Abstract
The CXC chemokine, CXCL1 (melanoma growth-stimulatory activity/growth-regulated protein α), plays a major role in inflammation, angiogenesis, tumorigenesis, and wound healing. Recently, chemokines have been extensively related to cellular transformation, tumor growth, homing, and metastasis. CXCL1 and its mouse homologue MIP-2 have been shown to be involved in the process of tumor formation. When chemokines such as CXCL1 and CXCL8 (IL-8) become disregulated so that they are chronically expressed, tissue damage, angiogenesis, and tumorigenesis can follow. This up-regulation of chemokines has been attributed to constitutive activation of NF-κB. The constitutive NF-κB activation is an emerging hallmark in various types of tumors including breast, colon, pancreatic, ovarian, as well as melanoma. Previous findings from our laboratory and other laboratories have demonstrated the role of endogenous activation of NF-κB in association with enhanced metastatic potential of malignant melanoma cells and suggest that targeting NF-κB may have potential therapeutic effects in clinical trials. An important step in this direction would be to delineate the important intracellular pathways and upstream kinases involved in up-regulation of NF-κB in melanoma cells. In this review, the signaling pathways involved in the disregulation of NF-κB and chemokine expression are discussed.
Keywords: chemokines, NF-κB, signaling
INTRODUCTION
Chemokines (chemotactic cytokines) are small peptides that are potent activators and chemoattractants for leukocyte sub-populations and some nonhematopoietic cells [1, 2]. They play a crucial role in immune and inflammatory reactions such as allergic disorders, autoimmune diseases, and in viral infections. Most chemokines cause chemotactic migration of leukocytes and affect angiogenesis, hematopoiesis, tumorigenesis, metastasis, and tumor rejection [3–6].
Chemokines and their receptors
The chemokines consist of two major families, CXC or α chemokines and CC or β chemokines, and two minor families, C or γ chemokines and CX3C or δ chemokine [7, 8]. The CXC family has an amino acid (aa) positioned between the first and second cysteine, whereas the CC family has two cysteines positioned side by side. The CXC chemokine family has been subdivided into two categories depending on presence of an ELR motif (glutamic acid-leucine-arginine) preceding the first cysteine residue in the protein. The C chemokine family is represented mainly by lymphotactin, and the CX3C family exhibits 3 amino acid between the first two cysteines and is represented by fractalkine or neurotactin.
Chemokines regulate angiogenesis, a process upon which tumors depend for growth, survival, invasion, and metastasis. CXC chemokines can regulate angiogenesis both positively and negatively depending on the presence or absence of ELR in their NH2 terminus [9–11]. Members of the CXC family that behave as angiogenic factors include CXCL8 [interleukin-8 (IL-8)], CXCL1-3 [melanoma growth-stimulating activity (MGSA) α, β, and γ], CXCL5 (epithelial-derived neutrophil-activating factor-78), CXCL6 [ (granulocyte chemotactic protein-2)], and CXCL7 (neutrophil-activating polypeptide-2). The overexpression of (ELR−) chemokines, such as CXCL9 [also known as monokine induced by interferon-γ (IFN-γ)] or CXCL10 (IFN-inducible protein 10) in human lymphomas, grown in nude mice or human non-small cell lung cancer grown in severe combined immunodeficiency (SCID) mice, leads to spontaneous regression that is directly related to impaired angiogenesis [12].
The specific effects of chemokines on their target cells are mediated by a family of 7-transmembrane (7TM) G-protein-coupled receptors (GPCR). These chemokine receptors are part of a much bigger superfamily of GPCR that include receptors for hormones, neurotransmitters, paracrine substances, and inflammatory mediators [13]. Chemokine receptors vary significantly in their expression, binding, and response to specific chemokines on different cell types. Chemokine receptors have also recently been implicated in several disease states including allergy, psoriasis, atherosclerosis, malaria, and AIDS [14–18]. Six receptors have been characterized for CXC chemokines (CXCR1–CXCR6), and 10 receptors for CC chemokines (CCR1–CCR10). XCR1 is the receptor for XCL1 (lymphotactin) and CX3CR1 for CX3CL1 (fractalkine) (see ref [19] for review). A chemokine-binding protein, also known as the Duffy antigen receptor for chemokines, has been shown to bind promiscuously to CXC and CC chemokines [20]. In addition, other 7TM GPCR, encoded by herpes- and poxviruses, have been identified [21, 22]. Significant advances have been made in understanding the regulation of chemokine receptor expression and the intracellular signaling mechanisms used in bringing about cell activation.
Chemokine and chemokine receptor expression in association with transformation
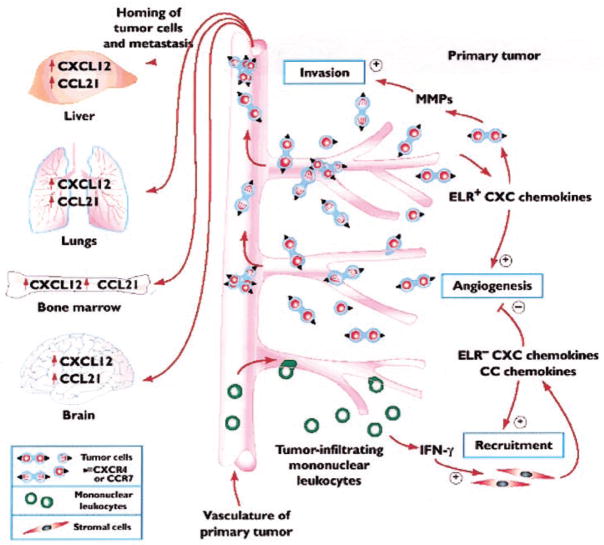
For more than a decade, chemokines have been recognized as important molecules for the homing of a population of leukocytes under conditions of homeostasis and inflammatory and/or immunological responses. However, recent studies are providing an equally important role for these chemotactic cytokines in tumor biology [23]. Chemokines display autocrine, paracrine, and hormonal roles in promoting tumorigenesis, invasion, homing, and metastasis to distant, preferential target organs (Fig. 1). An understanding of this expanded role in promoting tumor biology should open new doors to therapeutic intervention.
Fig. 1.
