Abstract
An ileal cannulation model was developed in conjunction with a flow cytometric assay to gain a better understanding of the mechanisms of immunopathogenesis of Johne's disease caused by Mycobacterium avium subsp. paratuberculosis. Initial studies with calves showed that M. avium subsp. paratuberculosis DNA is detectable by PCR in ileal biopsies during the first months following experimental infection. Inflammatory lesions were not detected on endoscopic evaluation up to 8 months postexperimental infection. M. avium subsp. paratuberculosis DNA was detected in multiple tissues at necropsy 8 months postinfection. Examination of the activation status of epithelial lymphocytes from the jejunum and ileum from infected and control animals at necropsy revealed that none of the major subsets of lymphocytes (NK, CD2+, and CD2− γδ T lymphocytes, or CD4 and CD8 αβ T lymphocytes) expressed activation molecules CD25, CD26, CD71, ACT1, or ACT16. Subsets of CD4 and CD8 T lymphocytes from control and infected animals expressed CD26. The majority of CD4 and CD8 T lymphocytes expressed CD45R0, the memory T-lymphocyte marker. An immune response to M. avium subsp. paratuberculosis was detected by 3 months postinfection, dominated by a strong proliferative response of CD4 memory T lymphocytes. The findings indicate an immune response develops following initial exposure to M. avium subsp. paratuberculosis that controls but does not eliminate the pathogen. This persistence of M. avium subsp. paratuberculosis possibly leads to erosion and dysregulation of protective immunity at later time points postinfection. Continuous access to the ileum offers an opportunity to elucidate the cellular and molecular events leading to immune dysregulation and development of chronic inflammatory ileitis.
Mycobacterium avium subsp. paratuberculosis, the causative agent of paratuberculosis (Johne's disease [JD]), has a broad host range. It has been isolated from multiple ruminant and nonruminant species including humans (1, 5, 14, 23, 31, 33, 35, 36). The disease has a worldwide distribution and is present in every country with significant livestock industries. The increasing recognition of the global prevalence of M. avium subsp. paratuberculosis has intensified concerns regarding animal and human health as well as its impact on economics and international trade (20). The most recent estimate of M. avium subsp. paratuberculosis prevalence in U.S. dairy herds reported in a survey conducted by the National Animal Health Monitoring System was 68%, a large increase over a 1996 estimate (43). Losses due to JD in the United States are estimated to exceed $250 million annually (26). Efforts to control JD have been impeded by the limited understanding of the immune response to M. avium subsp. paratuberculosis, the mechanisms of immunopathogenesis leading to dysregulation of protective immunity, and the lack of an effective vaccine. Progress in the development of a vaccine has been impeded by the lack of a method of obtaining data on the immune response to candidate vaccines predictive of their capacity to elicit a protective response in a short time course. To date, methods of evaluation have involved the use of terminal vaccination trials where efficacy of a vaccine has been estimated at necropsy or based on results from long-term field trials (reviewed in reference 13). In the present study, we examined the potential of using a bovine ileal cannulation model in conjunction with a flow cytometric (FC) assay to study the immune response to M. avium subsp. paratuberculosis and the mechanisms of pathogenesis that lead to dysregulation of protective immunity and to disease. The ileal cannulation model allows for continuous and long-term access to the ileum and monitoring of the local gut-associated immune responses without repeated surgical intervention or sacrifice of the animal. The model, in conjunction with an FC assay (11), greatly extends opportunities to study the mechanisms of immunopathogenesis and will be useful as a model for evaluating the local mucosal immune response to candidate vaccines.
MATERIALS AND METHODS
Animals.
Thirteen newborn Holstein bull calves were obtained from the Washington State University research dairy at different time points of the study. Six were obtained at the initiation of the study to standardize the surgical protocol for cannulation of the ileum. Three additional calves were used during this time course as negative controls. One calf was cannulated and maintained as a cannulated negative control. Two calves were maintained as nonsurgically altered negative controls. The dairy is a closed herd that is considered to be free of paratuberculosis since it has had no cases diagnosed for over 35 years and since the dairy performs periodic, whole-herd, enzyme-linked immunosorbent assay (ELISA) screening tests. All calves were fed 4 liters of maternally derived colostrum within 6 h of birth and taken to a biosecurity level 2 (BSL-2) isolation unit within the first 24 h of life. Calves were fed milk replacer, whey pellets, calf starter grain, and free-choice alfalfa hay. The two control calves without cannulas were moved to a clean barn away from the BSL-2 isolation units. The control calf with a cannula was maintained in a BSL-2 isolation unit.
All protocols and procedures were approved by the Washington State University Institutional Animal Care and Use Committee.
Ileal cannulation surgery.
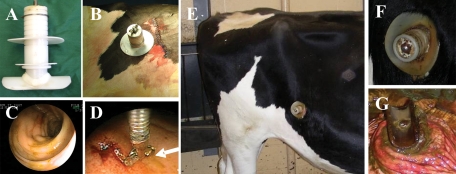
Calves were raised in isolation facilities for 8 to 9 weeks until they were weaned from milk replacer and deemed to be large enough for surgery. Prior to surgery, all calves were withheld from feed for a minimum of 24 h. Calves were induced by masking with isoflurane and oxygen prior to placement of an endotracheal tube and then maintained under anesthesia with isoflurane and oxygen. The cannulation technique was similar to that described by Streeter et al. (41), with some modifications. Briefly, the right flank caudal to the 13th rib was clipped and prepared aseptically for surgery. A 20-cm vertical incision was made in the center of the paralumbar fossa through the skin, muscle layers, and peritoneum. The cecum and distal ileum were exteriorized. A T-shaped polyethylene cannula (diameter, 1 in.; neck, 3 in.) (Ankom Technology, Macedon, NY) was inserted into a 5-cm incision on the antimesenteric side of the distal ileum approximately 20 cm from the ileocecal junction. The intestine was closed with a purse string suture around the cannula using a size 3-0 polydioxanone monofilament. Next, a 3-cm incision was made through the body wall, 5 cm caudal to the original incision to allow the neck of the cannula to exit the abdominal cavity. The cannula was held in place through the aid of a polyethylene retaining washer placed around the cannula neck against the outer skin. The paralumbar incision was closed in a conventional manner (Fig. 1).
FIG. 1.
Pictures showing the modified cannula used in the study (A), appearance of cannula after surgery (B), endoscopic field showing no inflammation present at the time of inoculation with M. avium subsp. paratuberculosis (C), and collection of pinch biopsy (D). Other images show the status of cannula 8 months postsurgery just before necropsy. Inflammation at the site of implantation was kept at a minimum by keeping the site clean (E and F). Inspection of the internal portion of the cannula showed minimal inflammation (G).
Postsurgery care.
Calves were administered ceftiofur hydrochloride (1.1 mg/kg of body weight, subcutaneously every 24 h) immediately prior to surgery and for 5 days postsurgery. Flunixin meglumine (1.1 mg/kg, intravenously every 12 h) was administered immediately prior to surgery and for 1 to 2 days postsurgery. Calves were monitored twice daily for temperature, pulse rate, respiratory rate, attitude, appetite, urination, defecation, and wound healing for 2 weeks postsurgery.
M. avium subsp. paratuberculosis preparations.
A strain of M. avium subsp. paratuberculosis, K10, and a substrain of K10 transformed to express the green fluorescent protein (GFP-K10) were generously provided by Raul Barletta, University of Nebraska, Lincoln, NE (12). Bacteria were grown in 7H9 broth medium (Difco, Lawrence, KS) supplemented with 6.7% para-JEM GS (Trek Diagnostic systems, Cleveland, OH), 2 μg/ml mycobactin J (Allied Monitor, Fayette, MO), and 0.05% Tween 80 at 37°C in a shaking incubator (100 rpm). When culture density reached an optical density at 600 nm (OD600) of 0.6 to 0.8, the master stocks were aliquoted into 1.5-ml microcentrifuge screw-top tubes (VWR, Batavia, IL), mixing 600 μl of broth culture with 600 μl of 30% glycerol, and stored at −80°C. To prepare the bacterial inoculum, 1 ml of frozen stock was cultured in 10 ml of 7H9 broth medium in a 50-ml tube at 37°C in a shaking incubator (100 rpm) until the density of the culture reached an OD600 of 0.5 (approximately 5 × 108 CFU/ml). One milliliter of the culture was centrifuged (12,000 × g for 10 min), and the pellet was resuspended in 1 ml of phosphate-buffered saline (PBS; pH 7.4) by passing it through a 20-gauge needle and then a 22-gauge needle, as previously recommended (13). Calf inocula were prepared by combining 200 μl of the resuspended preparation (1 × 108 CFU) with 20 ml of PBS.
