Abstract
Vaccination is a tool that could be beneficial in managing the high prevalence of brucellosis in free-ranging bison in Yellowstone National Park. In this study, we characterized immunologic responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 (RB51) or a recombinant RB51 strain overexpressing superoxide dismutase (sodC) and glycosyltransferase (wboA) genes (RB51+sodC,wboA). Bison were vaccinated with saline only or with 4.6 × 1010 CFU of RB51 or 7.4 × 1010 CFU of RB51+sodC,wboA (n = eight animals/treatment). Bison vaccinated with RB51 or RB51+sodC,wboA had greater (P < 0.05) antibody responses, proliferative responses, and production of gamma interferon to RB51 after vaccination than did nonvaccinates. However, bison vaccinated with RB51+sodC,wboA cleared the vaccine strain from draining lymph nodes faster than bison vaccinated with the parental RB51 strain. Immunologic responses of bison vaccinated with RB51+sodC,wboA were similar to responses of bison vaccinated with RB51. Pregnant bison were intraconjunctivally challenged in midgestation with 107 CFU of B. abortus strain 2308. Bison vaccinated with RB51, but not RB51+sodC,wboA vaccinates, had greater protection from abortion, fetal/uterine, mammary, or maternal infection than nonvaccinates. Our data suggest that the RB51+sodC,wboA strain is less efficacious as a calfhood vaccine for bison than the parental RB51 strain. Our data also suggest that the RB51 vaccine is a currently available management tool that could be utilized to help reduce brucellosis in free-ranging bison.
Although several newly infected herds have been recently identified, the United States achieved a milestone in 2008 in that all states were simultaneously declared free of cattle brucellosis. However, the persistence of Brucella abortus in bison and elk reservoirs in the greater Yellowstone area (GYA; areas adjacent to Yellowstone National Park) remains a potential threat for reintroduction of brucellosis into domestic livestock. The fact that recently identified Brucella-infected herds were from the GYA has emphasized the possibility of wildlife-to-cattle transmission and renewed interest in resolving the persistence of brucellosis in wildlife reservoirs.
Vaccination is a tool that can be used to prevent abortions and the transmission of brucellosis between wildlife and domestic livestock. Although numerous studies evaluating brucellosis vaccines in domestic livestock have been conducted over past decades, research efforts in recent years have begun to focus on developing vaccines for use in wildlife reservoirs. The B. abortus strain RB51 (RB51) was previously evaluated as a calfhood vaccine for bison and found to be efficacious in preventing abortion and fetal/uterine infection after experimental challenge (9). However, the efficacy of RB51 as a calfhood vaccine for bison appears to be slightly reduced compared to data obtained from similar studies with cattle (4). The previous Brucella vaccine for cattle, B. abortus strain 19 vaccine (S19), was reported to not be efficacious as a calfhood vaccine for bison (5). Because vaccination programs for free-ranging wildlife are likely to be difficult and expensive, vaccines that provide optimal safety and efficacy are needed.
Data suggest that domestic livestock and wildlife reservoirs have diverse immunologic responses to RB51 and S19 vaccines, with differing levels of protection (3, 4, 6, 8, 10). In an effort to develop new and more efficacious vaccines, Brucella strains have been generated via recombinant DNA techniques and screened through laboratory animal models. When the protective antigen superoxide dismutase (sodC) or glycosyltransferase (wboA) was overexpressed in RB51 (13, 14), vaccinated mice had greater protection against experimental challenge than mice vaccinated with the parental RB51 strain. Since immunologic responses to brucellosis vaccines and efficacy may differ between laboratory animals and ruminant reservoirs of brucellosis, the present study was initiated to evaluate the RB51 strain overexpressing sodC and wboA (RB51+sodC,wboA) as a possible vaccine for use in bison.
MATERIALS AND METHODS
B. abortus cultures.
A commercial vaccine derived from RB51 and an experimental vaccine containing B. abortus strain RB51 overexpressing sodC and wboA (RB51+sodC,wboA) were obtained in lyophilized forms (Colorado Serum Company, Denver). Vaccines were diluted in accordance with the manufacturer's recommendations and administered in 3-ml volumes subcutaneously. After vaccination, the concentration of viable bacteria within inocula was determined by standard plate counts.
For immunologic assays measuring mononuclear cell proliferation, RB51 suspensions (1 × 1012 CFU/ml) were inactivated by gamma irradiation (1.4 × 106 rads), washed in 0.15 M sodium chloride (saline), and stored at −70°C.
For the challenge portion of the experiment, B. abortus strain 2308 (S2308) was grown on tryptose agar for 48 h at 37°C. The bacteria were harvested from the agar by aspiration using saline. Suspensions of S2308 were adjusted by using a spectrophotometer, and the concentrations of viable bacteria were determined by standard plate counts.
Animals and inoculation.
Twenty-four ∼10-month-old bison heifers were obtained from a brucellosis-free herd. After acclimation for 4 weeks, bison were randomly assigned to three groups (n = 8 animals/group) for subcutaneous vaccination with saline (control), RB51, or RB51+sodC,wboA. All inoculations were administered in the cervical region drained by the superficial cervical (prescapular) lymph node.