The pleiotropic role that chemokines play in promoting cellular transformation, tumor growth, invasion and homing, and metastasis to distant preferential organs. CXC and CC chemokines play multifunctional roles in facilitating tumor cell growth and invasion by augmenting their local angiogenic environment and up-regulating the expression of local proteinases to aid tumor cell invasion and entry into the circulation. Display of chemokine receptors on tumor cells may facilitate homing and organs that produce the chemokine ligands for those receptors. (Photocopied with permission from [23].)
Molecules that regulate the metastatic spread of tumors to specific organs should be expressed constitutively at principal sites of metastasis and must be capable of mediating the invasion of cells into tissues. In addition, the distinct receptor repertoire should be expressed by the target cells. As chemokines play an important role in leukocyte trafficking and homeostasis, they are important molecules for the above process.
KSHV-GPCR (the human GPCR encoded by the Kaposi’s sarcoma herpesvirus) signals constitutively, and signaling is further augmented by the binding of CXC chemokine ligands such as CXCL1 [24]. Expression of this receptor is associated with transformation [25]. A point mutation of CXCR2, but not CXCR1, results in constitutive signaling of the receptor and transformation of transfected cells in a similar manner to the KSHV-GPCR [26]. Thus, CXC chemokine receptor CXCR2 is thought to participate in cellular transformation. This and several other studies support the hypothesis that expression of CXCR2 on certain cells in the presence of persistent autocrine and paracrine stimulation with specific CXC chemokine ligands can promote preneoplasticto-neoplastic cellular transformation.
Recently, Müller et al. [27] have shown that expression of specific chemokine receptors is an essential event that leads to the homing and metastasis of breast cancer. This occurs in a chemokine ligand and receptor-dependent manner. In breast cancer cells, signaling through CXCR4 or CCR7 was found to mediate actin polymerization and pseudopodia formation and subsequently, induce local chemotactic and invasive responses. Thus, it appears that chemokine ligands and their receptors dictate the precise destination of metastatic tumor cells to specific organs. Furthermore, the association of expression of CCR10 and its ligand CCL27/CTACK7 in malignant melanoma cells and the high incidence of skin metastases in this malignant disease support the involvement of chemokine receptors in metastasis [28]. In addition, other cancers of haematopoietic and nonhaematopoietic origin, including acute myeloid leukaemia, chronic lymphoblastic leukemia, chronic lymphocytic leukemia, non-Hodgkin’s B-cell lymphoma, ovarian cancer, and pancreatic cancer, express functionally active chemokine receptors that mediate tumor cell migration in vitro [29–32].
The CXC chemokines containing an ELR motif include CXCL1, 2, 3, 5, 6, 7, and 8 [33–35]. These chemotactic cytokines act through CXCR1 and/or CXCR2 receptors. Our laboratory focuses mainly on the role of chemokines in melanoma. CXCL1 and CXCL8 are members of the CXC chemokine subfamily and are associated with metastatic melanoma. The mouse CXCL1 homologues are keratinocytes and macrophage-inflammatory protein-2 (MIP-2). CXCL1 (MGSA) was first purified in our laboratory from human melanoma-conditioned medium [36]. CXCL1 is shown to be up-regulated in melanoma cells, and it is involved in pathogenesis of melanoma. We have shown previously that human-cultured nevi and melanoma continue to express CXCL1 mRNA in the absence of serum or exogenous growth factors, but cultured normal melanocytes express little CXCL1 [37–40]. In addition, studies by Norgauer et al. [41] have demonstrated that secretion of MGSA in melanoma cell lines was 6- to 16-fold higher than normal melanocytes. Norgauer et al. [41] also showed enhanced expression of CXCR2 in melanoma tumor cells as compared with normal melanocytes. The biological functions of CXCL1 are mediated through 7TM GPCR. CXCL1 binds and activates CXCR2. The biological functions include regulation of cell growth/cell viability and cell motility. CXCL1 modulates inflammation, angiogenesis, wound healing, and tumorigenesis [42–44]. In this review, we will focus mainly on chemokines as modulators of tumorigenesis.
Our laboratory and others have shown previously that over-expression of CXCL1 or CXCL8 in melanocytes is associated with enhanced growth, ability to form tumors in nude and SCID mice, and enhanced metastatic capacity in melanoma tumors [45–48]. Antibodies to these ligands or their receptor, CXCR2, can block these processes [41, 48]. Overexpression of CXCL1 in immortalized melanocytes transformed these cells such that they developed the capability to form tumors in nude and SCID mice [44]. Furthermore, antiserum to CXCL1 inhibits tumor growth by melan-a cells expressing CXCL1 proteins [43]. Thus it is clear that this chemokine has strong tumorigenic potential for melanocytes.
Malignant melanoma is the most dangerous skin cancer, which, if not detected early, may metastasize with fatal consequences. The prevalence of skin cancer and melanoma is increasing at an alarming rate. About 80,000 cases of melanoma are diagnosed each year [49]. A key event in development of melanoma is mutation of key cell regulatory genes resulting in loss of tumor suppressors combined with constitutive expression of oncogenes, chemotactic cytokines (chemokines), and other growth factors. Many laboratories, including our own, have shown disregulation of nuclear factor-κB (NF-κB)-dependent angiogenic chemokines such as CXCL1 and CXCL8 in human melanoma [50–53]. It is interesting that the NF-κB site is present not only in the promoter of angiogenic chemokines, but also in angiostatic chemokines such as the IFN-γ-inducible chemokine CXCL10. While NF-κB might potentially be involved in promoting transcription of angiogenic and angiostatic chemokines, NF-κB does not act alone in modulating chemokine expression. Its transcriptional activity is modulated by coactivators and repressors, which constitute the functional enhanceosome. For example, IFN-γ is the major activator of CXCL10 transcription [54], and NF-κB may further modify that IFN-γ-induced transcription. Thus, constitutive expression of NF-κB in tumor cells has the potential for facilitating immortalization of these tumors and escape from apoptosis, but this facilitation would be dependent in the context of other coactivators or repressors of transcription.