Endoscopic evaluation.
An Olympus CV-60 Gastroscope (Center Valley, PA) with an outer diameter of 12.2 mm and a channel diameter of 3.2 mm was used to visualize and document intestinal mucosal changes and perform biopsies. Prior to biopsy collection, the cannula plug was removed, and the ileum was flushed with water to remove digesta. The gastroscope was inserted through the cannula into the ileum and directed orally or aborally. Air was used to insufflate the intestinal lumen to facilitate advancement of the scope. Following visual examination, a 2.8-mm biopsy instrument was inserted via the endoscope port to retrieve 15 to 20 mucosal and submucosal biopsies (Fig. 1). The biopsies were taken from areas, both oral and aboral, within 10 cm of the cannula.
Study design.
The study was designed to evaluate the use of the ileal cannulation model to study the infection and immune response to M. avium subsp. paratuberculosis in live calves.
Six calves (numbers 100, 101, 119, 121, 136, and 139) were inoculated a single time with 1 × 108 CFU of M. avium subsp. paratuberculosis GFP-K10. Two calves (122 and 123) were inoculated twice, 7 days apart, with 1 × 108 CFU of strain GFP-K10. A single calf (124) was inoculated four times, 7 days apart, with 1 × 108 CFU of strain GFP-K10. Additional animals were used to compare FC data between experimentally inoculated (136, 137, and 139) and negative control (132, 142, and 143) animals. The experimentally inoculated animals were from an ongoing experiment to compare the immune response to wild-type M. avium subsp. paratuberculosis K10 (animals 136 and 139) and to M. avium subsp. paratuberculosis K10 (137) with a targeted disruption in relA, a global regulator gene (27). One control animal (132) was cannulated and housed in a BSL-2 facility. Two animals (142 and 143) were not cannulated and were housed in a clean barn.
Preinoculation biopsies were obtained 1 day prior to inoculation and stored in sterile microtubes for use in PCR, culture, and histopathology. The PCR samples were stored at −20°C until processed. Culture samples were processed immediately, and histopathology samples were stored in 10% neutral buffered formalin. Whole blood (330 ml) was aseptically collected from the jugular vein. Ten milliliters was placed into a plain BD Vacutainer (Franklin Lakes, NJ) tube to obtain serum for an Idexx HerdCheck paratuberculosis antibody test kit (Westbrook, ME), and 20 ml was placed in two 10-ml Vacutainer tubes containing sodium heparin for a gamma interferon (IFN-γ) assay (Bovigam enzyme immunoassay [EIA] kit; Parkville, Victoria, Australia). A total of 300 ml was placed into Vacutainer bottles containing 100 ml of acid-citrate-dextrose (ACD) for FC analysis as previously described (15). Five to 10 grams of feces was collected directly from the rectum for fecal culture, taking care not to induce bleeding.
To inoculate animals, a size 20 French Foley catheter was inserted into the cannula, directed orally, and inflated with air to obstruct ingesta flow through the ileum during the inoculation process. For inoculation, calves were administered their assigned treatments of M. avium subsp. paratuberculosis in 20 ml of PBS directly into the occluded ileum. The Foley catheter was left in place for 1 h to allow the inoculum to remain in contact with the isolated section of ileum. Previous studies demonstrated that M. avium subsp. paratuberculosis is taken up by enterocytes and microfold cells of the small intestines within 30 min after direct inoculation (37, 38).
Blood, serum, fecal, and biopsy samples were obtained on day 1 postinfection (p.i.) and then monthly until calves were euthanatized. The month postinoculation when each calf was euthanatized is shown in Table 1. At necropsy, tissue samples (duodenum, jejunum, ileum, ileocecal junction, cecum, rectum, mesenteric lymph nodes, ileocecal lymph nodes, liver, and spleen) were collected for histopathology, PCR, and culture for M. avium subsp. paratuberculosis. Intraepithelial lymphocytes (IEL) were obtained from segments of jejunum and ileum incised lengthwise to expose the mucosal surface. The mucosa was rinsed with PBS, followed by incubation in dithiothreitol (5 mM) in PBS for 30 min along with mechanical stripping with a gloved hand to break down mucous and free the IEL. Lymphocytes were separated from the resultant cell suspension by density gradient separation (Accupaque; 1.086 g/ml; Accurate Chemical and Scientific Corp., NY) and then labeled for FC analysis.
TABLE 1.
Biopsy schedule and IS900 PCR results
| Calfa | PCR detection of M. avium subsp. paratuberculosis at the indicated time pointb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Mo 1 | Mo 2 | Mo 3 | Mo 4 | Mo 5 | Mo 6 | Mo 7 | Mo 8 | Mo 9 | Mo 10 | |
| 132* | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Euthanized |
| 100** | Neg | Neg | Euthanized | ||||||||
| 101** | Neg | Neg | Neg | Pos | Pos | Neg | Neg | Neg | Euthanized | ||
| 119** | Neg | Neg | Euthanized | ||||||||
| 121** | Neg | Neg | Neg | Pos | Pos | Euthanized | |||||
| 122*** | Neg | Neg | Neg | Pos | Pos | Euthanized | |||||
| 123*** | Neg | Neg | Neg | Pos | Pos | Euthanized | |||||
| 124**** | Neg | Neg | Neg | Pos | Pos | Euthanized | |||||
| 136** | Pos | Pos | Pos | Pos | Euthanized | ||||||
| 137** | Pos | Pos | Pos | Pos | Euthanized | ||||||
| 139** | Pos | Pos | Pos | Pos | Euthanized | ||||||
| 142* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 143* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
*, uninoculated control; **, inoculated one time; ***, inoculated two times; ****, inoculated four times.
Animals 142 and 143 were noncannulated controls, and thus no biopsies were taken for these calves. NA, not available; Neg, negative; Pos, positive.
Histopathology.
Biopsy specimens and tissue samples obtained at necropsy were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for morphological evaluation as reported previously (32). Replicate sections were stained with Fite's acid fast stain and examined at a magnification of ×400. All histologic examinations were conducted by a single pathologist.
Sample processing for PCR (DNA extraction).
Biopsy and necropsy samples for PCR detection of M. avium subsp. paratuberculosis DNA were stored in microcentrifuge tubes at −20°C until processing. The DNA was extracted from tissues with DNeasy tissue kits (Qiagen, Valencia, CA) according to the manufacturer's instructions and as described by Quist et al. (29).
A second method of DNA extraction was used on tissues of calf 136 and calf 139. Tissue samples were transferred into 2-ml screw-cap tubes containing 600 μl of 0.85% of sodium chloride solution, 600 μl of phenol-chloroform-isoamyl alcohol (25:24:1), and 200 μl of 0.1-mm zirconia-silica beads (BioSpec Products, Inc., OK). The tubes were processed twice in a FastPrep-24 instrument (MP Biomedicals, CA) for 40 s at a setting of 6.0 and then centrifuged. The DNA was extracted from the aqueous phase by further phenol-chloroform-isoamyl alcohol extraction, followed by chloroform extraction. After ethanol precipitation, the DNA pellet was dissolved in nuclease-free water and stored at −20°C until needed.
PCR.