Superficial cervical lymph node biopsies.
Four RB51-vaccinated, four RB51+sodC,wboA-vaccinated, and two control bison were randomly selected for surgical removal of the right or left superficial cervical lymph node at 4, 8, or 12 weeks after inoculation. After surgical removal as previously described (3), each lymph node was divided into proximal, middle, and distal portions. Lymph node sections were weighed, triturated using a tissue grinder, serially diluted in saline, and placed on tryptose agar plates containing 5% bovine serum. After incubation at 37°C in 5% CO2, bacterial cell counts were made from each dilution by standard plate counts. Strains RB51 and RB51+sodC,wboA were identified based on colony morphology (1), growth characteristics, and a Brucella-specific PCR procedure (7). For each animal, the CFU/g was averaged over all three lymph node portions, and the average was used for statistical analysis.
Serologic evaluation.
Blood samples were collected by jugular venipuncture prior to vaccination and at 4-week intervals for up to 24 weeks postvaccination. Blood was allowed to clot for 12 h at 4°C and centrifuged. Serum was divided into 1-ml aliquots, frozen, and stored at −70°C.
Serological titers of animals to Brucella were determined by tube agglutination (1) and enzyme-linked immunosorbent assay (ELISA) procedures. For the ELISA procedure, RB51 was grown on tryptose agar for 48 h at 37°C and 10% CO2. Bacteria were harvested off the plates using phosphate-buffered saline (PBS) containing 0.001 M EDTA. After the bacteria were washed in PBS, the concentration was determined by using standard plate counts. Bacteria were killed by the addition of 0.5% formalin. After adjustment to 108 CFU/ml in carbonate-bicarbonate buffer, 100 μl/well was added to a microtiter plate, followed by incubation at 4°C overnight. After a washing step with PBS, 300 μl of saline containing 25 mg of casein/ml (PBS-casein) was added to each well. Plates were then incubated at room temperature for 2 h and washed three times with 300 μl of PBS containing 0.05% Tween 20 (PBS-Tween). Based on previous data (S. C. Olsen, unpublished data), serum samples were diluted 1:1,600 in PBS-casein, and 100 μl was added in quadruplicate to wells. After incubation at room temperature for 2 h, plates were washed three times with PBS-Tween. After the addition of 100 μl of a 1:2,500 dilution of peroxidase-conjugated rabbit anti-bovine immunoglobulin G (IgG) (Jackson Immunoresearch), the plates were incubated for 2 h at room temperature. After the addition of substrate (3,3′,5,5′-tetramethylbenzidine and H2O2 in 0.1 M citric buffer), the plates were incubated in the dark for 30 min, the reactions were stopped with 100 μl/well of 0.18 M sulfuric acid, and optical densities were read at 380 nm on a microtiter plate reader.
PBMC and lymph node cells for lymphocyte proliferation assays.
At all sampling times after vaccination, blood was obtained from the jugular vein of all bison and placed into an acid-citrate dextrose solution. Peripheral blood mononuclear cells (PBMC) were enriched by density centrifugation using a Ficoll-sodium diatrizoate gradient (Sigma Diagnostics, Inc., St. Louis, MO). PBMC were diluted in RPMI 1640 medium to 107 viable cells per ml as determined by trypan blue dye exclusion.
Then, 50 μl of each cell suspension, containing 5 × 105 cells, was added to flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated RB51 (109 to 105 bacteria/well). Wells containing 1 μg of pokeweed mitogen (Sigma Chemical Company)/ml were used as positive controls for proliferative responses. Microtiter plates contained duplicate wells for control, pokeweed, and all RB51 concentrations for each animal. Microtiter plates were prepared prior to initiation of the study and maintained at −70°C until use. Cell cultures were incubated for 7 days at 37°C under 5% CO2. After 7 days of incubation, cell cultures were pulsed with 1.0 μCi of [3H]thymidine per well for 18 h. Cells were harvested onto glass filter mats and counted for radioactivity in a liquid scintillation counter. Cell proliferation results were converted to stimulation indices (counts per minute [cpm] of wells containing antigen/cpm of wells without antigen) for statistical comparisons.
IFN-γ production.
In vitro production of gamma interferon (IFN-γ) by PBMC was measured at all sampling times after vaccination. Briefly, PBMC from each animal were isolated and adjusted to 107 viable cells per ml as described previously. Then, 50-μl portions of cell suspension, containing 5 × 105 cells, were added to flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated RB51 (2 × 108 bacteria/well). Cell cultures were incubated at 37°C under 5% CO2, and supernatants were removed at 72 h after the initiation of culture. Supernatants were held at −70°C until assayed for IFN-γ by using a commercially available kit (Cervigam; CSL Veterinary, Victoria, Australia). Standard dilutions of a purified bovine IFN-γ of known quantity (108 to 0.211 ng/ml) were included on each microtiter plate. Optical density measurements at 450 nm were made by using an ELISA plate reader (Molecular Devices, Sunnyvale, CA). Linear regression was used to prepare a standard curve from which the concentration of IFN-γ in each sample was determined. Antigen-specific net IFN-γ was determined for each sample by subtracting the concentration of IFN-γ in wells without antigen from IFN-γ concentrations in wells with antigen.
