK acts upstream of IKK. In contrast, kinase-inactive MEKK1 did not significantly effect NF-κB luciferase activity. In NHEM cells, neither of the two kinase-inactive constructs affected the basal NF-κB activity (Fig. 2A).
Fig. 2. NIK is involved in the regulation of NF-κB or CXCL1 promoter activity as compared with MEKK1.
A, the NHEM and Hs294T cell lines were transfected with NF-κB-luciferase promoter/CXCL1-luciferase promoter, the RSV-β-gal vector, and one of the following constructs: a reporter construct lacking NF-κB promoter (pGL2), a wild type NIK, or a kinase-dead NIK construct and finally MEKK1 dominant negative or wild type constructs. B, Hs294T and WM115 cell lines were transfected with NF-κB-luciferase promoter, the RSV-β-gal vector, and one of the following constructs: a reporter construct lacking NF-κB promoter (pGL2), a wild type NIK, or a kinase-dead NIK construct (0.2, 0.4, and 0.6 μg). In addition, Hs294T and WM115 were also transfected with AP1-luciferase promoter, the RSV-β-gal vector, and a wild type NIK or a kinase-dead NIK construct. 18 –20 h after transfection, cells were placed in serum-free medium and incubated for another 20 –24 h; cells were then harvested, and luciferase activity was measured. Values obtained were normalized to β-galactosidase activity. The experiments were performed 3–5 times in duplicate. Error bars represent S.D. Basal promoter activity for each construct when transfected with vector alone is set at 1. *, p ≤ 0.05.
To test the effect of overexpression of the kinase-dead mutant of NIK (KK-AA) in a dose-dependent manner, we co-transfected different concentrations of the NIK construct (0.2, 0.4, and 0.6 μg) with the NF-κB promoter-luciferase reporter construct and examined the effect on NF-κB promoter activity in two melanoma cell lines, Hs294T and WM115 (Fig. 2B). We choose these two melanoma cell lines, since they have high constitutive NF-κB binding and promoter activity. NF-κB-luciferase promoter activity was almost 90% inhibited by the overexpression of NIK mutant in both of the melanoma cell lines (p ≤ 0.05). The NIK wild type construct produced about a 2.8-fold increase in NF-κB luciferase activity in the WM115 cell line but had little effect on the luciferase reporter activity in Hs294T cells. To test the specificity of effect of the NIK kinase-dead mutant on NF-κB promoter activity, we examined the AP1 promoter activity in cells co-transfected with NIK mutant. AP1 activity was not affected by co-transfecting wild type or mutant NIK. All together, these findings led us to believe that NIK is required for activation of NF-κB expression in melanoma cell lines, Hs294T and WM115. The MEKK1 dominant negative, as expected, did not show any inhibition of NF-κB or CXCL1 promoter activity in these cells.
NIK Basal Expression and Association with IKK Is Higher in Melanoma Cells
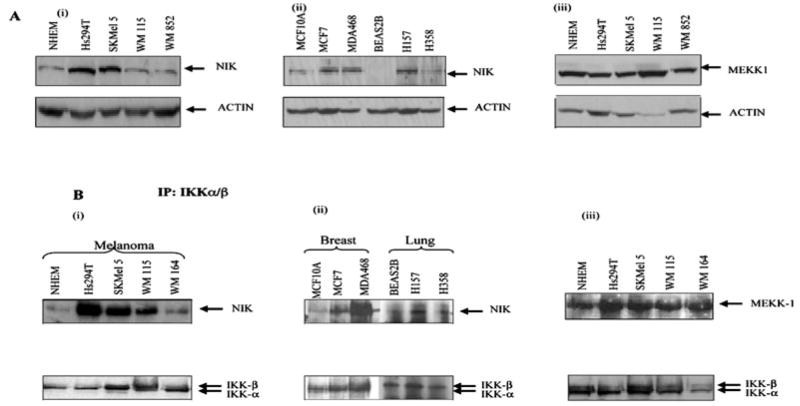
Based on the above findings, we wished to examine the differences, if any, in the basal protein expression levels of NIK and MEKK1 kinases in melanoma cells as compared with normal melanocytes. Cells were lysed in radioimmune precipitation buffer, and whole cell extracts were analyzed on 10% SDS-PAGE. To rule out the possibility of any differences in NIK or MEKK1 protein expression due to differences in growth rate, rapidly growing cultures of RPE cells were also examined (data not shown). Western blot analyses showed that NIK expression is significantly higher in the melanoma cell lines, Hs294T and SKMel 5, as compared with normal NHEM (Fig. 3A, i) or RPE cells (data not shown). No differences were observed in MEKK1 expression in Hs294T cells as compared with NHEM (Fig. 3A, iii). The equal loading of protein was confirmed by normalization using anti-β-actin antibody (Fig. 3A).