Oligonucleotide primers were derived from the DNA insertion sequence IS900, which is unique to M. avium subsp. paratuberculosis. Primers 150C and 921 (IS900/150C-Forward, 5′-CCGCTAATTGAGAGATGCGATTGG-3′; and IS900/921-Reverse 5′-AATCAACTCCAGCAGCGCGGCCTCG-3) were used to amplify a unique 229-bp fragment of the IS900 gene, as described by P. H. Vary et al. (45). The PCR mixture consisted of 25 μl of Go Taq Green Master Mix (Promega, Madison, WI), 1 μl of (25 pmol/μl) upstream primer, 1 μl of (25 pmol/μl) downstream primer, 1 μl of the DNA sample, and 22 μl of nuclease-free water. The PCR cycling conditions were the following: 35 cycles at 95°C for 1 min, 60°C for 1 min, and a final extension at 72°C for 1 min in a Thermal Cycler PCR System 9700 (Gene Amp, Foster City, CA). Negative controls consisted of sterile water and DNA extracted from intestinal mucosal samples taken from a JD-negative animal. Positive controls consisted of DNA extracted from intestinal mucosal samples taken from a cow with confirmed JD. The amplification product was analyzed using gel electrophoresis on a 2% agarose gel.
Culture of tissues and feces.
All biopsies, necropsy tissues, and fecal samples were submitted to Washington Animal Disease Diagnostic Laboratory for M. avium subsp. paratuberculosis culture using the following method. One to two grams of feces was suspended in 35 ml of sterile water. The tubes were shaken for 30 min and then allowed to settle for 30 min at room temperature. Ten to 15 ml of supernatant was placed in a sterile 50-ml conical tube and centrifuged at 1,730 × g (2,750 rpm) at room temperature for 20 min. Following removal of the supernatant, 12.5 ml of brain heart infusion (Difco Laboratories, Detroit, MI) broth and 12.5 ml of 1.8% hexadecyl pyridinium chloride broth were added to the fecal pellet as well as biopsies and necropsy tissues and then incubated overnight at 35°C. The samples were centrifuged for 20 min at 1,730 × g (2,750 rpm). The supernatants were discarded, and the fecal pellets and tissues were resuspended in 1 ml of antibiotic solution (5 mg of amphotericin B, 10 mg of vancomycin, and 10 mg of nalidixic acid dissolved in 50 ml of brain heart infusion broth and 50 ml of sterile water) overnight at 35°C. Three drops of sample were placed on four Herrold's egg yolk medium slant tubes with mycobactin J. The tubes were held for 13 weeks at 37°C and checked periodically for growth.
Due to poor recovery of M. avium subsp. paratuberculosis in tissue samples, a second method of tissue decontamination and culture modified from the method described by Schwartz et al. (34) was used on selected tissues from calves 100, 101, 119, and 121 to 124 and all tissues from calves 136, 139, and 132. Briefly, 500 mg of tissue from necropsy samples was cut into five pieces with a sterile scalpel blade and placed in a sterile 15-ml centrifuge tube containing 5 ml of a solution of NaOH-N-acetyl-l-cysteine, as described previously (16). The samples were kept at room temperature for 20 to 30 min and vortexed for 15 s twice. Ten milliliters of PBS was added to the sample and vortexed for 5 s. The samples were then centrifuged at 1,500 × g for 20 min. The supernatants were discarded, and 5 ml of PBS was added to each pellet and vortexed for 5 s. The samples were centrifuged at 1,500 × g for 5 min. The supernatants were discarded, and 5 ml of PBS was added to each pellet and vortexed for 5 s and then centrifuged at 1,500 × g for 5 min. The supernatants were again discarded, and the pellet was added to 10 ml of 7H9 liquid broth culture medium supplemented with 6.7% para-JEM GS with 2 μg/ml mycobactin J, 0.05% Tween 80, and para-JEM AS (antibiotic solution) (TREK Diagnostic Systems, Cleveland, OH) and incubated at 37°C in a shaking incubator (100 rpm). After the samples were incubated for 1 month, 100 μl of the samples was spread over 7H9 solid culture plates supplemented as described above and checked monthly for 5 months.
Johne's ELISA.
Serum samples were tested using an Idexx HerdCheck paratuberculosis antibody test kit. Test serum samples as well as duplicate positive and negative controls were run concurrently on the same plate as per kit instructions. A test run was considered valid if the difference between the positive control mean and negative control mean was ≥0.150 OD units and the negative control mean was ≤0.20 OD units. The presence or absence of antibody to M. avium subsp. paratuberculosis was determined based on the ratio of the OD value of the sample to that of the positive control (S/P ratio) for each sample. For diagnostic purposes, an S/P ratio of <0.25 was classified as negative, and an S/P ratio of ≥0.25 was classified as positive, as per instructions. Plates were read with a 96-well plate spectrophotometer (EL 808 Ultra Microplate Reader; Bio-Tek Instruments, Inc).
Bovine IFN-γ test.
Heparinized blood samples were utilized for a commercial EIA for the detection of IFN-γ (Bovigam EIA kit; Parkville, Victoria, Australia). The kit instructions were used to conduct the assay with the following modification. Briefly, 2 ml of heparinized blood was added to each of six wells of a 24-well tissue culture plate from each animal sampled. Nothing was added to the first two wells, 10 μg of Johnin purified protein derivative (PPD) was added to two wells, and 10 μg of concanavalin A was added to each of the last two wells. The plates were incubated at 37°C for 24 h. The two wells from each treatment were pooled and placed into 15-ml conical tubes and centrifuged at 1,500 × g for 10 min to obtain plasma, which was collected and stored at −20°C until processed. The remaining procedure was followed as described in the instruction manual from the Bovigam EIA kit.
Antigens.
M. avium subsp. paratuberculosis Johnin PPD was obtained from the National Veterinary Service Laboratory (Ames, IA). Soluble antigen was prepared from cultures of K10. Briefly, M. avium subsp. paratuberculosis was harvested from liquid culture by centrifugation at 1,500 × g for 10 min at 4°C, washed three times in PBS, suspended in PBS (pH 7.4) containing 0.01% Tween 80, and then irradiated (2.5 megarads) by exposure to 60Co as described previously (15). Following irradiation, preparations of M. avium subsp. paratuberculosis were sonicated and then mixed and vortexed with zirconia-silica beads in PBS (Biospec Products, Inc., Bartlesville, OK) to free soluble antigen (15). Phenylmethylsulfonyl fluoride was added to M. avium subsp. paratuberculosis soluble antigen at a final concentration of 1 mM to prevent protein degradation. Protein concentration of soluble antigen was calculated by measuring the OD280.
Blood processing for tissue culture and FC.
For analysis of the composition of peripheral blood at the time of initiation of culture, 10 ml of blood was collected in ACD and lysed in Tris-buffered NH4Cl to remove erythrocytes. The resultant cells were washed several times in PBS containing 20% ACD to remove excess platelets and then used in FC as described previously (15).
For analysis of the immune response to M. avium subsp. paratuberculosis antigens, peripheral blood mononuclear cells (PBMC) were obtained from buffy coat fractions of blood collected in ACD separated by density gradient centrifugation with Accupaque (density, 1.086 g/ml) (Accurate Chemical and Scientific Corp., Westbury, NY). Residual erythrocytes were lysed with H2O. Dead cells and debris were removed from cultured cells by density gradient centrifugation before use in FC.
PBMC were resuspended in RPMI 1640 medium supplemented with 13% bovine calf serum, 2-mercaptoethanol, and antibiotics and then distributed in T75 tissue culture flasks (3 × 106 cells/ml). One flask each was stimulated with PPD (20 μg/ml; RPMI 1640 complete medium) or soluble antigen (4 μg/ml). An additional flask was cultured without stimulation. Following culture for 6 days, cells were collected and subjected to density gradient centrifugation to remove dead cells and then labeled for FC as described previously (15).
FC analysis.
The monoclonal antibodies (MAbs) used in the present study are shown in Table 2. The three-color combinations used in the study are shown in Table 3. The combinations were designed to distinguish NK lymphocytes, αβ T lymphocytes, CD2+ and CD2− γδ T lymphocytes, and CD4 and CD8 T lymphocyte subsets. The CD2+ and CD2− γδ T lymphocytes correspond to the two major WC1− and WC1+ subsets of γδ T lymphocytes in ruminants (9). The MAbs specific for CD4 and CD8 were combined with CD45R0 (3) to distinguish naïve and memory T-lymphocyte subsets and MAbs specific for molecules upregulated on activated T lymphocytes: CD25, interleukin-2 alpha chain, CD26, dipetidyl peptidase IV ectoenzyme, CD71, transferrin receptor, ACT1 (activation molecule 1), and ACT16 (10, 21, 24). The same combinations of MAbs were used for labeling lymphocytes at the initiation and after 6 days of culture and also to label epithelial lymphocytes isolated from the jejunum and ileum at necropsy.
