Abstract
Human pythiosis is an emerging and life-threatening infectious disease caused by the fungus-like organism Pythium insidiosum. High rates of morbidity and mortality for patients with pythiosis are exacerbated by the lack of early diagnosis and an effective treatment. Here, we developed and evaluated an immunochromatographic test (ICT) for the diagnosis of human pythiosis, in comparison to a standard serological test of immunodiffusion (ID). Culture filtrate antigen of P. insidiosum was used to detect human anti-P. insidiosum antibody. Sheep anti-human immunoglobulin G-colloidal gold conjugate was used to generate an ICT signal. Thirty-three sera from patients with vascular (n = 27), ocular (n = 4), and cutaneous (n = 2) pythiosis and 181 control sera from healthy blood donors (n = 100), as well as patients with a variety of infectious (n = 56) and noninfectious (n = 25) diseases, were included in the test evaluation. The turnaround time for generating a result by the ICT was less than 30 min, while that for ID was ∼24 h. Based on the results for all sera of pythiosis patients and the control groups, the ICT showed 88% sensitivity and 100% specificity and ID showed 61% sensitivity and 100% specificity. By both tests, false-negative results for sera from all ocular pythiosis patients were obtained. In addition, the ID test yielded false-negative results for sera from eight patients with vascular pythiosis and one patient with cutaneous pythiosis. It was concluded that the ICT is a rapid, user-friendly, and reliable serological test for the early diagnosis of vascular and cutaneous pythiosis.
Pythiosis is a life-threatening infectious disease caused by the oomycete, fungus-like, aquatic organism Pythium insidiosum, which is the only Pythium species of the kingdom Stramenopila known to infect humans and some animals, such as horses, dogs, cats, and cattle, in tropical and subtropical countries (5, 11). Although microscopic features of oomycete organisms are similar to those of fungi, a phylogenic analysis shows that Pythium spp. are more closely related to diatoms and algae than to the true fungi (10). P. insidiosum inhabits swampy areas, where it exists in two stages: perpendicular branching hyphae and biflagellate zoospores (12). Infection has been proposed to occur by invasion of the zoospores into host tissue after attachment and germination (12).
Human pythiosis is endemic in Thailand, where the disease has been increasingly reported from all over the country (2, 3, 8, 9, 19-24, 26, 27). Four forms of human pythiosis have been described: (i) cutaneous pythiosis, affecting the face or limbs as a granulomatous and ulcerating lesion; (ii) vascular pythiosis, affecting arteries and resulting in arterial occlusion or an aneurysm; (iii) ocular pythiosis, causing corneal ulcers; and (iv) disseminated pythiosis, featuring the infection of internal organ (9). Vascular and ocular infections are the most common forms of pythiosis. The majority of vascular pythiosis patients have an affected leg amputated, while most ocular pythiosis patients have an infected eye removed (9). Many vascular pythiosis patients die from a ruptured aneurysm. Thalassemias and agriculture-related careers are known as predisposing factors (9, 21, 27).
Culture identification is a definite diagnostic method for pythiosis, but it is a time-consuming procedure and requires expertise and often hard-to-obtain internal tissue (1, 9, 11, 17, 23). Conventional antifungal drugs are not effective to control the infection (9). The main treatment option for pythiosis is surgery, which should be urgently performed to limit disease progression and ensure better prognoses for patients (9). Some serodiagnostic tests have been developed to facilitate the early diagnosis of pythiosis (4, 6, 7, 13-15, 18, 25). In-house enzyme-linked immunosorbent and Western blot assays show high degrees of sensitivity and specificity for the diagnosis of pythiosis (6, 7, 13). However, the tests require skilled personnel, stable and reproducible reagents, expensive equipment, and long turnaround times. Immunodiffusion (ID) (4, 14, 18) is a simple serological test that has been commonly used in laboratories for the diagnosis of pythiosis and is considered to be a standard serodiagnostic test for pythiosis. Although the ID test is easy to perform and has high specificity, it shows poor sensitivity and requires a long turnaround time, which may lead to a false-negative result and delayed treatment. Therefore, improvement in the diagnostic procedure is an important health care goal.
