Abstract
Varicella is a widespread disease of childhood resulting from primary infection with varicella-zoster virus (VZV). The objective of this study was to determine the kinetics of the decline of maternal anti-VZV antibodies in French infants between birth and the age of 15 months in order to estimate the duration of passively acquired maternal anti-VZV immunoglobulin G (IgG). This prospective multicenter study was conducted between October 2005 and January 2007 in the pediatric wards and/or pediatric emergency units of seven French hospitals scattered throughout the country. The level of anti-VZV IgG antibodies in serum was measured by a time-resolved fluorescence immunoassay (TRFIA) (the threshold considered positive is 150 mIU/ml). A total of 345 infants were included. Seventy-seven percent of mothers reported a history of varicella. A rapid decline in the prevalence of anti-VZV antibodies was observed during the first few months of life, with the mean antibody titer decreasing from 536 mIU/ml at birth and through 1 month to below the 150-mIU/ml threshold at 3 to 4 months. The half-life of passively acquired maternal immunoglobulins was around 6 weeks. Based on a large number of subjects, this study clearly demonstrated, for the first time in France, high levels of passively acquired maternal antibodies during the neonatal period, and it allowed us to estimate the duration of passively acquired maternal anti-VZV IgG in French infants. After 4 to 5 months, infants had very low levels of maternal anti-VZV IgG, below the 150-mIU/ml cutoff of the VZV IgG TRFIA.
Varicella (chickenpox) is a widespread disease of childhood resulting from a primary infection with varicella-zoster virus (VZV). In France, an age-specific prevalence study reported seroprevalence rates of about 50% by the age of 4 years and 90% by 8 years (11). The disease is usually benign but may lead to severe complications and occasionally death (6, 17).
In France, varicella vaccines have been available since 2004 but are not yet recommended on a routine basis (9).
When mothers have experienced varicella or received VZV vaccination, infants are considered protected during the first months of life by passive transfer of maternal anti-VZV antibodies (1). The antibody titer in the newborn has been shown to be proportional to the level in the mother (22). However, passive immunity declines rapidly, and the exact duration and extent of protection remain uncertain. In other countries, some studies have shown that maternal antibodies were no longer detectable at 6 months (7) or even as early as 4 months (19).
The objective of this study was to determine the kinetics of the decline of maternal anti-VZV antibodies in French infants between birth and the age of 15 months in order to estimate the duration of passively acquired maternal anti-VZV immunoglobulin G (IgG) in French infants. This could be useful, in countries in which routine vaccination is recommended, for assessing the optimal age for varicella vaccination in infants.
MATERIALS AND METHODS
This prospective multicenter study was conducted between October 2005 and January 2007 in the pediatric wards and/or pediatric emergency units of seven French hospitals scattered throughout the country. Each center had to consecutively include 6 infants in each of the following 9 age categories: newborn to 3 months; 4 to 6 months; 7, 8, 9, 10, 11, and 12 months; and 13 to 15 months.
Inclusion criteria.
To be included, infants had to be 15 months old or younger. They had to be hospitalized or seen in an outpatient department with a scheduled blood sampling. Furthermore, they had to be born after at least 37 gestational weeks with a birth weight of at least 2,800 g. One or both parents had to sign the written informed-consent form.
Exclusion criteria.
Infants with a history of varicella or anti-VZV immunization were excluded, as were those for whom contact with a VZV-infected individual within 3 weeks before inclusion was reported. Infants with known or suspected immunodeficiencies or histories of immune globulin or blood transfusion and those whose mothers were transfused during pregnancy were also excluded.
Data collection.
For each infant, the following information was collected: recruitment site (pediatric emergency unit, pediatric inpatient or outpatient department), date of birth, gender, birth weight, gestational age, maternal age, and maternal history of varicella or anti-varicella vaccination.
Antibody level measurement.
