Abstract
A goal of the current study was to evaluate serological applications of Toxoplasma gondii GRA2 and rhoptry protein 1 (ROP1) antigens. Soluble recombinant GRA2 and ROP1 antigens as fusion proteins containing six histidyl residues at the N and C terminals were obtained using an Escherichia coli expression system. Purification by one-step metal affinity chromatography allowed recovery of milligram amounts of pure recombinant proteins per liter of culture. The usefulness of these antigens for diagnosis of human infections was tested on 167 serum samples obtained during routine diagnostic tests. A panel of 37 serum samples from patients with acute toxoplasmosis was compared to a panel of 90 serum samples from individuals with past infection. The results indicated that both GRA2 and ROP1 recombinant antigens detected antibodies more frequently in samples from individuals with acute infections (100% and 94.6%, respectively) than in samples from individuals with chronic infections (22.5% and 15.5%, respectively). These results suggest that immunoglobulin G antibodies against GRA2 and ROP1 antigens are produced during the acute stage of toxoplasmosis but are uncommon in the chronic phase of the infection. Hence, these recombinant proteins can be used as specific molecular markers to differentiate between acute and chronic infections.
Toxoplasma gondii is an obligate intracellular parasite that invades nucleated cells of warm-blooded animals, including humans. This pathogen is one of the most widespread parasites with respect to both its hosts and its geographical distribution. Human infection (toxoplasmosis) in immunocompetent adults is usually asymptomatic. The immune system of a healthy person is effective in controlling a tachyzoite infection (the invasive form of T. gondii), but the bradyzoite form persists for life in tissue cysts in the central nervous system and in cardiac and skeletal muscles. In immunocompromised individuals, including patients with AIDS and organ transplant recipients, reactivation of this latent infection may occur, causing toxoplasmic encephalitis. Moreover, toxoplasmosis acquired by women during pregnancy poses a significant threat to the fetus. Congenital infection may cause spontaneous abortion or severe fetal abnormalities. These facts emphasize the importance of being able to make an accurate distinction between primary infection and reactivation (especially during pregnancy).
The serological diagnosis of toxoplasmosis relies on the detection of specific antibodies in the serum samples of infected patients. The outcome of these tests depends on the types of antigen and methods of detection. The enzyme-linked immunosorbent assay (ELISA) for the detection of immunoglobulins (Igs) is an easy test to perform, and many manual and automated systems are commercially available.
Acute toxoplasmosis can be identified by serological markers for anti-T. gondii-specific IgM or the demonstration of a significant increase in the specific IgG antibody titers in paired serum samples. As IgM antibodies may remain detectable for more than 1 year after the initial infection, the presence of these markers cannot be used to estimate the stage of toxoplasmosis (17). Currently, distinguishing between acute and chronic infection is based primarily on IgG avidity in that a low level of antibody avidity is specific for acute-phase serum samples, whereas high avidity is indicative of past infection (8, 28). In addition, the use of the PCR and its modification (PCR-DNA enzyme immunoassay and real-time PCR) in conjunction with other diagnostic methods have significantly improved both the prenatal diagnosis of congenital toxoplasmosis and the detection of acute disease in the immunocompromised host (3, 7, 21). For these reasons, finding new diagnostic markers for differentiation of toxoplasmosis is an active area of research. Recombinant T. gondii proteins represent one such family of new specific molecular markers and with careful selection could differentiate between acute and chronic stages of the infection.
As a member of the phylum Apicomplexa, T. gondii possesses specialized, secretory organelles called rhoptries, micronemes, and dense granules. Proteins secreted by T. gondii from these organelles are considered to play an essential role in intracellular parasitism of this protozoan (5). Rhoptry proteins (ROP) may facilitate formation of the parasitophorous vacuole (PV) and mediate its clustering with host cell organelles (27). Dense granule antigens (GRA) are major components of both the vacuole surrounding tachyzoites and the cyst wall surrounding slower-growing bradyzoites (6), making GRA and ROP promising diagnostic tools and important protective antigens. At present, many investigators are studying the serological applications and the protective immunity induced by secretory organelles (9, 10, 15).