Fig. 3. The level of NIK protein and IKK-associated NIK is higher in some melanoma cells.
A, the normal cells, melanoma, breast cancer, and lung cancer cell lines were incubated in serum-free media overnight. The proteins were resolved on a 10% SDS-PAGE under reducing conditions and transferred to nitrocellulose membrane. The membrane was blotted with NIK and MEKK1 antibody and was stripped and reprobed with actin antibody to monitor equal loading of protein. This figure is representative of three separate experiments. B, co-immunoprecipitation assays. Whole cell extracts were made from normal and malignant cells (melanoma, breast, and lung cancer), and then 800 μg of protein from each sample was used for immunoprecipitation using IKKα and -β antibody (2 μg) to monitor the endogenous levels. These immunoprecipitated extracts were then used to monitor the NIK or MEKK1 associated with IKKαβ by Western blot as described under “Materials and Methods.” The same membrane was blotted with IKKαβ for normalization. This figure is a representative of three different experiments.
Since NIK and MEKK1 were differentially expressed in melanoma cell lines, as compared with normal melanocytes, we further examined the association of NIK and MEKK1 with IKKαβ in order to evaluate the difference, if any, in the activity of these enzymes in the signalosome complex. Whole cell lysates were prepared, and immunoprecipitations were performed as described under “Materials and Methods.” Briefly, IKKα and IKKβ were immunoprecipitated from cell extracts with appropriate antibodies and analyzed for associated NIK and MEKK1 by blotting the membrane with anti-NIK and MEKK1 antibodies, respectively. Immunoblot analysis of the same blot confirmed the presence of similar quantities of IKK proteins in each of the extracts used for immunoprecipitates. As shown in Fig. 3B, a higher degree of NIK and IKK association was observed in Hs294T, SKMel 5, and WM115 as compared with NHEM (Fig. 3B, i). There was no difference in MEKK1 and IKK association between melanoma cell lines and NHEM cells (Fig. 3B, iii).
To determine whether this pathway is activated in cancer cells other than melanoma, we tested the expression of NIK and its association with IKK in breast and lung cancer cell lines. Interestingly, we observed that NIK expression was up-regulated in breast cancer cell lines such as MCF-7 and MDA468 as well as SCLC lung cancer cell lines H157 and H358 compared with normal controls (Fig. 3, A and B, ii). Taken together, the above results suggest that the elevation in basal NIK expression and IKKαβ-associated activity may be a common event in malignancy.
NIK Activates ERK1/2 via MEK1-ERK1/2 Kinase Pathway
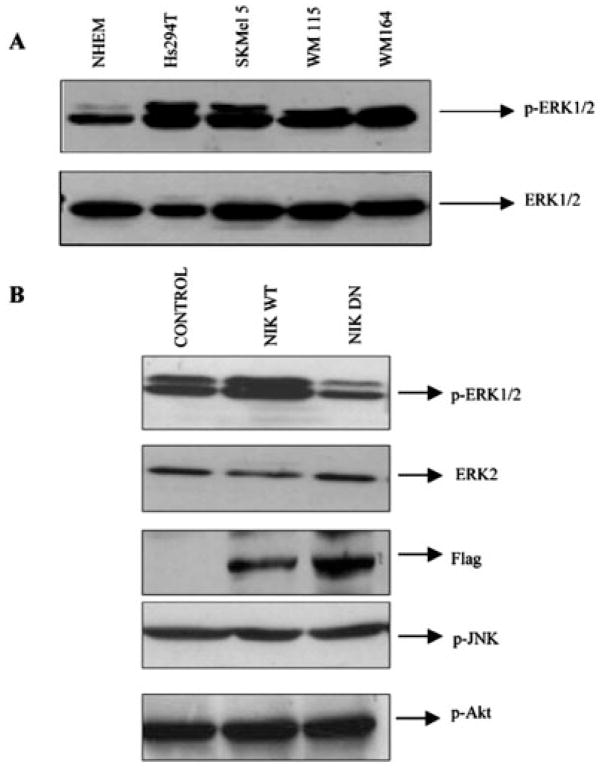
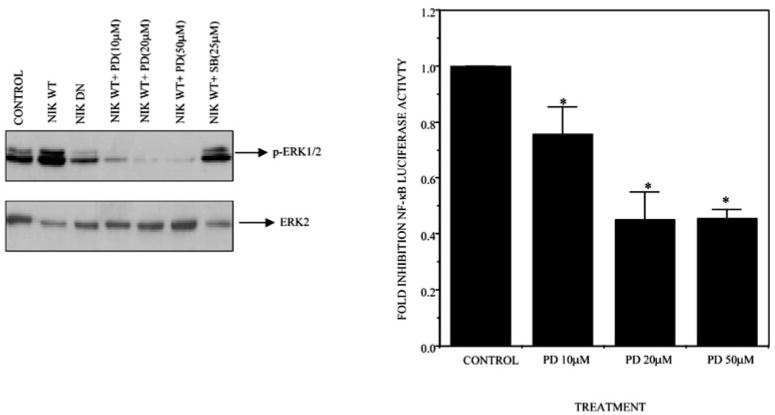
MAPKs have been shown to regulate cell proliferation and cell survival. Of the three MAPKs, ERK1/2, p38 MAPK, and JNK, ERK1/2 has specifically been associated with cell survival; therefore, we examined the activity of ERK1/2 in melanoma cells as compared with normal cells. Interestingly, we observed a marked increase in ERK activity in melanoma cell lines compared with NHEM cells (Fig. 4A). This increase in ERK activity was also apparent in breast cancer cell lines (data not shown), suggesting enhanced ERK activity in cancers. Tumor cells derived from pancreas, colon, lung, ovary, and kidney tissues showed especially high frequencies of MAPK activation, while those derived from brain, esophagus, stomach, liver tissues, and hematopoietic cells showed low frequencies with only a limited degree of MAPK activation (42). Many tumor cells, in which point mutations of ras genes were detected, showed constitutive activation of MAPKs. However, there were also many exceptions to this observation; thus, MAPK activation can be ras/raf-dependent or -independent (42). In a recent report, NIK has been shown to activate the MAPK signaling pathway (32). Since overexpression of a kinase-dead mutant of NIK