Abstract
Human interferon-inducible protein 10 (IP-10; HGMW-approved gene symbol CXCL10) is an ELR− CXC chemokine that contains binding domains for both the chemokine receptor CXCR3 and glycosaminoglycans. IP-10 has been recently demonstrated to be a potent angiostatic protein in vivo. Whether IP-10 exerts its angiostatic function through binding to CXCR3, glycosaminoglycans, or both, is not clear. To clarify this issue, we created expression constructs for mutants of IP-10 that exhibit partial (IP-10C) or total (IP-10C22) loss of binding to CXCR3 or loss of binding to glycosaminoglycans (IP-10H and IP-10C22H). The A375 human melanoma cell line was transfected with these expression vectors, and stable clones were selected and inoculated subcutaneously into nude mice. As expected, tumor cells secreting wild-type IP-10 showed remarkable reduction in tumor growth compared to control vector-transfected tumor cells. Surprisingly, mutation of IP-10 resulting in partial loss of receptor binding (IP-10C), or loss of GAG binding (IP-10H), did not significantly alter the ability to inhibit tumor growth. This tumor growth inhibition was associated with a reduction in microvessel density, leading to the observed increase in both tumor cell apoptosis and necrosis. In contrast, expression of the IP-10C22 mutant failed to inhibit melanoma tumor growth. These data suggest that CXCR3 receptor binding, but not glycosaminoglycan binding, is essential for the tumor angiostatic activity of IP-10. We conclude that the arginine 22 amino acid residue of IP-10 is essential for both CXCR3 binding and angiostasis.
Introduction
IP-101 was identified as a 10-kDa secreted protein induced by interferon-γ [1] and lipopolysaccharide [2] in a variety of cell types, including endothelial cells, keratinocytes, fibroblasts, mesangial cells, astrocytes, monocytes, and neutrophils [3]. IP-10 belongs to the superfamily of chemokines and was initially characterized as a chemoattractant for activated T lymphocytes. Based upon its amino acid composition, IP-10 belongs to the CXC subgroups of chemokines and it binds to the CXC chemokine receptor 3 (CXCR3) [4]. Three interferon-induced chemokines, IP-10 (HGMW-approved gene symbol CXCL10), monokine induced by interferon-γ (Mig/CXCL9), and interferon-inducible T cell α chemoattractant (I-TAC/CXCL11), share the same G-protein-coupled receptor, CXCR3. CXCR3 is preferentially expressed on Th1 lymphocytes, and display of this receptor is important for inflammatory and T-cell-dependent anti-tumor effects of the interferon-induced chemokines [5]. IP-10 has been demonstrated to be hyperexpressed in many Th1-type inflammatory conditions such as atherosclerosis [6], multiple sclerosis [7], and transplant rejection [8]. IP-10 can also bind heparan sulfated glycosaminoglycans (GAGs) [9]. Chemokine binding to GAGs appears to be involved in the control of tumor cell replication, migration, invasion, and vascularization [10–13].
IP-10 is recognized as having potent angiostatic activity in vivo. IP-10 can inhibit a number of angiogenic activities such as basic fibroblast growth factor-induced neovascularization in athymic mice [14] and human tumor-derived angiogenesis [15–17]. Due to the angiogenesis requirement for sustained growth of tumors, antiangiogenic treatment has become a key strategy for treatment of cancer patients. Whether CXCR3 is required for IP-10 angiostatic activity is still under controversy. The positive evidence for the requirement for CXCR3 in angiostasis is that the inhibitory effect of IP-10 on the proliferation of primary cultures of human microvascular endothelial cells expressing CXCR3 was blocked by anti-CXCR3 monoclonal antibody [18]. Even though IP-10 has a direct effect on some endothelial cells, endothelial cells such as human umbilical vein endothelial cells do not express detectable CXCR3 mRNA or cell surface CXCR3 protein [19]. These data have been interpreted by some investigators to mean that CXCR3 does not mediate the IP-10 angiostatic process. To clarify this issue, mutations in IP-10 were made to alter its binding to CXCR3, to GAG, or to both. The following amino acid substitutions to alanine were made: (1) N-terminal IP-10 amino acid residues in the CXCR3 binding and triggering domain (3LSRTVR8), (2) the basic residues of the 40s loop GAG binding domain of IP-10 (46KKKGEKR52), and (3) arginine 22 at the amino terminus. These individual mutants, and combinations thereof, were evaluated for angiostatic and anti-tumor activities in a melanoma tumor xenograft model. Results show that IP-10 inhibition of angiogenesis in melanoma tumor is dependent on binding to CXCR3, but not GAG.
Results
In Vitro Characteristics of the Expression of IP-10 Proteins
For cloning of human IP-10, we stimulated peripheral monocytes isolated from human blood with recombinant human interferon-γ at a final concentration of 100 IU/ml, extracted the total RNA, and reverse transcribed it to cDNA with primers based upon published human IP-10 sequence (Accession No. X02530) with the addition of a Kozak consensus sequence upstream of the start codon.