TABLE 2.
MAbs used in the present study
| MAb | Ig isotype | Specificity | Reference or source |
|---|---|---|---|
| AKS1 | IgG1 | CD335 | 40 |
| MUC2A | IgG2a | CD2 | 18 |
| ILA11A | IgG2a | CD4 | 2 |
| 7C2B | IgG2a | CD8 | 15 |
| GB21A | IgG2b | TCR1 δ chain specifica | 9 |
| ILA116A | IgG3 | CD45R0 | 4 |
| CACT116A | IgG1 | CD25 | 22 |
| CACT114A | IgG2b | CD26 | Sangun Lee |
| ILA77A | IgM | CD71 | 21 |
| CACT200A | IgG1 | ACT1 | 10 |
| GB110A | IgM | ACT16 | 10 |
T-cell receptor 1.
TABLE 3.
MAb combinations used to analyze the composition and activation status of lymphocyte subsetsa
| Well no. | MAb combination |
|---|---|
| 1 (control) | |
| 2 | CD335, CD2, and TCR1 δ |
| 3 | CD25, CD4, and CD45R0 |
| 4 | CD26, CD25, and CD45R0 |
| 5 | CD71, CD25, and CD45R0 |
| 6 | ACT1, CD25, and CD45R0 |
| 7 | ACT16, CD25, and CD45R0 |
| 8 | CD25, CD8, and CD45R0 |
| 9 | CD26, CD8, and CD45R0 |
| 10 | CD71, CD8, and CD45R0 |
| 11 | ACT1, CD8, and CD45R0 |
| 12 | ACT16, CD8, and CD45R0 |
Each well contains three antibodies of differing isotypes. With the use of isotype-specific second antibodies conjugated to different fluorochromes, cell subsets labeled with the antibodies appear in separate cytometer channels, as noted in the figures. The control well contains secondary antibodies but no primary antibodies. The control well is used to show that the secondary antibodies do not label cells nonspecifically.
Briefly, 106 lymphocytes were incubated for 15 min in 96-well conical bottom assay plates with combinations of the MAbs listed in Table 3. A total of 0.7 μg of each MAb was used in a final volume of 200 μl of PBS containing 20% ACD, 0.5% horse serum, and 0.09% azide. The lymphocytes were then washed three times and incubated for an additional 15 min in cocktails of isotype-specific second-step reagents conjugated with fluorescein, phycoerythrin, or phycoerythrin-Cy5 (Caltag, Burlingame, CA; Southern Biotechnology Associates Birmingham, AL). Following incubation, the lymphocytes were washed two times in PBS-20% ACD, fixed in 2% PBS-buffered formaldehyde, and kept at 7°C until examined.
A FACSort flow cytometer equipped with argon and red lasers, a Macintosh 5 computer, and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA) were used to collect data. FCS Express software (De Novo software, Thornton, Ontario, Canada) was used to analyze the data.
FC analysis of the proliferation response to M. avium subsp. paratuberculosis antigens.
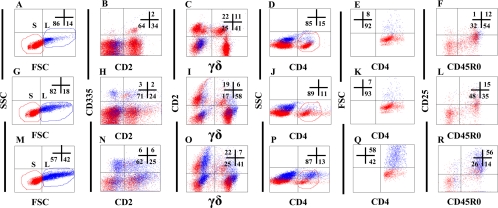
Blood was collected every 2 weeks for the first month after inoculation with M. avium subsp. paratuberculosis and then every month thereafter and processed for lymphocyte culture and FC as described previously (15). Selective gating was used to obtain results on specific subpopulations of lymphocytes. For time zero, data were collected on 25,000 mononuclear cells using gates placed on mononuclear cells, and side scatter (SSC) versus forward light scatter (FSC) was measured (Fig. 2). Granulocytes and nonspecific signal were excluded at the time of data acquisition. The S gate was used to define small lymphocytes (resting unactivated lymphocytes). The L gate was used to define monocytes and large lymphocytes at the start of culture and large lymphocytes undergoing blastogenesis and proliferation on day 6 of culture. For analysis of cultured lymphocytes, data were collected with and without additional gates to collect data on specific populations of lymphocytes. Only gates S and L were used to obtain data on all populations of lymphocytes: NK lymphocytes and αβ and γδ T lymphocytes. An additional gate was used to electronically isolate CD4+ and CD8+ T lymphocytes at the time of analysis to determine the pattern of expression of activation molecules on naïve and memory T lymphocytes.
FIG. 2.
Gating strategies used to follow the development of a CD4 T-lymphocyte response to M. avium subsp. paratuberculosis antigens following exposure to M. avium subsp. paratuberculosis through the cannula. (A to F) Lymphocytes at time zero. (G to L) lymphocytes cultured in RPMI medium alone for 6 days. (M to R) Lymphocytes cultured with PPD for 6 days. The numbers in the upper-right quadrant show the relative percentages of cells in each quadrant. The S and L gates in the plots in panels A, G, and M show the change in the relative proportions of activated proliferating cells that occurred following culture with and without antigen stimulation. The color coding of the cells in the S and L gates was used to track and distinguish resting (red, unactivated) cells from activated, proliferating (blue) cells. The color coding facilitated observing the actual change in the proportion of activated cells shown in the respective plots for NK cells (B, H, and N), γδ T cells (C, I, and O), and CD4 T cells (D, F, J, L, P, and R). Putting a third selective gate on CD4 let us enumerate the proportion of activated CD4 cells present at T0 and after culture with and without antigen (compare plots E, K, and Q).
Statistical analysis.
Representative FC profiles demonstrate how the data were obtained for statistical evaluation (Fig. 2). CD4 and CD8 T cells were electronically isolated and evaluated for a proliferative response to M. avium subsp. paratuberculosis antigens. Since the proliferative responses to soluble antigen and PPD were similar, results are shown only for PPD. The two-sample t test with equal variances on Statistix, version 7.0 (Analytical Software, Tallahassee, FL), was used to compare the response to PPD over time in experimentally inoculated versus uninfected, negative control animals. To compare the negative control group to the experimentally inoculated group, the percentages of activated CD4 T lymphocytes measured at time zero (preinoculation) were subtracted from the percentages of activated CD4 T lymphocytes measured 3 to 4 months postinoculation. The difference pre- and postinoculation for the experimental group was then compared to the difference before and after a period of 3 to 4 months in the negative controls.
RESULTS
Cannulation and health of animals.
Most of the animals had uneventful recoveries from surgery and returned to normal behavior and health soon after surgery. The ileal mucosa typically appeared normal at the time of endoscopic evaluation at 2 weeks postsurgery. A rare postsurgical complication included adhesion formation between the cannulated ileum and other abdominal structures, resulting in intestinal blockage. Calves suffering intestinal blockage were removed from the study. An infrequent minor complication was excessive granulation tissue around the cannula. In the initial experiments where smaller (0.5-in.) cannulas were used, we noted that, as the calves grew, skin surrounding the cannula overgrew the neck of the cannula. This problem was resolved by using 1-in. cannulas. The use of the larger cannulas also made it easier to pass and maneuver the endoscope. Thus far, cannulated calves with the larger cannulas have survived for up to 10 months without complications.
Histopathology and PCR.