significantly suppressed the NF-κB reporter activity (Fig. 2A), we examined the possibility that NF-κB is regulated through the NIK-MEK-MAPK-NF-κB signaling pathway. First, the effect of overexpression of kinase-active (WT) or kinase-dead NIK plasmid constructs on ERK1/2 activation was examined in Hs294T melanoma cells. Cells were transiently transfected with the respective constructs, cultured for 36 h, and lysed, and then whole cell extracts were resolved on a reducing 10% SDS-PAGE. Phosphorylation of ERK at Tyr204 is involved in activation of ERK. Western blot analysis using an antibody that only recognizes the Tyr204-phosphorylated form of ERK revealed a marked increase in ERK1/2 phosphorylation in cells transfected with WT NIK, while the cells transfected with the kinase-dead NIK exhibited a decrease in the ERK phosphorylation (Fig. 4B). The same membrane was stripped and probed with ERK2, showing equal loading of proteins. In contrast to ERK activation, JNK and Akt activation were unaffected, showing the effects were specific for ERK activation. The same extracts were also probed with anti-FLAG antibody to confirm the expression of these constructs by transfection (Fig. 4B). These observations suggest that NIK can also activate the MAPK pathway.
Fig. 4. The endogenous ERK activity is higher in most of the melanoma cells.
A, the whole cell extracts from normal and melanoma cell lines were resolved on an SDS-PAGE reducing gel and then tested for expression of p-ERK1/2. The same blot was stripped and reprobed with ERK2 for normalization of proteins. B, NIK affects the activity of ERK but has no effect on JNK or Akt activity. Hs294T cells were transfected with wild type or dominant negative NIK, and then 20 h after transfection, cells were cultured in serum-free medium and incubated for another 24 h. The same extracts were tested for expression of p-ERK1/2, p-JNK, and p-Akt. The p-ERK1/2 membrane was then stripped and reprobed with ERK2 for confirming the equal loading of protein. The data shown in this figure represent the mean of three experiments.
PD98059 Inhibits ERK Activation and NF-κB Promoter Activity
To determine whether this activation is through the MEK-ERK signaling pathway, we treated the Hs294T cells transfected with the WT-NIK construct with increasing concentrations of PD98059, an inhibitor of MEK1 (Fig. 5, right panel). WT-NIK led to an increase in ERK activation, which was effectively inhibited by incubation with PD98059 in a concentration-dependent manner (10, 20, and 50 μM). However, incubation with the p38 inhibitor, SB-202190, had no effect on ERK1/2 activity. Probing the blot with anti-ERK2 confirmed the equal loading of the protein.
Fig. 5. The specific inhibitor of MEK1/2, PD98059, can inhibit the up-regulated ERK expression as well as NF-κB promoter activity.
A, Hs294T cells were transfected with wild type and dominant negative NIK. Twenty hours after transfection, cells were placed in serum-free medium and incubated for 20 h, after which cells transfected with the NIK wild type expression construct were treated with 10, 20, and 50 μM PD98059 for 30 min. The cells were then lysed, and protein was estimated. 50 μg of protein were loaded in each lane, and SDS-PAGE analysis under reducing conditions was performed followed by transblot and immunodetection. ERK1/2 activity was determined by probing the Western blot with phosphospecific ERK antibody. The same membrane was striped and reprobed with ERK2 to normalize the loading of proteins. B, Hs294T cells were co-transfected with the NF-κB-luciferase-reporter construct and RSV-β-gal. Ten hours after transfection, medium was replaced with medium containing either Me2SO or PD98059 within a range of 10 –50 μM. Cells were harvested 48 h after transfection, and luciferase activity was determined. The luciferase activity was normalized with β-galactosidase, and -fold induction was calculated. The figure represents a mean of three experiments ± S.D. *, p ≤ 0.05.