The immunochromatographic test (ICT) has been popularly applied for the serodiagnosis of many infectious diseases because of its user-friendly format, rapid result generation, and high degrees of detection sensitivity and specificity. Most importantly, the test can be used in remote areas or areas where pythiosis is endemic which lack diagnostic facilities. In the present study, we aimed to develop an in-house ICT for the rapid detection of specific human anti-P. insidiosum immunoglobulin G (IgG) in serum samples. The performance of the ICT was evaluated in comparison to that of an ID test for the serodiagnosis of pythiosis.
MATERIALS AND METHODS
Microorganism and growth conditions.
The P. insidiosum strain CBS119452, isolated from Thai patients with vascular pythiosis, was used to prepare antigen in this study. The organism had been maintained on Sabouraud dextrose agar at 37°C until antigen preparation.
Antigen preparation.
The P. insidiosum CBS119452 isolate was subcultured on Sabouraud dextrose agar and incubated at 37°C for 2 days. Several small agar pieces containing hyphal elements from the growing culture were transferred into 200 ml of Sabouraud dextrose broth and shaken (150 rpm) at 37°C for 1 week. Thimerosal (Merthiolate; final concentration, 0.02% [wt/vol]) was added to kill the cultures before they were filtered through a Durapore membrane filter (0.22-μm pore size; Millipore, County Cork, Ireland). Phenylmethylsulfonyl fluoride (0.1 mg/ml) and EDTA (0.3 mg/ml) were added to minimize protein degradation in the filtered broth before it was concentrated ∼80-fold using an Amicon Ultra-15 centrifugal filter (nominal molecular weight limit, 10,000; Millipore, Bedford, MA). The concentrated filtered broth was referred to as culture filtrate antigen (CFA) and was measured for protein concentration by a spectrophotometer. The CFA was stored at −20°C until use.
Serum samples.
A total of 33 sera from patients with known cases of human pythiosis (27 vascular, 4 ocular, and 2 cutaneous) were used for the test evaluation. The diagnosis of human pythiosis was based on previously reported criteria (9), as follows: (i) culture isolation of P. insidiosum (n = 15), (ii) serodiagnosis (n = 9), and (iii) the presence of the unique clinicopathological features of vascular pythiosis (n = 9). An additional 181 sera were collected for use in four control groups. The first group included 100 sera randomly collected from healthy blood donors who came to the Blood Bank Division, Ramathibodi Hospital. The second group included sera from 19 healthy thalassemic patients with no clinical evidence of pythiosis. The third group included sera from six patients with noninfectious diseases (five with highly positive antinuclear antibody titers and one with thromboangiitis obliterans [TAO]). The fourth group included sera from 56 patients with other infections (7 with penicilliosis, 7 galactomannan positive, 6 with cryptococcosis, 5 with malaria, 4 with aspergillosis, 4 with mycoplasmosis, 3 with zygomycosis, 3 with histoplasmosis, 3 with syphilis, 3 positive for anti-human immunodeficiency virus [HIV] antibody, 2 with toxoplasmosis, 2 with leptospirosis, 2 with melioidosis, 1 with amoebiasis, 1 with disseminated candidiasis, 1 positive for anti-hepatitis A virus antibody, 1 positive for anti-hepatitis B virus antibody, and 1 positive for anti-hepatitis C virus antibody). All sera were kept at −20°C until use.
ICT. (i) Conjugation of antibody to colloidal gold.
The 40-nm-particle colloidal gold suspension (Arista, Allentown, PA) was adjusted to pH 9.65 by using 0.2 M Na2CO3. To each 500 μl of colloidal gold, 3 μg of rabbit anti-human IgG (Dako, Glostrup, Denmark) was added, and the mixture was incubated for 30 min at room temperature. The residual surfaces of the colloidal gold particles were blocked by incubation with 5% (wt/vol) bovine serum albumin (Sigma, St. Louis, MO) for 10 min. The conjugate was centrifuged at 6,000 × g for 15 min, and the supernatant was then discarded. The conjugate pellet was washed in 0.5% (wt/vol) casein and centrifuged at 6,000 × g for 15 min. After the removal of the supernatant, the conjugate was resuspended in a solution of 0.5% (wt/vol) casein and 20% (wt/vol) sucrose in 0.02 M Tris-HCl (pH 8.0) with 40 times less volume than the original suspension. A piece of 2.5- by 2.5-mm glass fiber (GF33; Whatman Schleicher & Schuell, Dassel, Germany) was impregnated with this IgG-colloidal gold conjugate (2.5 μl) and dried in a dehumidifier cabinet for an hour.
