Abstract
Background
Invasive mold infections (IMIs) are common in individuals who have undergone hematopoietic stem cell transplantation (HSCT). We sought to determine clinical and biological risk factors for different IMIs during each period (early and late) after allogeneic HSCT.
Methods
Cases of proven and probable IMI diagnosed in HSCT recipients at the Fred Hutchinson Cancer Research Center (Seattle, WA) from 1 January 1998 through 31 December 2002 were included. Survival was estimated with Kaplan-Meier curves, and Cox regression models were used for multivariable analyses.
Results
During the study period, 1248 patients underwent allogeneic HSCT; 163 (13.1%) received a diagnosis of probable or proven IMI. The majority of cases were caused by Aspergillus species (88%). The incidence of IMI caused by other molds remained low (<2%) over the 4-year study period. Risk factors for IMI early after HSCT and late after HSCT differed, with host variables (age) and transplant variables (human leukocyte antigen match) predominating as early risk factors and other clinical complications (graft-versus-host disease and cytomegalovirus disease) predominating later. Biological risk factors that were important during all periods included multiple cytopenias (neutropenia, lymphopenia, and monocytopenia) and iron overload.
Conclusions
Risk factors for invasive aspergillosis after allogeneic HSCT are multifactorial and differ according to timing after HSCT. Increased attention should be placed on understanding the immunopathogenesis of fungal disease after HSCT.
Invasive fungal infections are now a major cause of morbidity and mortality after hematopoietic stem cell transplantation (HSCT) [1-3]. After the introduction of fluconazole prophylaxis in the 1990s, the incidence of candidemia decreased, coinciding with the emergence of invasive mold infections (IMIs), particularly invasive aspergillosis (IA) [1, 4]. The epidemiology of IMI continues to evolve; recently, the emergence of non-Aspergillus molds, such as Zygomycetes, Fusarium species, and Scedosporium species, has been noted [5-8].
Although the clinical risk factors associated with IA (e.g., underlying disease and graft-versus-host disease [GVHD]) during different periods after HSCT have been described, we have little understanding of the biological basis of clinical associations [1, 6, 9-11]. For instance, results of more-recent studies suggest that at least some of the variables associated with specific underlying diseases and other conditions, such as severe GVHD, surpass pharmacological immune suppression and include quantifiable “biological factors” that might impact immunity, such as iron overload, diabetes, and malnutrition [12-14]. These risk factors are recognized by laboratory tests and, therefore, may enable development of more-tailored preventative strategies. Specific goals of this study were (1) to analyze risk factors associated with the development of IMI after allogeneic HSCT, including risk factors for the development of infection due to non-Aspergillus molds; (2) to differentiate risk factors for early IMI from risk factors for late IMI; and (3) to evaluate the impact of biological risk factors on the development of IMI after HSCT.
PATIENTS AND METHODS
Study patients and definitions
We retrospectively reviewed data from the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) for all patients who underwent allogeneic HSCT during the 5-year period from 1 January 1998 through 31 December 2002; this period predated the performance of a randomized trial to evaluate voriconazole as prophylactic therapy after allogeneic HSCT. All patients were identified by searching a prospectively maintained FHCRC database. Case patients with a diagnosis of proven or probable IMI were compared with patients without proven or probable IMI. This study was approved by the FHCRC institutional review board.
Only patients with IMI documented as proven or probable according to standardized definitions [15] were considered to be case patients. In brief, proven disease required clinical signs and symptoms compatible with IMI and histopathologic or microbiologic documentation of disease from biopsied tissue samples. Infection was considered to be probable if the fungus was identified by culture of bronchoalveolar lavage fluid or sputum samples and if the patient had signs and symptoms consistent with IMI. The day of diagnosis of IMI was the day on which the first diagnostic test was performed. For patients whose diagnosis was obtained from postmortem examination, the day of death was considered to be the day of diagnosis. IMI was considered to be early if diagnosed <40 days after HSCT, late if diagnosed 40−100 days after HSCT, and very late if diagnosed >100 days after HSCT. The 1-year cumulative incidence of IMI was calculated according to year of transplantation. Patients with known IMI diagnosed before HSCT were excluded from the analysis. Patients who underwent >1 transplantation were also excluded from the study, with the exception of planned tandem transplantation (autologous HSCT followed by allogeneic HSCT).
Conditioning regimens, GVHD prophylaxis, and supportive care for allograft recipients at FHCRC have been described elsewhere [16-19]. Standard care for prevention of candidiasis and cytomegalovirus (CMV) disease included fluconazole (400 mg per day) administered until day 75 after HSCT and administration of ganciclovir based on monitoring for CMV pp65 antigenemia. Patients were housed in rooms equipped with high-efficiency particulate air filtration. Patients who developed fever while they had neutropenia routinely received ceftazidime monotherapy, with the addition of an aminoglycoside and vancomycin when clinically indicated. If fever persisted >96 h despite administration of broad-spectrum antibiotics, amphotericin B (1.0 mg/kg or an equivalent dose of a lipid preparation) was added to the patient's regimen until resolution of fever and neutropenia.