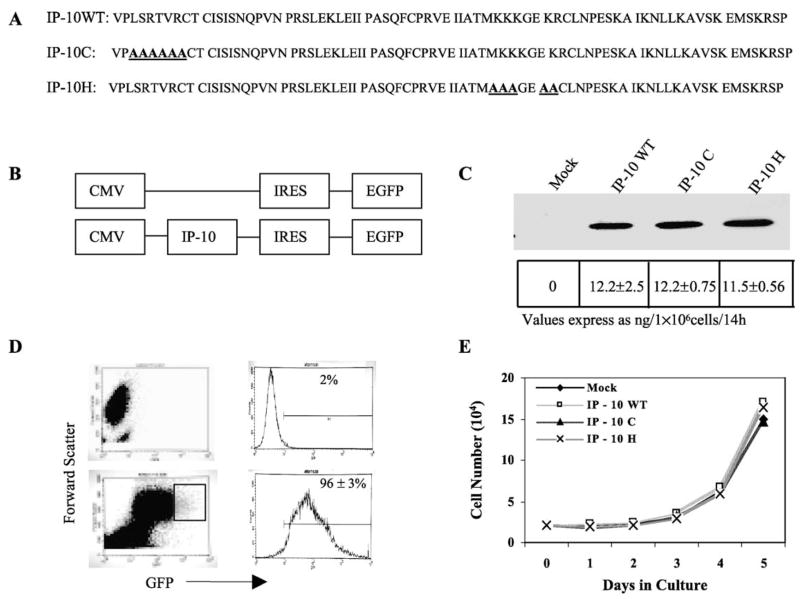
The N-terminal region of IP-10 has been reported to be critical for receptor activation [20–22]. To determine whether this region is required for the angiostatic activity of IP-10, we replaced the amino acid sequence 3LSRTVR8 with alanines (Fig. 1A, line 2). We did not replace the first two valine and proline residues since they must be conserved for cleavage of the signal peptide. The GAG binding motif, BBBXXB or BBXB [23,24], is located in the C-terminal region of chemokines. To abrogate GAG binding, we replaced the basic amino acids on the 40s loop of IP-10, 46KKKGEKR51, with alanines (Fig. 1A, line 3). To create the melanoma cell lines stably expressing IP-10 (mutant and wild type) and to monitor protein expression in the cells, we used the pIRES2-EGFP expression vector (Fig. 1B). After transfection and selection with G418, we visualized the A375 melanoma cells expressing IP-10 proteins by EGFP expression microscopically (data not shown) or by flow cytometry (Fig. 1D). In contrast to nontransfected cells (Fig. 1D, top), approximately 96% of the transfected cells expressed EGFP (bottom) as an indirect indicator of IP-10 protein expression. Moreover, we verified the secretion of IP-10 proteins into the culture medium by Western blot (Fig. 1C, top) and by ELISA. The ELISA data revealed that 9.6 × 105 IP-10-transfected cells secreted 11.5 to 12.2 ng IP-10 protein over a 14-h period, but no IP-10 was detectable in the culture medium of empty vector-transfected cells over the same time frame (Fig. 1C, bottom). To determine whether cell proliferation was affected by the IP-10 gene transfection, we performed cell number assays in vitro. We observed that the in vitro growth of cells expressing IP-10 was not altered among the different IP-10 mutants and wild-type transfected tumor cells (Fig. 1E).
FIG. 1.
Mutagenesis and expression of IP-10 protein. (A) Protein sequence of human IP-10 wild type and C and H mutants used in this study. (B) The cDNAs of IP-10 (wild type or mutant) were cloned into the pIRES2-EGFP vector and the empty vector served as control. (C) The secretion of human IP-10 proteins by A375 human melanoma cells transfected with IP-10 expression vectors was evaluated by Western blot with anti-IP-10 antibody (top) and ELISA (bottom). (D) The nontransfected (top) or IP-10-transfected cells (bottom) were identified by EGFP expression using fluorescence flow cytometry. (E) In vitro proliferation of the transfected A375 cells was examined by cell number assays in serum-free medium over 5 days by hemocytometer counting. Data shown are from two separate experiments performed in duplicate each time.
In Vitro Characteristics of Receptor Binding Activity of Mutant IP-10
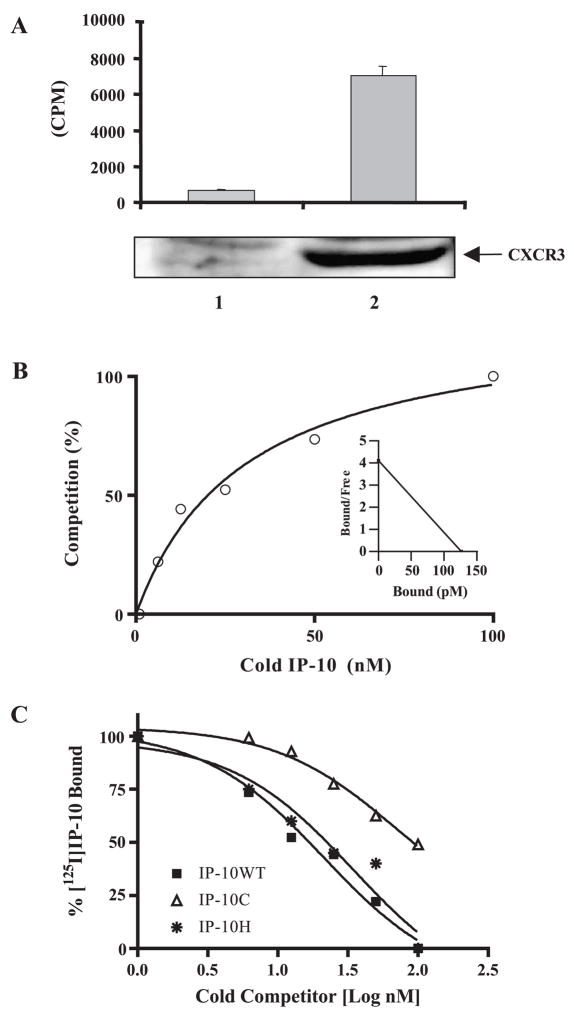
The IP-10 anti-tumor activity occurs through at least two mechanisms: (1) activation of the CXCR3 receptor on T cells or (2) inhibition of angiogenesis. To determine whether IP-10 inhibition of angiogenesis required CXCR3 binding or GAG binding, we mutated the activation domain of IP-10 and examined its receptor binding activity. To verify loss of receptor binding and eliminate complication from proteoglycan binding influence, we chose the GAG-deficient CHO cells and stably transfected them with human CXCR3 (Fig. 2A, lane 2, bottom). In contrast to null-transfected cells (Fig. 2A, lane 1), 125I-labeled IP-10 binding in CXCR3-expressing cells (Fig. 2B, lane 2, top) was remarkably increased. The expression of CXCR3 receptor on the cell membrane was also verified using anti-CXCR3-PE (R&D Systems, Inc., Minneapolis, MN, USA) by FACS analysis (data not shown). The CXCR3-expressing cells were then used to perform a competitive binding assay with increasing concentrations of the unlabeled IP-10 (WT or mutant) proteins. The affinity constant of CXCR3 binding to iodinated IP-10 was analyzed using the program GraphPad Software Prism 4 (GraphPad Software, Inc., San Diego, CA, USA). The ratio of sites bound with 125I-IP-10 to the total number of binding sites per 106 cells (% fraction bound) and the corresponding concentrations of total ligand were corrected for specific binding. Fig. 2B shows the addition of unlabeled wild-type IP-10 to compete with 125I-labeled IP-10 in a one-binding-site model. The percentage of maximal specific binding of 125I-labeled IP-10 was calculated after subtraction of nonspecific binding. The inset in Fig. 2b shows the transformed data. The EC50 value is the effective concentration of unlabeled IP-10 (wild type or mutants) that produces half-maximal binding of iodinated IP-10 to CXCR3. We determined the EC50 by nonlinear least-square fits of the measured binding data according to a one-binding-site model. We also analyzed binding curves from competition of mutant IP-10 with 125I-IP-10 WT by nonlinear least-square fits to calculate EC50. In contrast to the receptor binding affinity of wild-type IP-10 (EC50 = 21.4 ± 1.31 nM), the mutant IP-10C showed a 3.28-fold weaker receptor binding affinity (EC50 = 70.3 ± 1.54 nM). In addition, IP-10H, with a mutation in the GAG-binding sites, showed no significant change in the receptor binding affinity (EC50 = 21.1 ± 0.26 nM) (Fig. 2C).