MECHANISM OF DISREGULATION OF CXCL1 EXPRESSION IN MELANOMA
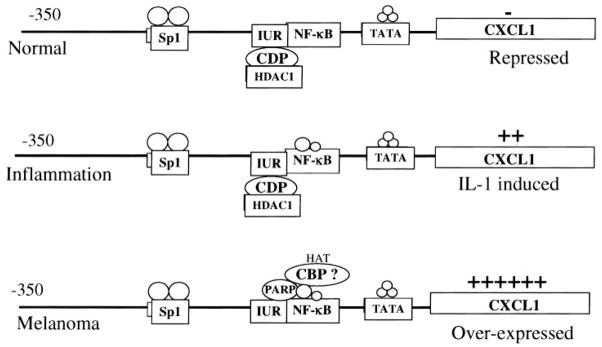
CXCL1 is not expressed constitutively in normal retinal pigment epithelial (RPE) cells or normal human epidermal melanocytes (NHEM), but it can be induced by IL-1, lipopolysaccharide (LPS), and tumor necrosis factor α(TNF-α) [55]. In contrast, malignant melanoma cells exhibit high constitutive levels of CXCL1 mRNA and proteins (Fig. 2). IL-1 treatment does not significantly increase the elevated levels of the gene, although it does appear to stabilize CXCL1 mRNA [51]. Transcription of the CXCL1 gene is regulated largely through a 306-bp minimal promoter situated immediately upstream of the transcription start site. The four cisacting elements comprising the minimal promoter include a TATA box (25–30 nt), a NF-κB-binding site (67–77 nt), an AT-rich high mobility group protein I (HMGI) (Y)-binding element nested within the NF-κB site, an immediate upstream region (IUR; 78–93 nt), and a GC-rich SP1-binding site (117–128 nt) [56]. The IUR is an ~20-bp sequence that is located immediately upstream of the NF-κB element in the CXCL1 promoter.
Fig. 2.
Transcription of CXCL1 is regulated through several cis elements including NF-κB, HMGI (Y), Sp1, and IUR. The IUR contains a binding site for the negative regulator, CDP, and PARP, an activator of transcription. In normal cells, CXCL1 is not induced, but it can be induced by IL-1β, LPS, and TNF-α during inflammation. During IL-1 induction and tumorigenesis, there is an increase in the nuclear levels of p65 and p50 subunits of NF-κB. In melanoma cells, NF-κB is activated constitutively. PARP displaces CDP, and CBP is proposed to bind NF-κB and Sp1 to stabilize the enhanceosome and keep the chromatin in an acetylated and active state.
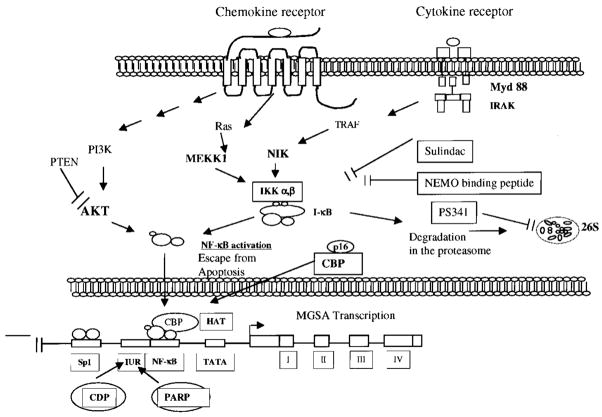
NF-κB constitutes a family of proteins, which are regulated at the level of transcription, translation, or post-translational processing. Disregulation of NF-κB transcription machinery and constitutive expression of chemotactic cytokines are factors thought to be early events in malignant tumor progression. Rel/NF-κB, a family of structurally related DNA-binding proteins, has been implicated in the regulation of cell growth and oncogenesis based on its induction of proliferative and anti-apoptotic gene products. In nonstimulated, nontransformed cells, NF-κB is sequestered in the cytoplasm and is complexed with IκB, a family of inhibitory proteins, which bind to NF-κB and mask its nuclear localization signal, thereby preventing nuclear transport of NF-κB [57]. On activation, IκB becomes phosphorylated, ubiquitinated, and degraded, freeing the NF-κB p65/p50 or p65/p52 complex to move to nucleus and bind specific DNA promoter sequences. The cytokine-induced IκB phosphorylation and subsequent degradation are regulated by activation of a recently described macromolecular complex, the “signalosome” called IκB kinase or IKK (700–900 kDa) [58–63]. The IKK complex consists of two catalytic units, IKK-α and IKK-β (also referred to as IKK-1 and IKK-2), which can directly phosphorylate IκB, as well as a regulatory subunit IKKγ or NF-κB essential modulator (NEMO) [64]. IKK-1 and -2 can phosphorylate IκB-α at serine 32 and 36 in vitro. Furthermore, recent studies of transient overexpression have suggested that some mitogen-activated protein kinase kinase kinases (MAPKKKs), including NF-κB-inducing kinase (NIK) and MEKK1–3, are involved in the activation of the IKK complex [65–68]. NF-κB is also regulated by other kinases, including phosphatidylinositol 3 kinase (PI3K) and Akt [69, 70] (Fig. 3).
Fig. 3.
Model of potential components/upstream kinases involved in constitutive activation of NF-κB and thus chronic expression of CXCL1 in melanoma. The activation of these kinases can occur in an autocrine (by CXCL1) or paracrine (by cytokines and growth factors) manner. This constitutive expression can be blocked with the IKK inhibitor, NEMO-binding peptide, or PS341, a proteasome inhibitor and target tumor cells for apoptosis.