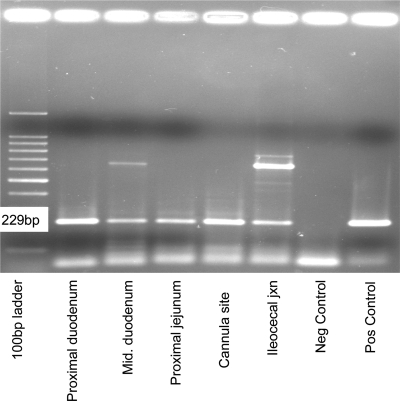
M. avium subsp. paratuberculosis cells tagged with GFP were detected in ileal mucosal macrophages by fluorescence microscopy of biopsies at 24 h p.i. Infected macrophages were more difficult to detect in subsequent biopsies. However, M. avium subsp. paratuberculosis DNA was detected in biopsied tissues by PCR throughout the study. Seven of the nine experimentally infected calves had positive biopsy samples for the IS900 probe at 3 months postinoculation. The two calves that did not have positive biopsies were euthanized at 3 months and had positive samples at necropsy. The number of tissue samples positive for M. avium subsp. paratuberculosis DNA taken at necropsy varied depending on how long the calves had been infected prior to necropsy. Calf 101 was infected for 8 months and had 10 PCR-positive samples collected at necropsy from the proximal duodenum, middle duodenum, distal duodenum, proximal jejunum, cannula site, distal ileum, ileocecal junction, ileocecal lymph node, distal colon, and liver (Fig. 3). Calves infected for 4 months (121 to 124) had fewer (four to five) tissue sites that were PCR positive including the distal jejunum, proximal ileum, middle ileum, cannula site, distal ileum, ileocecal junction, mesenteric lymph nodes, ileocecal lymph node, spleen, and liver.
FIG. 3.
PCR of M. avium subsp. paratuberculosis DNA isolated from the indicated tissues at necropsy.
Culture.
Initial attempts to culture M. avium subsp. paratuberculosis from biopsies and feces using the first method described yielded negative results. Subsequent modification of the isolation and culture techniques facilitated isolation of M. avium subsp. paratuberculosis from biopsy samples at four different time points and two different time points from calves 136 and 139, respectively. At necropsy calf 139 had three positive culture sites, and calf 136 had five positive culture sites. Samples from calves 100, 101, 119, and 121 to 124 were reprocessed for culture after 2 years of frozen storage. M. avium subsp. paratuberculosis was successfully cultured from calf 124 necropsy tissues where the initial culture technique had yielded no growth.
ELISA and IFN-γ.
Antibody activity was not detected by ELISA in sera from the infected calves at any time point during the study. Results of the IFN-γ assay did not show a significant difference between experimental calves and negative control calves (data not shown).
FC analysis of PBMC.
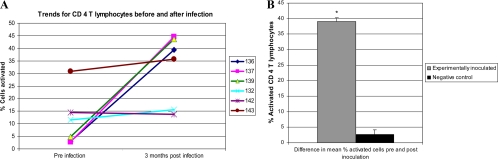
Direct inoculation of M. avium subsp. paratuberculosis via the ileum elicited a response similar to the response elicited by inoculation by the oral route (15). As noted previously, NK lymphocytes comprised 1 to 3% of PBMC at the initiation of cultures (Fig. 2B). The extent of proliferation was variable in cultures with and without antigen. There appeared to be more extensive proliferation in cultures with antigen. About half of the proliferating NK lymphocytes were CD2− in cultures with and without antigen (Fig. 2H and N). γδ T lymphocytes comprised 50 to 60% of the lymphocytes at the initiation and after 6 days of culture. Most of the γδ T lymphocytes were CD2−. The CD2− γδ T lymphocytes expressed CD25 at a low level at time zero (data not shown). Expression increased following 6 days of culture, predominantly in cultures stimulated with antigen. Expression was highest on proliferating γδ T lymphocytes. The CD2+ γδ T lymphocytes expressed minimal or no CD25 at the initiation and following 6 days of culture. The majority of the lymphocytes remained small, indicating that few of the lymphocytes were activated. B lymphocytes were present and persisted in the cultures, as noted previously (15). Their presence is inferred from the results shown in Fig. 2C, I, and O (lower-left quadrants). The lower-left quadrant of plot B (time zero) also contained monocytes. CD4 T lymphocytes comprised about 15% of the lymphocytes at the initiation of culture and 11% and 13% following 6 days of culture in RPMI medium alone or with antigen, respectively (Fig. 2D, J, and P). Some CD45R0+ memory CD4 T lymphocytes exhibited a low level of expression of CD25 at the initiation of culture, predominantly on cells in the small gate (Fig. 2F). Expression was similar on memory T lymphocytes following culture in RPMI medium for 6 days (compare Fig. 2F and L) but markedly increased on proliferating blast cells in antigen-stimulated cultures (compare plots L and R). The difference in the proportions of activated proliferating cells (large gate) was more evident when the CD4 population was electronically isolated for evaluation. Although the total populations of CD4 cells present at the beginning and end of culture appeared similar, approximately 58% of the cells in the antigen-stimulated culture were blast cells (Fig. 2Q; since the data for PPD and soluble antigen stimulation were very similar, data are shown only for PPD). All the blast cells (large gate) expressed CD25, as noted previously (15). The increase in expression was predominantly on enlarged proliferating lymphocytes, readily evident using electronic gates S and L to distinguish resting and activated lymphocytes (compare Fig. 2F, L, and R). Comparison of activated cells from uninfected calves at the initiation of culture with cells cultured with and without antigen showed that the proportion of activated cells at the initiation of culture varied from a few percent as shown in Fig. 2 or up to 30% for CD4 T cells (Fig. 4) and 27% for CD8 T cells (data not shown). Examination of the response to PPD over time showed little change in the control negative animals (Fig. 4A). In contrast, there was a marked increase in the response of animals experimentally inoculated with wild-type and mutant M. avium subsp. paratuberculosis (Fig. 4A). The difference in CD4 T-lymphocyte activation in PBMC poststimulation with antigen was significantly larger (P = 0.0001) in experimentally inoculated animals than in noninfected controls (Fig. 4B).
FIG. 4.
Comparison of the response to PPD in uninfected and experimentally infected calves. There was a significant difference (P = 0.0001) between activated CD 4 T lymphocytes in the experimentally inoculated group (animals 136, 137, and 139) compared to the negative control group (132, 142, and 143) over time. Neg, negative; Pos, positive.
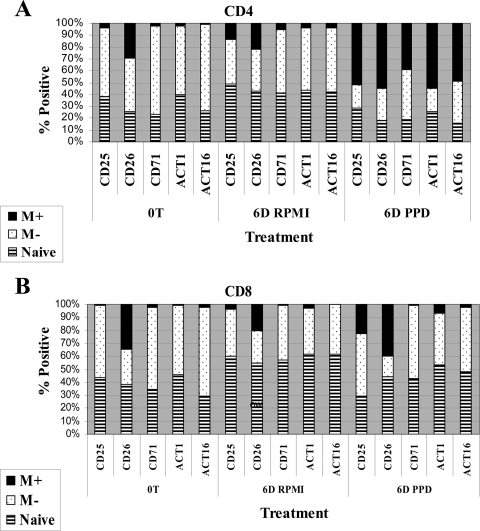
As shown in Fig. 5, expression of additional activation molecules was also upregulated. CD26 was expressed on a subset of CD4 T lymphocytes at the initiation of culture and on the majority of CD4 T lymphocytes after stimulation. CD25 and the other molecules (CD71, ACT1, and ACT16) were upregulated only on antigen-stimulated lymphocytes. The relative proportions of naïve and CD25− memory T lymphocytes decreased with the increase in CD45R0+ CD4 memory T lymphocytes, reflecting the increase in the proportion of proliferating CD25+ CD4 memory T cells in the culture (Fig. 5A).
FIG. 5.
Comparison of the relative proportions of naïve and memory CD4 and CD8 T lymphocytes expressing five activation molecules at the initiation of culture (time zero, 0T) and following 6 days (6D) of culture in RPMI medium alone or stimulation with PPD. M+, CD25+ memory T cells; M−, CD25− memory T cells.
CD8 T lymphocytes comprised about 8% of the lymphocytes at the initiation of culture and 10% at 6 days with and without antigen (Fig. 5B). In contrast to CD4 T lymphocytes, CD25 and CD26 were the predominant molecules clearly upregulated on CD8 T lymphocytes (Fig. 5B). The activation molecules were expressed on memory T lymphocytes present in the L gate.
FC analysis of intraepithelial lymphocytes.