(ii) Immobilization of antigen and antibody onto a nitrocellulose membrane.
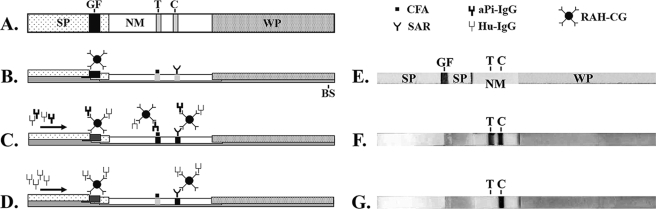
A 1.5-cm-wide nitrocellulose membrane (AE99; Whatman Schleicher & Schuell, Dassel, Germany) was lined with CFA (1:5 dilution; the test line) and sheep anti-rabbit IgG (150 μg/ml in 50 mM ammonium acetate buffer, pH 4.5; the control line) at 1 μl/cm by a dispenser (ZX 1000; BioDot, Irvine, CA) (Fig. 1A and B). The membrane was dried, blocked with 1% (wt/vol) bovine serum albumin, and dried again in a dehumidifier cabinet.
FIG. 1.
Schematic diagrams of an ICT strip: top view (A, E, F, and G) and side view (B, C, and D). (A and B) An ICT strip consists of a plastic backing support (BS), two sample pads (SP), a glass fiber (GF; containing rabbit anti-human IgG-colloidal gold conjugate), a nitrocellulose membrane (NM; containing test [T] and control [C] lines), and a wicking pad (WP). (C) Positive result. The test and control lines are visible. (D) Negative result. Only the control line is visible. (E) Actual ICT strip corresponding to the diagrams in panels A and B. (F) Actual ICT strip with a positive result, corresponding to the diagram in panel C. (G) Actual ICT strip with a negative result, corresponding to the diagram in panel D. Arrows show the direction of serum flow. SAR, sheep anti-rabbit antibody; aPi-IgG, anti-P. insidiosum IgG; Hu-IgG, human IgG; RAH-CG, rabbit anti-human IgG-colloidal gold conjugate.
(iii) Assembly of ICT strips.
The immobilized nitrocellulose membrane, the glass fiber with the colloidal gold conjugate, the sample pad (903 paper; Whatman Schleicher & Schuell, Dassel, Germany), and the wicking pad (3MM chromatography paper; Whatman, Maidstone, England) were assembled onto a backing of plastic, which was then cut into 2.5-mm-wide strips by a strip-cutting machine (CM 4000 R; BioDot, Irvine, CA) (Fig. 1A and B).
(iv) Detection of human anti-P. insidiosum antibody by the ICT.
Each individual sample was diluted 1:10,000 in phosphate buffer (pH 7.4). The ICT was evaluated in duplicate with 100 μl of diluted serum in a 96-well microtiter plate. The test signal of each ICT strip was read by the naked eye at 30 min by three independent laboratory personnel. To quantify the ICT signal, each strip was scanned by a scanner (Epson Perfection 1670 photo scanner; Seiko Epson Corp., Japan) to obtain a tagged image file format picture. Test and background signal intensities were measured by the Quantity-One program (Bio-Rad). The intensity value derived from a test signal after the subtraction of the background signal was referred to as the ICT value (IV). Sensitivities and specificities for all cutoff levels of IVs were calculated and graphically displayed in receiver-operating characteristic (ROC) curves by using the Stata version 10 program (StataCorp, TX).
ID test.