Blood samples were collected during the infants' clinical visit or hospitalization. An additional 0.5 ml of blood was collected in a dry tube and centrifuged for 10 to 15 min at 3,000 rpm. After centrifugation, serum was extracted and stored at −20°C. At the end of the inclusion period, all serum samples were centralized at the Virus Reference Department at the Health Protection Agency, London, United Kingdom, for assessment of anti-VZV antibody titers. The level of anti-VZV IgG antibodies in serum was measured by the time-resolved fluorescence immunoassay (TRFIA) technique. The VZV TRFIA is a quantitative VZV IgG assay calibrated against the British standard VZV antibody (15, 16), and VZV IgG levels were interpreted as VZV IgG negative (VZV IgG level, <100 mIU/ml), VZV IgG equivocal (100 mIU/ml to <150 mIU/ml), and VZV IgG positive (150 mIU/ml or greater). For the calculation of the mean anti-VZV antibody titers (geometric mean titers [GMT]), all antibody titer results were taken into account.
Statistical analysis.
GMT were given for each 1-month interval from birth to the age of 15 months. The antibody titer was compared according to infant gender, birth weight, gestational age, and maternal history of varicella. Qualitative variables were compared using the chi-square test, and quantitative variables were compared using the Student t test or the nonparametric Cochran-Mantel-Haenszel test. A P value below 0.05 was considered statistically significant.
Sample size estimation was based on the results of Linder et al. (14), who observed that 24% of 2-month-old babies carried maternal anti-VZV antibodies. The minimum sample size required for detection of a prevalence of 24% with a precision of 5% (alpha = 0.05) was 281. This number was increased to 378, representing 54 infants per center and 42 for each age category, to compensate for possible recruitment anomalies. Statistical analysis was performed by Mapi-Naxis, Lyon, France, using SAS software, version 8.
A birth weight between 2,600 g and 2,800 g was considered a minor protocol deviation, and such infants were thus included in the analysis.
Ethical considerations.
This study complies with the Declaration of Helsinki. Written informed consent was obtained from at least one of the parents (or from the infant's legal representative), and the study protocol was approved by the local ethics committee. According to French legislation, informed consent from one of the parents is sufficient if the study protocol presents no risk to the child.
RESULTS
Population characteristics.
A total of 353 infants were initially included. Eight were excluded because of missing information (five had no information on antibody titers, two had received anti-VZV vaccination, and one had no case report form). Thus, 345 infants enrolled from pediatric inpatient departments (48%), pediatric emergency units (34%), or pediatric outpatient departments (18%) were analyzed. The median age at inclusion was 8.7 months (range, 0.03 to 15.6 months). The median gestational age was 39 weeks, and the median birth weight was 3,330 g. These two variables did not vary according to age (P, 0.71 and 0.67, respectively). Fifty-two percent of infants were males, but this proportion decreased significantly with age, from 67% in the age category between birth and 3 months to 36% in the 12- to 15-month-old group (P = 0.04).
The mothers' median age was 29 years (range, 17 to 46 years). Among all mothers, 264/345 (76.5%) reported having had varicella while 45 (13%) did not report having developed the disease or having received vaccination. Thirty-six women (10.4%) could not provide the information.
Anti-VZV antibody titers.
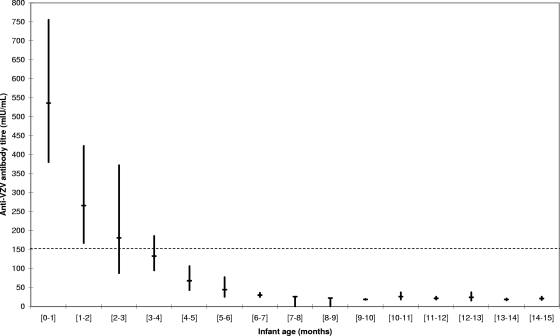
The mean anti-VZV antibody titers according to infants' age are presented in Fig. 1. For each 1-month class, the antibody levels are given as GMT together with the 95% confidence interval (95% CI). A significant difference was observed according to age, with the mean antibody titer decreasing from 536 mIU/ml at birth and through 1 month to below the 150-mIU/ml threshold at 3 to 4 months (133 mIU/ml; P < 0.001 by the Cochran-Mantel-Haenszel test). Above the age of 6 months, the GMT remained almost constant between 18 and 30 mIU/ml. Table 1 gives the proportion of infants in each 3-month age category with anti-VZV antibody titers above the 150-mIU/ml threshold. This proportion decreased significantly, from 83% in infants between birth and the age of 3 months to 29.5% in infants 3 to 6 months old. In the 6- to 9-month category, only one infant (1.1%) had a GMT above the threshold. In the 9- to 12-month and 12- to 15-month age categories, this proportion increased slightly, to 3.5% and 2.2%, respectively.