Next, to evaluate the role of MAPK in regulating the constitutive NF-κB activation in vivo, Hs294T melanoma cells were co-transfected with a NF-κB-dependent luciferase reporter and the pRSV-β-galactosidase expression construct and then treated with specific chemical inhibitors for ERK1/2 (PD98059) at the indicated concentration. A decrease of 20, 49, and 50% in NF-κB transactivation was observed at 10, 20, and 50 μM of PD98059, respectively. This inhibition was statistically significant based upon the paired Student’s t test (p ≤ 0.05) (Fig. 5, left panel).
ERK1/2 Is Involved in NIK-mediated NF-κB Transactivation in Melanoma Cells
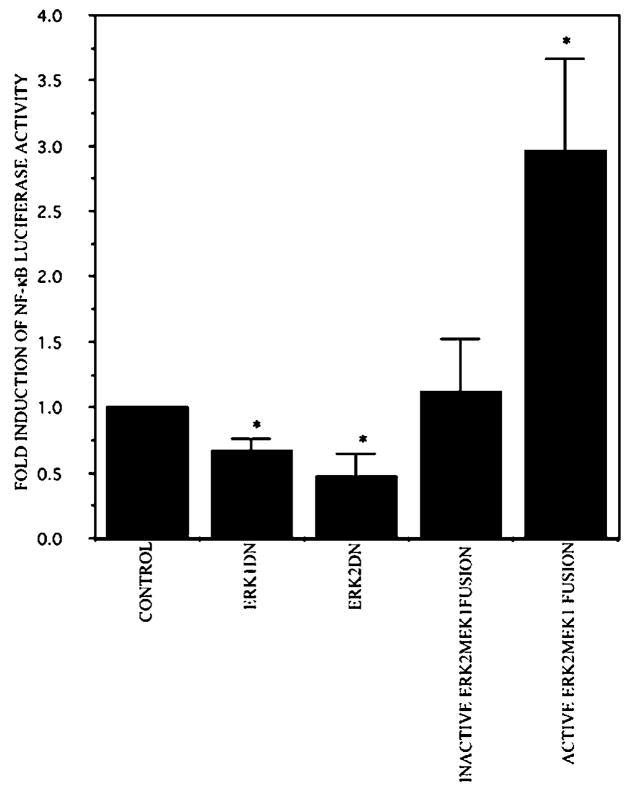
To further test the hypothesis that ERK1/2 is involved in NIK-mediated NF-κB transactivation, we determined the effect of overexpression of ERK1 and ERK2 on NF-κB luciferase activity. Hs294T cells were co-transfected with the NF-κB-luciferase reporter plasmid DNA and either empty vector, ERK1 (dominant negative), or ERK2 constructs (dominant negative or constitutive active). As expected, the basal NF-κB luciferase activity was reduced by 40 and 50% in the presence of the dominant negative ERK1 and ERK2 expression vectors, respectively, as compared with empty vector controls. A significant increase of about 3.2-fold was observed in cells transfected with the constitutively active ERK2 vector, as compared with empty vector transfections based upon the paired Student’s t test (p ≤ 0.05) (Fig. 6).
Fig. 6. ERK mediates NIK-enhanced NF-κB activation.
Dominant negative ERK decreases NF-κB transactivation, whereas the dominant active form enhances the transactivation. The Hs294T cells were co-transfected with the NF-κB luciferase reporter construct, with either dominant negative or active ERK and the RSV-β-gal expression construct. Twenty hours after transfection, the medium was replaced with serum-free medium. Cells were harvested 48 h after transfection, and luciferase and β-galactosidase activity were measured. The relative luciferase activity represents the luciferase activity of the sample that was normalized by β-galactosidase activity from three different experiments. The results are reported as the mean ± S.D. of -fold induction considering 1 as the relative luciferase activity of the cells transfected with corresponding empty vector. *, p ≤ 0.05.