The ID test was modified from the method of Pracharktam et al. (18). Briefly, agar gel diffusion was carried out on a 5-cm-diameter petri dish with 2% agar in barbital (Veronal) buffer (0.9% [wt/vol] C8H11N2NaO3, 0.05% [wt/vol] NaN3, pH 8.6). The CFA and serum to be tested were each added to 4-mm-diameter wells separated by 4 mm. The petri dish was incubated in a moist chamber at room temperature for 24 h. The appearance of a precipitation line visible to the naked eye was considered a positive test result.
RESULTS
Development of the ICT results.
The components of an ICT strip are depicted in Fig. 1. CFA was blotted onto a nitrocellulose membrane (indicated as the test line) and used as the specific P. insidiosum antigen for detecting anti-P. insidiosum IgG in serum samples. Sheep anti-rabbit IgG (indicated as the control line) was blotted distally from CFA. When human IgGs in serum moved upward by capillary action through the glass fiber, they formed complexes with the rabbit anti-human IgG-colloidal gold conjugate. The complexes migrated through the nitrocellulose membrane. Immune complexes containing human anti-P. insidiosum IgG bound CFA and developed a purple signal at the test line. In contrast, immune complexes lacking human anti-P. insidiosum IgG passed through the test line without developing a signal. The sheep anti-rabbit IgG bound the remaining immune complexes containing rabbit anti-human IgG-colloidal gold conjugate and exhibited an internal test validation signal at the control line.
Diagnostic performance of the ICT in comparison to ID.
ICT and ID results were read by three independent laboratory personnel. Based on the results for all sera from pythiosis patients (27 with vascular pythiosis, 4 with ocular pythiosis, and 2 with cutaneous pythiosis) and the control groups, the ICT showed 88% sensitivity, 100% specificity, 100% positive predictive value, and 98% negative predictive value while ID showed 61% sensitivity, 100% specificity, 100% positive predictive value, and 93% negative predictive value. False-negative ICT results were obtained for sera from all ocular pythiosis patients. False-negative ID results were obtained for sera from all ocular pythiosis patients, eight patients with vascular pythiosis, and one patient with cutaneous pythiosis.
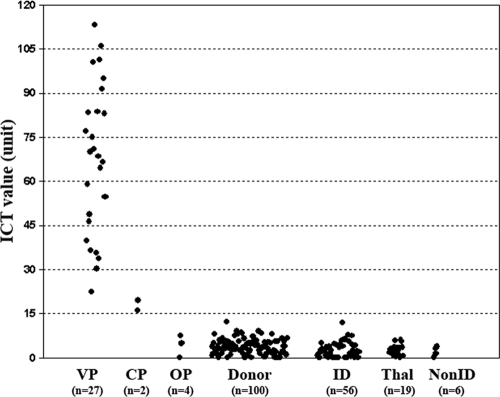
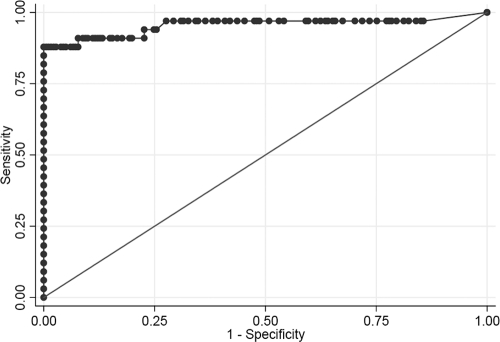
ICT signals generated from all sera were quantified and converted to IV units (see Materials and Methods) (Fig. 2). The mean IV for vascular pythiosis patients was 67.2 U (range, 22.4 to 113.3 U), whereas that for cutaneous pythiosis patients was 17.7 U (range, 16.0 to 19.4 U), that for ocular pythiosis patients was 4.3 U (range, 0 to 7.4 U), that for blood donors was 3.8 U (range, 0 to 12.3 U), that for patients with other infectious diseases was 2.5 U (range, 0 to 11.9 U), that for thalassemic patients was 2.7 U (range, 0 to 5.9 U), and that for patients with autoimmune diseases or TAO was 2.1 U (range, 0 to 3.8 U). As an alternative to the determination of results by three independent laboratory personnel, the IV cutoff point was selected by a ROC analysis to differentiate between patients with and without human pythiosis. The ICT has very good discriminative power for identifying patients with human pythiosis, as shown by the large area (0.95) under the ROC curve (Fig. 3). The IV of 16.0 was selected as the cutoff value, because it gave the highest sensitivity (88%) and specificity (100%). For comparison, the IV cutoff value of 12.3 yielded a sensitivity of 88% and a specificity of 99% and the IV cutoff value of 19.4 yielded a sensitivity of 85% and a specificity of 100%.