FIG. 1.
Anti-VZV antibody titers in 345 French infants between birth and the age of 15 months. Antibody levels are given as GMT. Vertical bars indicate 95% CI. Dashed line indicates the threshold of protection.
TABLE 1.
Median anti-VZV antibody titers and proportions of newborn infants with anti-VZV antibody titers above the 150-mIU/ml threshold according to age
| Age group (mo) | No. (%) in group | Mean (95% CI) anti-VZV antibody titer (mIU/ml) | % of infants with an antibody titer of ≥150 mIU/ml (95% CI) |
|---|---|---|---|
| 0-3 | 48 (13.9) | 333.8 (253-440) | 83.3 (69.8-92.5) |
| 3-6 | 44 (12.8) | 83.6 (63.4-110) | 29.5 (16.8-45.2) |
| 6-9 | 94 (27.2) | 25.9 (22.5-29.8) | 1.1 (0-5.8) |
| 9-12 | 114 (33.0) | 21.6 (19.1-24.5) | 3.5 (1.0-8.7) |
| ≥12 | 45 (13.0) | 22.1 (16.9-28.7) | 2.2 (0.1-11.8) |
Looking in more detail at the first few months after birth, it appears that the percentage of infants with anti-VZV antibody titers above the 150-mIU/ml threshold reached 95% between birth and 1 month but decreased to 75% in the 1- to 3-month category, to 50% in the 3- to 4-month category, and to 0% at 4 months (data not shown). Our data indicate that the half-life time of the anti-VZV antibodies is approximately 6 weeks.
In infants under the age of 6 months, the proportion of those with antibody titers above 150 mIU/ml was significantly higher when the mother reported a clinical history of varicella (64.8% versus 33.3%; P = 0.038). In contrast, no difference was observed in infants above the age of 6 months according to the maternal history of varicella (data not shown).
DISCUSSION
Based on a large number of subjects, this study clearly demonstrated high levels of passively acquired maternal antibodies during the neonatal period and allowed us to estimate, for the first time in France, the duration of passively acquired maternal VZV IgG in full-term infants.
A rapid decline in the level of anti-VZV maternal antibodies was observed during the first few months of life, with a substantial decrease even between the birth to 1-month and 1- to-2-month groups (Fig. 1). The percentage of infants with anti-VZV antibody titers above the threshold considered to be protective decreased drastically, from 83% between birth and 3 months to 1% between 6 and 9 months, and after 4 months, most infants seemed no longer to be protected by maternal anti-VZV antibodies (Table 1). These results are in accordance with those reported in the literature (Table 2). It is noteworthy that in the study of preterm and full-term infants by Leineweber et al. (12), anti-VZV antibody persistence was tested only at 1 to 3 months and 6 to 12 months, making it impossible to assess antibody levels between 4 and 6 months. Among preterm infants, 56% had antibody persistence at 1 to 3 months versus only 5% (1/21) at 6 to 12 months. Among full-term infants, 13% had antibody persistence at 6 to 12 months. This decline in the level of maternal antibody titers over the first months of life has already been documented for other infectious diseases (5, 12, 13). The half-life of anti-VZV antibodies calculated in our study (6 weeks) is in accordance with the results of a previous study reporting a half-life of 45 days for passively transferred anti-VZV IgG (21).
TABLE 2.