Neutropenia was defined as a neutrophil count <500 cells/mL, lymphopenia was defined as a lymphocyte count <300 cell/mL, and monocytopenia was defined as a monocyte count <10 cell/mL. Hyperglycemia was defined as a glucose level >200 g/dL, hypoalbuminemia was defined as a serum albumin level <3 g/dL, and acidosis was defined as a serum pH <7.35. Infection due to respiratory viruses (parainfluenza, respiratory syncytial virus, and influenza viruses) was defined by the presence of signs and symptoms of either upper respiratory tract infection or pneumonia and identification of the virus with direct fluorescent antibody staining, shell viral culture, or conventional culture of respiratory secretions. Only those infections occurring before IMI were included as potential risk factors. To determine iron status, we recorded serum ferritin level, transferrin saturation, and bone marrow iron level. The relationship between serum ferritin and liver iron concentrations has been proved, and serum ferritin is an acceptable, valuable, indirect method for hepatic iron evaluation [20]. Blood transfusion frequency was also included as a marker of iron overload.
Variables and statistical analysis
Candidate host variables included sex, age, underlying disease and remission status, history of diabetes mellitus, laboratory analyses (iron studies and tests for hyperglycemia, acidosis, and malnutrition at time of diagnosis of IMI), and CMV serostatus of the donor and the recipient. Underlying disease was classified as good or poor risk: good-risk disease included acute leukemia in first remission, chronic myeloid leukemia in the chronic phase, myelodyplasia-refractory anemia, aplastic anemia, and nonmalignant hematologic diseases, whereas poor-risk disease included all other diagnoses. To identify risk factors related to HSCT, we evaluated conditioning regimen, stem cell source, myeloablative versus nonmyeloablative conditioning regimen, tandem transplantation, and donor type. Composite variables for stem cell source (bone marrow or peripheral blood stem cells) and donor type (unrelated, HLA-matched related, or HLA-mismatched related) were entered into the model. Cord blood recipients were identified as an independent variable, without accounting for HLA matching.
Quantification of RBC and platelet transfusions and laboratory markers of iron overload, cytopenias, acute GVHD (grade ≥3), chronic GVHD (clinically extensive), CMV disease (CMV detected by direct fluorescent antibody or cultured from bronchoalveolar lavage fluid or tissue samples), hyperglycemia, hypoalbuminemia, acidosis, and infection with respiratory viruses were included as time-dependent covariates. The highest daily dose of corticosteroids in prednisone equivalents (≤1.9 mg/kg per day, 2.0−2.9 mg/kg per day, or ≥3.0 mg/kg per day) was calculated and entered into the risk factor model as a time-dependent variable.
Associations in 2 × 2 tables were analyzed by use of χ2 and Fisher's exact tests, when applicable. To identify risk factors associated with IMI after HSCT, multivariable Cox regression models were performed. Risk factors for early and late diseases were identified with 2 additional multivariable Cox regression models for early infection (diagnosed <40 days after HSCT) and late infection (diagnosed 40−100 days after HSCT). Forty days was chosen as a cutoff point to ensure that the early model reflected the immediate postengraftment period of neutropenia and to be consistent with previous analyses [1].
RESULTS
Over the study period, a total of 1248 patients who underwent allogeneic HSCT were evaluated. One hundred sixty-three cases of IMI were diagnosed. The 1-year cumulative incidence of proven or probable IMI is shown in figure 1; incidence did not significantly vary from year to year (data not shown).
Figure 1.
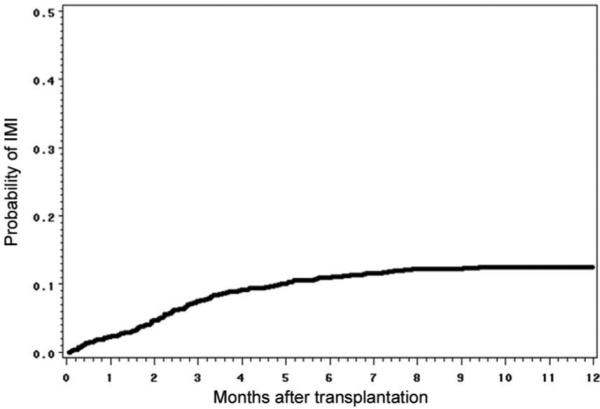
One-year cumulative incidence of invasive mold infections (IMIs) among hematopoietic stem cell transplant recipients at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−2002. Day 0 is the day of receipt of stem cells.
Among patients with IMI, Aspergillus species was the most common pathogen (found in 142 [87%] of the 163 patients), followed by Fusarium species (6; 4%), Zygomycetes (5; 3%), Scedosporium species (1; 1%), and Acremonium species (1; 1%). Six patients (4%) had mixed mold infections (Scedosporium species and Aspergillus species in 1 patient, Acremonium species and Aspergillus species in 1 patient, Zygomycetes and Aspergillus species in 3 patients, and Fusarium species and Aspergillus species in 1 patient). The overall incidence of IMI caused by molds other than Aspergillus species remained low during both the 1990s and the early 2000s (figure 2). Clinical characteristics of patients with and patients without a diagnosis of IMI are shown in table 1.