FIG. 2.
IVs of all sera from patients with vascular pythiosis (VP), cutaneous pythiosis (CP), and ocular pythiosis (OP), from blood donors, and from patients with a variety of infectious diseases (ID) and noninfectious diseases, including thalassemia (Thal) and other noninfectious diseases (nonID).
FIG. 3.
ROC curve. Pythiosis and control serum groups (total, 214 samples) are included in the ROC analysis. The cutoff value of 16 gave the sensitivity and specificity of 88 and 100%, respectively. The area under the ROC curve was 0.9546.
DISCUSSION
The ID test has high specificity but poor sensitivity and requires a long turnaround time. Because early and accurate diagnosis would improve the clinical outcomes for patients with pythiosis, the ICT was developed to address this issue. Thirty-three pythiosis patient sera and 181 control sera from healthy blood donors and patients with thalassemia (a predisposing factor for pythiosis), various infectious diseases, autoimmune diseases, and TAO, which clinically mimics vascular pythiosis, were used for the comparison of ICT and ID performances. Pythiosis can be misdiagnosed as aspergillosis and zygomycosis because the causative agents have microscopic morphologies similar to that of P. insidiosum (16). Sera from patients with aspergillosis, zygomycosis, and other endemic infectious diseases (such as melioidosis, HIV infection, and malaria) were tested for any background or cross-reactivity. All control sera tested negative by the ICT and ID, demonstrating 100% specificity for both tests.
The sensitivity of the ICT to detect anti-P. insidiosum IgG in all pythiosis sera was greater than that of ID (88% for the ICT; 61% for ID). Sera from all ocular pythiosis patients tested negative by both the ICT and ID. The failure to detect anti-P. insidiosum antibodies in these patients was due likely to poorly induced antibody responses to localized infections of the eye (6). Therefore, the serodiagnosis of ocular infection should be avoided, because of an expected high rate of false-negative results. When ocular pythiosis patient sera were excluded from the evaluation, the sensitivity of the ICT increased to 100% and that of ID increased to 69%. The ICT is a ready-to-use test, while ID is complicated by a need to prepare a fresh diffusion gel right before performing the test. The turnaround time of the ICT was remarkably shorter than that of ID (30 min for the ICT; ∼24 h for ID). Therefore, the ICT shows better sensitivity and is more convenient than ID. It is suitable for the serodiagnosis of vascular and cutaneous pythiosis but not ocular pythiosis.
The two highest IVs among the controls (Fig. 2) were obtained for a blood donor (12.3 U) and a patient with penicilliosis (11.9 U). The ICT results for the control sera from these subjects were reported as negative by the readers, as no test signal was grossly detected. Among patients positive for pythiosis (Fig. 2), two cutaneous pythiosis patients had the lowest IVs (16.0 and 19.4 U). Low anti-P. insidiosum IgG levels in these cutaneous pythiosis patients were already expected because one patient had advanced HIV infection and a low CD4 cell count (52 cells/μl) and the other had an acute P. insidiosum infection with a few-day history of symptoms prior to hospital admission. Nevertheless, the sera were reported to be positive, as faint test lines were consistently and unambiguously detected by the three readers. These findings indicated that the ICT had good discriminating power for negative and weakly positive samples.
In conclusion, the in-house ICT had higher sensitivity and specificity, required a shorter turnaround time, and was a more user-friendly test than ID. In addition, the ICT is suitable for use at the bedside, as well as in remote hospitals where skilled personnel or diagnostic materials are lacking.