Details of studies that have reported the decay of maternal anti-VZV antibodies and the age at which anti-VZV antibodies disappear in infantsa
| Study (reference) | Country | Study period | Birth | No. of infants | Assay used to measure anti-VZV Ab | Age (mo) at anti-VZV Ab disappearance |
|---|---|---|---|---|---|---|
| Present study | France | 2005-2006 | Full term | 345 | TRFIA | 4 |
| Ozaki et al. (19) | Japan | 1980 | Full term | 24 | NT | 4 |
| Heininger et al. (10) | Switzerland | 1994-1999 | Full term | 240 | ELISA | 4-6 |
| Linder et al. (14) | Israel | 1997 | Preterm | 120 | IFAMA | 2-6 |
| Leineweber et al. (12) | Switzerland | 1999-2000 | Preterm | 66 | ELISA | <6 |
| Full term | 31 | ELISA | >6 | |||
| van der Zwet et al. (22) | The Netherlands | 1995-2000 | Preterm | 27 | VIDAS | 3 |
Ab, antibodies; NT, neutralization test (described previously by Asano and Takahashi [1a]); ELISA, enzyme-linked immunosorbent assay; IFAMA, immunofluorescent antibodies to membrane antigen assay; VIDAS, the Vitek immunodiagnostic assay system, an automated enzyme-linked fluorescent immunoassay.
Our study has some limitations. In order to describe the actual kinetics of maternal antibodies, it would have been more accurate to follow a cohort of newborn infants up to the age of 15 months and to repeatedly assess the anti-VZV antibody titers during this period. However, repeated blood sampling of the same infants was considered unethical, and our data provide a reasonable estimation of the proportion of infants considered to be protected by maternal antibodies during the first months of life. Nevertheless, there are no reliable correlates of protection provided by passively acquired anti-VZV IgG, and breakthrough infections can occur. The 150-mIU/ml threshold for positivity used in the VZV TRFIA is based on correlation with the Merck glycoprotein enzyme immunoassay and the recommendations of the U.S. Advisory Committee on Immunization Practices (15).
Although it has been shown to be cost-effective (3, 4), anti-VZV vaccination is not recommended on a routine basis in France. In this context, our study reflects passive transfer of maternal anti-VZV antibodies produced after a clinical episode of varicella rather than after anti-VZV vaccination. The absence in our study of mothers with histories of varicella vaccination did not allow us to compare the antibody titers in infants whose mothers had histories of varicella infection with those in infants whose mothers had varicella vaccination only.
In our study, the proportion of infants from birth to 1 month with anti-VZV antibody titers below the threshold considered to be protective (5%) suggested that a small portion of French mothers of childbearing age probably are not immune to VZV and are susceptible to contracting infection during pregnancy or around birth. This is in line with data regarding the seroprevalence of anti-VZV antibodies in the French population (11). In another study conducted in France at the end of 2005, 1.2% of 486 pregnant women included were seronegative for VZV (20). Congenital varicella syndrome is rare, but the risk is approximately 2% when infection occurs at 13 to 19 weeks of gestation. Congenital infection results in a wide clinical spectrum, which may include low birth weight, and multiple congenital abnormalities, leading in a number of cases to death in the first years of life. Maternal varicella occurring within 5 days before and 2 days after birth is also associated with severe neonatal varicella, with a high fatality rate for newborns (18). Vaccination of women of childbearing age without clinical histories of varicella is a way to decrease the morbidity associated with VZV. Since 2007, the Haut Conseil de la Santé Publique has recommended vaccination in France for women of childbearing age, particularly those who intend to become pregnant and have no history of varicella infection (9).
Besides congenital and neonatal VZV, the burden of disease in young infants is important. Data extracted from the French Medical Information System (Programme de Médicalisation des Systèmes d'Information Médecine Chirurgie Obstétrique), which covers all public and private hospitals in the country, indicate that 21,179 hospital stays were related to varicella between 1997 and 2002, representing an average of 3,500 hospitalizations a year (2). Among all cases, 7,058 (33%) had complications and 159 (0.8%) patients died, 14 of them before the age of 1 year. More recently, a national surveillance network based on 165 pediatric wards in hospitals located throughout France reported 1,575 hospitalizations related to varicella between March 2003 and July 2005, including 38 (2.4%) cases requiring intensive care. This survey showed that complications, especially cutaneous superinfections, were the major reasons for hospitalizations due to VZV infection and that the number of such complications increased steadily between the ages of 3 months and 1 year (8). VZV disease could be less complicated before 3 months due to the protection conferred by maternal VZV antibodies. From an infant vaccine perspective, precise knowledge of the kinetics of maternal antibodies is important in order to properly estimate the optimal age of vaccination. Indeed, when live attenuated vaccines are injected, the presence of maternal IgG antibodies may neutralize vaccine viruses, thereby inhibiting the vaccine-specific immune response. Therefore, another way to decrease the burden of VZV disease in infants is to reduce the gap of immunity between the disappearance of transmitted maternal antibodies and the age of initiation of active immunization for countries with routine VZV vaccination programs.