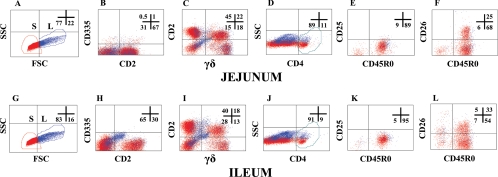
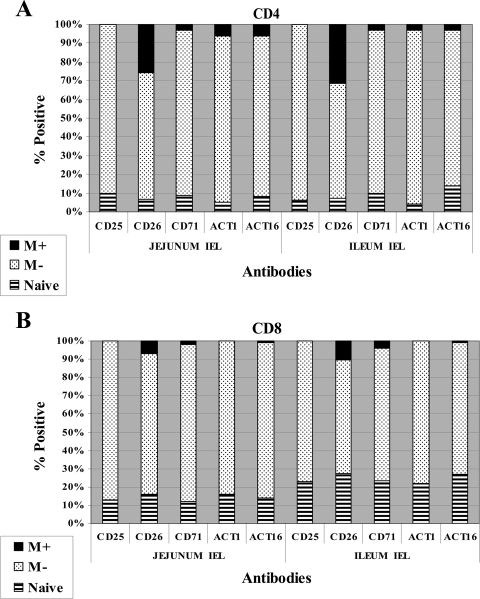
FC profiles of lymphocytes (IELs) in the jejunum and ileum were obtained at necropsy. The method of preparation yielded a large number of epithelial lymphocytes but no macrophages. As shown in Fig. 6B and H, NK lymphocytes comprised about 1% or less of the lymphocytes in the IEL in the jejunum and ileum. αβ T lymphocytes comprised 30 to 45% of the T lymphocytes while γδ T lymphocytes comprised 25 to 40%. In contrast to peripheral blood, the proportions of CD2+ and CD2− γδ T lymphocytes were almost equal. CD4 and CD8 T lymphocytes comprised about 10% each of the lymphocytes (data not shown for CD8). B lymphocytes comprised about 15% in the jejunum and 28% in the ileum in these representative profiles (inferred from the percentage of cells present in the lower-left quadrants in plots C and I of Fig. 6). Except for CD26, there was little or no expression of the other activation molecules examined in this study (Fig. 7). The compositions and of lymphocyte populations and patterns of expression of activation molecules were similar on preparations of cells collected from control animals (data not shown).
FIG. 6.
Gating strategies used to determine the relative proportion and activation status of NK (CD335), γδ T lymphocytes, and CD4 T lymphocytes in intraepithelial lymphocytes isolated from the jejunum and ileum from a calf 5 months p.i.
FIG. 7.
Comparison of the relative proportion of naïve and memory CD4 and CD8 T IEL expressing five activation molecules in the jejunum and ileum. M+, CD25+ memory T cells; M−, CD25− memory T cells.
DISCUSSION
Development of the ileal cannulation model and an FC assay to characterize the composition of PBMC before and after antigenic stimulation has afforded an opportunity to analyze the immune response to M. avium subsp. paratuberculosis and the early events of infection and pathogenesis in the ileum in greater detail (11). The model and assay now offer opportunities to extend studies to later stages of infection to detail the cellular and molecular events associated with dysregulation of protective immunity and the development of clinical disease. The model may also afford a method to assess the potential efficacy of candidate vaccines. Previous studies have shown that calves are readily infected by the oral route and that M. avium subsp. paratuberculosis can be detected in multiple tissues in the first weeks p.i. (15, 42, 46). Detection becomes progressively more difficult at later times p.i. as infection comes under immune control. Direct analysis of the interaction of M. avium subsp. paratuberculosis with ileal tissue in cattle and goats, using segmented ileal loop and everted ileum models, has shown that M. avium subsp. paratuberculosis is rapidly taken up within minutes by microfold cells, enterocytes, macrophages, and most likely dendritic cells (19, 28, 37-39, 44). Examination of mesenteric lymph nodes and other peripheral tissue following direct inoculation into the ileum, using a surgical intervention model, revealed that M. avium subsp. paratuberculosis can be detected in lymph nodes within an hour p.i. (48). Bacteria were detected in different tissues at 8 months p.i. in this study (48). Although it proved difficult to culture bacteria from tissues in the present study, use of the cannulation model has shown that M. avium subsp. paratuberculosis persists in ileal tissue at a low level detectable by PCR, indicating that a chronic infection is established following exposure. The immune response that develops during this time period is able to control infection but unable to eliminate the pathogen. Endoscopic evaluation demonstrated that the presence of M. avium subsp. paratuberculosis at a low level does not initially induce a visible inflammatory response for at least 8 months p.i. Consistent with this observation, histological examination did not reveal any signs of lymphocytic infiltration or accumulations of macrophages characteristic of lesion development.
In addition, no difference was evident in the compositions of epithelial lymphocyte subset populations present in the jejunum and ileum of uninfected and infected animals. The majority of CD4 and CD8 lymphocytes expressed the memory T-cell marker CD45R0. Except for CD26, none of the activation molecules examined in this study was upregulated on either population. CD26 was expressed on both CD4 and CD8 subsets. Recent efforts to culture and stimulate mucosal lymphocytes from infected animals have shown that cells do not proliferate in response to antigenic stimulation (unpublished observations).
Without culture of M. avium subsp. paratuberculosis from the tissues or feces, it is reasonable to question whether M. avium subsp. paratuberculosis infections were established in these study animals. Evidence that supports establishment of infection includes the fact that it became easier to detect M. avium subsp. paratuberculosis DNA from biopsies as time from inoculation progressed and that M. avium subsp. paratuberculosis DNA was detected from different tissue sites, suggesting spread from the ileal inoculation site. These findings were similar to those described by Wu et al. (48). With new culture techniques, we were able to culture M. avium subsp. paratuberculosis from the last two study calves infected with wild-type M. avium subsp. paratuberculosis as well as from tissues of a calf that was previously culture negative. We are confident that incorporation of the subsequent culture technique would have greatly enhanced our ability to obtain positive cultures from all study animals.
Improvements in analytical software have facilitated the use of multiparameter FC to study the immune response to pathogenic mycobacteria (11, 15). As reported here, multicolor FC has provided the means to examine the immune response to M. avium subsp. paratuberculosis in greater detail and to help resolve one of the difficulties encountered in comparing the immune response to M. avium subsp. paratuberculosis in control and experimentally infected animals. It is possible to use combinations of electronic gates and artificial color coding to isolate populations of cells for further analysis. Activated proliferating lymphocytes increase in size. This permits the use of two electronic gates in SSC and FSC to define resting and activated cells as well as to monitor the extent of cell proliferation following culture with and without antigenic stimulation. The use of a third electronic gate permits the isolation of a cell population to determine the extent of proliferation and expression of molecules upregulated following culture. The use of FSC versus a fluorescence channel permits comparison of the relative proportion of resting and activated cells present in the respective gated cell populations. The two populations of cells can then be analyzed to evaluate expression of two or more additional molecules. The use of a single gate in SSC versus FSC in conjunction with the gated population permits examination of the expression of activation molecules (e.g., CD25) on resting and activated cells before and after culture. We have used these various gating strategies in the present study with the cannulated ileum model to extend our previous observations.
Examination of the immune response demonstrated that direct inoculation leads to the development of an immune response similar to the response elicited by exposure to M. avium subsp. paratuberculosis by the oral route (15, 46). The response is characterized by a vigorous CD4 memory T-lymphocyte response to PPD and soluble antigen. CD25, CD26, CD71, and nine additional molecules are upregulated on CD4 memory T lymphocytes (15). CD8 memory T lymphocytes also proliferate in response to antigenic stimulation, but the response is less vigorous. The studies completed thus far suggest that the CD8 response may develop later than the CD4 response. In addition, there may be a difference in the pattern of expression of some activation molecules on activated CD4 and CD8 memory T lymphocytes, as noted in the present study. Further studies are needed to extend these observations.