The role of the CCAAT displacement protein (CDP) and poly(ADP-ribose) polymerase (PARP) in cooperation with NF-κB in CXCL1 promoter activation
Previously, we demonstrated that in addition to the NF-κB element, the IUR element is essential for basal as well as cytokine-induced expression of the CXCL1 gene. In particular, point mutations within a putative TCGAT motif of the IUR element abolished basal and IL-1-induced transcription in reporter gene assays with RPE and Hs294T cells. Furthermore, in electrophoretic mobility shift assays (EMSA), these mutations blocked the ability of this element to compete with a constitutive, IUR-specific complex in RPE and Hs294T nuclear extracts [54]. UV cross-linking and southwestern blot analyses revealed that at least one protein, having a relative molecular size of 115 kDa, bound the IUR element in a sequence-specific manner [71]. Purification of the 115-kDa IUR-specific protein by oligonucleotide-affinity chromatography revealed its identity as the PARP and demonstrated that it plays a role in the activation of the CXCL1 promoter [72]. PARP is a 114–115-kDa nuclear DNA-binding protein, which catalyzes the transfer of long, branched ADP-ribose chains to itself or different classes of target proteins involved in chromatin decondensation, DNA replication, DNA repair, and gene expression [73, 74]. ADP-ribosylated PARP can affect a cellular processes such as apoptosis, necrosis, cellular differentiation, and malignant transformation [75]. To determine whether the 20-bp IUR element is a binding site for known transcription factors, the CXCL1 promoter was also analyzed within a transcriptional element database (Transfac) using a Web-based search engine, the Transcription Element String Search (TESS). The search identified the CXCL1 IUR element as a putative binding site for the human CDP, which is highly homologous to the Drosophila CCAAT displacement protein homologue (CUT) protein [76]. The human CDP is a homeodomain protein, which is composed of an N-terminal, coiled-coiled domain, three highly homologous ~70-aa long CUT repeat domains, a C-terminal homeodomain, and two transcription-repression domains [77, 78]. CDP is an active repressor of cell cycle-dependent or differentiation-specific genes including the gp91-phox, p21WAF1/CIP1/SDI1, osteocalcin, thymidine kinase, cystic fibrosis-related transconductance receptor, and c-myc [79–83]. CDP is a 170-kDa protein. In EMSAs, recombinant CDP polypeptides bound the IUR element in a sequence-selective manner [76]. In cotransfection experiments, overexpression of the CDP protein inhibited CXCL1 promoter activity, whereas overexpression of antisense CDP mRNA induced CXCL1 promoter activity fivefold over the control [76]. These results indicate that the transcription of the CXCL1 gene is negatively regulated by the CDP. It is possible that CDP-mediated repression may also involve displacement of other transactivating factors that bind to the CXCL1 promoter, such as NF-κB, Sp1, HMGI (Y), PARP, or factors contributing to the stability of the CXCL1 enhanceosome. However, there is no direct evidence to support this hypothesis.
Enhanceosome models for cytokine gene expression, analogous to the CXCL1 paradigm, have been proposed for the regulation of IL-6 and CXCL8 (IL-8) promoters [84]. Both promoters have binding sequences for the NF-κB, CCAAT/enhancer binding protein, and TATA binding protein. The strongest promoter activation relies on the p65 NF-κB subunit, which specifically recruits cyclic AMP response element-binding (CREB) protein (CBP/p300) to the site.
Engagement of CBP/p300 in the enhanceosome and its histone acetylase activity have been proposed to stabilize the enhanceosome and stimulate transcription from these promoters. In an independent study, CDP has been shown to interact physically with CBP/p300 and is a target for acetylation at specific residues near the homeodomain [85]. These models strongly implicate antagonistic roles for CBP/p300 and CDP in the regulation of IL-6 and CXCL8 transcription, although direct involvement of CDP in regulation of either promoter has not been established. Transcription repression by CDP may also involve its ability to recruit a histone deacetylase activity, HDAC1, leading to deacetylation of histones, a phenomenon consistent with transcriptionally inert chromatin [86]. Similar interactions among NF-κB, CBP/p300, and CDP may be involved in the regulation of CXCL1 gene regulation. The relevance of the IUR binding factors, CDP and PARP, is of potential interest in disorders such as chronic inflammatory conditions and malignancy, where constitutive overexpression of the CXCL1 gene contributes to disease etiology. Interactions of CDP with the IUR cis-acting element may allow for tight repression of the CXCL1 gene. The loss or displacement of CDP may be an important phenomenon in the short-term induction of the CXCL1 gene, usually associated with acute inflammatory responses, or in the constitutive, high-level expression of CXCL1 observed in chronic inflammatory processes, tumorigenesis, and malignant melanoma.
Upstream signals that lead to endogenous activation of NF-κB in melanoma cells
Our laboratory has demonstrated that melanoma cells exhibit endogenous nuclear activation of NF-κB through EMSAs and luciferase reporter assays. In addition, IκB is degraded more rapidly in melanoma cells than in normal cells [87]. Transfection of melanoma cells with the dominant negative inhibitor of NF-κB (IκB ΔN) reduced the tumor growth, reduced the metastatic potential, reduced IL-8 production, and reduced growth of CD31-positive blood vessel in the tumor. These data support the role for endogenous activation of NF-κB in association with enhanced metastatic potential for malignant melanoma cells.
The level of IKK activity was assessed in eight melanoma cell lines by immunoprecipitating IKK-α and -β using glutathione-S-transferase-IκBα (aa 1–54) as a substrate. The results showed that, compared with NHEM, the activities of IκB kinases were 3- to 14-fold higher in melanoma cells [88]. Thus, melanoma cells exhibit constitutively high IKK activity, persistent overexpression of nuclear NF-κB p65/p50, and increased basal CXCL1 transcription.
NIK is up-regulated in melanoma cells
NIK was identified by means of its association with TNF receptor-associated factor 2 (TRAF2) and has been shown to potently activate NF-κB when overexpressed [65]. Expression of kinase-defective forms of NIK blocks NF-κB activation in response to most inducers. NIK has been hypothesized to be involved directly in TNF-α-induced activation of NF-κB and has been suggested to be involved in NF-κB activation in response to other stimuli, especially IL-1 [89]. However, NIK also interacts with other TRAF proteins, including TRAF3, which appears not to be involved in NF-κB activation [90]. That NIK interacts strongly and preferentially with IKKα and β and activates their phosphorylation has been confirmed using the yeast two-hybrid system as well as protein interaction studies [91]. However, recent results from IKK and NIK knockout studies demonstrate that IKKα and NIK are not required for IKK activation by TNF-α [92]. The signaling pathways involved in regulation of cell proliferation, survival, and oncogenesis are of prime interest in cancer biology. Since its discovery, Rel/NF-κB has been the focus of intensive research, especially the mechanism(s) that control its activation. More than 60% of the melanoma cells studied to date showed higher expression of CXCL1, CXCL8, IL-1β, IL-6, basic fibroblast growth factor, IL-7, platelet-derived growth factor, IL-10, granulocyte macrophage-colony stimulating factor, insulin-like growth factor-1, nerve growth factor, vascular endothelial growth factor, epidermal growth factor, and transforming growth factor-β at mRNA level [93]. The majority of these genes contain a NF-κB element in their inducible promoter. As mentioned above, our laboratory has previously shown a higher level of CXCL1 expression in malignant melanoma cells as compared with normal melanocytes [88]. This increase in IKK activity is responsible for increased IκB phosphorylation and degradation, thereby increasing NF-κB activation and nuclear localization, which finally leads to increased expression of CXCL1. So far, proinflammatory cytokines have been shown to activate NF-κB through an NIK/MEKK-IKK-IκB signaling pathway in many cell types. However, the proteins responsible for regulating IKK activation in melanoma cells are not known. Preliminary data suggest that NIK kinase is required for the up-regulation of NF-κB activity in melanoma cells.