Flow cytometry.
At all sampling times after vaccination, PBMC suspensions, containing 2 × 107 cells, were centrifuged, and the supernatant was discarded. The cells were stained with PKH-67 green fluorescent dye (Sigma) in accordance with the manufacturer's instructions. Cells were adjusted to 107 viable cells per ml as described above. A 50-μl portion of each cell suspension, containing 5 × 105 cells, was added to each of eight separate flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or 1640 medium containing gamma-irradiated RB51 (2 × 108 bacteria per well). Cell cultures were incubated for 7 days at 37°C under 5% CO2. Approximately 2 × 105 pooled cells in 200 μl of culture media were added to individual wells of round-bottom microtiter plates, centrifuged (15 min, 400 × g), and resuspended in 100 μl of primary antibody (1 μg/well) in PBS containing 1% fetal bovine serum and 0.1% sodium azide (fluorescence-activated cell sorting buffer). Primary antibodies (VMRD, Pullman, WA) included anti-CD4 (17D1-IgG), anti-CD8 (ST8-IgM), anti-B cell (PIG45A-IgG2b), anti-γδTCR (GB21A-IgG2b), and anti-WC1 (BAQ4A-IgG1). After a 15-min incubation at room temperature, cells were centrifuged (15 min, 400 × g) and resuspended in 100 μl each of peridinin chlorophyll protein (1 μg/ml)-conjugated rat anti-mouse IgG1 (Becton Dickinson, Franklin Lakes, NJ) and phycoerythrin (1 μg/well)-conjugated goat anti-mouse IgM or IgG2b (Southern Biotechnology Associates, Birmingham, AL). Cells in secondary antibody were incubated for 15 min at room temperature in the dark, washed with fluorescence-activated cell sorting buffer, resuspended in 200 μl of PBS containing 0.04% sodium azide, and analyzed on a flow cytometer (FACScan; Becton Dickinson). The data were analyzed by using commercially available software (CellQuest [Becton Dickinson] and Modfit [Verity Software House, Inc., Topsham, ME]).
Experimental Brucella challenge.
Animals were raised to adulthood and pasture bred at ∼30 months of age. Breeding dates were determined by rectal palpation between 40 and 90 days of gestation. At ∼5 months of gestation, pregnant bison were transferred to a biolevel 3 containment facility, where they were individually housed for the duration of the study. At between 170 and 180 days of gestation, the bison were fasted for 24 h prior to being anesthetized with carfentanil (Wildnil; 0.007 to 0.008 mg/kg of body weight; Wildlife Pharmaceuticals, Ft. Collins, CO) and xylazine (0.10 to 0.13 mg/kg; Mobay Corp, Shawnee, KS) administered intramuscularly via pneumatic dart (Pneudart, Williamsburg, PA). A prechallenge sample of blood was obtained via jugular venipuncture. After intraconjunctival challenge with 107 CFU of S2308 (50 μl of inoculum per eye), the anesthetic was reversed with an intravenous injection of naltrexone (0.88 to 0.97 mg/kg; Mallinckrodt, St. Louis, MO).
Immediately after abortion, or within 48 h of parturition, cows were euthanized by intravenous administration of sodium pentobarbitol. Maternal samples obtained at necropsy included blood, milk from all four quarters, lymph nodes (bronchial, hepatic, internal iliac, mandibular, parotid, prescapular, retropharyngeal, and supramammary), mammary gland tissue from all four quarters, placentome or caruncle, spleen, lung, liver, and vaginal swab. Fetal samples obtained included spleen, lung, blood, bronchial lymph node, gastric contents, and rectal swabs. Swabs and fluid samples were inoculated directly on tryptose agar plates containing 5% bovine serum. Tissue samples were triturated in 0.15 M NaCl by using a tissue grinder and plated on both tryptose agar containing 5% bovine serum and Kuzdas and Morse media. For four target tissues (placentome and supramammary, parotid, and prescapular lymph nodes) the samples were weighed, triturated using a tissue grinder, and serially diluted in saline, and the colonization (CFU/g) was determined by bacterial cell counts made from each dilution by standard plate counts. All samples were incubated at 37°C and 5% CO2. B. abortus bacteria were identified on the basis of colony morphology, growth characteristics, and a Brucella-specific PCR procedure (1, 7).
Abortion was defined as the premature birth of a Brucella-infected, nonviable fetus after S2308 challenge. Dams and calves were considered to be infected if a single colony of B. abortus was recovered from any sample obtained at necropsy. Mammary or fetal/uterine infection was defined as the recovery of the S2308 challenge strain from supramammary lymph node, milk, mammary gland, placentome, vaginal swab, or any fetal sample.
Statistical analysis.