Preparations of PBMC also contain NK and γδ T lymphocytes. NK lymphocytes comprise ∼2 to 3% of the lymphocytes in freshly isolated PBMC (17). The CD2+ and CD2− γδ T lymphocytes may comprise ∼50 to 60%. The CD2+ subset usually comprises a smaller proportion of the cells than the CD2− subset in young animals(9). The role of NK and γδ T lymphocytes in immunity and immunopathogenesis of JD is unclear, but they may be a source of cytokines that modulate immune responses (6, 25, 30, 47). The majority of freshly isolated bovine NK cells express CD2. As noted here, some NK cells may be activated and proliferate when cultured in medium alone and lose expression of CD2. Activation and proliferation are more pronounced in cultures stimulated with antigen. Activated NK cells express CD25. Further studies are needed to show whether there is an antigen-specific response important in JD (25). The CD2− subset of γδ T lymphocytes expresses CD25 at a low level in fresh preparations of PBMC. Following culture, this subset becomes activated, especially in the presence of antigen. The CD2+ γδ T-lymphocyte subset expresses CD25 after antigen stimulation, but the relative concentration of CD2+ γδ T lymphocytes never exceeds the CD2− population. In contrast to the relative proportions of these populations in peripheral blood, the proportions are similar in jejunal and ileal epithelial lymphocytes. The relative proportion of these subsets may be equal to or greater than the proportions of CD4 and CD8 T lymphocytes in the jejunum and ileum. Further studies are needed to clarify the role of these cell subsets in the immunopathogenesis of JD. Previous studies have suggested that they could play a role in regulation of the immune response to M. avium subsp. paratuberculosis (7, 8).
Although the FC assay has proved useful in the further characterization of the immune response to M. avium subsp. paratuberculosis, previous and ongoing studies show that the assay is best used with experimental animals kept in BSL-2 holding facilities. Some animals maintained in open facilities develop a response to PPD, presumably as a result of exposure to environmental mycobacteria (Fig. 4). The control animals that we have maintained in BSL-2 facilities have had a minimal response to PPD and soluble antigen. We have noted, however, that control animals maintained in clean but open facilities can develop a response to M. avium subsp. paratuberculosis antigens that is difficult to distinguish from the early response induced in experimentally infected calves. As shown here, comparison of the proliferative response to PPD in experimentally infected calves maintained in BSL-2 facilities showed that there is a significant difference between the responses of calves maintained in open facilities and those in BSL-2 facilities. The response to PPD in experimentally infected calves increases over time while the response to PPD in uninfected calves persists at the same level.
In summary, we have developed a cannulated ileum model for the study of JD as well as other enteric pathogens and used it to study the early and late stages of infection and JD pathogenesis. The present and ongoing studies have now demonstrated that cannulated animals can be maintained for more than a year without loss of access to the ileum. Initial studies have shown that direct exposure to M. avium subsp. paratuberculosis leads to a persistent low level of infection that does not induce pathological changes in the ileum for up to a year. The immune response to M. avium subsp. paratuberculosis following direct inoculation was similar to the response elicited after exposure by the oral route. The early response to M. avium subsp. paratuberculosis is dominated by CD4 T cells, with the CD8 T-cell response occurring later. NK and γδ T cells are present and may proliferate, especially in antigen-stimulated cultures. In contrast to the composition in peripheral blood, CD2+ and CD2− γδ T cells are present in equal proportions in the jejunum and ileum. The majority of CD4 and CD8 T cells expressed CD45R0. Consistent with the pathological findings, few cells expressed the activation molecules examined in this study.
It is also possible that this model could be used to study other enteric diseases such as salmonella, clostridial, and protozoal enteritis, where serial sampling directly from infected tissues could be beneficial.
Acknowledgments
The assistance of Amanda Grimm and Laura Drader is acknowledged and greatly appreciated.
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract number N01-AI-30055; the JDIP program (USDA-CSREES-NRI-CAP award no. 2007-01019); and the WSU Monoclonal Antibody Center.
Footnotes
Published ahead of print on 18 February 2009.
REFERENCES
- 1.Autschbach, F., S. Eisold, U. Hinz, S. Zinser, M. Linnebacher, T. Giese, T. Löffler, M. W. Büchler, and J. Schmidt. 2005. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn's disease. Gut 54944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, C. L., A. J. Teale, J. G. Naessens, B. M. Goddeeris, N. D. MacHugh, and W. I. Morrison. 1986. Characterisation of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J. Immunol. 1364385-4391. [PubMed] [Google Scholar]
- 3.Bembridge, G. P., N. D. MacHugh, D. McKeever, E. Awino, P. Sopp, R. A. Collins, K. I. Gelder, and C. J. Howard. 1995. CD45RO expression on bovine T cells: relation to biological function. Immunology 86537-544. [PMC free article] [PubMed] [Google Scholar]
- 4.Bembridge, G. P., K. R. Parsons, P. Sopp, N. D. MacHugh, and C. J. Howard. 1993. Comparison of monoclonal antibodies with potential specificity for restricted isoforms of the leukocyte common antigen (CD45R). Vet. Immunol. Immunopathol. 39129-136. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, R. W., J. I. Keenan, R. B. Gearry, M. A. Kennedy, M. L. Barclay, and R. L. Roberts. 2008. Incidence of Mycobacterium avium subspecies paratuberculosis in a population-based cohort of patients with Crohn's disease and control subjects. Am. J. Gastroenterol. 1031168-1172. [DOI] [PubMed] [Google Scholar]
- 6.Boysen, P., I. Olsen, I. Berg, S. Kulberg, G. M. Johansen, and A. K. Storset. 2006. Bovine CD2−/NKp46+ cells are fully functional natural killer cells with a high activation status. BMC Immunol. 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiodini, R. J., and W. C. Davis. 1992. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb. Pathog. 13447-463. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, R. J., and W. C. Davis. 1993. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4+ helper and CD8+ immunoregulatory T lymphocytes which down-regulate gamma/delta+ T-cell cytotoxicity. Microb. Pathog. 14355-367. [DOI] [PubMed] [Google Scholar]
- 9.Davis, W. C., W. C. Brown, M. J. Hamilton, C. R. Wyatt, J. A. Orden, A. M. Khalid, and J. Naessens. 1996. Analysis of monoclonal antibodies specific for the γδ TcR. Vet. Immunol. Immunopathol. 52275-283. [DOI] [PubMed] [Google Scholar]
- 10.Davis, W. C., J. Naessens, W. C. Brown, J. A. Ellis, M. J. Hamilton, G. H. Cantor, J. I. R. Barbosa, W. Ferens, and G. A. Bohach. 1996. Analysis of monoclonal antibodies reactive with molecules upregulated or expressed only on activated lymphocytes. Vet. Immunol. Immunopathol. 52301-311. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrmann, S., M. Streitz, and F. Kern. 2008. How flow cytometry is changing the study of TB immunology and clinical diagnosis. Cytometry A 731100-1106. [DOI] [PubMed] [Google Scholar]
- 12.Harris, N. B., D. K. Zinniel, M. K. Hsieh, J. D. Cirillo, and R. G. Barletta. 2002. Cell sorting of formalin-treated pathogenic Mycobacterium paratuberculosis expressing GFP. BioTechniques 32522-527. [DOI] [PubMed] [Google Scholar]
- 13.Hines, M. E. II., J. R. Stabel, R. W. Sweeney, A. M. Talaat, D. Bakker, G. Benedictus, W. C. Davis, G. W. de Lisle, I. A. Gardner, R. A. Juste, V. Kapur, A. Koets, J. McNair, G. Pruitt, and R. H. Whitlock. 2007. Experimental challenge models for Johne's disease: a review and proposed international guidelines. Vet. Microbiol. 122197-122. [DOI] [PubMed] [Google Scholar]