Tumor expression of oncogenes is associated with the hyperactivation of growth and survival pathways. This causes constitutive activation of these signaling pathways without requirement of exogenously derived signals. In spite of the numerous tropic factors and receptors that govern the survival of specific cells, many of these receptors use common intracellular signaling molecules and pathways to mediate their signals. Of these, the two pathways that have a central role in the survival signaling are the PI3K/Akt, the Ras/MAPK pathways.
Ras activates NF-κB and protects cells from apoptosis
Ras family members play important roles in cell growth, differentiation, transformation, and apoptosis. It has been demonstrated that overexpression of any of three normal Ras genes, N-Ras, H-Ras, or K-Ras, leads to in vitro transformation [94]. In vivo overexpression of normal N-Ras is associated with development of hyperplasia and tumors in transgenic mice [95]. A newly described form of ras, called M-Ras, is closely related to R-Ras, Tc21, H-Ras, K-Ras, and N-Ras [96]. Overexpression of activated Ras in melanocytes null for p16 INK4a/ARF p19 induces overexpression of mutant-activated M-Ras and induces transforming foci in NIH3T3 cells, although the ability of M-Ras to induce transforming foci is weaker than that of Ha-Ras [97]. Activation of the PI3K/Ras/Raf/Soc/MEK/ERK pathway is common for GPCR [98]. Enhanced Ras activity results in increased myc expression, G1/S phase transition, and enhanced NF-κB and AP1 activity. Thus, Ras might be activating NF-κB and hence chemokine expression. The up-regulation of chemokine expression has potential for tumor progression. Activating mutations of Ras are also prevalent in 90% of pancreatic adenocarcinomas and in 50% of colon and thyroid tumors.
Unpublished data from our laboratory has shown that N-Ras is up-regulated in most melanoma cells, and H-, K-, and R-Ras expression is not altered substantially. In an attempt to identify CXCL1/GRO-regulated genes, which may be involved in CXCL1/GRO-induced melanocyte transformation, we found, using differential display, that continuous expression of CXCL1 or CXCL3 up-regulates the expression of M-Ras at the mRNA and protein levels. The ELR motif is required for receptor activation by CXCL1. The melan-a clones expressing the ELR motif mutant forms of CXCL1 failed to exhibit increased Ras protein expression. An in vitro transformation assay demonstrated that M-Ras could induce cellular transformation in a manner similar to CXCL1 in control melan-a cells [99]. Overexpression of dominant-negative M-Ras in CXCL1 expressing melan-a cells blocked transformation. Thus, CXCL1-mediated transformation requires Ras activation in melanocytes. CXCL1 expressing melan-a clones exhibited enhanced NF-κB and AP-1 activity. In vitro transformation assays demonstrated that M-Ras overexpression induced cellular transformation in a manner similar to CXCL1 in control melan-a cells. Conversely, overexpression of dominant-negative M-Ras in CXCL1 expressing melan-a s cells blocked transformation. Thus, CXCL1-mediated transformation requires Ras activation in melanocytes. Previous studies have shown that NF-κB activation suppresses apoptosis [100]. To test whether CXCL1-induced NF-κB was facilitating transformation by allowing melanocytes to escape from apoptosis, the super repressor of NF-κB (IκB-α ΔN) was overexpressed in immortalized murine melanocyte clones. These cells exhibited a fivefold loss in cell viability and a fivefold increase in apoptosis, compared with cells transfected with control vector. Thus overall, the data suggest that NF-κB activation protects against Ras-mediated apoptosis.
Akt is activated constitutively in some melanoma cells leading to activation of NF-κB
As ras-activating factors are secreted by melanoma cells, ras activation might lead to enhanced PI3K activity in melanoma cell lines, which would result in constitutive activation of protein kinase B (PKB) or Akt. PKB/Akt is the cellular homologue of the transforming viral oncogene v-Akt and bears significant homology to PKA and PKC [101]. Akt is a serine/threonine protein kinase involved in regulation of cell survival signals. The three mammalian isoforms all contain an N-terminal PH domain, a central kinase domain with an activation loop, and a C-terminal domain. Akt function is controlled by localization to the membrane, which is dependent on available phosphotidylinositol phosphates (PIPs), and by the level of its phosphorylation. Akt is phosphorylated at two sites, the Thr-308 phosphorylation site in the kinase domain and a conserved, regulatory serine phosphorylation site, Ser-473, near the C terminus [102]. Receptor protein tyrosine kinase activation leads to production of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 at the inner leaflet of the membrane. Subsequently, these membrane lipids recruit Akt to the inner leaflet of plasma membrane, where PDK-1 is located. With lipid/membrane association, there is a conformational change in Akt, exposing Ser-473 and Thr-308. Subsequently, PDK-1 phosphorylates Thr-308 in Akt, stabilizing the activation loop. Phosphorylation of Thr-308 is a prerequisite for kinase activation, but phosphorylation of Ser473 in the C-terminal hydrophobic residue is required for full activation of Akt kinase. The identity of the kinase responsible for phosphorylating the Ser-473 site (putatively termed PDK-2) remains elusive [103]. In a later phase through unknown mechanisms, activated Akt is translocated to the nucleus, where several of its substrates reside [104]. Thus far, at least 13 Akt substrates have been identified in mammalian cells, and they fall into two main classes: regulators of apoptosis and regulators of cell growth, including protein synthesis and glycogen metabolism, and cell-cycle regulation on the other. All identified substrates are phosphorylated within the same basic motif, R-X-R-X-X-S/T. The Akt substrates involved in cell-death regulation include members of the forkhead family of transcription factors, the proapoptotic factor, BAD, the nuclear factor CREB, the pro-apoptotic protease caspase 9, and IKK linking to transcription factor NF-κB [105]. Akt is activated in several different carcinomas such as ovarian, breast, and pancreatic cancers. It has been suggested previously that Akt/PKB might be involved in NF-κB activation by a pathway dependent/independent of IKK activation [106–108].