Serologic, IFN-γ, proliferation, and colonization data were analyzed as the logarithms of their values. Serologic data were compared over all sampling times using a two-way analysis of variance model, whereas differences between treatments for flow cytometric, [3H]thymidine incorporation, tissue colonization, and net IFN-γ data at each sampling time were compared by a general linear model procedure (SAS Institute, Inc., Cary, NC). Means for individual treatments were separated by use of a least-significant-difference procedure (P < 0.05). Chi-square analysis was used to compare the incidences of abortion and S2308 infection between vaccinated and nonvaccinated animals after experimental challenge.
RESULTS
Vaccine and challenge dosages.
Standard plate counts indicated that mean vaccination dosages for bison in RB51 and RB51+sodC,wboA treatments were 4.6 × 1010 and 7.4 × 1010 CFU, respectively. The mean challenge dose was 1.51 × 107 ± 0.57 × 107 CFU of S2308.
Evaluation of clearance from the superficial cervical lymph node.
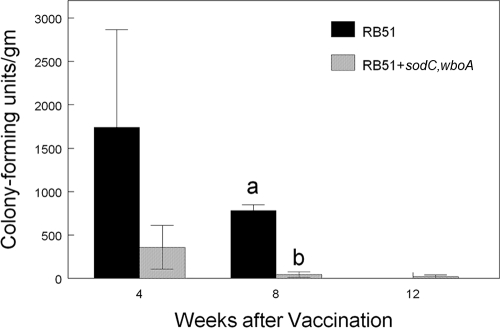
Both RB51 and RB51+sodC,wboA were recovered from the superficial cervical lymph nodes of all vaccinated bison at 4 and 8 weeks postvaccination (Fig. 1). The respective vaccine strains were also recovered from two of four RB51-vaccinated bison and one of four RB51+sodC,wboA-vaccinated bison at 12 weeks after inoculation. Although there was a trend (P > 0.05) for RB51 vaccinates to have higher colonization in the draining lymph node at 4 weeks after vaccination, only at the 8-week-postinfection sampling was the CFU/g in the superficial cervical lymph node reduced (P < 0.05) in RB51+sodC,wboA vaccinates compared to bison inoculated with the parental RB51 strain. Neither vaccine strain was recovered from the superficial cervical lymph node of nonvaccinated bison at 4, 8, or 12 weeks.
FIG. 1.
Colonization (CFU/g) of superficial cervical lymph nodes at 4, 8, or 12 weeks after vaccination of bison with 4.6 × 1010 of RB51 or 7.4 × 1010 CFU of RB51+sodC,wboA (n = 4 animals/treatment/time). After surgical removal, lymph nodes were weighed, triturated using a tissue grinder, serially diluted in saline, and plated on tryptose agar plates containing 5% bovine serum. Colonization was determined by standard plate counts. Responses are presented as mean titers ± the standard errors of the mean. Means with different superscripts are significantly different (P < 0.05).
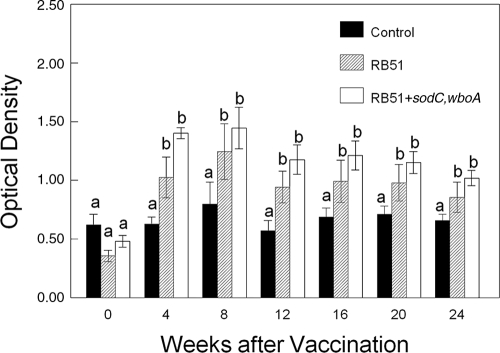
Serologic evaluation.
Serum from bison vaccinated with RB51 or RB51+sodC,wboA remained negative by the tube agglutination test in samples obtained up to 24 weeks after vaccination. Bison vaccinated with RB51 or RB51+sodC,wboA had greater (P < 0.05) antibody titers on the RB51 ELISA at 4, 8, 12, 16, 20, and 24 weeks after vaccination than bison inoculated with saline (Fig. 2). The antibody titers of RB51- and RB51+sodC,wboA-vaccinated bison did not differ (P > 0.05) at any sampling time.
FIG. 2.
Serologic responses of bison vaccinated with either RB51 or RB51+sodC,wboA or of control bison to gamma-irradiated RB51 in an ELISA. Bison were subcutaneously vaccinated with saline only, 4.6 × 1010 CFU of RB51, or 7.4 × 1010 CFU of RB51+sodC,wboA (n = 8 animals/treatment). Responses are presented as mean titers ± the standard errors of the mean. Means marked with different letters (a and b) are significantly different (P < 0.05).
All RB51- and RB51+sodC,wboA-vaccinated bison were negative by the tube agglutination test prior to challenge and demonstrated seroconversion at necropsy. Tube agglutination titers at necropsy did not differ (P > 0.05) between control and vaccinated treatments (data not shown).
Lymphocyte proliferation assays.