Figure 2.
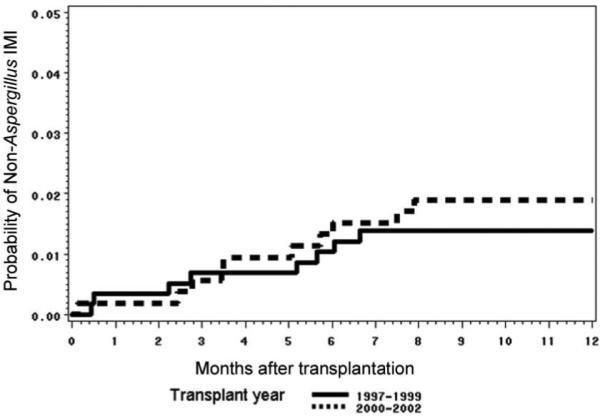
One-year cumulative incidence of invasive mold infections (IMIs) caused by molds other than Aspergillus species, among hematopoietic stem cell transplant recipients at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−1999 and 2000−2002. Day 0 is the day of receipt of stem cells.
Table 1.
Demographic and clinical characteristics of allogeneic hematopoietic stem cell transplant recipients with and without a diagnosis of invasive mold infection at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−2002.
| Variable | Case patients (n = 163) | Control subjects (n = 1085) |
|---|---|---|
| Female sex | 62 (38) | 483 (45) |
| Age, median years (range) | 48 (18−69) | 44 (18−73) |
| Underlying disease | ||
| Aplastic anemia | 3 (1.8) | 18 (1.7) |
| Acute lymphocytic leukemia | 13 (8.0) | 83 (7.7) |
| Acute myeloid leukemia | 36 (22.1) | 256 (23.6) |
| Chronic lymphocytic leukemia | 5 (3.1) | 25 (2.3) |
| Chronic myeloid leukemia | 43 (26.4) | 349 (32.2) |
| Hodgkin diesease | 5 (3.1) | 10 (1.0) |
| Myelodysplastic syndromes | 25 (15.3) | 144 (13.3) |
| Multiple myeloid | 10 (6.1) | 45 (4.2) |
| Myeloproliferative disorder | 5 (3.1) | 40 (3.7) |
| Non-Hodgkin lymphoma | 12 (7.4) | 73 (6.7) |
| Other cancer | 2 (1.2) | 13 (1.2) |
| Other leukemia | 3 (1.8) | 15 (1.4) |
| Othera | 1 (0.6) | 14 (1.3) |
| Myeloablative conditioning | 127 (77.9) | 910 (83.9) |
| ATG in conditioning | 5 (3.1) | 33 (3.0) |
| Peripheral blood as stem cell source | 75 (46.5) | 513 (47.3) |
| HLA-matched graft | 131 (80.4) | 917 (84.5) |
| CMV serostatus | ||
| R+D+ | 50 (30.7) | 297 (27.4) |
| R+D- | 43 (26.4) | 259 (23.9) |
| R-D+ | 17 (10.4) | 154 (14.2) |
| R-D- | 53 (32.5) | 367 (33.8) |
| TBI | ||
| None | 46 (28.2) | 407 (37.5) |
| Low doseb | 37 (22.7) | 184 (17.0) |
| High dosec | 80 (49.1) | 494 (45.5) |
| Hyperglycemia | 107 (65.6) | 771 (71.1) |
| Hypoalbuminemia | 103 (63.2) | 896 (82.6) |
| Neutropenia | 41 (25.2) | 132 (12.2) |
| Lymphopenia | 107 (65.6) | 565 (52.1) |
| Monocytopenia | 63 (38.7) | 235 (21.7) |
| Acute GVHD, grade 3 | 69 (42.3) | 221 (20.4) |
| Chronic GVHD, clinically extensive | 39 (23.9) | 594 (54.7) |
| Acidosis | 6 (3.7) | 54 (5.0) |
| Received transfusion | 158 (96.9) | 1019 (93.9) |
| Influenza infection | 6 (3.7) | 40 (3.7) |
| Parainfluenza infection | 19 (11.7) | 68 (6.3) |
| RSV infection | 8 (4.9) | 34 (3.1) |
| CMV disease | 31 (19.0) | 72 (6.6) |
| High ferritin level | 13 (8.0) | 91 (8.4) |
| Steroids, maximum dose | ||
| 0.1−1.9 mg/kg/day | 44 (27.0) | 448 (41.3) |
| 2.0−2.9 mg/kg/day | 81 (49.7) | 334 (30.8) |
| ≥3.0 mg/kg/day | 10 (6.1) | 76 (7.0) |
NOTE. ATG, antithymocyte globulin; CMV, cytomegalovirus; D-, donor negative; D+, donor positive; GVHD, graft-versus-host disease; R-, recipient negative; R+, recipient positive; RSV, respiratory syncytial virus; TBI, total body irradiation.