The tumor suppressor phosphatase and tensin homologue deleted from chromosome 10 (PTEN), also referred to as mutated in multiple advanced cancers, has specificity for 3′-phosphorylated PIPs [109]. PTEN is an important lipid phosphatase that plays a role in deactivation of Akt. This phosphatase regulates the PI3K/Akt signaling pathway, and loss of PTEN in tumor cells correlates with activation and phosphorylation of Akt. Although inactivating mutations of PTEN render cells resistant to apoptosis, overexpression of wild-type PTEN sensitizes cells to death following detachment from its extracellular matrix [110]. This potentially explains the frequency of PTEN mutations in late-stage, invasive tumors. PTEN mutations have been previously described in melanoma [111]. These findings suggest the importance of the PI3K/Akt pathway in tumor progression. Preliminary findings from our work implicate the PI3K/Akt/PTEN pathway in melanoma cells.
Thus, different melanoma cell lines have disturbance in one or more than one upstream signaling pathway, but the common thread on which they all finally converge is NF-κB, which is involved in regulation of chemokines such as CXCL1, as well as escape from apoptosis for the cancerous cells.
CXCL1 overexpression in normal melanocytes is associated with tumor formation in vivo
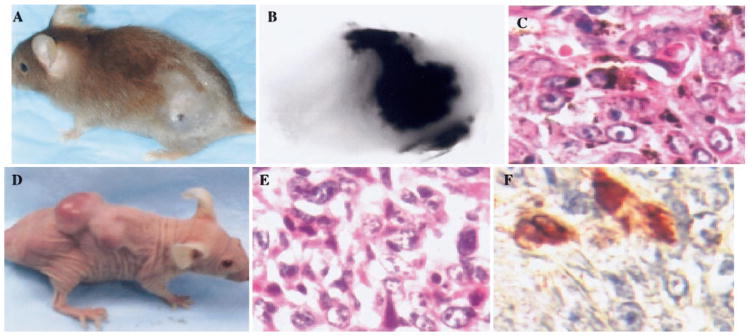
INK4a/p16 is a tumor suppressor gene that is often inactivated in families with hereditary melanoma. P16INK4a associates with cyclin-dependent kinase CDK4 and inhibits the CDK4 and -6 kinases, which are responsible for phosphorylation of the retinoblastoma protein (RB) [112, 113]. Overexpression of p16INK4a inhibits the phosphorylation of RB by CDK4/cyclin D and facilitates cell cycle arrest in G1 [114, 115]. In addition to p16INK4a, this locus encodes a growth inhibitor protein, termed p19ARF, through alternate reading frames of the first exon. p19ARF also functions as a negative regulator of cell cycle progression [116]. Many tumor suppressor genes have been associated with predisposition to develop melanoma, but only INK4a/ARF has been identified as a true melanoma susceptibility gene after almost two decades of effort. Several other oncogenes such as ras, c-Met, SV40, and CXCL1 have been related to genesis and progression of human melanoma. CXCL1 is overexpressed in human malignant melanoma cells and is linked to transformation of immortalized murine melanocytes. To study the direct role of CXCL1 on the genesis of primary melanoma lesions, transgenic mouse lines were established. These cell lines express the murine homologue of CXCL1, MIP-2, under the transcriptional control of tyrosinase promoter/enhancer in mice that were deficient or not deficient for INK4a/ARF (Fig. 4). After treatment with 7,12-dimethylbenz(a) anthracene, cutaneous melanoma formed in 12% of Tyr-MIP-2 transgene-positive mice, while only 2% of the Tyr-MIP-2 transgene-negative mice developed melanoma. In addition, when melanocytes cultured from MIP-2 transgenic mice null for INK-4a/ARF were transplanted to the nude mice, melanoma formation occurred in 83% of the cases, with the latency period of 3 months. However, no melanoma lesions arose in nude mice injected with INK4a/ARF −/− melanocytes not expressing the MIP-2 transgene. Thus, it appears that the loss of INK-4a/ARF coupled with MIP-2 transgene expression in melanocytes results in melanoma tumor formation in the nude mice xenograft model. Based on these observations, we suggest that enhanced MIP-2 expression in cooperation with loss of INK-4a/ARF may play a potent role in induction of melanoma in vivo [117].
Fig. 4.
Histological analysis of melanoma lesions that developed in association with overexpression of MIP-2 and loss of p16. (A) Typical cutaneous-pigmented melanoma lesion arising pigmented melanoma lesion arising in MIP-2-transgenic mice heterozygous for INK4a/ARF. (B) Morphology of the melanoma. (C) H&E staining of tissue section from a typical pigmented melanoma arising in MIP-2 transgenic mice. Melanoma formation in nude mice transplanted with MIP-2-transgenic melanocytes that were null for INK4a/ARF. Two million epidermal melanocytes derived from MIP-2-transgenic, newborn mouse completely deficient for INK4a/ARF were injected in the subscapular region of nude mice. (D) After 101 days of latency, skin melanoma lesions were observed. (E) H&E staining reveals the histological characteristics of a melanocytic tumor lesion. S-100 immunostaining of tumor cells in lung. (Photocopied with permission from ref. [117].)
SUMMARY
Chemokines play an important role in tumor biology. The disregulation of the transcription factor NF-κB leads to constitutive expression of certain chemokines and cytokines. NF-κB is involved in regulation of cell growth, oncogenesis, and escape from apoptosis. Several other coactivators and repressors such as PARP, CDP, and NF-κB are involved in the regulation of CXCL1. In addition, some signaling components such as NIK and Akt might be altered, leading to activation of NF-κB in melanoma cells. Overall, our work has demonstrated the importance of loss of tumor suppressor function, disregulation of NF-κB, and constitutive chemokine/chemokine receptor expression in tumor progression. The next important step would be to test the inhibitors of NF-κB, Akt, and/or chemokine receptors alone or in combination with chematherapeutic agents in order to determine efficiency for treatment of melanoma. We are examining the use of PS-341 [118], a proteasome inhibitor, and NEMO binding peptide [119], an IKK inhibitor, to reduce constitutive NF-κB activity and the growth of melanoma.
Acknowledgments
We express our gratitude to fellows and students in the laboratory who contributed to the studies discussed here: C. S. Nirodi, Jinming Yang, YingChun Yu, Jing Luan, and Dingzhi Wang. We also acknowledge Amar B. Singh for critically reviewing the manuscript and providing helpful suggestions.