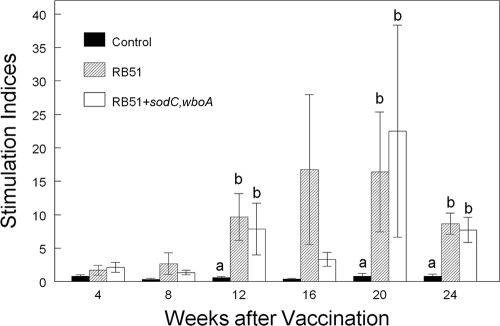
Bison vaccinated with RB51 or RB51+sodC,wboA had greater proliferative responses (P < 0.05) to gamma-irradiated whole RB51 than did nonvaccinates at 12, 20, and 24 weeks after vaccination (representative data are presented in Fig. 3). Proliferative responses of PBMC from bison vaccinated with RB51+sodC,wboA did not differ (P > 0.05) at any sampling time from the responses of PBMC from bison inoculated with RB51.
FIG. 3.
Proliferative responses to 108 CFU of gamma-irradiated RB51 by PBMC from bison vaccinated with saline only, 4.6 × 1010 CFU of RB51, or 7.4 × 1010 CFU of RB51+sodC,wboA (n = 8 animals/treatment). Cells were incubated at 37°C and 5% CO2 for 7 days and pulsed for 18 h with [3H]thymidine. The results are expressed as mean stimulation indices. Means within a sampling time marked with different lowercase letters (a and b) are significantly different (P < 0.05). The mean stimulation indices of bison PBMC incubated in the absence of antigen were 9,713 ± 1,984 cpm.
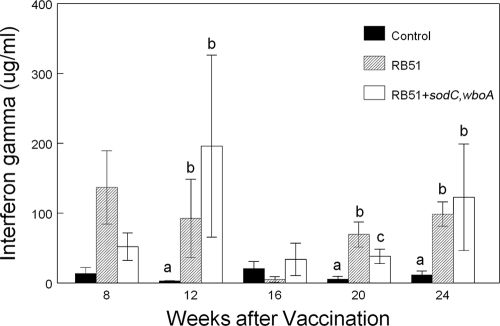
IFN-γ production.
The production of IFN-γ by PBMC increased (P < 0.05) with longer in vitro incubation times, with the highest mean IFN-γ concentrations in both vaccine treatments observed in samples obtained after 72 h of incubation (data not shown). Dependent upon the length of incubation in vitro, the mean IFN-γ concentrations produced by the PBMC from bison vaccinated with RB51 or RB51+sodC,wboA were significantly greater (P < 0.05) than mean concentrations produced by the PBMC of nonvaccinates beginning at 8 weeks after vaccination (representative data are given in Fig. 4). In samples obtained later than 8 weeks after vaccination, the mean IFN-γ production from PBMC of RB51 vaccinates or RB51+sodC,wboA vaccinates was greater (P < 0.05) at 12, 20, and 24 weeks than the responses of PBMC obtained from nonvaccinated bison. With the exception of the 20-week sampling, the mean IFN-γ concentrations for both vaccine treatments did not differ (P > 0.05) at any sampling time.
FIG. 4.
IFN-γ production by bison PBMC. Bison were vaccinated with saline only, 4.6 × 1010 CFU of RB51 or 7.4 × 1010 CFU of RB51+sodC,wboA (n = 8 animals/treatment). Cells were incubated at 37°C and 5% CO2 for 72 h in the presence or absence of 108 CFU of gamma-irradiated RB51. The results are expressed as mean net IFN-γ production (production in wells containing RB51 − production in wells without antigen). Means within a sampling time with different superscripts are significantly different (P < 0.05).
Flow cytometry.
Although there was a trend for RB51 vaccinates to demonstrate increased proliferation in response to gamma-irradiated RB51 in all leukocyte subsets at all sampling times, only the total cells proliferating at 20 weeks and the CD4 cells proliferating at 12 and 16 weeks differed statistically (P < 0.05) from the responses of PBMC from control bison (Table 1). For RB51+sodC,wboA-vaccinated bison, flow cytometric analysis suggested a trend for increasing responses to gamma-irradiated RB51 in most leukocyte subsets at 20 and 24 weeks, although only the total cell proliferation at 24 weeks statistically differed (P < 0.05) from the responses of control bison. With the exception of total cell proliferation at 20 weeks after vaccination, flow cytometric analysis suggested that the responses of PBMC from RB51 and RB51+sodC,wboA vaccinates to gamma-irradiated RB51 did not differ (P > 0.05).
TABLE 1.
Flow cytometric analysis of responses to B. abortus strain RB51 after vaccinationa
| Time postvaccination and vaccination group | Mean no. of proliferating cells/10,000 PBMC ± SEMb
|
||||
|---|---|---|---|---|---|
| Total | CD4+ | CD8+ | γδTCR+ | B cells | |
| 12 wk | |||||
| Control | 1 ± 1 | 65 ± 48 | 183 ± 138 | 618 ± 351 | 531 ± 477 |
| RB51 | 739 ± 477 | 976 ± 351* | 702 ± 380 | 886 ± 393 | 1,743 ± 613 |
| RB51+sodC,wboA | 96 ± 61 | 148 ± 91 | 19 ± 19 | 378 ± 353 | 496 ± 255 |
| 16 wk | |||||
| Control | 0 ± 0 | 30 ± 29 | 205 ± 191 | 573 ± 426 | 1,214 ± 602 |
| RB51 | 913 ± 473 | 760 ± 301* | 614 ± 408 | 427 ± 376 | 2,158 ± 141 |
| RB51+sodC,wboA | 803 ± 440 | 342 ± 176 | 814 ± 441 | 882 ± 595 | 1,119 ± 309 |
| 20 wk | |||||
| Control | 643 ± 401 | 271 ± 105 | 76 ± 76 | 20 ± 20 | 936 ± 453 |
| RB51 | 1,949 ± 699* | 867 ± 605 | 1,181 ± 700 | 788 ± 552 | 1,002 ± 468 |
| RB51+sodC,wboA | 816 ± 266 | 780 ± 638 | 487 ± 273 | 225 ± 128 | 904 ± 187 |
PKH-67-labeled PBMC from bison subcutaneously vaccinated with saline only, RB51, or RB51+sodC,wboA (n = 8 animals/treatment) were incubated with 108 CFU of gamma-irradiated B. abortus strain RB51 at 37°C and 5% CO2 for 7 days, labeled with monoclonal antibodies, and analyzed in a flow cytometer.