Acknowledgments
This work was supported by research grants from Chulabhorn Research Institute (K.R., S.I., and A.I.); the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (T.K.); and the Thailand Research Fund (T.K.).
We thank Amnuay Thithapandha for reviewing the manuscript. We are grateful to Mongkol Kunakorn, Boonmee Sathapatayavongs, Pimpan Tadthong, Piriyaporn Chongtrakool, Savittree Piromsontikorn, Kim Wongcharoenrat, Thanyasiri Jindayok, Piroon Mootsikapun, Angkana Chaiprasert, Nongnuch Vanittanakom, Sunsanee Chaiyaroj, and Ariya Chindamporn for their help and suggestions, as well as material support.
Footnotes
Published ahead of print on 18 February 2009.
REFERENCES
- 1.Chaiprasert, A., S. Samerpitak, W. Wanachiwanawin, and P. Thasnakorn. 1990. Induction of zoospore formation in Thai isolates of Pythium insidiosum. Mycoses 33317-323. [DOI] [PubMed] [Google Scholar]
- 2.Chetchotisakd, P., C. Pairojkul, O. Porntaveevudhi, B. Sathapatayavongs, P. Mairiang, K. Nuntirooj, B. Patjanasoontorn, O. T. Saew, A. K. Chaiprasert, and M. R. Haswell-Elkins. 1992. Human pythiosis in Srinagarind Hospital: one year's experience. J. Med. Assoc. Thai. 75248-254. [PubMed] [Google Scholar]
- 3.Imwidthaya, P. 1994. Human pythiosis in Thailand. Postgrad. Med. J. 70558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imwidthaya, P., and S. Srimuang. 1989. Immunodiffusion test for diagnosing human pythiosis. Mycopathologia 106109-112. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman, L. 1998. Penicilliosis marneffei and pythiosis: emerging tropical diseases. Mycopathologia 1433-7. [DOI] [PubMed] [Google Scholar]
- 6.Krajaejun, T., M. Kunakorn, S. Niemhom, P. Chongtrakool, and R. Pracharktam. 2002. Development and evaluation of an in-house enzyme-linked immunosorbent assay for early diagnosis and monitoring of human pythiosis. Clin. Diagn. Lab. Immunol. 9378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krajaejun, T., M. Kunakorn, R. Pracharktam, P. Chongtrakool, B. Sathapatayavongs, A. Chaiprasert, N. Vanittanakom, A. Chindamporn, and P. Mootsikapun. 2006. Identification of a novel 74-kilodalton immunodominant antigen of Pythium insidiosum recognized by sera from human patients with pythiosis. J. Clin. Microbiol. 441674-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krajaejun, T., R. Pracharktam, S. Wongwaisayawan, M. Rochanawutinon, M. Kunakorn, and S. Kunavisarut. 2004. Ocular pythiosis: is it under-diagnosed? Am. J. Ophthalmol. 137370-372. [DOI] [PubMed] [Google Scholar]
- 9.Krajaejun, T., B. Sathapatayavongs, R. Pracharktam, P. Nitiyanant, P. Leelachaikul, W. Wanachiwanawin, A. Chaiprasert, P. Assanasen, M. Saipetch, P. Mootsikapun, P. Chetchotisakd, A. Lekhakula, W. Mitarnun, S. Kalnauwakul, K. Supparatpinyo, R. Chaiwarith, S. Chiewchanvit, N. Tananuvat, S. Srisiri, C. Suankratay, W. Kulwichit, M. Wongsaisuwan, and S. Somkaew. 2006. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 43569-576. [DOI] [PubMed] [Google Scholar]
- 10.Kwon-Chung, K. J. 1994. Phylogenetic spectrum of fungi that are pathogenic to humans. Clin. Infect. Dis. 19S1-7. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza, L., L. Ajello, and M. R. McGinnis. 1996. Infection caused by the oomycetous pathogen Pythium insidiosum. J. Mycol. Med. 6151-164. [Google Scholar]