We focused our efforts on two secretory antigens: GRA2 (28 kDa) and ROP1 (66 kDa). Soluble ROP1 is secreted into the PV during entry of T. gondii into host cells before quickly disappearing (26). This rapid disappearance suggests that ROP1 plays a role in early invasion. Dense GRA2, within the PV, is specifically targeted to the tubulovesicular network which forms connections with the vacuole membrane. In 1998, Mercier et al. (19) investigated the role of GRA2 in intracellular survival during infection in mice. Their results indicate that GRA2 protein plays an important role during in vivo infection of T. gondii, because the absence of that protein partially attenuates the virulence of the parasite during the acute phase of infection. Furthermore, immunization with purified GRA2 has been shown to induce both a vigorous antibody response and a T-cell response (11, 20, 25). In addition, GRA2 and ROP1 allowed the detection of T. gondii-specific IgG in humans with toxoplasmosis (2, 10, 23). Taken together, these data suggest that the GRA2 and ROP1 antigens represent promising diagnostic tools for detection of toxoplasmosis in humans.
In the present study, we evaluated the usefulness of the T. gondii recombinant GRA2 (r-GRA2) and r-ROP1 antigens in diagnostic tests. The antigenicity of recombinant antigens against human serum samples was confirmed by Western blotting and ELISA analysis. Our results suggested that these proteins might be useful for differential detection of the early phase of T. gondii infection.
MATERIALS AND METHODS
Construction of the recombinant plasmids.
The nucleotide sequences of the T. gondii genes encoding GRA2 and ROP1 antigens were obtained from the GenBank database (accession no. M99392 and M71274, respectively). Tachyzoites from the T. gondii RH strain were used to isolate genomic DNA, which was used as the template for amplification of gra2 and rop1 genes by the use of a standard PCR amplification protocol and Pwo DNA polymerase (DNA-Gdańsk II, Poland). The PCR products were inserted into pUET1 vector (DNA-Gdańsk II, Poland).
The DNA fragment of rop1 (corresponding to nucleotides 252 to 1188) encoding ROP1 was obtained by PCR using the following primers: 5′-GTGCCAGATCTAGCGTCGCATTCTCATTCG-3′ (forward) and 5′-CCAAAGCTTTTGCGATCCATCATCCTGCTCTG-3′ (reverse). The primers contained the BglII and HindIII recognition sequences (underlined) to facilitate cloning. The PCR product was digested with both BglII and HindIII and inserted into the BglII and HindIII sites of pUET1. The resulting pUET-ROP1 construct retained the open reading frame encoding amino acid residues 85 to 396 of the ROP1 protein and a cluster of six histidine residues for purification of the recombinant protein by metal affinity chromatography at the N and C termini.
The DNA fragment of gra2 exon 1 (corresponding to nucleotides 802 to 881) encoding the N-terminal part of dense GRA2 was obtained by PCR using the following primers: 5′-CCGGTAGATCTTGCCGAGTTTTCCGGAGTTG-3′ (forward) and 5′-GGTGTATGTTCACCTTTTCCCCCAACTGCTCTCTC-3′ (reverse) that were designed to contain BglII and fragments of gra2 exon 2 sequences (underlined). The DNA fragment of gra2 exon 2 (corresponding to nucleotides 1128 to 1532) encoding the C-terminal part of the protein was obtained by PCR using the following primers: 5′-GATGAGAGAGCAGTTGGGGGAAAAGGTGAACATAC-3′ (forward) and 5′-GCAGTCAAGCTTCTGCGAAAAGTCTGGGACG-3′ (reverse) that were designed to contain fragments of exon 1 and HindIII sequences. Next, products of PCR1 and PCR2 were used as the templates for amplification of the gra2 gene fragment (nucleotides 802 to 1532, without the intron sequence) by the use of the following primers: 5′-CCGGTAGATCTTGCCGAGTTTTCCGGAGTTG-3′ (forward) and 5′-GCAGTCAAGCTTCTGCGAAAAGTCTGGGACG-3′ (reverse) that were designed to contain BglII and HindIII sequences. The PCR product was digested with BglII and HindIII and inserted into the BglII and HindIII sites of pUET1. The resulting pUET-GRA2 construct retained the open reading frame encoding amino acid residues from 24 to 185 of the GRA2 protein and a cluster of six histidine residues at the N and C termini.
The nucleotide sequences of the recombinant plasmids were verified using the dideoxy termination sequencing method.
Expression and purification of recombinant antigens.
r-GRA2 and r-ROP1 antigens of T. gondii were expressed in Escherichia coli cells as fusion proteins containing a cluster of six histidine residues for purification by metal affinity chromatography at the N and C termini and purified as previously described (14).