Values denoted with an asterisk (*) are significantly different (P < 0.05) from the control treatment.
Challenge.
The RB51 vaccine strain was not recovered at any time from maternal or fetal samples obtained at necropsy.
After experimental challenge with S2308, bison vaccinated with RB51 had reduced (P < 0.05) incidence of abortion, fetal/uterine infection, mammary infection, and maternal infection compared to nonvaccinated bison (Table 2). Bison vaccinated with the RB51+sodC,wboA strain did not differ (P > 0.05) from control bison in the incidence of abortion, fetal/uterine/mammary infection, or maternal infection after S2308 challenge.
TABLE 2.
Efficacy of B. abortus RB51 or RB51+sodC,wboA strain in protecting against experimental challenge at midgestation with 107 CFU of B. abortus strain 2308
| Vaccination group | Vaccination dose (CFU) | Rate (%) of abortion or infection (no. aborted or infected/total no.)a
|
|||
|---|---|---|---|---|---|
| Abortion | Fetal/uterine infection | Mammary infection | Maternal infection | ||
| RB51 | 4.26 × 1010 | 33 (2/6)* | 50 (3/6)* | 33 (2/6)* | 50 (3/6)* |
| RB51+sodC, wboA | 7.4 × 1010 | 66 (4/6) | 83 (5/6) | 66 (4/6) | 100 (6/6) |
| Control | 100 (8/8) | 100 (8/8) | 100 (8/8) | 100 (8/8) | |
Means denoted with an asterisk (*) are significantly different (P < 0.05) from the control treatment.
Compared to nonvaccinates, bison vaccinated with the RB51 or RB51+sodC,wboA strain had reduced (P < 0.05) S2308 colonization in parotid and prescapular lymph node samples obtained at necropsy after experimental challenge (Table 3). Although not statistically significant (P > 0.05), RB51 and RB51+sodC,wboA vaccinates also tended to have lower S2308 colonization in the suprammamary lymph nodes and placentomes than the nonvaccinates. This nonsignificant trend also indicated reduced colonization in tissues obtained at necropsy from RB51 vaccinates compared to colonization in tissues from bison vaccinated with the RB51+sodC,wboA strain.
TABLE 3.
Colonization of B. abortus in bison lymph nodes and placentome taken at necropsy after midgestational challenge with B. abortus strain 2308 in bison vaccinated with saline only (control) or with the RB51 or RB51+sodC,wboA strain
| Vaccination group | Vaccination dose (CFU) | No. of bison | Mean S2308 CFU/g of tissue ± SEMa
|
|||
|---|---|---|---|---|---|---|
| Parotid LN | Prescapular LN | Supramammary LN | Placentome | |||
| RB51 | 4.26 × 1010 | 6 | 0.44 ± 0.28a | 0 ± 0a | 1.06 ± 0.53 | 4.17 ± 1.88 |
| RB51+sodC,wboA | 7.4 × 1010 | 6 | 1.34 ± 0.46a | 0.30 ± 0.30a | 1.51 ± 0.63 | 5.57 ± 1.77 |
| Control | 8 | 2.85 ± 0.38b | 1.93 ± 0.42b | 2.85 ± 0.38 | 8.01 ± 0.25 | |
LN, lymph nodes. Means with different superscript letters are significantly different (P < 0.05).
When mean numbers of S2308 culture-positive tissues at necropsy were compared, RB51 vaccinates had fewer culture-positive maternal and fetal tissues than did control bison (Table 4). Bison vaccinated with the RB51+sodC,wboA strain had fewer (P < 0.05) maternal tissues culture positive for the S2308 challenge strain than did nonvaccinates, but the mean numbers of culture-positive fetal tissues did not differ (P > 0.05). Bison vaccinated with the RB51 or RB51+sodC,wboA strain did not differ (P > 0.05) in mean numbers of maternal or fetal tissues from which S2308 was recovered.
TABLE 4.