Includes renal cell carcinoma (n = 10), biphenotypic leukemia (n = 10), paroxysmal noctural hematuria (n = 6), Waldenstrom macroglobulinemia (n = 3), acute megaloblastic leukemia (n = 3), malignancy not further differentiated (n = 2), systemic sclerosis (n = 2), plasma cell leukemia (n = 2), eosinophilic syndrome (n = 1), immunodeficiency not otherwise identified (n = 1), amyloidosis (n = 1), lymphoproliferative disorder (n = 1), medulloblastoma (n = 1), cervical carcinoma (n = 1), melanoma (n = 1), Ewing sarcoma (n = 1), acute leukemia not differentiated (n = 1), hairy cell leukemia (n = 1), and erythroblastic leukemia (n = 1).
≤200 cGy.
>200 cGy.
Risk factors for any IMI (during any period), identified by univariate and multivariable analyses, are shown in table 2. Multiple host variables, transplant variables, and transplant complications were identified as potentially important risk factors in univariate analysis (table 2). In multivariable analysis, the only host variable independently associated with IMI was older age. After controlling for other variables, there was a trend toward higher risk observed with receipt of an HLA-mismatched graft. Multiple clinical transplantation complications independently increased the risk of IMI, including CMV disease, influenza and parainfluenza infection, and severe acute GVHD (grade ≥3). All cell-line cytopenias (neutropenia, lymphopenia,and monocytopenia) independently increased the risk of IMI. Other biological risk factors (hyperglycemia, high ferritin levels, and acidosis) were not statistically significant in the model that included other clinical complications. However, a high frequency of blood transfusions remained statistically significant in the overall multivariable model.
Table 2.
Risk factors for invasive mold infection among 1248 patients who received allogeneic hematopoietic stem cell transplants at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−2002.
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
| Recipient-related factors | ||||
| Age, by 10-year increase | 1.2 (1.0−1.4) | .01 | 1.3 (1.1−1.5) | <.001 |
| Poor-risk diseasea | 1.5 (1.1−2.0) | <.01 | NS | NS |
| Transplantation-related factors | ||||
| High-dose TBI | 1.7 (1.1−2.7) | .01 | NS | NS |
| Related HLA mismatched vs. related HLA matched | 1.9 (1.0−3.7) | .04 | 1.8 (0.9−3.4) | .09 |
| Transplantation complications | ||||
| Hyperglycemia | 2.0 (1.4−2.8) | <.001 | NS | NS |
| Cytopenias | ||||
| Neutropenia | 2.1 (1.3−3.3) | <.01 | 2.2 (1.3−3.6) | <.01 |
| Lymphopenia | 1.9 (1.3−2.6) | <.01 | 1.4 (1.0−2.0) | .05 |
| Monocytopenia | 2.3 (1.6−3.3) | <.001 | 1.8 (1.3−2.6) | <.01 |
| Acute GVHD, grade ≥3 | 3.8 (2.8−5.3) | <.001 | 2.4 (1.7−3.4) | <.001 |
| Chronic GVHD | 1.8 (1.1−2.9) | .02 | NS | NS |
| Corticosteroid dosage | ||||
| 0.1−1.9 mg/kg/day | 2.2 (1.3−3.8) | <.01 | 1.7 (1.0−3.1) | .07 |
| 2.0−2.9 mg/kg/day | 5.2 (3.1−8.7) | <.001 | 3.1 (1.7−5.4) | <.001 |
| ≥3.0 mg/kg/day | 4.9 (2.3−10.6) | <.001 | 2.3 (1.0−5.2) | .04 |
| Acidosisb | 4.0 (1.8−9.1) | <.001 | NS | NS |
| Transfusions, by 1 U increase | 1.8 (1.5−2.3) | <.001 | 1.4 (1.1−1.8) | <.01 |
| Ferritin level >2000 ng/mL | 1.8 (0.9−3.4) | .09 | NS | NS |
| CMV disease | 9.5 (6.4−14.1) | <.001 | 6.9 (4.6− 10.4) | <.001 |
| Influenza infection | 2.7 (1.2−6.2) | .02 | 2.5 (1.1−5.8) | .03 |
| Parainfluenza infection | 3.2 (2.0−5.2) | <.001 | 2.6 (1.6−4.3) | <.001 |
| RSV infection | 2.4 (1.2−4.9) | .02 | NS | NS |
NOTE. CMV, cytomegalovirus; GVHD, graft-versus-host disease; NS, not significant; RSV, respiratory syncytial virus; TBI, total body irradiation.
Good-risk disease included acute leukemia in first remission, chronic myeloid leukemia in the chronic phase, myelodysplasia-refractory anemia, aplastic anemia, and nonmalignant hematologic diseases. Poor-risk disease included all other diagnoses.
pH < 7.35.
Infections were classified according to timing after HSCT, and risk factors were analyzed among the different periods. Of 163 cases of IMI, 36 (22%) were diagnosed early (<40 days after HSCT), 65 (40%) were diagnosed late (40−100 days after HSCT), and 62 (38%) were diagnosed very late (>100 days after HSCT). Among the subset of patients with non-Aspergillus infection, no cases were diagnosed early. The majority of patients with invasive zygomycosis (88%) received a diagnosis very late, whereas fusariosis was diagnosed late in 43% of cases and very late in 57% (figure 3).