FIG. 2.
Competitive CXCR3 receptor binding assay. (A) Glycosaminoglycan-deficient Chinese hamster ovary cells (pgsA-745) were stably transfected with empty expression vector (lane 1, bottom) or with the human CXCR3 expression vector (lane 2, bottom), and the CXCR3 expression in the cell lysate was verified by Western blot with anti-CXCR3 antibody. Binding of 100 pM 125I-labeled IP-10 to the cells without (lane 1, top) or with CXCR3 expression (lane 2, top) was measured as described under Materials and Methods. (B) The percentage of cold IP-10 competition binding with specific 125I-labeled IP-10 binding was calculated after subtraction of nonspecific binding. The input of 125I-labeled IP-10 is 100 pM and the increasing concentrations of unlabeled IP-10 range from 0 to 100 nM. Each point represents the mean of three experimental. The inset is the transformed data of 125I-labeled binding to a one-binding-site model of CXCR3. (C) The GAG-deficient CHO cells expressing CXCR3 were used to test the competition of the 125I-labeled IP-10 binding to CXCR3 receptor with unlabeled wild-type or mutant IP-10 in increasing concentrations of up to 1000-fold. Data shown are representative of three independent experiments.
In Vitro Characteristics of GAG-Mutant IP-10 Binding Activity
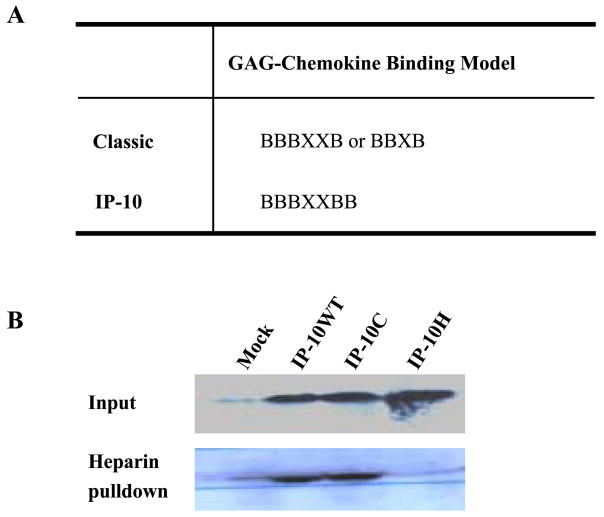
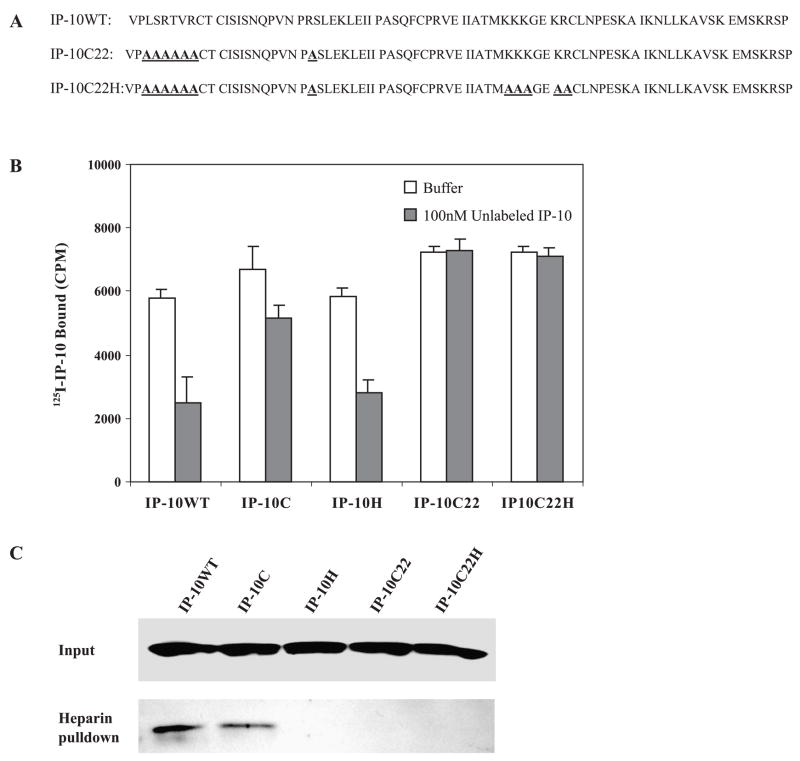
Chemokines bind selectively to GAGs such as heparin, chondroitin sulfate, and dermatan sulfate. There is a common BBBXXB or BBXB motif in heparin-binding proteins [9,23,24]. We have mutated the basic residues of 46KKKGEKR51 on the 40s cluster of IP-10 to alanine (Fig. 3A). When we performed a heparin–Sepharose (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) pull-down binding assay on cell supernatants from cell lines stably expressing IP-10H, the mutant IP-10H demonstrated an abrogated heparin binding capacity, whereas the wild-type or N-terminal mutant IP-10C fully retained heparin binding capacity (Fig. 3B).
FIG. 3.
GAG-mutant IP-10 binding assay. (A) Defined GAG-chemokine binding motifs and proposed GAG-IP-10 binding motif. (B) Cleared culture medium supernatants from A375 cells stably expressing IP-10 proteins or mock-transfected A375 cells were incubated with heparin – Sepharose in 0.9% NaCl at 4°C and rotated for 4 h, and the heparin – Sepharose was washed with 0.5 M NaCl. The washed heparin beads (lower band) and cell supernatants (upper band), which had been concentrated with Microcon YM-3 (cutoff Mr 3000) membranes, were resolved on a 16% SDS – PAGE reducing gel and transferred to nitrocellulose. The membrane was blotted with anti-human IP-10 antibody and visualized by enhanced chemiluminescence assay as described under Materials and Methods.