Toxoplasma lysate antigen (TLA) from tachyzoites (strain RH) was prepared according to the method used by Pietkiewicz et al. (24).
Electrophoresis and Western blot analysis.
The protein fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 12% polyacrylamide gel stained with Coomassie blue. The concentrations of these recombinant proteins were measured using the Bradford assay with bovine serum albumin as a standard. After electrophoresis, purified proteins were transferred onto nitrocellulose membranes. For immunoblot analysis with human serum samples, the membranes were saturated with 5% fat-free milk in phosphate-buffered saline-0.05% Tween 20 (PBS-0.05% Tween 20) for 1 h, incubated with serum samples diluted 1:100, washed, and incubated with peroxidase-conjugated goat anti-human IgG secondary antibodies (Sigma) used at 1:4,000. A peroxidase activity was detected with H2O2 and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) as a chromogenic substrate.
ELISA.
MaxiSorp multiwell plates (Nunc, Denmark) were coated with 0.1 ml of r-GRA2 and r-ROP1 proteins and TLA at a final concentration of 1 μg/ml for each antigen in a coating buffer (0.05 M carbonate buffer; pH 9.6). Control plates were coated with a protein sample of E. coli BL21(DE3) pLysS transformed with pUET1 vector obtained with the same purification method and dilution as that used for the recombinant T. gondii antigens. After overnight incubation at 4°C, plates were washed three times with PBS-0.1% Triton X-100 and blocked at 37°C for 1 h using blocking solution (1% bovine serum albumin-1% Triton X-100-PBS). Subsequently, they were incubated for 1 h at 37°C with human serum samples diluted 1:100 in the blocking solution. Plates were then washed three times with PBS-0.1% Triton X-100 and anti-human IgG peroxidase-labeled conjugates (Sigma) (1 mg/ml), diluted 1:4,000 in blocking solutions, and added to each well. Next, the plates were incubated for 35 min at 37°C and then washed. Finally, enzymatic activity was revealed by incubating the plates with o-phenylenediamine dihydrochloride (Sigma) chromogenic substrate. After 40 min at 37°C in darkness, the color development reaction was stopped by adding 0.1 ml of 1 M sulfuric acid and the color intensity was measured using a microtiter plate reader (Victor 3V; Perkin Elmer) at 490 nm.
Each serum sample was examined twice, and the results were determined for each serum sample by calculating the mean value of the optical density (OD) reading for duplicate wells. A positive result was defined as any value higher than the average OD reading plus three standard deviations (cutoff) obtained with 10 serum samples from the negative-control serum group. The cutoff values were as follows: 0.157 for r-GRA2, 0.236 for r-ROP1, and 0.223 for TLA. An ELISA performed with the control E. coli BL21(DE3) pLysS pUET1 antigen revealed an OD consistently below 0.18 (data not shown), which showed that possible contaminations of the recombinant antigens with E. coli proteins did not influence the ELISA results.
Serum samples.
All serum samples used in this study were received from routine toxoplasmosis screenings. A total of 167 healthy human serum samples were analyzed and divided into three groups based on serological profiles previously characterized by conventional laboratory assays that made it possible to classify the samples as follows. Group I consisted of 37 human serum samples from patients in the acute phase of toxoplasmosis. The presence of specific IgM antibodies was measured by IgM-ELISA Vidas (bioMérieux, France) and Toxo-immunosorbent agglutination assay plus IgM/IgA tests. All serum samples had positive IgG antibodies (IgG-ELISA Vidas; bioMérieux, France), with 33 at low avidity and 4 at borderline avidity as determined by a commercial antibody avidity test (Vidas Toxo-IgG avidity; bioMérieux, France). Group II consisted of 90 human serum samples from patients with indicative infections acquired in the distant past (chronic toxoplasmosis). All those serum samples had positive IgG antibodies (IgG-ELISA Vidas; bioMérieux, France), high avidity as determined by a commercial antibody avidity test (Vidas Toxo-IgG avidity; bioMérieux, France), and an absence of specific IgM antibodies. Group III (the control group) included 40 human serum samples from seronegative individuals.
Statistical analysis.
Statistical analysis of the ELISA results was performed with the Microsoft Excel 2003 program for evaluation of the χ2 test.
RESULTS
Expression, purification, and reactivity of the recombinant antigens.