Colonization of B. abortus in tissues taken at necropsy after midgestational challenge with B. abortus strain 2308 from bison vaccinated with saline (control) or with the B. abortus RB51 or RB51+sodC,wboA strain
| Vaccination group | Vaccination dose (CFU) | Mean no. of tissues ± SEM from which S2308 was recovereda
|
|||
|---|---|---|---|---|---|
| Maternal
|
Fetal
|
||||
| Positive | Negative | Positive | Negative | ||
| RB51 | 4.26 × 1010 | 6.8 ± 3.3a | 15.2 ± 3.3a | 2.8 ± 1.5a | 4.2 ± 1.5a |
| RB51+sodC, wboA | 7.4 × 1010 | 10.3 ± 2.9a | 11.7 ± 2.9a | 4.5 ± 1.4ab | 2.5 ± 1.4ab |
| Control | 17.3 ± 1.5b | 4.7 ± 1.5b | 6.5 ± 0.2b | 0.5 ± 0.2b | |
Means with different superscript letters are significantly different (P < 0.05).
DISCUSSION
The results of this study indicate that the recombinant RB51+sodC,wboA vaccine strain is not as efficacious in protecting bison from challenge with virulent B. abortus as the RB51 parental strain. This may be due to characteristics of the vaccine strain in vivo, as we noted a reduction in the colonization of lymphatic tissues and a failure to stimulate immunologic responses that were greater than that associated with the RB51 parent strain. Data obtained in the present study suggest that the RB51+sodC,wboA strain is safe in bison, since no adverse effects, persistent colonization, or adverse tissue localization was noted. Although our data suggest it is not ideal for use in calfhood vaccination of bison, the in vivo properties of RB51+sodC,wboA may make it useful for management procedures such as booster or adult vaccination. However, additional studies would be required to characterize its efficacy and safety as a booster or adult vaccine.
The finding that the recombinant RB51+sodC,wboA strain was cleared faster in vivo and appears to be less efficacious in bison than the parental RB51 strain was unexpected. Our results differ from those of a previous study of mice that reported that the clearance of RB51+sodC,wboA from the spleens of vaccinated mice did not differ from the clearance of the parental RB51 strain (12). That study and others found that mice vaccinated with RB51 overexpressing sodC and/or wboA had increased protection to experimental challenge compared to mice vaccinated with RB51 (12-14). The sodC gene encodes a Cu/Zn superoxide dismutase that catalyzes the dismutation of oxygen radicals (2, 11), and the wboA gene encodes a glycosyltransferase essential for O-side chain biosynthesis on the lipopolysaccharide (15). Both sodC and wboA genes are native to B. abortus, although an IS711 insertion element disrupts the wboA gene in RB51 (2, 11, 15). Since overexpression of wboA in RB51 leads to accumulation of the O-side chain predominantly in the cytoplasm, with small amounts expressed on the lipopolysaccharide (13), one possible explanation for our observation is that the accumulation in the cytoplasm altered the ability of the strain to survive in vivo in bison but not in mice. In any case, the immunologic and in vivo characteristics of the vaccine obviously differ when administered to bison compared to mice.
The present study reaffirms the need to evaluate potential vaccine strains in the targeted species. Although laboratory animals (mice and guinea pigs) have traditionally been used to screen new Brucella vaccine strains for protective characteristics, this model typically involves peritoneal vaccination and challenge, with protection generally assessed via hepatic and splenic colonization. In large ruminants, vaccines are usually administered subcutaneously or intramuscularly, with challenges or field exposures occurring through mucosal surfaces. Transmission of B. abortus occurs laterally through abortions or the distribution of Brucella-infected parturition materials or vertically through shedding in milk. Therefore, vaccine protection in large ruminants determined using Brucella midgestation challenge models is primarily assessed through the prevention of abortions or uterine/mammary infections and secondarily assessed by reduction or elimination of lymph node colonization at parturition, after mucosal exposure with an infectious dose of a virulent strain of Brucella.
It should be noted that current brucellosis vaccines for cattle (RB51 and S19) are very effective in preventing clinical symptoms of brucellosis (i.e., abortions or weak Brucella-infected calves). Because they prevent Brucella colonization in fetal or uterine tissues and the mammary gland, they are also very effective at reducing transmission. However, current brucellosis vaccines are less effective in preventing infection at parturition after midgestational S2308 challenge than in protecting against abortion and transmission. Of regulatory importance is the fact that current brucellosis vaccines provide minimal protection against seroconversion after exposure to virulent B. abortus strains, although experimental data (Olsen, unpublished) suggest that the titers of vaccinated cattle may decline more rapidly after experimental challenge than those of nonvaccinates. Since the U.S. eradication program uses seroprevalence to monitor for brucellosis, vaccination will not eliminate seropositive responses on surveillance tests if animals are exposed to infectious dosages of B. abortus field strains.
Because the delivery of brucellosis vaccines to free-ranging wildlife will most likely be difficult and expensive, a vaccine with the greatest efficacy in the species of interest is essential. Current brucellosis management plans for free-ranging bison include only vaccination and do not currently include test and removal procedures. Since it is unlikely that a vaccine can be effectively delivered to the entire targeted wildlife population, a percentage of the population will remain capable of being infected with and transmitting B. abortus. Therefore, even with a highly efficacious vaccine and effective delivery program, it is anticipated that some bison would still transmit B. abortus and cause seroconversion in bison protected by vaccination. It is anticipated that the seroprevalence would be slow to decline and, in the absence of test and removal procedures, would be a poor indicator of the program's effectiveness in reducing the prevalence of brucellosis. Parameters other than seroprevalence will most likely need to be monitored to truly characterize the effectiveness of a vaccination-only program on disease prevalence.