In Vivo Tumor Growth of Mock- and IP-10-Transfected A375 Cells
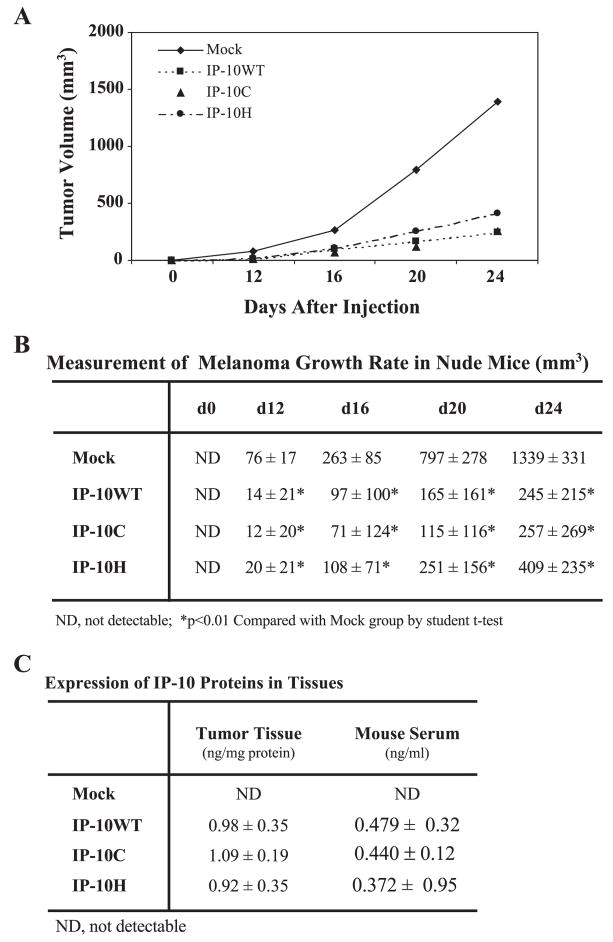
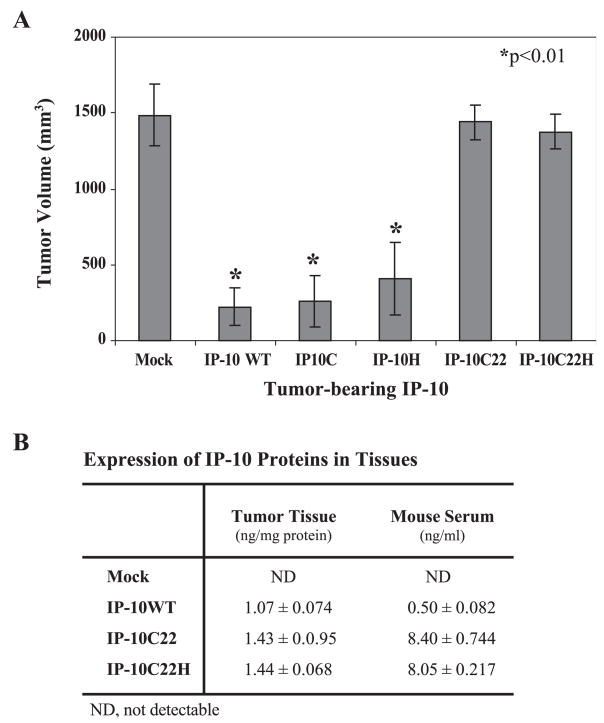
IP-10 is an immunomodulatory chemokine widely recognized to be a potent inhibitor of both IL-8- and bFGF-induced angiogenic activity [25]. IP-10 can regulate non-small-cell lung cancer-derived angiogenesis, tumor growth, and spontaneous metastasis [15]. Moreover, the A375 human melanoma cells expressing the human IP-10 gene secreted sufficient IP-10 to inhibit angiogenesis and tumor growth in vivo in a nude mouse model [17]. However, it is unclear whether the CXCR3 receptor, or proteoglycan binding, is responsible for this process. To clarify this issue, we used the pIRES2-EGFP vector, which would allow both the IP-10 gene and the EGFP gene to be translated from a single bicistronic mRNA. Using this system, we transfected mutant IP-10C and IP-10H, which impaired CXCR3 binding and GAG binding, respectively, into A375 human melanoma cells and selected stable polyclonal cell lines expressing these mutant or wild-type IP-10 chemokines. Approximately 96% of the transfected cells stably expressed EGFP per cell line and we sorted out the brightest cells (10% of the total EGFP-expressing cells) for further selective expansion. We inoculated 106 cells from each of these stable clones subcutaneously into the flank region of nude mice and monitored tumor growth rate for 24 days. Results showed that cells expressing either receptor-binding mutant IP-10 or heparin-binding mutant IP-10 proteins retained the capacity to inhibit tumor growth (3.2- and 5.2-fold, respectively), in contrast to mock-transfected cells not expressing the human IP-10 (Fig. 4A). There was no significant difference between tumor growth in mutant versus wild-type IP-10-expressing A375 melanoma clones (Fig. 4B). At the endpoint of the experiment, anti-human IP-10 ELISA demonstrated that approximately 1 ng human IP-10 (wild type or mutant) was present per milligram of tumor tissue protein and approximately 0.4 ng human IP-10 was observed per milliliter of serum in the tumor-bearing mice (Fig. 4C).
FIG. 4.
In vivogrowth of the transfected human A375 cells. (A) Human A375 melanoma cells (1 × 106) stably producing IP-10 proteins were injected subcutaneously into nude mice and tumors were measured in two dimensions using digital calipers at the indicated time. (B) The tumor volume was calculated according to [17] and data are expressed as mean mm3 ± standard deviation (SD) of five mice per group per experiment. The entire experiment was performed twice. Comparisons between groups were performed using Student’s t test. Two-tailed P values ≤0.01 were considered significant. (C) The IP-10 expression levels in tumor xenografts and serum were determined by ELISA at termination of experiments. IP-10 protein levels are expressed as mean ng ± SD per milligram tissue protein or per milliliter serum from five mice per group.