Figure 3.
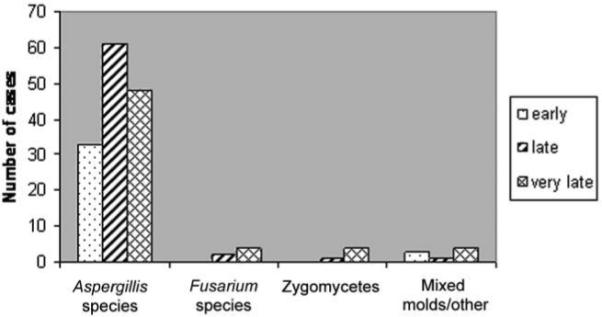
Timing of invasive mold infections (IMIs). Early IMI refers to infection diagnosed from day 0 through day 40; late IMI refers to infection diagnosed from day 41 through day 100; very late IMI refers to infection diagnosed after day 100.
Subanalyses were performed to determine risk factors associated with the development of early versus late IMI (table 3). Because there were a limited number of events for multivariable analysis, time-dependent covariates were added singly to a baseline model that included receipt of antithymocyte globulin in conditioning, poor-risk underlying disease, and receipt of HLA-mismatched graft. In this model to predict risk factors for early IMI, poor-risk underlying disease, receipt of an unrelated-donor or HLA-mismatched HSCT, and receipt of antithymocyte globulin in conditioning were independently associated with IMI. When time-dependent covariates were added singly to the model, hyperglycemia, lymphopenia, elevated ferritin level, receipt of transfusions, CMV disease, and receipt of corticosteroids were all associated with increased risk of early IMI.
Table 3.
Multivariate analysis of risk factors for early (days 1−39 after transplantation) and late (days 40−100 after transplantation) invasive mold infection (IMI) among recipients of allogeneic hematopoietic stem cell transplants at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−2002.
| Early IMI (n = 36) |
Late IMI (n = 65) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||
| Variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Female sex | NS | NS | NS | NS | 0.6 (0.4−1.0) | .07 | 0.6 (0.3−1.0) | .04 |
| Age, by 10-year increase | NS | NS | NS | NS | 1.4 (1.2−1.8) | <.01 | 1.6 (1.2−1.9) | <.001 |
| CMV status, R+D- vs. R-D- | NS | NS | NS | NS | 1.8 (1.0−3.4) | .06 | NS | NS |
| Poor-risk diseasea | NS | NS | NS | NS | NS | NS | NS | NS |
| Unrelated vs. related donor | 2.4 (1.1−5.3) | .03 | 2.6 (1.2−5.6) | .02 | NS | NS | NS | NS |
| HLA matched vs. HLA mismatched | 6.7 (2.4−18.3) | <.01 | 6.7 (2.4−18.3) | <.01 | NS | NS | NS | NS |
| ATG in conditioning | 3.2 (1.0−10.3) | .06 | 4.9 (1.2−19.5) | .02 | NS | NS | NS | NS |
| TBI in conditioning | 2.3 (1.1−5.3) | .04 | NS | NS | NS | NS | NS | NS |
| Hyperglycemiab | 4.8 (2.3−9.9) | <.001 | 4.2 (2.0−8.7) | <.001 | 1.6 (1.0−2.7) | .07 | NS | NS |
| Lymphopeniab | 6.7 (2.2−20.2) | <.01 | 6.3 (2.1−19.2) | .001 | 2.5 (1.5−4.3) | <.001 | NS | NS |
| Monocytopeniab | NS | NS | NS | NS | 2.4 (1.4−4.0) | .001 | NS | NS |
| Hypoalbuminemiab | NS | NS | NS | NS | 0.6 (0.4−1.0) | .04 | NS | NS |
| Ferritin level >2000 ng/mL | 5.8 (1.6−20.6) | <.01 | 4.7 (1.3−17.1) | .02 | NS | NS | NS | NS |
| CMV disease | 19.7 (1.3−2.9) | <.001 | 27.5 (9.1−83.3) | <.001 | 9.7 (5.3−17.8) | <.001 | 7.1 (3.8−13.1) | <.001 |
| Transfusionb | 1.9 (1.3−2.9) | .001 | 1.8 (1.2−2.7) | .003 | 1.8 (1.3−2.4) | <.001 | 1.4 (1.1−1.9) | .02 |
| Acute GVHD b | NS | NS | NS | NS | 5.0 (3.1−8.2) | <.001 | 2.9 (1.7−4.7) | <.001 |
| Corticosteroid dosage | ||||||||
| 0.1−1.9 mg/kg/day | 4.8 (2.0−11.2) | <.01 | 4.6 (1.9−10.8) | <.001 | NS | NS | NS | NS |
| 2.0−2.9 mg/kg/day | 2.9 (1.1−8.2) | .04 | NS | NS | 18.0 (4.4−74.3) | <.001 | 9.8 (2.3−41.1) | .001 |
| >3.0 mg/kg/day | 7.5 (2.1−26.9) | <.01 | 6.1 (1.7−22.3) | <.01 | 9.2 (1.5−54.8) | .02 | NS | NS |
NOTE. ATG, antithymocyte globulin; CMV, cytomegalovirus; D-, donor negative; GVHD, graft-versus-host disease; HR, hazard ratio; NS, not significant; R-, recipient negative; R+, recipient positive; TBI, total body irradiation.