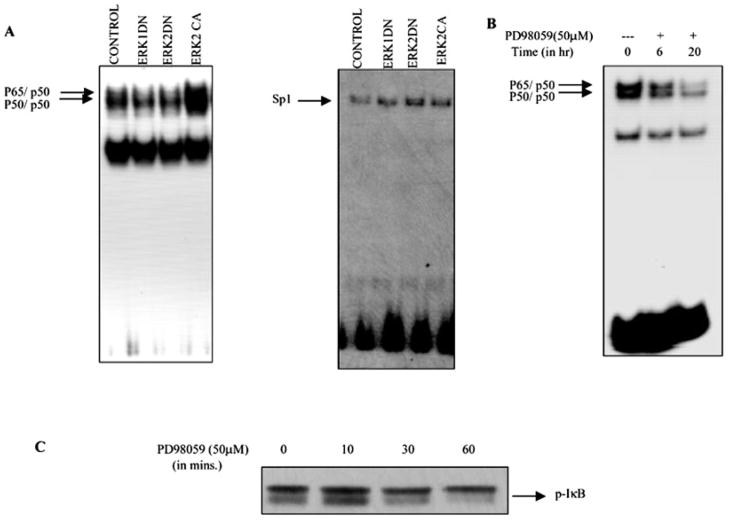
Erk Is Involved in the Constitutive NF-κB DNA Binding Activity in Melanoma Cells
Recently, constitutive activation of the MEK-ERK has been shown to negatively regulate NF-κB-dependent transcription through modulation of TATA-binding protein activation in LPS-stimulated THP-1 cells (37). This finding prompted us to determine whether ERK’s regulation of NF-κB activation in melanoma is through regulation of its DNA binding activity. We performed EMSA to evaluate the NF-κB DNA binding activity in Hs294T cells transiently transfected with ERK constructs (dominant negative and constitutive active). The 32P-labeled consensus NF-κB DNA binding sequence was used as a probe. As shown in Fig. 7, EMSA revealed that while overexpression of constitutively active ERK2 resulted in an approximately 2-fold increase of the nuclear NF-κB-DNA complexes, the ERK1 and ERK2 dominant negative transfected cells showed a 50% decrease in nuclear NF-κB-DNA binding. In contrast to NF-κB binding, overexpression of ERK constructs (dominant negative and constitutive active) did not affect the Sp1 binding, suggesting that the effect on NF-κB nuclear localization and DNA binding is not the result of a generalized increase in transcription activation. We also examined the effect of PD98059 on NF-κB binding, and, as expected, a decrease in DNA binding was observed. The observation that neither overexpression of the ERK dominant negative mutant constructs nor PD98059 could inhibit the NF-κB binding completely indicates that more than one pathway is responsible for regulating NF-κB activation in melanoma cells. In addition, to investigate the role of IκB phosphorylation and degradation in ERK-mediated NF-κB activation, we tested the effect of PD98059 on IκB phosphorylation. In agreement with our hypothesis, PD98059 blocked the phosphorylation of IκB in a time-dependent manner and hence degradation and nuclear translocation, thereby decreasing NF-κB binding and transactivation. Taken together, our data suggest that NIK-induced MAPK activation is involved in constitutive activation of NF-κB in Hs294T cells.
Fig. 7. ERK is involved in the NF-κB binding to DNA.
A, Hs294T melanoma cells were transfected with either empty vector or ERK dominant negative or dominant active constructs. Twenty hours after transfection, cells were placed in serum-free medium. Forty-eight hours after transfection, cells were harvested, and nuclear extracts were made. 10 μg of nuclear extracts were incubated with 32P-labeled NF-κB or Sp1 oligonucleotide probe in the presence of 1–2 μg of poly(dI-dC). The reaction mixture was electrophoresed on a 6% native gel, which was dried and processed for autoradiography. The arrow indicates the specific complexes associated with the NF-κB and the Sp1 probe. The EMSA shown is representative one of three different experiments. Results were quantitatively similar in all three experiments. B, Hs294T cells were incubated with PD98059 (50 μM) for the indicated time periods, nuclei were extracted, and an NF-κB EMSA was performed as described for A. C, Hs294T melanoma cells were preincubated with proteosome inhibitor MG115 and cycloheximide for 2 h and then treated with PD98059 (50 μM) for 10, 30, and 60 min, respectively. The cells were then lysed using radioimmune precipitation buffer, and protein was estimated. 50 μg of protein were loaded in each lane, and SDS-PAGE analysis under reducing conditions was performed. The effect of PD98059 on phosphorylation of IκB was determined using phosphospecific Iκβ antibody.
DISCUSSION
The signaling pathways involved in regulation of cell proliferation, survival and oncogenesis are of prime interest in cancer biology. Since its discovery, Rel/NF-κB has been the focus of intensive research, especially the mechanism(s) that control its activation. More than 60% of the melanoma cells studied to date showed higher expression of CXCL1, CXCL8, IL-1β, IL-6, basic fibroblast growth factor, IL-7, platelet-derived growth factor-α, IL-10, granulocyte-macrophage colony-stimulating factor, insulin-like growth factor, nerve growth factor, vascular endothelial growth factor, epidermal growth factor, and transforming growth factor-β at the mRNA level. The majority of these genes contain an NF-κB element in their inducible promoter (43). It has been reported previously that NF-κB activation is required for CXCL1/GRO-induced melanocyte transformation (44). Previous work from our laboratory has shown a higher level of CXCL1 expression in Hs294T malignant melanoma cells as compared with normal melanocytes, and this increase in CXCL1 has been attributed to higher IKK activity and thus NF-κB activity (7–9). However, the proteins responsible for regulating IKK activation in melanoma cells are not known.