Acknowledgments
We thank the coinvestigators of this study (I. Abadie, L. Abalea, F. Audic-Gérard, C. Bailly-Botuha, F. Babre, S. Berberian, L. Berthomieu, C. Boscher, V. Brossard, I. Ceruti-Hazart, N. Delaperrière, N. Fargier, H. Feghaki, E. Fleurence, C. Fourmaux-Poulain, S. Gaubicher, C. Gay, N. Godon, M. Grall-Lerosey, L. Kohen-Couderc, E. Lachassine, D. Mamireau, C. Lardennois, P. Lemoine, F. Madhi, C. Metz, B. Peyret, N. Remus, O. Richer, C. Roumegoux, E. Sabbagh-Helali, J. Sarlangue, L. Sarthou, J.-F. Segura, A. Seiz, and L. Tripodi) and the personnel of the seven French hospital laboratories who participated in the study (S. Capdepont, M. Gueudin, P. Ledudal, V. Narbonne, S. Pillet, I. Poilane, C. Robin, and P. Volle). We also thank Christel Saussier and Remi Gauchoux (Mapi-Naxis) for data analysis.
This work was supported by Sanofi Pasteur MSD.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Asano, Y., Y. Hiroishi, N. Itakura, S. Hirose, Y. Kajita, S. Suga, and T. Yazaki. 1988. Placental transfer of IgG subclass-specific antibodies to varicella-zoster virus. J. Med. Virol. 261-6. [DOI] [PubMed] [Google Scholar]
- 1a.Asano, Y., and M. Takahashi. 1978. Studies on neutralization of varicella-zoster virus and serological follow-up of cases of varicella and zoster. Biken J. 2115-23. [PubMed] [Google Scholar]
- 2.Bonmarin, I., B. Ndiaye, E. Seringe, and D. Levy-Bruhl. 2005. Épidémiologie de la varicelle en France. Bull. Épidémiol. Hebdom. 830-31. [Google Scholar]
- 3.Coudeville, L., A. Brunot, T. D. Szucs, and B. Dervaux. 2005. The economic value of childhood varicella vaccination in France and Germany. Value Health 8209-222. [DOI] [PubMed] [Google Scholar]
- 4.Coudeville, L., F. Paree, T. Lebrun, and J. Sailly. 1999. The value of varicella vaccination in healthy children: cost-benefit analysis of the situation in France. Vaccine 17142-151. [DOI] [PubMed] [Google Scholar]
- 5.Evans, H. E., S. O. Akpata, and L. Glass. 1971. Serum immunoglobulin levels in premature and full-term infants. Am. J. Clin. Pathol. 56416-418. [DOI] [PubMed] [Google Scholar]
- 6.Galil, K., C. Brown, F. Lin, and J. Seward. 2002. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr. Infect. Dis. J. 21931-935. [DOI] [PubMed] [Google Scholar]
- 7.Gershon, A. A., R. Raker, S. Steinberg, B. Topf-Olstein, and L. M. Drusin. 1976. Antibody to varicella-zoster virus in parturient women and their offspring during the first year of life. Pediatrics 58692-696. [PubMed] [Google Scholar]
- 8.Grimprel, E., C. Levy, F. de La Rocque, R. Cohen, B. Soubeyrand, E. Caulin, T. Derrough, A. Lecuyer, P. d'Athis, and J. Gaudelus. 2007. Paediatric varicella hospitalisations in France: a nationwide survey. Clin. Microbiol. Infect. 13546-549. [DOI] [PubMed] [Google Scholar]
- 9.Haut Conseil de la Santé Publique. 22 April 2008. Vaccination schedule for 2008. Recommendations from the Haut Conseil de la Santé Publique. Bull. Épidémiol. Hebdom. 16-17:129-148. (In French.) [Google Scholar]