Good-risk disease included acute leukemia in first remission, chronic myeloid leukemia in the chronic phase, myelodysplasia-refractory anemia, aplastic anemia, and nonmalignant hematologic diseases. Poor-risk disease included all other diagnoses.
Covariates were added singly to the base model containing poor-risk disease, ATG in conditioning, and HLA matching.
Risk factors associated with late IMI were also analyzed (table 3). In this model, advanced age and female sex were host-related risk factors for late infection. No transplant-related factors were independently associated with IMI, whereas a number of transplant complications posed significant risks, including acute GVHD (grade ≥3), CMV disease, corticosteroid dose, and high frequency of blood transfusions. Notably, other markers of iron overload did not appear to be significant independent risk factors for late IMI.
There were too few IMIs caused by molds other than Aspergillus species to allow for multivariable models. To evaluate risk factors, we compared the 20 patients who had non-Aspergillus mold infections with patients without IMI and calculated risk in univariate analysis (table 4). Multiple transplant variables and complications were associated with high risk of IMI. Although receipt of blood transfusions was associated with a high risk of IMI, other biological indicators of iron overload, such as high bone marrow iron and ferritin levels, were marginally significant.
Table 4.
Risk factors associated with non-Aspergillus invasive mold infection in 20 patients who received allogeneic hematopoietic stem cell transplants at Fred Hutchinson Cancer Research Center (Seattle, WA), 1998−2002.
| Variable | Unadjusted HR (95% CI) | P |
|---|---|---|
| Nonmyeloablative conditioning | 2.7 (1.1−6.8) | .03 |
| High dose TBI in conditioning | 3.1 (1.1−8.9) | .04 |
| Neutropenia | 4.1 (1.4−12.3) | .01 |
| Ferritin level >2000 ng/mL | 5.8 (1.0−34.7) | .05 |
| Bone marrow iron level increased | 3.5 (0.9−13.6) | .06 |
| Transfusions, per 1 U increase | 3.2 (1.2−8.9) | .02 |
| Acute GVHD, grade ≥3 | 5.1 (2.1−12.5) | <.01 |
| Chronic GVHD, clinically extensive | 6.2 (1.5−25.3) | .01 |
| Corticosteroid dosage 2.0−2.9 mg/kg/day | 5.5 (1.1−28.5) | .04 |
NOTE. GVHD, graft-versus-host disease; HR, hazard ratio; TBI, total body irradiation.
DISCUSSION
From 1998 through 2002, 163 (13.1%) the of allogeneic HSCT recipients at our center received a diagnosis of IMI, with the majority of IMIs being caused by Aspergillus species. However, because of the strict definition of invasive disease used in our study and variable diagnostic aggressiveness, these numbers may represent an underestimate. We did not witness an overt increase in the incidence of non-IA IMIs over the study period. The vulnerability of HSCT recipients to IMI appears to be multifactorial, encompassing both classically recognized clinical risk factors (e.g., advanced age, receipt of HLA-mismatched cells, and late complications, such as CMV disease and GVHD) and other biological risk factors that can impact antifungal defenses (most notably, deficiency in multiple cell lines and iron overload). The risk of infection during the early and late periods after HSCT differs.
Consistent with other studies, our data show that a high proportion of IMIs develop late after HSCT. The shift from early to late IA after HSCT was noted in the 1990s [6, 10] and is probably attributable to changing transplantation practices, such as the use of peripheral blood as a stem cell source and nonmyeloablative conditioning, which result in attenuated cytopenias. We examined risk factors for the development of early (<40 days after HSCT) and late (40−100 days after HSCT) IMI. Early IMI is impacted by underlying disease and transplant-related factors, such as unrelated and mismatched HSCT and receipt of antithymocyte globulin with conditioning. Biological risk factors associated with early IMI include hyperglycemia, parameters of iron overload, and lymphopenia. It follows that complications of HSCT should impact late IMI to a greater degree; these include CMV disease, a high frequency of transfusions, severe acute GVHD, and receipt of high doses of corticosteroids.
Previous studies have noted lymphopenia, neutropenia, and/or monocytopenia as risk factors for IA and/or IMI; although these cytopenias have not been consistently identified in multivariable models [1, 9, 11, 21], animal models have demonstrated that antifungal defenses involve more than neutrophil killing. For instance, Aspergillus-specific CD4+ T cell responses have long been recognized as important in regulating effective pulmonary inflammation and potentially adding antifungal effector activity [22-24].
CMV disease was associated with the development of early and late IMI (a finding that has been noted in previous studies) [1, 9, 10]. The exact mechanism is not known; immune modulation of the virus [25] and/or ganciclovir-induced neutropenia probably contribute. We found a relationship between influenza and parainfluenza virus infection after HSCT and the development of IMI, although these covariates were no longer statistically significant in the subanalyses of early and late infection. Infection due to these viruses may function to affect local defenses against invasive molds [26, 27]. Alternatively, infection with these viruses could act as an indicator for other host risk factors that are as yet undefined and not controlled for in the analyses.