- 14.Jeyanathan, M., D. C. Alexander, C. Y. Turenne, C. Girard, and M. A. Behr. 2006. Evaluation of in situ methods used to detect Mycobacterium avium subsp. paratuberculosis in samples from patients with Crohn's disease. J. Clin. Microbiol. 442942-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo, H. C., Y. H. Park, M. J. Hamilton, G. M. Barrington, C. J. Davies, J. B. Kim, J. L. Dahl, W. R. Waters, and W. C. Davis. 2004. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 726870-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubica, G. P., W. E. Dye, M. L. Cohn, and G. Middlebrook. 1963. Sputum digestion and decontamination with N-acetyl-l-cysteine-sodium hydroxide for culture of mycobacteria. Am. Rev. Respir. Dis. 87775-779. [DOI] [PubMed] [Google Scholar]
- 17.Kulberg, S., P. Boysen, and A. K. Storset. 2004. Reference values for relative numbers of natural killer cells in cattle blood. Dev. Comp. Immunol. 28941-948. [DOI] [PubMed] [Google Scholar]
- 18.Larsen, R. A., M. L. Monaghan, Y. H. Park, M. J. Hamilton, J. A. Ellis, and W. C. Davis. 1990. Identification and characterization of monoclonal antibodies reactive with bovine, caprine, and ovine T-lymphocyte determinants by flow microfluorimetry. Vet. Immunol. Immunopathol. 25195-208. [DOI] [PubMed] [Google Scholar]
- 19.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25131-137. [DOI] [PubMed] [Google Scholar]
- 20.Nacy, C., and M. Buckley. 2008. Mycobacterium avium paratuberculosis: infrequent human pathogen or public health threat? Report of a colloquium, 15 to 17 June 2007, Salem, MA. American Academy of Microbiology, Washington, DC. [PubMed]
- 21.Naessens, J., and W. C. Davis. 1996. Ruminant cluster CD71. Vet. Immunol. Immunopathol. 52257-258. [DOI] [PubMed] [Google Scholar]
- 22.Naessens, J., M. Sileghem, N. MacHugh, Y. H. Park, W. C. Davis, and P. Toye. 1992. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology 76305-309. [PMC free article] [PubMed] [Google Scholar]
- 23.Naser, S. A., G. Ghobrial, C. Romero, and J. F. Valentine. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 3641039-1044. [DOI] [PubMed] [Google Scholar]
- 24.Ohnuma, K., N. H. Dang, and C. Morimoto. 2008. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 29295-301. [DOI] [PubMed] [Google Scholar]
- 25.Olsen, I., P. Boysen, S. Kulberg, J. C. Hope, G. Jungersen, and A. K. Storset. 2005. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect. Immun. 735628-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40179-192. [DOI] [PubMed] [Google Scholar]
- 27.Park, K. T., J. L. Dahl, J. P. Bannantine, R. G. Barletta, J. Ahn, A. J. Allen, M. J. Hamilton, and W. C. Davis. 2008. Demonstration of allelic exchange in the slow-growing bacterium Mycobacterium avium subsp. paratuberculosis, and generation of mutants with deletions at the pknG, relA, and lsr2 loci. Appl. Environ. Microbiol. 741687-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel, D., L. Danelishvili, Y. Yamazaki, M. Alonso, M. L. Paustian, J. P. Bannantine, L. Meunier-Goddik, and L. E. Bermudez. 2006. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 742849-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quist, C. F., V. F. Nettles, E. J. Manning, D. G. Hall, J. K. Gaydos, T. J. Wilmers, and R. R. Lopez. 2002. Paratuberculosis in key deer (Odocoileus virginianus clavium). J. Wildl. Dis. 38729-737. [DOI] [PubMed] [Google Scholar]
- 30.Roark, C. L., P. L. Simonian, A. P. Fontenot, W. K. Born, and R. T. L. O'Brien. 2008. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 20353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero, C., A. Hamdi, J. F. Valentine, and S. A. Naser. 2005. Evaluation of surgical tissue from patients with Crohn's disease for the presence of Mycobacterium avium subspecies paratuberculosis DNA by in situ hybridization and nested polymerase chain reaction. Inflamm. Bowel Dis. 11116-125. [DOI] [PubMed] [Google Scholar]
- 32.Rosadio, R. H., M. D. Lairmore, H. I. Russell, and J. C. DeMartini. 1988. Retrovirus-associated ovine pulmonary carcinoma (sheep pulmonary adenomatosis) and lymphoid interstitial pneumonia. I. Lesion development and age susceptibility. Vet. Pathol. 25475-483. [DOI] [PubMed] [Google Scholar]
- 33.Scanu, A. M., T. J. Bull, S. Cannas, J. D. Sanderson, L. A. Sechi, G. Dettori, S. Zanetti, and J. Hermon-Taylor. 2007. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn's disease and Johne's disease: common neural and immune pathogenicities. J. Clin. Microbiol. 453883-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz, D., I. Shafran, C. Romero, C. Piromalli, J. Biggerstaff, N. Naser, W. Chamberlin, and S. A. Naser. 2000. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn's disease patients. Clin. Microbiol. Infect. 6303-307. [DOI] [PubMed] [Google Scholar]
- 35.Sechi, L. A., M. Mura, E. Tanda, A. Lissia, G. Fadda, and S. Zanetti. 2004. Mycobacterium avium subsp. paratuberculosis in tissue samples of Crohn's disease patients. Microbiologica 2775-77. [PubMed] [Google Scholar]
- 36.Sechi, L. A., A. M. Scanu, P. Molicotti, S. Cannas, M. Mura, G. Dettori, G. Fadda, and S. Zanetti. 2005. Detection and isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. Am. J. Gastroenterol. 1001529-1536. [DOI] [PubMed] [Google Scholar]
- 37.Sigurdardóttir, Ó. G., A. M. Bakke-McKellep, B. Djønne, and Ø. Evensen. 2005. Mycobacterium avium subsp. paratuberculosis enters the small intestinal mucosa of goat kids in areas with and without Peyer's patches as demonstrated with the everted sleeve method. Comp. Immunol. Microbiol. Infect. Dis. 28223-230. [DOI] [PubMed] [Google Scholar]
- 38.Sigurdardóttir, Ó. G., C. M. Press, and Ø. Evensen. 2001. Uptake of Mycobacterium avium subsp. paratuberculosis through the distal small intestinal mucosa in goats: an ultrastructural study. Vet. Pathol. 38184-189. [DOI] [PubMed] [Google Scholar]
- 39.Sigurdardóttir, Ó. G., M. Valheim, and C. M. Press. 2004. Establishment of Mycobacterium avium subsp. paratuberculosis infection in the intestine of ruminants. Adv. Drug Deliv. Rev. 56819-834. [DOI] [PubMed] [Google Scholar]
- 40.Storset, A. K., S. Kulberg, I. Berg, P. Boysen, J. C. Hope, and E. Dissen. 2004. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 34669-676. [DOI] [PubMed] [Google Scholar]
- 41.Streeter, M. N., S. J. Barron, D. G. Wagner, C. A. Hibberd, F. N. Owens, and F. T. McCollum. 1991. Technical note: a double L intestinal cannula for cattle. J. Anim. Sci. 692601-2607. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney, R. W., J. Uzonna, R. H. Whitlock, P. L. Habecker, P. Chilton, and P. Scott. 2006. Tissue predilection sites and effect of dose on Mycobacterium avium subsp. paratuberculosis organism recovery in a short-term bovine experimental oral infection model. Res. Vet. Sci. 80253-259. [DOI] [PubMed] [Google Scholar]
- 43.USDA Animal and Plant Health Inspection Service, Veterinary Services. 2008. Johne's disease on U.S. dairies, 1991-2007. USDA Animal and Plant Health Inspection Service, Ames, IA.
- 44.Valheim, M., Ó. G. Sigurðardóttir, A. K. Storset, L. G. Aune, and C. M. Press. 2004. Characterization of macrophages and occurrence of T cells in intestinal lesions of subclinical paratuberculosis in goats. J. Comp. Pathol. 131221-232. [DOI] [PubMed] [Google Scholar]
- 45.Vary, P. H., P. R. Andersen, E. Green, J. Hermon-Taylor, and J. J. McFadden. 1990. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J. Clin. Microbiol. 28933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 715130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolk, K., and R. Sabat. 2006. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17367-380. [DOI] [PubMed] [Google Scholar]
- 48.Wu, C. W., M. Livesey, S. K. Schmoller, E. J. Manning, H. Steinberg, W. C. Davis, M. J. Hamilton, and A. M. Talaat. 2007. Invasion and persistence of Mycobacterium avium subsp. paratuberculosis during early stages of Johne's disease in calves. Infect. Immun. 752110-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]