In order to evaluate antigenicity of recombinant antigens, the proteins were expressed in E. coli and purified by a one-step chromatography procedure using metal affinity chromatography with Ni2+ bound to iminodiacetic acid-agarose (Novagen). The GRA2 and ROP1 antigens were expressed as soluble proteins with calculated molecular masses of 23 kDa and 39 kDa, respectively. The E. coli expression system applied produced about 28 mg and 16 mg of purified r-GRA2 and r-ROP1 proteins from 1 liter of induced culture, respectively. The purification resulted in electrophoretically homogeneous preparations of recombinant proteins (approximately 97% purity; results not shown).
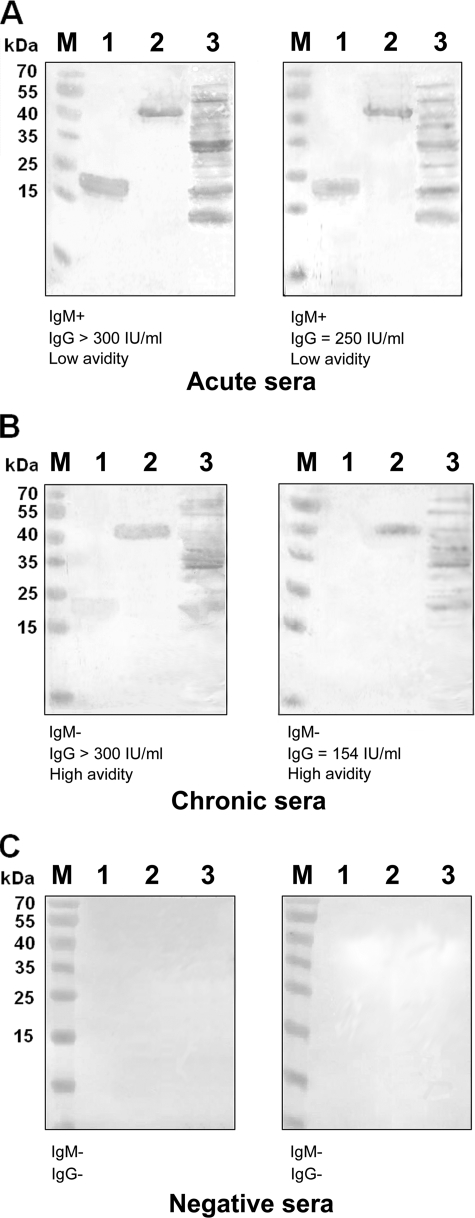
The immunoreactivity of the purified antigens (r-GRA2 and r-ROP1) was tested by Western blot analysis with six human serum samples chosen from each of the three serum sample groups included in this study. IgG antibodies present in serum samples from patients in the acute phase of toxoplasmosis (group I) efficiently recognized the r-GRA2 and r-ROP1 antigens (Fig. 1A). In contrast, IgGs from human serum samples from patients in the chronic phase of infection (group II) reacted weakly with these proteins (Fig. 1B). Furthermore, immunoreactivity of the r-GRA2 and r-ROP1 antigens with serum samples from healthy patients (group III) was not observed (Fig. 1C).
FIG. 1.
Representative results of Western blotting analyses. Purified r-GRA2 (lane 1), r-ROP1 (lane 2), and TLA (lane 3) were tested with anti-T. gondii human serum samples from group I (A), group II (B), and group III (C). M, molecular mass marker (Fermentas, Lithuania).
Reactivity of IgG antibodies from human serum samples with recombinant antigens (r-GRA2 and r-ROP1) and TLA in ELISA.