Histopathological Characteristics of Mock-Transfected and Wild-Type or Mutant IP-10-Transfected Melanoma Tumor
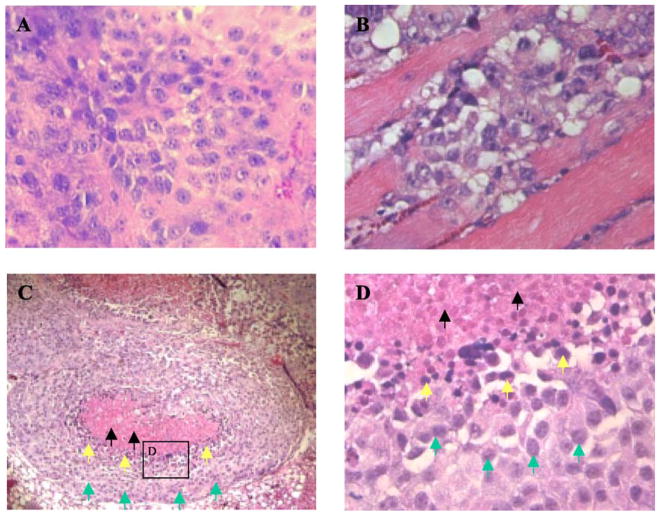
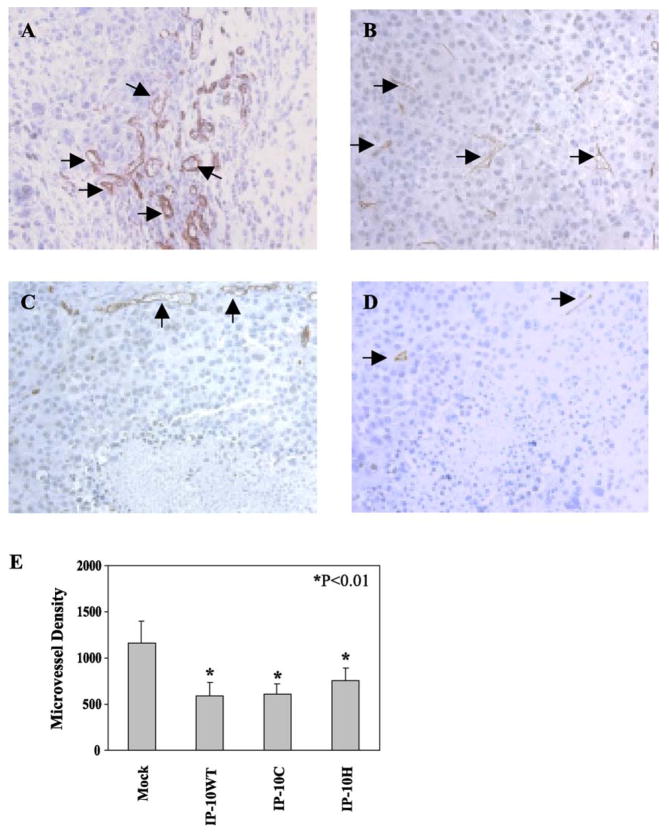
We excised all tumors if they reached a size of approximately 1500 mm3. H&E-stained sections of vector-alone-transfected tumors revealed large areas of confluent tumor cells with active nuclear mitosis (Fig. 5A). These large tumors occasionally exhibited limited invasion of tumor cells into surrounding muscles (Fig. 5B). In contrast, IP-10 (wild type or mutants)-transfected tumors were considerably smaller and tumor sections demonstrated central necrosis surrounded by a rim of very basophilic cells (Fig. 5C). At high magnification (400×) (Fig. 5D), it is clear that the cells in the center of the tumor exhibit signs of necrosis and pyknotic nuclei are visible. Tumor sections stained for immunoreactive CD31 revealed poor microvessel distribution in the central area of tumors, where there was apoptosis and necrosis. We observed more blood vessels in the sections of mock-transfected tumor (Fig. 6A) than in the IP-10-expressing tumors (Fig. 6C); similarly, well-formed microvessel networks were distributed throughout the mock control tumors (Fig. 6B), in contrast to the IP-10 (both wild type and mutants)-transfected tumors (Fig. 6D). Moreover, we determined tumor vessel distribution quantitatively by random measurement of vessel plus capillary density and statistically analyzed it (Fig. 6E), demonstrating that IP-10 wild type and mutant proteins played an inhibitory role on tumor-induced angiogenesis.
FIG. 5.
Histopathological alterations of the transfected melanoma tumors. (A) H&E-stained sections of control tumors showed mitotic activity and (B) occasionally the tumor cells invaded the muscles; (C) the IP-10-transfected melanomas showed central necrosis and a pyknotic ring of tumor cells around the necrotic zone. (D) High magnification of box in C showing pyknotic nuclei. Green, yellow, and black arrows indicate viable, apoptotic or pyknotic, and dead cells, respectively.
FIG. 6.
Vessel and microvessel distributions of the transfected melanoma tumors. To evaluate the endothelial cell content of tumors, the fixed, embedded tumor tissues were sectioned and stained for immunoreactive CD31 in control tumors (A) and (B) and IP-10-transfected melanoma tumors (C) and (D). (E) Microvessel density was digitally measured from five random fields using the Image-Pro Plus program (Media Cybernetics, LP).
In Vivo Characteristics of Double Mutant IP-10 Anti-tumor Activity
Mutant forms of IP-10 with decreased binding affinity to the activation domain of CXCR3 or impaired heparin binding still maintained anti-tumor activity. Since the arginine residue 22 on the N loop of IP-10 is important for its binding to both CXCR3 receptor and heparin [22], we went on to examine the role of R-22 in the angiostatic activity of IP-10. We created additional mutations by replacement of arginine residue 22 with alanine in the mutant IP-10C (Fig. 7A, line 2, named IP-10C22) and combined these mutants with mutations of the heparin-binding domain (46AAAGEAA52) (Fig. 7A, line 3, named IP-10C22H). To assess the binding activity of these mutant IP-10 proteins to CXCR3 receptors, we used the stably expressed CXCR3 molecular model of GAG-deficient CHO cells described in the legend to Fig. 2. The competition binding of IP-10C22 or IP-10C22H to CXCR3 receptor was too low to obtain the EC50 value. Moreover, mutant IP-10 proteins purified from either culture medium or from bacterial expression identically lost heparin-binding capacity (Fig. 7C). Interestingly, both mutant IP-10 proteins (IP-10C22 and IP-10C22H) lost anti-tumor activity and angiostatic activity in vivo (Fig. 8A), even though protein expression levels were relatively high in tumor tissues and serum compared to mice bearing tumors expressing wild-type IP-10 (Fig. 8B).
FIG. 7.
Binding analysis of double mutant IP-10 protein. (A) Protein sequence of human wild-type IP-10 (line 1); IP-10C22, an arginine 22 to alanine substitution at the N loop of IP-10C (line 2); and an additional double mutant, IP-10C22H. (B) The glycosaminoglycan-deficient Chinese hamster ovary cells expressing CXCR3 were used to evaluate the competition of 125I-labeled IP-10 binding to CXCR3 receptor with excess unlabeled wild-type or mutant IP-10 over increasing concentrations (up to 1000-fold). (C) Heparin-binding assay for wild-type or mutant IP-10 was performed under physiological conditions as described in the legend to Fig. 3.
FIG. 8.