Acute GVHD, which typically presents in the first 30−60 days after HSCT, was independently associated with late IMI. Both acute and chronic GVHD were associated with non-Aspergillus IMI in univariate analysis. There are a number of reasons why we cannot directly compare these risk factor analyses. First, the numbers of cases of non-Aspergillus IMI did not support multivariable modeling; in IA models, different effects of GVHD are seen in adjusted models that contain treatment variables (e.g., corticosteroid use). Also, the non-Aspergillus IMI analysis was not restricted to day 100, because these infections predominately occurred late after HSCT. A related observation is the association between non-Aspergillus IMI and nonmyeloablative conditioning, which likely represents risk factors associated with severe GVHD late after HSCT.
Receipt of corticosteroids also increased the risk of IMI; this has been well established in earlier studies [1, 6, 9-11]. Corticosteroids affect the host immune response to Aspergillus species by preventing alveolar macrophage killing of phagocytosed Aspergillus fumigatus conidia [28] and by blunting alveolar macrophage production of proinflammatory cytokines (e.g., IL-1α and TNF-α) and chemokines (MIP-1α) that are important for recruiting neutrophils and monocytes [29]. Results of this study show alternative associations between steroids administered at different doses during the periods of risk (early vs. late IA). This may reflect the number of patients evaluated in each group or potentially reflect the intent of steroid administration. For instance, steroids may be administered at different doses depending on the underlying condition (e.g., GVHD vs. bronchiolitis obliterans organizing pneumonia), and the association could reflect the biologic impact of the underlying disease itself.
One of the aims of this study was to evaluate the impact of iron overload on the development of IMI after HSCT. Unfortunately, our analysis was limited by incomplete data for bone marrow iron and transferrin levels. Thus, the most valid predictors of iron overload included the number of blood and/or platelet transfusions, which is a relatively crude surrogate for potential iron overload, and serum ferritin levels. In all analyses, a high number of blood and/or platelet transfusions was independently associated with an increased risk of IMI. However, elevated ferritin level, which is a potentially better measure of iron overload, was identified as a risk factor only in the univariate and limited multivariable models, specifically for early IMI. It is important to note that ferritin levels can also be elevated during acute inflammatory conditions. Also, blood transfusions are associated with a down-regulation of the immune response and have been associated with increased risk of infection after liver transplantation; this finding is thought to be attributable to an immune modulating effect of the trans-fusion itself [30], rather than to a long-term impact on iron levels. Most of the evidence for iron overload impacting on the risk of IMI comes from studies of zygomycosis. Maertens et al. [12] reported 5 cases of zygomycosis in allogeneic HSCT recipients, and iron overload was present in all 5 cases. In a study involving 365 patients with myeloma who underwent autologous HSCT, bone marrow iron level was an independent risk factor for the development of severe infection [13]. It may be that, in our cohort, frequency of transfusions and iron overload is a surrogate for underlying disease; for example, patients with myelodysplastic syndromes are likely to have a greater frequency of pre-HSCT transfusions. More studies should be performed to define the biologic explanation(s) underlying these associations.
In our study, we describe the impact of several laboratory-defined risk factors (“biological risk factors”), because these function to increase our understanding of the pathobiology of IA and may enable development of more-specific preventative strategies that target mold infections. Results of other studies are uncovering associations between IA in HSCT recipients and polymorphisms in genes encoding proteins involved in immune responses to Aspergillus species. For instance, a polymorphism in donor Toll-like receptor 4 gene appears to increase the risk of IA after HSCT, as does a polymorphism in the recipient gene for plasminogen [31, 32]. How these polymorphisms impact pathogen-specific immunity and/or the development of underlying risk factors (e.g., GVHD) remains unanswered. It may be possible to use genetic information to develop more-targeted strategies to prevent mold infections in a more efficient manner than waiting for the development of disease or other (later) risk factors, such as GVHD.
The cohort of patients with data analyzed here underwent HSCT from 1998 through 2002; this period was chosen to predate the institutional use of voriconazole prophylaxis as part of a large, randomized trial. As an effect, contemporary risk factors for IMI, which depend on evolving transplantation and supportive-care strategies, may be different than those outlined. It would be of interest to evaluate risk factors in more-recent years, subsequent to changes in transplantation practices (e.g., increased use of nonmyeloablative conditioning) and adoption of different preventative strategies.
There are limitations to this study. Patients who received a diagnosis of possible IMI were included in the control group; however, because of the difficulty in establishing a proven or probable diagnosis, some of these patients may have had IMI. Thus, significant differences between the 2 groups may have been diluted. Also, the design of the study did not enable analysis of environmental exposure to the organisms, which may have had a role in the risk of IA.
In conclusion, our single-center experience during the period 1998−2002 demonstrates a high incidence of IA and a stable, low incidence of other mold infections, including zygomycosis. Risk factors for early and late infection include host variables and complications of HSCT. Several biologic risk factors are identified; these include cytopenias, as expected, and iron overload. Recognition of these risk factors for IMI may enable the development of more-tailored strategies to prevent mold infections after allogeneic HSCT.