Using various melanoma cell lines (Hs294T, SKMel 5, WM115, and WM852), we observed constitutively increased NF-κB (p50/p65 and p50/p50) complex formation in melanoma cells as compared with NHEM. In search of kinase(s) upstream of IKK, we first explored NIK and MEKK1 as possible candidates. In melanoma as well as breast and lung cancer cells, the basal protein expression level of NIK as well as its IKK-associated activity was higher compared with normal controls, whereas no difference was observed in MEKK1 expression. Transient overexpression of a kinase-dead NIK (KK/AA) plasmid construct significantly reduced not only basal NF-κB but also CXCL1 promoter activity in melanoma cells, whereas transfection with the same construct had no effect in NHEM cells. These findings clearly show that NIK is involved in the up-regulation of NF-κB activity in melanoma cells. Moreover, up-regulation of this signaling cascade is a common phenomenon in number of cancer cells such as breast, lung, and melanoma. Thus, further studies are required for understanding this pathway important for regulation of NF-κB in cancer cells.
A recent report by Kouba et al. (45) demonstrated that epidermal keratinocytes utilize NIK-dependent NF-κB activation pathways. In addition, proinflammatory cytokines such as interleukin-1 and tumor necrosis factor-α have been shown to activate NF-κB in many cell types through the NIK/MEKK-IKK-IκB signaling pathway (24, 25), while some of the recent studies have shown that NIK is selectively required for gene transcription induced through ligation of the LTβ receptor but not the TNF receptor (31). In Hs294T cells, IL-1 is not involved in NF-κB activation as determined by using dominant negative Myd88 and IRAK plasmid constructs (data not shown). It is possible that some other cytokine/growth factor may be responsible for NIK up-regulation/activation. However, in two of four melanoma cell lines, the basal protein expression level of NIK is significantly higher than in normal cells. We hypothesize that this increase in protein expression in a subset of malignant melanomas could contribute to the higher NIK kinase activity and thereby higher IKK activity, which finally leads to higher NF-κB activation. However, we observed enhanced NIK activity in three of four melanoma cell lines, suggesting that factors other than increased protein levels are involved. It remains to be determined whether this increased NIK activity is because of an activating mutation or increased availability of other activating factors/adaptors.
Previous studies in our laboratory demonstrated that the IKK activation alone could not account for the total NF-κB activity in Hs294T cells (6 –8). In the present study, apart from showing a requirement of NIK in the endogenous NF-κB activation, we provide evidence for the first time that NIK/MAP3K regulates NF-κB activation through a novel MAPK pathway in addition to the conventional IKK-IκB-NF-κB pathway in Hs294T melanoma cells. In the course of our studies, we observed a higher basal ERK activation in Hs294T cells compared with normal melanocytes. Based on our hypothesis that more than one signaling pathway is involved in the regulation of NF-κB activation, we tested whether NIK is an upstream kinase in the MEK-ERK pathway. Interestingly, we not only found that NIK is upstream of ERK but also that it regulates NF-κB activation through ERK phosphorylation. Transient overexpression of NIK-WT increased ERK phosphorylation, whereas kinase-dead NIK abrogated this activation. A recent report by Foehr et al. (32) showing NIK as a MEK1-dependent activator of the MAPK pathway, regulating the differentiation of PC12 cells, supports this finding (32). At the same time, transient overexpression of constitutively active and dominant negative ERK plasmid constructs increased and decreased NF-κB promoter activity, respectively. PD98059 inhibited the NIK-mediated ERK activation as well as the NF-κB reporter activity, but the inhibition was only 50% of the total reporter activity. Interestingly, ERK activity was completely inhibited at this dose of PD98059 (50 μM). Taken together, these data support our hypothesis that activation of more than one pathway downstream of NIK is involved in the regulation of NF-κB activation. Transcriptional activation often requires more than one signaling pathway; for example, recently three distinct kinase cascades were shown to be required for maximum up-regulation of IL-8 (46). We postulate that the inhibition of both pathways (NIK/IKK-IκB and NIK/MEK-ERK-NF-κB) can provide total inhibition of NF-κB activation.
Signal transducers can positively regulate NF-κB activation by increasing nuclear translocation and DNA binding, affecting transactivating capacity of NF-κB or by modulating TATA-binding protein activation. Many studies have described a close association between ERK1 activity and phosphorylation and degradation of IκB protein, thus leading to increased nuclear translocation of NF-κB, increased DNA binding, and hence NF-κB activation in other cell systems. For instance, in vitro phosphorylation of glutathione S-transferase-IκBα by ERK1 activated by okadaic acid was reported by Sonoda et al. (47). Moreover, in the lymphoblastoid cell line CEM, overexpression of either MEK1 or ERK1 resulted in a constitutive nuclear localization of NF-κB DNA binding activity (48).