In vivo growth of the transfected human A375 cells with double mutant IP-10. (A) Human A375 melanoma cells (1 × 106) stably producing mutant IP-10 proteins were inoculated subcutaneously into nude mice (five mice per group) and tumors were measured in two dimensions using digital calipers at the indicated time. The entire experiment was performed twice with similar results each time. The tumor volume was calculated according to [17] and data from one of the repeat experiments are expressed as mean mm3 ± SD. Comparisons between groups were performed using the Student t test. Two-tailed P values ≤0.01 were considered significant. (B) The IP-10 expression levels in tumor tissues and mouse serum were determined by ELISA at the termination of the experiments.
Discussion
In the present study we expressed mutant and wild-type forms of IP-10 protein to define the role of CXCR3 and glycosaminoglycan binding in the angiostatic properties of IP-10 during melanoma tumor growth. IP-10 has potent in vivo T-cell-dependent anti-tumor effects [26] that are mediated through activation of CXCR3 [27] as well as angiostatic activities that appear to be mediated by direct effects on endothelial cells [14]. In vitro studies have indicated that CXCR3 and an uncharacterized IP-10 receptor are expressed on some endothelial cells [18,19]. While it is also possible that IP-10 may induce T cells to adhere to activated endothelial cells [4], this in vitro finding remains controversial [28]. To clarify the role of CXCR3 in IP-10 angiostatic activity in the absence of T cell involvement, athymic nude mice were used in these studies. Using chemokine mutation analysis, a cluster of amino acids on the N-terminus of IP-10, which serves as an activation domain (3LSRTVR8), was replaced with alanine. Although the binding of mutant IP-10C to receptor was not completely abrogated (Fig. 2C), the signal transduction mediated through CXCR3 in response to this mutant was expected to be greatly impaired, based on prior work suggesting that mutation of a single amino acid residue at the N-terminus preceding the first cysteine remarkably impaired receptor signaling and chemotaxis [20–22]. Human melanoma A375 cells expressing and secreting mutant IP-10C formed smaller tumors in nude mice (nu/nu) than those expressing empty vector alone. Although IP-10C exhibited a 1.8-fold weaker binding affinity to CXCR3 compared to IP-10 wild-type protein, at the level the protein was expressed in tumor tissue and serum, receptor binding was expected to be saturated. Thus, it is not surprising that this mutant retained the capacity to inhibit angiogenesis despite reduced binding affinity for CXCR3. The inhibitory effect of IP-10 (mutant and wild type) was related directly to the ability of IP-10 to inhibit the melanoma tumor-induced angiogenesis or vascularization (Fig. 6), since IP-10 did not inhibit the proliferation of A375 melanoma cells (Fig. 1E). Furthermore, the combination of mutation of IP-10C with alanine exchange of arginine 22 on the N loop, IP-10C22 (3LSRTVR8 → 3AAAAAA8 plus R22A), abrogated binding to CXCR3 as well as GAGs, and simultaneously there was a total loss of IP-10 anti-tumor activity. Whether this additional arginine 22 mutation also alters the essential IP-10 conformational events that are required for the angiostatic process is not clear.
The selective recruitment of leukocytes to an inflammatory site by chemotactic factors is believed to require immobilization of chemokines on proteoglycans of the extracellular matrix and on the GAGs of endothelial cells [28]. Recombinant IP-10 purified from Escherichia coli transformed cells or secreted from A375 melanoma cells can bind to heparin and heparan sulfate glycosaminoglycans [9] in vitro under physiological salt conditions (Fig. 3B, lane 2). The glycosaminoglycan moieties that bind chemokines include heparin, heparan sulfate, chondroitin sulfate, and dermatan sulfate [23]. The interaction of chemokines with soluble GAGs can prevent binding of chemokines to their appropriate receptor [29], or in contrast, interaction with membrane-bound GAGs can potentiate the activity of chemokines [30]. We have clarified the role of GAG binding in the capacity of IP-10 to inhibit angiogenesis in a melanoma xenograft model. The motifs BBXB and BBBXXB, where B is a basic residue and X is a hydropathic residue, are found in several chemokines known to bind heparin, and the critical spacing of the hydropathic residue(s) between the basic residues may confer the protein/GAG specificity and affinity [24]. Inspection of the human IP-10 sequence revealed that it has a BBBXXBB cluster between the 40s loop and the β3 strand. We mutated the basic amino acids (B) to A to yield an AAAXXAA motif (IP-10H). This mutant IP-10H completely lost its GAG binding capacity. When the IP-10H-expressing melanoma cells were inoculated into nude mice, this mutant IP-10H exerted the same inhibitory role on tumor growth as IP-10WT did (Fig. 4), suggesting that GAG binding is not essential for the ability of IP-10 to inhibit tumor growth. However, we cannot rule out the possibility that the GAG binding domain of IP-10 is important under conditions under which IP-10 concentrations are low, where GAG binding may be able to increase the local concentration of IP-10. In our experiments, the levels of IP-10 (mutant and wild type) in the tumor tissues were between 920 ± 350 and 1090 ± 190 pg/mg protein (Fig. 4C). In contrast, VEGF and CXCL1 levels in the tumor tissues were 290 ± 26 and 820 ± 181 pg/mg protein for VEGF and CXCL1, respectively.
Our in vivo data suggested that IP-10 inhibition of melanoma tumor-induced angiogenesis was the result of activation of a signaling mechanism through binding to the CXCR3 receptor but not heparin, since mutation of the GAG binding domain (IP-10H) did not result in loss of anti-tumor capacity. However, IP-10 anti-tumor activity was lost with the IP-10C22 mutant, which failed to bind to receptor as well as GAG. Although the potent angiostatic effects of the interferon-inducible proteins such as IP-10, Mig, and I-TAC are well documented, the receptors on human microvascular endothelial cells that bring about angiostatic response to chemokines are unclear. It has recently been shown that two forms of CXCR3 exist due to alternative splicing events [31]. CXCR3-A [18,32,33] is thought to modulate T cell chemotactic responses and CXCR3-B is proposed to modulate angiostatic activity [31]. An unidentified receptor responsible for angiostatic activity is currently under investigation [19]. Gene therapy that allows induction or delivery of IP-10 mimetics is particularly attractive due to the immunologic anti-tumor/antiangiogenesis properties in preclinical models. Further efforts to explore the IP-10 functional domain and specific receptor for these anti-tumor properties of IP-10 will be of importance for future therapies for cancers.