Recognizing that it would be preferable to have greater efficacy than what was demonstrated here, our data suggest that RB51 would be preferable to the RB51+sodC,wboA strain for use as a calfhood vaccine in bison. The efficacy of brucellosis vaccines under field conditions is generally greater than that noted under experimental conditions, where all animals are pregnant and receive an infectious dose of virulent B. abortus during midgestation, when they are most susceptible to brucellosis. Therefore, until a more efficacious vaccine is developed, the RB51 vaccine is a currently available management tool that could potentially be utilized to help reduce the prevalence of brucellosis in free-ranging bison.
Acknowledgments
We thank Deb Buffington, Aileen Hudspith, Doug Ewing, Todd Holtz, John Kent, Todd Pille, Darl Pringle, Jay Steffan, and Dennis Weuve for technical assistance.
Product names are necessary to report factually on available data; however, the U.S. Department of Agriculture neither guarantees nor warrants the standard of the product, and the use of the name by the U.S. Department of Agriculture implies no approval of the product to the exclusion of others that may also be suitable.
The Veterinary Technologies Corp. has international patents and trademarks on B. abortus strain RB51.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Bacteriological methods serological methods, p. 17-136. In Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France.
- 2.Bricker, B. J., L. B. Tabatabai, B. A. Judge, B. L. Deyoe, and J. E. Mayfield. 1990. Cloning and expression and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect. Immun. 582935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheville, N. F., A. E. Jensen, S. M. Halling, F. M. Tatum, D. C. Morfitt, S. G. Hennager, W. M. Frerichs, and G. Schurig. 1992. Bacterial survival, lymph node changes and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am. J. Vet. Res. 531881-1888. [PubMed] [Google Scholar]
- 4.Cheville, N. F., S. C. Olsen, A. E. Jensen, M. G. Stevens, and M. V. Palmer. 1996. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am. J. Vet. Res. 571153-1156. [PubMed] [Google Scholar]
- 5.Davis, D. S. 1993. Summary of bison/brucellosis research conducted at Texas A&M University 1985-1993, p. 347-361. In R. E. Walker (ed.), Proceedings of North American Public Bison Herds Symposium. National Bison Association, Denver, CO.
- 6.Kreeger, T. J., W. E. Cook, W. H. Edwards, P. H. Elzer, and S. C. Olsen. 2002. Brucella abortus strain RB51 vaccination in elk. II. Failure of high dosage to prevent abortion. J. Wildl. Dis. 3827-31. [DOI] [PubMed] [Google Scholar]
- 7.Lee, L.-K., S. C. Olsen, and C. A. Bolin. 2001. Effects of exogenous recombinant interleukin-12 on immune responses and protection against Brucella abortus in a murine model. Can. J. Vet. Res. 65223-228. [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen, S. C., S. J. Fach, M. V. Palmer, R. E. Sacco, W. C. Stoffregen, and W. R. Waters. 2006. Immune responses of elk to initial and booster vaccinations with Brucella abortus strain RB51 or 19. Clin. Vaccine Immunol. 131098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen, S. C., A. E. Jensen, W. C. Stoffregen, and M. V. Palmer. 2003. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Res. Vet. Sci. 7417-22. [DOI] [PubMed] [Google Scholar]
- 10.Olsen, S. C., T. J. Kreeger, and W. Schultz. 2002. Immune responses of bison to ballistic or hand vaccination with Brucella abortus strain RB51. J. Wildl. Dis. 38738-745. [DOI] [PubMed] [Google Scholar]
- 11.Sriranganathan, N., S. M. Boyle, G. G. Schurig, and H. Misra. 1990. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet. Microbiol. 26359-366. [DOI] [PubMed] [Google Scholar]
- 12.Vemulapalli, R., A. Contreras, N. Sanakkayala, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2004. Enhanced efficacy of recombinant Brucella abortus RB51 vaccines against B. melitensis infection in mice. Vet. Microbiol. 102237-245. [DOI] [PubMed] [Google Scholar]
- 13.Vemulapalli, R., Y. He, L. S. Buccolo, S. M. Boyle, N. Sriranganathan, and G. G. Schurig. 2000. Complementation of Brucella abortus RB51 with a functional wboA gene results in O-antigen synthesis and enhanced vaccine efficacy but no change in rough phenotype and attenuation. Infect. Immun. 683927-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vemulapalli, R., Y. He, S. Cravero, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2000. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun. 683286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vemulapalli, R., J. R. McQuiston, G. G. Schurig, N. Sriranganathan, S. M. Halling, and S. M. Boyle. 1999. Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin. Diagn. Lab. Immunol. 6760-764. [DOI] [PMC free article] [PubMed] [Google Scholar]