References
- 1.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 2.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking and inflammation. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 5.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses Annu. Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 9.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–3853. [PubMed] [Google Scholar]
- 12.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- 14.Wells TN, Proudfoot AE. Chemokine receptors and their antagonists in allergic lung disease. Inflamm Res. 1999;48:353–362. doi: 10.1007/s000110050472. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto J, Adachi Y, Onoue Y, Kanegane H, Miyawaki T, Toyoda M, Seki T, Morohashi M. CD30 expression on circulating memory CD4+ T cells as a Th2-dominated situation in patients with atopic dermatitis. Allergy. 2000;55:1011–1018. doi: 10.1034/j.1398-9995.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 16.Mach F. The role of chemokines in atherosclerosis. Curr Atheroscler Rep. 2001;3:243–251. doi: 10.1007/s11883-001-0067-y. [DOI] [PubMed] [Google Scholar]
- 17.Tkachuk AN, Moormann AM, Poore JA, Rochford RA, Chensue SW, Mwapasa V, Meshnick SR. Malaria enhances expression of CC chemokine receptor 5 on placental macrophages. J Infect Dis. 2001;183:967–972. doi: 10.1086/319248. [DOI] [PubMed] [Google Scholar]
- 18.Loetscher P, Moser B, Baggiolini M. Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv Immunol. 2000;74:127–180. doi: 10.1016/s0065-2776(08)60910-4. [DOI] [PubMed] [Google Scholar]
- 19.International Union of Immunological Societies/World Health Organization Subcommittee on Chemokine Nomenclature. Chemokine/chemokine receptor nomenclature. J Leukoc Biol. 2001;70:465–466. [Google Scholar]
- 20.Tang T, Owen JD, Du J, Walker CL, Richmond A. Molecular cloning and characterization of a mouse gene with homology to the Duffy-antigen receptor for chemokines. DNA Seq. 1998;9:129–143. doi: 10.3109/10425179809072188. [DOI] [PubMed] [Google Scholar]
- 21.Yang YT, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkilde MM, Waldhoer M, Lüttichau HR, Schwartz TW, Rosenkilde MM. Virally encoded 7TM receptors. Oncogene. 2001;20:1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- 23.Strieter RM. Chemokines: not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001;2:285–286. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]
- 24.Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Investig. 1998;102:1469–1472. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 26.Burger M, Burger JA, Hoch RC, Oades Z, Takamori H, Schraufstatter IU. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi’s sarcoma herpesvirus-G protein-coupled receptor. J Immunol. 1999;163:2017–2022. [PubMed] [Google Scholar]
- 27.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 28.Mohle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H, Brugger W, Kanz L. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol. 2000;110:563–572. doi: 10.1046/j.1365-2141.2000.02157.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1) Leukemia. 1999;13:1954–1959. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 30.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64:323–332. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 31.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 32.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 33.Moser B, Schumacher C, von Tscharner V, Clark-Lewis I, Baggiolini M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991;266:10666–10671. [PubMed] [Google Scholar]
- 34.Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci USA. 1992;89:10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 36.Richmond A, Thomas HG. Melanoma growth stimulatory activity: isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988;36:185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- 37.Bordoni R, Fine R, Murray D, Richmond A. Characterization of the role of melanoma growth stimulatory activity (MGSA) in the growth of normal melanocytes, nevocytes, and malignant melanocytes. J Cell Biochem. 1990;44:207–219. doi: 10.1002/jcb.240440403. [DOI] [PubMed] [Google Scholar]
- 38.Chenevix-Trench G, Martin NG, Ellem KA. Gene expression in melanoma cell lines and cultured melanocytes: correlation between levels of c-src-1, c-myc and p53. Oncogene. 1990;5:1187–1193. [PubMed] [Google Scholar]
- 39.Moser B, Barella L, Mattei S, Schumacher C, Boulay F, Colombo MP, Baggiolini M. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J. 1993;294:285–292. doi: 10.1042/bj2940285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodeck U, Melber K, Kath R, Menssen HD, Varello M, Atkinson B, Herlyn M. Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J Investig Dermatol. 1991;97:20–26. doi: 10.1111/1523-1747.ep12477822. [DOI] [PubMed] [Google Scholar]
- 41.Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996;156:1132–1137. [PubMed] [Google Scholar]
- 42.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Investig Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- 46.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 47.Singh RK, Gutman M, Reich R, Bar-Eli M. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin 8. Cancer Res. 1995;55:3669–3674. [PubMed] [Google Scholar]
- 48.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–2675. [PubMed] [Google Scholar]
- 49.Shelton RM. Skin cancer: a review and atlas for the medical provider. Mt Sinai J Med. 2001;68:243–252. [PubMed] [Google Scholar]
- 50.Anisowicz A, Messineo M, Lee SW, Sager R. An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J Immunol. 1991;147:520–527. [PubMed] [Google Scholar]
- 51.Shattuck RL, Wood LD, Jaffe GJ, Richmond A. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993;150:4996–5012. [PubMed] [Google Scholar]
- 53.Ohmori Y, Fukumoto S, Hamilton TA. Two structurally distinct kappa B sequence motifs cooperatively control LPS-induced KC gene transcription in mouse macrophages. J Immunol. 1995;155:3593–3600. [PubMed] [Google Scholar]
- 54.Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GRO alpha gene requires both NF-kappa B and novel constitutive factors. J Biol Chem. 1995;270:30619–30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 56.Wood LD, Farmer AA, Richmond A. HMGI(Y) and Sp1 in addition to NF-kappa B regulate transcription of the MGSA/GRO alpha gene. Nucleic Acids Res. 1995;23:4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 58.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 59.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 60.Regnier CH, Song HY, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 61.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin MA. Cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 62.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 63.Israel A. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 2000;10:129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 64.Li XH, Fang X, Gaynor RB. Role of IKKgamma/nemo in assembly of the Ikappa B kinase complex. J Biol Chem. 2001;276:4494–4500. doi: 10.1074/jbc.M008353200. [DOI] [PubMed] [Google Scholar]