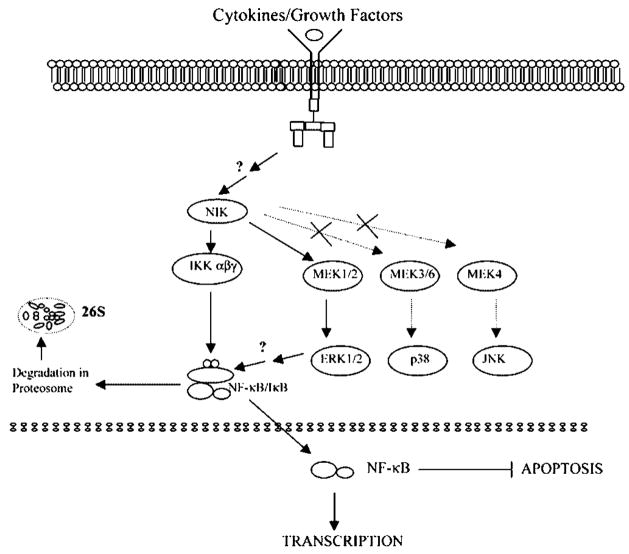
Our Western blot and EMSA analyses showed that in Hs294T cells, ERK regulation of NF-κB activation involves increased IκB phosphorylation and increased NF-κB DNA binding activity. An increase in IκB phosphorylation is responsible for enhanced degradation and thereby leads to increased nuclear localization and DNA binding. PD98059 inhibited the IκB phosphorylation, thus decreasing the nuclear translocation of NF-κB. Thus, we conclude that NIK regulates NF-κB activation through a novel NIK/MEK/ERK/NF-κB signaling pathway in addition to the classical NIK/IKK/IκB/NF-κB pathway (Fig. 8). Moreover, this regulation results in modulation of its nuclear translocation and hence DNA binding activity of NF-κB p50/p65.
Fig. 8. Proposed model for the regulation of NF-κB activation by NIK through a novel NIK/MEK/ERK/NF-κB signaling pathway in addition to the classical NIK/IKK/IκB/NF-κ B pathway.
Three possible mechanisms for constitutive activation of NIK have been considered: 1) NIK could be mutated such that it is constitutively active; 2) NIK could be activated by upstream activators; or 3) NIK could be activated through an autocrine loop. Current data suggest that if an autocrine loop contributes to the constitutive activation of NIK, it probably does not involve CXCR2, a Gαi-coupled receptor, or the IL-1 receptor, since CXCR2 blocking antibody, CXCL1 antibody, or pertussis toxin did not affect the constitutive NIK activity in three melanoma cell lines. Moreover, dominant negative MyD88 and dominant negative IRAK did not affect the constitutive NIK activity, ruling out an IL-1 receptor-mediated autocrine loop. Since transfection with wild type NIK did not increase the NF-κB luciferase activity, if NIK is activated by a novel upstream activator, the novel factor would probably be rate-limiting. We therefore postulate that NIK may undergo an activating mutation(s) in tumor cells, leading to constitutive NF-κB activation.
A clear understanding of the molecular mechanisms involved in the constitutive NF-κB activation in tumor cells will allow the targeting of critical components to block events such as protection of tumor cells from apoptosis.
Acknowledgments
We acknowledge Dr. Amar B. Singh for critically reviewing the manuscript and providing helpful suggestions and Neepa Ray for technical assistance.
Footnotes
This work was supported by a Department of Veterans Affairs Career Scientist Award (to A. R.) and by NCI, National Institutes of Health (NIH), Grants CA56704 and CA34590 (both to A. R.) as well as NIH Grants CA 68485 and P30 AR 41943.
The abbreviations used are: IKK, IκB kinase; NHEM, normal human epidermal melanocytes; RPE, retinal pigment epithelial cells; NIK, NF-κB-inducing kinase; MEKK1, MAPK kinase kinase; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated protein kinase; MEK, MAPK/ERK kinase; JNK, c-Jun NH2-terminal kinase; PD98059, 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one; TRAF, TNF receptor-associated factor; IL, interleukin; TNFα, tumor necrosis factor-α; Raf-1, Ras-associated factor-1; TNF, tumor necrosis factor; EMSA, electrophoretic mobility shift assay; WT, wild type.