Three separate IgG ELISAs were developed using r-GRA2, r-ROP1, and TLA as a coating antigen to evaluate the potential of each of these antigens for the serodiagnosis of toxoplasmosis. A total of 37 serum samples from group I (consisting of patients in the acute phase of toxoplasmosis), 90 serum samples from group II (patients in the chronic phase of toxoplasmosis), and serum samples from 40 seronegative individuals (group III; negative controls) were examined. Ten serum samples from the latter group were used to obtain relative absorbance values for each serum sample and the cutoff values, and the remaining 30 serum samples were used to determine the specificity of IgG ELISAs. None of the negative serum samples was found to score above the cutoff, resulting in a specificity of 100% for ELISAs (Table 1). The sensitivity of IgG ELISA calculated from all positive serum samples tested in this study (groups I and II) was at 88.9% for TLA and was lower for both the r-GRA2 and r-ROP1 antigens (46.4% and 38.8%, respectively). However, the results obtained for IgG ELISAs with recombinant antigens differed depending on the group of serum samples used. Serum samples from patients with acute toxoplasmosis (with low or borderline avidity) reacted with the r-GRA2 and r-ROP1 proteins with high sensitivities (100% and 94.6%, respectively), whereas much lower sensitivities (22.5% and 15.5%, respectively) were observed with serum samples from patients with chronic infection (serum samples with high avidity) (Table 1). The sensitivity of the IgG ELISA with TLA was similar to that seen with recombinant antigens for serum samples from group I (100%) but was significantly higher (85.5%) with serum samples from group II. Statistical analysis confirmed a high sensitivity of IgG ELISAs with r-GRA2 and r-ROP1 for serum samples from acute toxoplasmosis, and these results were statistically significant (χ2 = 57.176 and χ2 = 70.011; P = 0.0001). The results obtained for TLA were found to be not statistically significant (χ2 = 4.485; P > 0.034).
TABLE 1.
Immunoreactivities of the recombinant antigens (r-GRA2 and r-ROP1) and TLA compared using IgG antibodies from T. gondii-infected individuals with acute and chronic toxoplasmosis and from serum samples from healthy patients
| Serum sample groupa | Antigen | No. (%) of reactive serum samples | Absorbance value(s)b
|
|
|---|---|---|---|---|
| Mean | Range | |||
| I (acute toxoplasmosis [low or borderline avidity]) (n = 37) | r-GRA2 | 37c (100) | 0.414 | 0.174-0.863 |
| r-ROP1 | 35d (94.6) | 0.629 | 0.129-1.412 | |
| TLA | 37 (100) | 1.887 | 0.861-2.985 | |
| II (chronic toxoplasmosis [high avidity]) (n = 90) | r-GRA2 | 22c (22.5) | 0.155 | 0.150-0.329 |
| r-ROP1 | 14d (15.5) | 0.209 | 0.111-0.775 | |
| TLA | 77 (85.5) | 0.498 | 0.148-1.176 | |
| Total (n = 127) | r-GRA2 | 59 (46.4) | 0.284 | 0.150-0.863 |
| r-ROP1 | 49 (38.6) | 0.419 | 0.111-1.412 | |
| TLA | 113 (88.9) | 1.187 | 0.148-2.985 | |
| III (control group negative for anti-T. gondii antibodies) (n = 30) | r-GRA2 | 0 | 0.126 | 0.113-0.141 |
| r-ROP1 | 0 | 0.158 | 0.128-0.207 | |
| TLA | 0 | 0.284 | 0.168-0.278 | |
n, number of serum samples examined.
The cutoff values were 0.157 for r-GRA2, 0.236 for r-ROP1, and 0.223 for TLA.
Differences between the r-GRA2 results for groups I and II were statistically significant (χ2 = 57.176 [P = 0.0001]).
Differences between the r-ROP1 results for groups I and II were statistically significant (χ2 = 70.011 [P = 0.0001]).
DISCUSSION
At present, serologic kits for diagnosis of T. gondii infections rely principally on the use of whole extracts of tachyzoites. The recombinant antigenic proteins are promising tools that can be used in diagnostic assays for the detection of specific antibodies against T. gondii. Moreover, these proteins may allow not only for more economical and more accurately standardized tests but also for the creation of new kits capable of differentiating between acute and chronic infections.
In this paper we report the bacterial production of T. gondii soluble r-GRA2 and r-ROP1 antigen proteins by the use of the efficient expression system developed in our laboratory (12, 13, 14). This expression system and the method of purification by a one-step metal affinity chromatography led to the production of milligram amounts of pure recombinant proteins (28 mg of r-GRA2 and 16 mg of r-ROP1) per liter of culture. The production efficiency of this system is much higher than that which produced the thioredoxin-His fusion GRA2 and rGRA2 proteins obtained by others (10, 11).