- 65.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 66.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 67.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemoto S, DiDonato JA, Lin A. Coordinate regulation of IkappaB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-kappaB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 72.Nirodi C, NagDas S, Gygi SP, Olson G, Aebersold R, Richmond A. Role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J Biol Chem. 2001;276:9366–9374. doi: 10.1074/jbc.M009897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindahl T. Recognition and processing of damaged DNA. J Cell Sci Suppl. 1995;19:73–77. doi: 10.1242/jcs.1995.supplement_19.10. [DOI] [PubMed] [Google Scholar]
- 74.Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- 75.Lindahl T, Satoh MS, Poirier GG, Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995;20:405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- 76.Nirodi C, Hart J, Dhawan P, Moon NS, Nepveu A, Richmond A. The role of CDP in the negative regulation of CXCL1 gene expression. J Biol Chem. 2001;276:26122–26131. doi: 10.1074/jbc.M102872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blochlinger K, Bodmer R, Jack J, Jan LY, Jan YN. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988;333:629–635. doi: 10.1038/333629a0. [DOI] [PubMed] [Google Scholar]
- 78.Neufeld EJ, Skalnik DG, Lievens PM, Orkin SH. Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut. Nat Genet. 1992;1:50–55. doi: 10.1038/ng0492-50. [DOI] [PubMed] [Google Scholar]
- 79.Luo W, Skalnik DG. Interferon regulatory factor-2 directs transcription from the gp91phox promoter. J Biol Chem. 1996;271:18203–18210. doi: 10.1074/jbc.271.38.23445. [DOI] [PubMed] [Google Scholar]
- 80.Coqueret O, Berube G, Nepveu A. The mammalian cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 1998;17:4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Gurp MF, Pratap J, Luong M, Javed A, Hoffmann H, Giordano A, Stein JL, Neufeld EJ, Lian JB, Stein GS, Van Wijnen AJ. The CCAAT displacement protein/cut homeodomain protein represses osteocalcin gene transcription and forms complexes with the retinoblastoma protein-related protein p107 and cyclin A. Cancer Res. 1999;59:5980–5988. [PubMed] [Google Scholar]
- 82.Kim EC, Lau JS, Rawlings S, Lee AS. Positive and negative regulation of the human thymidine kinase promoter mediated by CCAAT binding transcription factors NF-Y/CBF, dbpA, and CDP/cut. Cell Growth Differ. 1997;8:1329–1338. [PubMed] [Google Scholar]
- 83.Miller H, Asselin C, Dufort D, Yang JQ, Gupta K, Marcu KB, Nepveu A. A cis-acting element in the promoter region of the murine c-myc gene is necessary for transcriptional block. Mol Cell Biol. 1989;9:5340–5349. doi: 10.1128/mcb.9.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 85.Li S, Aufiero B, Schiltz RL, Walsh MJ. Regulation of the homeodomain CCAAT displacement/cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP. Proc Natl Acad Sci USA. 2000;97:7166–7171. doi: 10.1073/pnas.130028697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li S, Moy L, Pittman N, Shue G, Aufiero B, Neufeld EJ, LeLeiko NS, Walsh MJ. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. J Biol Chem. 1999;274:7803–7815. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- 87.Shattuck-Brandt RL, Richmond A. Enhanced degradation of I-kappaB alpha contributes to endogenous activation of NF-kappaB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 88.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 89.Nasuhara Y, Adcock IM, Catley M, Barnes PJ. Differential IkappaB kinase activation and IkappaBalpha degradation by interleukin-1beta and tumor necrosis factor-alpha in human U937 monocytic cells. Evidence for additional regulatory steps in kappaB-dependent transcription. J Biol Chem. 1999;274:19965–19972. doi: 10.1074/jbc.274.28.19965. [DOI] [PubMed] [Google Scholar]
- 90.Takaori-Kondo A, Hori T, Fukunaga K, Morita R, Kawamata S, Uchiyama T. Both amino- and carboxyl-terminal domains of TRAF3 negatively regulate NF-kappaB activation induced by OX40 signaling. Biochem Biophys Res Commun. 2000;272:856–863. doi: 10.1006/bbrc.2000.2860. [DOI] [PubMed] [Google Scholar]
- 91.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 92.Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, Takeda K, Akira S, Matsumoto M. Essential role of nuclear factor (NF)-kappaB-inducing kinase and inhibitor of kappaB (IkappaB) kinase alpha in NF-kappaB activation through lymphotoxin beta receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 94.Crespo P, Leon J. Ras proteins in the control of the cell cycle and cell differentiation. Cell Mol Life Sci. 2000;57:1613–1636. doi: 10.1007/PL00000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mangues R, Seidman I, Gordon JW, Pellicer A. Overexpression of the N-ras proto-oncogene, not somatic mutational activation, associated with malignant tumors in transgenic mice. Oncogene. 1992;7:2073–2076. [PubMed] [Google Scholar]
- 96.Matsumoto K, Asano T, Endo T. Novel small GTPase M-Ras participates in reorganization of actin cytoskeleton. Oncogene. 1997;15:2409–2417. doi: 10.1038/sj.onc.1201416. [DOI] [PubMed] [Google Scholar]
- 97.Quilliam LA, Castro AF, Rogers-Graham KS, Martin CB, Der CJ, Bi C. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J Biol Chem. 1999;274:23850–23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- 98.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 99.Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, Tsubakimoto K, Endo T, Richmond A. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, Benoit V, Merville MP. Nuclear factor-kappa B, cancer, and apoptosis. Biochem Pharmacol. 2000;60:1085–1089. doi: 10.1016/s0006-2952(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 101.Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, Cheng JQ, Testa JR, Tsichlis PN. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. [PubMed] [Google Scholar]
- 102.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toker A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell. 2000;103:185–188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 104.Meier R, Hemmings BA. Regulation of protein kinase. Recept Signal Transduct Res. 1999;19:121–128. doi: 10.3109/10799899909036639. [DOI] [PubMed] [Google Scholar]
- 105.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 106.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 107.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 108.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998;58:5667–5672. [PubMed] [Google Scholar]
- 111.Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- 112.Xiong Y, Zhang H, Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993;7:1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- 113.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 114.Serrano M, Gomez-Lahoz E, DePinho RA, Beach D, Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995;267:249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- 115.Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 116.Sherr CJ. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 117.Yang J, Luan J, Yu Y, Li C, DePinho RA, Chin L, Richmond A. Induction of melanoma in murine macrophage inflammatory protein 2 transgenic mice heterozygous for inhibitor of kinase/alternate reading frame. Cancer Res. 2001;61:8150–8157. [PubMed] [Google Scholar]
- 118.Elliott PJ, Ross JS. The proteasome: a new target for novel drug therapies. Am J Clin Pathol. 2001;116:637–646. doi: 10.1309/44HW-5YCJ-FLLP-3R56. [DOI] [PubMed] [Google Scholar]
- 119.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]