Material and Methods
Cloning the human IP-10 gene and creating mutant IP-10 genes
Human peripheral monocytes were isolated by Ficoll – Paque centrifugation and stimulated with 100 IU/ml recombinant human interferon-γ (R&D Systems) for 6 h. The total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was obtained from RNA treatment with M-MLV reverse transcriptase (Life Technologies, Gaithersburg, MD, USA). The PCR primers (sense, GATCCTCGAGCCACCATGAATCAAACTGCGATTCTG; antisense, ATCGAATTCTTATTAAGGAGATCTTTTAGACATTTC) were designed based upon the published sequence of IP-10 (GenBank Accession No. X02530), incorporating a Kozak consensus sequence upstream of the start codon.
IP-10 becomes a mature chemokine after cleavage of the signal peptide, leaving 8 amino acids preceding the CXC motif. These 8 amino acids are essential for binding and activation of CXCR3. The inverse PCR technique was used to mutate the possible binding domains of IP-10 to the CXCR3 receptor and/or proteoglycan. Specifically, the N-terminal amino acids 3 – 8 of secreted IP-10 were replaced with alanine, and this mutant was named IP-10C; lysine residues 46, 47, 48, and 51 and arginine residue 52 distributed between the 40s loop and the β3 strand were replaced with alanine, and this mutant was named IP-10H. These mutant IP-10 genes were cloned into pBluescript SK and the sequence was confirmed by DNA sequence analysis.
Generation of IP-10 constructs and stable transfection of melanoma cells
The IP-10 cloning vectors were digested with restriction enzymes XhoI and EcoRI (New England Biolabs, Beverly, MA, USA) and subcloned into the multiple cloning site of the pIRES2-EGFP vector (Clontech, Palo Alto, CA, USA). The A375 human melanoma cell line (American Type Culture Collection (ATCC), Manassas, VA, USA) was transfected with the pIRES2-EGFP vector alone or with the expression vector containing an insert for IP-10 WT, IP-10C, IP-10H, IP-10C22, or IP-10C22H. Stable polyclonal lines were selected based upon neomycin resistance (neomycin 800 μg/ml; Life Technologies). The highest EGFP-expressing cells were sorted by flow cytometry for further selection of IP-10-expressing cells (Fig. 1D, cells in the right upper square of lower left graph).
Cell culture
Cell lines used in this study were obtained from ATCC. Human melanoma A375 cells were cultured in DMEM/F12 medium as previously described [34]. The GAG-deficient CHO cells were cultured in F12K medium supplemented with 3 mM glutamine and 10% heat-inactivated fetal bovine serum (Invitrogen).
Western blot analysis
Western blot was performed following the protocols from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Whole-cell extracts were prepared from cultured cells, and chemokines in culture supernatants were concentrated with Microcon YM-3 (Millipore Corp., Billerica, MA, USA). Briefly, for whole-cell extracts, cells were mechanically released from tissue culture plates by scraping, washed in cold PBS, and lysed in RIPA buffer (1 × PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) with protease inhibitor cocktail (Boehringer Mannheim Corp., Indianapolis, IN, USA) and 0.2 mM sodium orthovanadate. Cell extracts were cleared by microcentrifugation (10,000g) at 4°C for 10 min, supernatants were collected, and protein content was determined by Bio-Rad assay. Equal protein aliquots were solubilized in loading buffer and resolved on 10 or 16% SDS – PAGE reducing gel, transferred to nitrocellulose membrane (Bio-Rad Labs), blotted in TBS-T with 5% fat-free instant milk (Carnation), probed with the appropriate antibodies, and visualized by enhanced chemiluminescence assay.
Protein expression and purification
Recombinant human IP-10 beginning with the mature NH2-terminal codon 132 encoding valine and terminating with the COOH-terminal codon 363 encoding proline was engineered by PCR using the IP-10 cloning vectors described above as templates. The primers used for IP-10 WT and IP-10H were CGGGATCCATGTACCTCTCTCTAGAACCG (sense) and GGAATTCAGGAGATCTTTTAGACATTTCCT (antisense) and for IP-10C were AGGGATCCCTGCAGCTGCTGCAGCCTG (sense) and GGAATTCAGGAGATCTTTTAGACATTTCCT (antisense). The PCR products were digested with BamHI and EcoRI, purified, and subcloned into the multiple cloning site of pGEX-5X3. The bacteria expressing GST-tagged IP-10 proteins were purified with glutathione – Sepharose 4B, and the GST fusion proteins were cleaved with Factor Xa (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA). The cleaved IP-10 proteins were separated from Factor Xa by high-performance liquid chromatography using a C18 column and eluting with a 6%:94% to 60%:40% acetonitrile:water gradient containing 0.05% trifluoroacetic acid. The purified proteins were concentrated by vacuum centrifugation at room temperature and confirmed by Western blot with monoclonal antibody to human IP-10 and quantified by ELISA (R&D Systems).
Melanoma tumor formation in nude mice
Animal experiments were conducted according to IACUC-approved protocols. Nine-week-old female nude mice (Charles River Laboratories, Wilmington, MA, USA) were inoculated with 106 pIRES2-EGFP vector-transfected A375 melanoma cells, as a control, or IP-10 (WT or mutant)-expressing A375 cells. One million cells suspended in 100 μl PBS were subcutaneously injected into the right flank. Developing tumors were measured in two dimensions using digital calipers at regular intervals and tumor volume was calculated based upon the formula volume = width2 × length [17]. Animals were randomly grouped and each group consisted of five animals. The entire experiment was repeated once using a different set of nude mice.
Evaluation of histopathology and immunohistochemistry
Experiments were terminated when tumor volume reached 1500 mm3. Animals were sacrificed by CO2 asphyxiation. Tumor tissues were removed, measured, and fixed in buffered 4% paraformaldehyde for 24 h and then paraffin embedded. The sections were stained with Mayer’s hematoxylin and eosin or immunostained with CD31 antibody (Research Diagnostics, Inc., Flanders, NJ, USA). Blood vessels were visualized by immunohistochemically labeling endothelial cells. Five-micrometer sections of paraffin-embedded tissues were placed on charged slides and deparaffinized. The sections were rehydrated and placed in heated Target Retrieval Solution (Dako, Inc.) for 20 min. Endogenous peroxidase was quenched with 0.03% hydrogen peroxide and samples were treated with diluted rabbit serum prior to primary antibody addition. Tissues were incubated with goat anti-CD31 diluted in 1:400 for 45 min. Sections without primary antibody served as negative controls. The Vectastain ABC Elite (Vector Laboratories, Inc.) system and DAB+ (Dako, Inc.) were used to produce localized, visible staining. Slides were lightly counterstained with Mayer’s hematoxylin, dehydrated, and coverslipped.