- 10.Heininger, U., D. Desgrandchamps, and U. B. Schaad. 2006. Seroprevalence of varicella-zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine 243258-3260. [DOI] [PubMed] [Google Scholar]
- 11.Khoshnood, B., M. Debruyne, F. Lancon, C. Emery, F. Fagnani, I. Durand, and D. Floret. 2006. Seroprevalence of varicella in the French population. Pediatr. Infect. Dis. J. 2541-44. [DOI] [PubMed] [Google Scholar]
- 12.Leineweber, B., V. Grote, U. B. Schaad, and U. Heininger. 2004. Transplacentally acquired immunoglobulin G antibodies against measles, mumps, rubella and varicella-zoster virus in preterm and full term newborns. Pediatr. Infect. Dis. J. 23361-363. [DOI] [PubMed] [Google Scholar]
- 13.Linder, N., L. Sirota, Y. Aboudy, B. German, T. Lifshits, B. S. Barnea, B. Lieberman, E. Mendelson, and A. Barzilai. 1999. Placental transfer of maternal rubella antibodies to full-term and preterm infants. Infection 27203-207. [DOI] [PubMed] [Google Scholar]
- 14.Linder, N., I. Waintraub, Z. Smetana, A. Barzilai, D. Lubin, E. Mendelson, and L. Sirota. 2000. Placental transfer and decay of varicella-zoster virus antibodies in preterm infants. J. Pediatr. 13785-89. [DOI] [PubMed] [Google Scholar]
- 15.Maple, P. A., J. J. Gray, K. Brown, and D. W. Brown. 2009. Performance characteristics of a quantitative, standardised varicella-zoster IgG time resolved fluorescence immunoassay (VZV TRFIA) for measuring immunity following natural infection. J. Virol. Methods 15790-92. [DOI] [PubMed] [Google Scholar]
- 16.Maple, P. A. C., J. Gray, J. Breuer, G. Kafatos, S. Parker, and D. Brown. 2006. Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial enzyme immunoassays and Merck glycoprotein enzyme immunoassay. Clin. Vaccine Immunol. 13214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer, P. A., J. F. Seward, A. O. Jumaan, and M. Wharton. 2000. Varicella mortality: trends before vaccine licensure in the United States, 1970-1994. J. Infect. Dis. 182383-390. [DOI] [PubMed] [Google Scholar]
- 18.Mirlesse, V., J. F. Magny, Y. Sole, F. Jacquemard, F. Forestier, and F. Daffos. 1998. VZV infection concerning pregnant women and new-borns. Med. Mal. Infect. 28782-790. [Google Scholar]
- 19.Ozaki, T., H. Nagai, T. Kimura, T. Ichikawa, S. Suzuki, H. Kito, and Y. Asano. 1980. The age distribution of neutralizing antibodies against varicella-zoster virus in healthy individuals. Biken J. 239-14. [PubMed] [Google Scholar]
- 20.Saadatian-Elahi, M., Y. Mekki, C. Del Signore, B. Lina, T. Derrough, E. Caulin, J. Thierry, and P. Vanhems. 2007. Seroprevalence of varicella antibodies among pregnant women in Lyon-France. Eur. J. Epidemiol. 22405-409. [DOI] [PubMed] [Google Scholar]
- 21.Sarvas, H., I. Seppala, S. Kurikka, R. Siegberg, and O. Makela. 1993. Half-life of the maternal IgG1 allotype in infants. J. Clin. Immunol. 13145-151. [DOI] [PubMed] [Google Scholar]
- 22.van der Zwet, W. C., C. M. Vandenbroucke-Grauls, R. M. van Elburg, A. Cranendonk, and H. L. Zaaijer. 2002. Neonatal antibody titers against varicella-zoster virus in relation to gestational age, birth weight, and maternal titer. Pediatrics 10979-85. [DOI] [PubMed] [Google Scholar]