Acknowledgments
Financial support. National Institutes of Health (AI51468, AI54736, CA 18029, and CA15704) and a Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) grant.
Footnotes
Potential conflict of interest. K.A.M. has served as a consultant and/or participated in advisory boards for Pfizer, Astellas, Basilea, Merck, and Schering-Plough and has received research funding from Merck and Enzon. All other authors: no conflicts.
References
- 1.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 2.Singh N. Impact of current transplantation practices on the changing epidemiology of infections in transplant recipients. Lancet Infect Dis. 2003;3:156–61. doi: 10.1016/s1473-3099(03)00546-2. [DOI] [PubMed] [Google Scholar]
- 3.Wingard JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant. 1999;5:55–68. doi: 10.1053/bbmt.1999.v5.pm10371357. [DOI] [PubMed] [Google Scholar]
- 4.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation: a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–52. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 6.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 7.Nucci M. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr Opin Infect Dis. 2003;16:607–12. doi: 10.1097/00001432-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Nucci M, Marr KA, Queiroz-Telles F, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38:1237–42. doi: 10.1086/383319. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 10.Grow WB, Moreb JS, Roque D, et al. Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29:15–9. doi: 10.1038/sj.bmt.1703332. [DOI] [PubMed] [Google Scholar]
- 11.Thursky K, Byrnes G, Grigg A, Szer J, Slavin M. Risk factors for post-engraftment invasive aspergillosis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:115–21. doi: 10.1038/sj.bmt.1704543. [DOI] [PubMed] [Google Scholar]
- 12.Maertens J, Demuynck H, Verbeken EK, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24:307–12. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 13.Miceli MH, Dong L, Grazziutti ML, et al. Iron overload is a major risk factor for severe infection after autologous stem cell transplantation: a study of 367 myeloma patients. Bone Marrow Transplant. 2006;37:857–64. doi: 10.1038/sj.bmt.1705340. [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis D, Wessel V, Bodey G, Rolston K. Zygomycosis in the 1990s in a tertiary care center. Clin Infect Dis. 2000;30:851–6. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 16.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 17.Buckner CD, Clift RA, Appelbaum FR, et al. Effects of treatment regimens in patients allografted for acute and chronic myelogenous leukemia. Bone Marrow Transplant. 1991;7(Suppl 2):6–8. [PubMed] [Google Scholar]
- 18.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–43. [PubMed] [Google Scholar]
- 19.Nash RA, Pineiro LA, Storb R, et al. FK506 in combination with methotrexate for the prevention of graft-versus-host disease after marrow transplantation from matched unrelated donors. Blood. 1996;88:3634–41. [PubMed] [Google Scholar]
- 20.Brissot P, Bourel M, Herry D, et al. Assessment of liver iron content in 271 patients: a reevaluation of direct and indirect methods. Gastroenterology. 1981;80:557–65. [PubMed] [Google Scholar]
- 21.Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;36:621–9. doi: 10.1038/sj.bmt.1705113. [DOI] [PubMed] [Google Scholar]
- 22.Rivera A, Epps HV, Hohl T, Rizzuto G, Pamer E. Distinct CD4+ T cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect Immun. 2005;73:7170–9. doi: 10.1128/IAI.73.11.7170-7179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera A, Hohl T, Pamer EG. Immune responses to Aspergillus fumigatus infections. Biol Blood Marrow Transplant. 2006;12(Suppl 1):47–9. doi: 10.1016/j.bbmt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–9. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 25.Boeckh M, Nichols WG. Immunosuppressive effects of β-herpesviruses. Herpes. 2003;10:12–6. [PubMed] [Google Scholar]
- 26.Franke-Ullmann G, Pfortner C, Walter P, et al. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–80. [PubMed] [Google Scholar]
- 27.Kauth M, Grage-Griebenow E, Rohde G, et al. Synergistically upregulated interleukin-10 production in cocultures of monocytes and T cells after stimulation with respiratory syncytial virus. Int Arch Allergy Immunol. 2007;142:116–26. doi: 10.1159/000096381. [DOI] [PubMed] [Google Scholar]
- 28.Philippe B, Ibrahim-Granet O, Prevost MC, et al. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–42. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brummer E, Kamberi M, Stevens DA. Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J Infect Dis. 2003;187:705–9. doi: 10.1086/368383. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295–307. doi: 10.1378/chest.127.1.295. [DOI] [PubMed] [Google Scholar]
- 31.Zaas A, Liao G, Chien J, et al. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genetics. 2008;4:e1000101. doi: 10.1371/journal.pgen.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochud P, Chien J, Janer M, Marr K, Aderem A, Boeckh M. Donor's Toll-like receptor 4 haplotypes increase the incidence of invasive mould infections in hematopoietic stem cell transplant recipients.. 46th International Conference for Antimicrobial Agents and Chemotherapy (San Francisco, CA).; Washington, DC. 2006. American Society for Micro-biology. [Google Scholar]