- 12.Mendoza, L., F. Hernandez, and L. Ajello. 1993. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J. Clin. Microbiol. 312967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza, L., L. Kaufman, W. Mandy, and R. Glass. 1997. Serodiagnosis of human and animal pythiosis using an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza, L., L. Kaufman, and P. G. Standard. 1986. Immunodiffusion test for diagnosing and monitoring pythiosis in horses. J. Clin. Microbiol. 23813-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza, L., V. Nicholson, and J. F. Prescott. 1992. Immunoblot analysis of the humoral immune response to Pythium insidiosum in horses with pythiosis. J. Clin. Microbiol. 302980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza, L., S. H. Prasla, and L. Ajello. 2004. Orbital pythiosis: a non-fungal disease mimicking orbital mycotic infections, with a retrospective review of the literature. Mycoses 4714-23. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza, L., and J. Prendas. 1988. A method to obtain rapid zoosporogenesis of Pythium insidiosum. Mycopathologia 10459-62. [DOI] [PubMed] [Google Scholar]
- 18.Pracharktam, R., P. Changtrakool, B. Sathapatayavongs, P. Jayanetra, and L. Ajello. 1991. Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. J. Clin. Microbiol. 292661-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasertwitayakij, N., W. Louthrenoo, N. Kasitanon, K. Thamprasert, and N. Vanittanakom. 2003. Human pythiosis, a rare cause of arteritis: case report and literature review. Semin. Arthritis Rheum. 33204-214. [DOI] [PubMed] [Google Scholar]
- 20.Pupaibool, J., A. Chindamporn, K. Patarakul, C. Suankratay, W. Sindhuphak, and W. Kulwichit. 2006. Human pythiosis. Emerg. Infect. Dis. 12517-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathapatayavongs, B., P. Leelachaikul, R. Prachaktam, V. Atichartakarn, S. Sriphojanart, P. Trairatvorakul, S. Jirasiritham, S. Nontasut, C. Eurvilaichit, and T. Flegel. 1989. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J. Infect. Dis. 159274-280. [DOI] [PubMed] [Google Scholar]
- 22.Tanphaichitra, D. 1989. Tropical disease in the immunocompromised host: melioidosis and pythiosis. Rev. Infect. Dis. 11S1629-S1643. [DOI] [PubMed] [Google Scholar]
- 23.Thianprasit, M., A. Chaiprasert, and P. Imwidthaya. 1996. Human pythiosis. Curr. Top. Med. Mycol. 743-54. [PubMed] [Google Scholar]
- 24.Thitithanyanont, A., L. Mendoza, A. Chuansumrit, R. Pracharktam, J. Laothamatas, B. Sathapatayavongs, S. Lolekha, and L. Ajello. 1998. Use of an immunotherapeutic vaccine to treat a life-threatening human arteritic infection caused by Pythium insidiosum. Clin. Infect. Dis. 271394-1400. [DOI] [PubMed] [Google Scholar]
- 25.Vanittanakom, N., J. Supabandhu, C. Khamwan, J. Praparattanapan, S. Thirach, N. Prasertwitayakij, W. Louthrenoo, S. Chiewchanvit, and N. Tananuvat. 2004. Identification of emerging human-pathogenic Pythium insidiosum by serological and molecular assay-based methods. J. Clin. Microbiol. 423970-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanachiwanawin, W., L. Mendoza, S. Visuthisakchai, P. Mutsikapan, B. Sathapatayavongs, A. Chaiprasert, P. Suwanagool, W. Manuskiatti, C. Ruangsetakit, and L. Ajello. 2004. Efficacy of immunotherapy using antigens of Pythium insidiosum in the treatment of vascular pythiosis in humans. Vaccine 223613-3621. [DOI] [PubMed] [Google Scholar]
- 27.Wanachiwanawin, W., M. Thianprasit, S. Fucharoen, A. Chaiprasert, N. Sudasna, N. Ayudhya, N. Sirithanaratkul, and A. Piankijagum. 1993. Fatal arteritis due to Pythium insidiosum infection in patients with thalassaemia. Trans. R. Soc. Trop. Med. Hyg. 87296-298. [DOI] [PubMed] [Google Scholar]