The histological features of H&E- and immunostained sections from tumors in each group were microscopically analyzed. Microvascular density of each tumor section based on anti-CD31 immunostaining was calculated as the mean vessel density of five random fields (20× magnification). The vessel density was determined by Image-Pro Plus image analysis software (Media Cybernetics LP, Silver Spring, MD, USA).
Binding of IP-10 to CXCR3 receptor assay
Binding assays of wild-type and mutant forms of IP-10 to the CXCR3 receptor were performed according to the method as described by Soejims [19]. Briefly, the GAG-deficient CHO cell line named pgs-745 was transfected with a CXCR3 expression vector and stable neomycin-resistant cell lines were selected with G418 (800 μg/ml). CXCR3 expression in the polyclonal cell line was confirmed by Western blot using anti-human CXCR3 monoclonal antibody (R&D Systems) at a dilution of 1:1000 and secondary antibody diluted at 1:3000 (Chemicon International, Inc., Temecula, CA, USA). CXCR3-expressing GAG-deficient CHO cells (1.5 × 105) were plated in 24-well culture plates and incubated overnight in complete medium for adhesion. Cells were washed twice with washing buffer (50 mM Hepes, 1 mM CaCl2, 5 mM MgCl2, 0.5% BSA, and 0.5 M NaCl, pH 7.2) and once with binding buffer (washing buffer without NaCl). Cells were then incubated in 200 μl of binding buffer containing 100 pM 125I-labeled IP-10 (Amersham) in the presence of increasing concentrations of unlabeled IP-10 (up to 1000-fold molar excess) at room temperature for 1.5 h. Cells were washed three times with washing buffer and lysed in 1 ml of 1 M NaOH. Radioactivity was determined in a gamma counter (Gamma 5500B; Beckman). The binding buffer conditions were optimized to minimize chemokine self-association and to avoid osmotic lysis as described by Van Riper [35].
ELISA
IP-10-transfected A375 cells (1 × 106) were seeded on six-well plates in DMEM/F12 medium containing 10% FBS and incubated 6 h for adhesion. The monolayers were washed in serum-free culture medium and then incubated in 1 ml of serum-free medium for an additional 14 h at 37°C, at which time the supernatant was collected and cleared by centrifugation. Tumor tissues were homogenized in PBS with protein inhibitors and cleared by centrifugation. The protein content was determined by Bio-Rad assay. Aliquots were then subjected to anti-human IP-10 ELISA (R&D Systems) according to the manufacturer’s protocol.
Acknowledgments
We thank Andrew D. Luster (Harvard Medical School, Boston, MA, USA) for the human CXCR3 construct and Linda Horton, Evemie Schutyser, and Yingchun Yu for helpful discussions and excellent technical assistance. This work was supported by a Department of Veterans Affairs Career Scientist Award and Merit Award (to A.R.), NCI Grant CA34590 (to A.R.), and Grant 5P30 AR41943 from the Skin Disease Research Center.
Footnotes
Abbreviations used: IP-10, interferon-inducible protein of 10 kDa; GAG, glycosaminoglycan; CXCR, CXC chemokine receptor; CHO, Chinese hamster ovary; EGFP, enhanced green fluorescence protein; H&E, hematoxylin and eosin.
References
- 1.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672 – 676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 2.Zaks-Zilberman M, Zaks TZ, Vogel SN. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15:156 – 165. doi: 10.1006/cyto.2001.0935. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084 – 1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub DD, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809 – 1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975 – 980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mach F, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041 – 1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873 – 6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao DX, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-gamma-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell alpha chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J Immunol. 2002;169:1556 – 1560. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- 9.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219 – 231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlodavsky I, Goldshmidt O. Properties and function of heparanase in cancer metastasis and angiogenesis. Haemostasis. 2001;31 (Suppl 1):60 – 63. [PubMed] [Google Scholar]
- 11.Vlodavsky I, et al. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol. 2002;12:121 – 129. doi: 10.1006/scbi.2001.0420. [DOI] [PubMed] [Google Scholar]
- 12.Vlodavsky I, et al. Molecular properties and involvement of heparanase in cancer progression and normal development. Biochimie. 2001;83:831 – 839. doi: 10.1016/s0300-9084(01)01318-9. [DOI] [PubMed] [Google Scholar]
- 13.Marchisone C, Del Grosso F, Masiello L, Prat M, Santi L, Noonan DM. Phenotypic alterations in Kaposi’s sarcoma cells by antisense reduction of perlecan. Pathol Oncol Res. 2000;6:10 – 17. doi: 10.1007/BF03032652. [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo AL, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155 – 162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arenberg DA, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981 – 992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertl U, et al. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol. 2001;166:6944 – 6951. doi: 10.4049/jimmunol.166.11.6944. [DOI] [PubMed] [Google Scholar]
- 17.Feldman AL, et al. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer. 2002;99:149 – 153. doi: 10.1002/ijc.10292. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani P, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53 – 63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soejima K, Rollins BJ. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol. 2001;167:6576 – 6582. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- 20.Proost P, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554 – 3561. doi: 10.1182/blood.v98.13.3554. [DOI] [PubMed] [Google Scholar]
- 21.Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure – function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289 – 295. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- 22.Campanella GS, Lee EM, Sun J, Luster AD. CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10) J Biol Chem. 2003;278:17066 – 17074. doi: 10.1074/jbc.M212077200. [DOI] [PubMed] [Google Scholar]
- 23.Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. Glycosaminoglycan – protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156 – 167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Proudfoot AE, et al. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J Biol Chem. 2001;276:10620 – 10626. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]
- 25.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51 – 57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 26.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057 – 1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569 – 577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301 – 314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Sai J, Richmond A. Cell surface heparan sulfate participates in CXCL1-induced signaling. Biochemistry. 2003;42:1071 – 1077. doi: 10.1021/bi026425a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci USA. 1993;90:7158 – 7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasagni L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537 – 1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenberg DA, Zlotnick A, Strom SR, Burdick MD, Strieter RM. The murine CC chemokine, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol Immunother. 2001;49:587 – 592. doi: 10.1007/s002620000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salcedo R, et al. Differential expression and responsiveness of chemokine receptors (CXCR1 – 3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055 – 2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901 – 4909. [PubMed] [Google Scholar]
- 35.Van Riper G, Siciliano S, Fischer PA, Meurer R, Springer MS, Rosen H. Characterization and species distribution of high affinity GTP-coupled receptors for human RANTES and monocyte chemoattractant protein 1. J Exp Med. 1993;177:851 – 856. doi: 10.1084/jem.177.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]