References
- 1.Richmond A, Balentien E, Thomas HG, Flaggs G, Barton DE, Spiess J, Bordoni R, Francke U, Derynck R. EMBO J. 1988;7:2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisowicz A, Bardwell L, Sager R. Proc Natl Acad Sci U S A. 1987;84:7188 –7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balentien E, Han JH, Thomas HG, Wen DZ, Samantha AK, Zachariae CO, Griffin PR, Brachmann R, Wong WL, Matsushima K, Richmond A, Derynck R. Biochemistry. 1990;29:10225–10233. doi: 10.1021/bi00496a011. [DOI] [PubMed] [Google Scholar]
- 4.Shattuck RL, Wood LD, Jaffe GJ, Richmond A. Mol Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood LD, Farmer AA, Richmond A. Nucleic Acids Res. 1995;23:4210 –4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood LD, Richmond A. J Biol Chem. 1995;270:30619 –30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 7.Shattuck-Brandt RL, Richmond A. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 8.Devalaraja MN, Wang DZ, Ballard DW, Richmond A. Cancer Res. 1999;59:1372–1377. [PubMed] [Google Scholar]
- 9.Yang J, Richmond A. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 10.Baldwin AS., Jr Annu Rev Immunol. 1996;14:649 –683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 11.Beg AA, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 12.Perkins ND. Trends Biochem Sci. 2000;25:434 –440. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 13.Stancovski I, Baltimore D. Cell. 1997;91:299 –302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 14.Karin M. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 15.Regnier CH, Song HY, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 16.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. Science. 1997;278:866 –869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 17.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. Nature. 1997;388:548 –554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 18.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 19.Israel A. Trends Cell Biol. 2000;10:129 –133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 20.Li XH, Fang X, Gaynor RB. J Biol Chem. 2001;276:4494 –4500. doi: 10.1074/jbc.M008353200. [DOI] [PubMed] [Google Scholar]
- 21.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. Nature. 1997;385:540 –544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 22.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Proc Natl Acad Sci U S A. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemoto S, DiDonato JA, Lin A. Mol Cell Biol. 1998;18:7336 –7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer C, Page S, Weber M, Eisele T, Neumeier D, Brand K. J Biol Chem. 1999;274:24625–24632. doi: 10.1074/jbc.274.35.24625. [DOI] [PubMed] [Google Scholar]
- 25.Nasuhara Y, Adcock IM, Catley M, Barnes PJ, Newton R. J Biol Chem. 1999;274:19965–19972. doi: 10.1074/jbc.274.28.19965. [DOI] [PubMed] [Google Scholar]
- 26.Takaori-Kondo A, Hori T, Fukunaga K, Morita R, Kawamata S, Uchiyama T. Biochem Biophys Res Commun. 2000;272:856 –863. doi: 10.1006/bbrc.2000.2860. [DOI] [PubMed] [Google Scholar]
- 27.Arch RH, Gedrich RW, Thompson CB. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 28.Ling L, Cao Z, Goeddel DV. Proc Natl Acad Sci U S A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delhase M, Hayakawa M, Chen Y, Karin M. Science. 1999;284:309 –313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 30.Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, Takeda K, Akira S, Matsumoto M. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, Schreiber RD. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 32.Foehr ED, Bohuslav J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O’Mahony A, Greene WC. J Biol Chem. 2000;275:34021–34024. doi: 10.1074/jbc.C000507200. [DOI] [PubMed] [Google Scholar]
- 33.Minden A, Lin A, Smeal T, Derijard B, Cobb MH, Davis RJ, Karin M. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. J Exp Med. 1996;183:2017–2023. doi: 10.1084/jem.183.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter AB, Hunninghake GW. J Biol Chem. 2000;275:27858 –27864. doi: 10.1074/jbc.M003599200. [DOI] [PubMed] [Google Scholar]
- 36.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. J Biol Chem. 1999;274:30858 –30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 37.Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. Br J Pharmacol. 2000;131:99 –107. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Robbins DJ, Christerson LB, English JM, Vanderbilt CA, Cobb MH. Proc Natl Acad Sci U S A. 1996;93:5291–5295. doi: 10.1073/pnas.93.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- 40.Van Antwerp DJ, Verma IM. Mol Cell Biol. 1996;16:6037–6045. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pomerantz JL, Baltimore D. EMBO J. 1999;18:6694 –6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno1 M. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 43.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Richmond A. J Biol Chem. 2001;276:3650 –3659. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kouba DJ, Nakano H, Nishiyama Y, Kang J, Uitto J, Mauviel A. J Biol Chem. 2001;276:6214 –6224. doi: 10.1074/jbc.M004511200. [DOI] [PubMed] [Google Scholar]
- 46.Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, Malinin NL, Cooper JA, Resch K, Kracht M. Mol Cell Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonoda Y, Kasahara T, Yamaguchi Y, Kuno K, Matsushima K, Mukaida N. J Biol Chem. 1997;272:15366 –15372. doi: 10.1074/jbc.272.24.15366. [DOI] [PubMed] [Google Scholar]
- 48.Briant L, Robert-Hebmann V, Sivan V, Brunet A, Pouyssegur J, Devaux C. J Immunol. 1998;160:1875–1885. [PubMed] [Google Scholar]