In this work we also evaluated the diagnostic value of T. gondii recombinant antigenic proteins (r-GRA2 and r-ROP1). We tested the r-GRA2 and r-ROP1 reactivities by the use of two different groups of human serum samples representing acute and chronic infections. Both groups were analyzed using Western blot and ELISA techniques. IgG antibodies in serum samples from patients in the acute phase of toxoplasmosis (group I; serum samples with positive IgM and IgG at low avidity) reacted with the recombinant antigens more strongly than those in serum samples from patients in the chronic phase of toxoplasmosis (group II; serum samples with negative IgM and positive IgG at high avidity). In contrast, when TLA was used in ELISAs, no significant differences between the reactivities of IgG antibodies in groups I and II were observed. Thus, our results with r-GRA2 and r-ROP1 antigens suggest that these proteins can be used as serological markers of recently acquired infection.
The results for r-GRA2 are consistent with the results obtained by other investigators who have indicated that IgG antibodies from acute-phase serum samples reacted more avidly with GRA2 than those from chronic serum samples. Golkar et al. (10) employed thioredoxin-His-GRA2 protein for the detection of specific anti-T. gondii IgG in ELISAs, and their results revealed higher sensitivity (95.8% and 100%) of the IgG ELISA for two groups of acute-phase serum samples (collected in Iran and in France) than for two groups of chronic serum samples (71.4% and 65.7%). r-GRA2 used in our study allowed for wider differentiation between the serum samples from acute and chronic infections. We observed 100% sensitivity with serum samples from patients with acute toxoplasmosis and 22.5% with serum samples from humans with chronic infection. In contrast, Murray et al. (20), using the C-terminal fragment of GRA2 protein with a glutathione S-transferase domain, obtained similar sensitivity values with ELISA for serum samples from patients with acute toxoplasmosis (82.6%) and for serum samples from persons with an infection that had been acquired in the distant past (75%). This difference is probably due to the nature of the recombinant protein used. The fragment of r-GRA2 described by Murray et al. (20) was much shorter than the full-length antigen used in our research and by Golkar et al. (10).
The r-ROP1 antigen was shown previously to be sensitive in its ability to detect IgG antibodies in serum samples from patients with toxoplasmosis (2, 18, 23). In the study by Aubert et al. (2), the reactivity of this protein was higher for the serum samples that contained T. gondii-specific IgA, IgM, and IgG (87.6% [78 out of 89 samples]) than for those from the group of chronic serum samples (21.9% [23 out of 105 samples]). Thus, our results were similar to those reported previously by Aubert et al. (2). An avidity immunoblot assay with P66 antigen (ROP1) was also described by Marcolino et al. (18). Their results suggested that the protein band corresponding to P66 was recognized by both low-avidity IgG and high-avidity IgG. Furthermore, the r-ROP1 protein was used in a recently introduced recombinant line assay (recomLine Toxoplasma; Microgen, Germany) as demonstrated by Pfrepper et al. (23). In their study, the sensitivities of a new test for the detection of anti-ROP1 IgG antibodies in serum samples from patients with acute toxoplasmosis were 50.0% (11 out of 22 samples collected at the time of infection), 52.9% (29 out of 50 serum samples taken 3 months after the first sample), and 40.0% (7 out of 10 serum samples taken between 3 and 6 months after the first sample). Our study indicated a much higher (94.6%) sensitivity for the detection of specific IgGs in serum samples from individuals with recently acquired infection.
Results presented in this study show that specific IgG anti-GRA2 and anti-ROP1 antibodies are present in serum samples from patients at the acute phase of T. gondii infection. Furthermore, it is known that these proteins play an important role in the process of early invasion of parasites into the host cell. Also, some authors have described the usefulness of r-GRA2 and r-ROP1 antigens for immunization (11, 20). Taken together, these data suggest that GRA2 and ROP1 antigens are particularly promising candidates for effective vaccine against toxoplasmosis.
The successful use of recombinant antigenic proteins for the detection of anti-T. gondii-specific antibodies has been reported by a number of authors (1, 2, 4, 16, 18, 22, 23, 24). The recombinant antigens were assessed either separately or by combining several proteins to increase diagnostic sensitivity. In our previous studies, we proposed four recombinant antigens of T. gondii (MAG1, GRA7, GRA6, and P35) that more frequently detected IgG antibodies in human serum samples from patients with acute toxoplasmosis (12, 13, 14, 24). The values for reactivity of r-GRA2 (100%) and r-ROP1 (94.6%) were similar to those obtained earlier for r-MAG1 (97.3%) as tested with the same serum samples from patients with acute toxoplasmosis. Furthermore, these antigens had very low reactivities in patients suspected of or having chronic toxoplasmosis (22.5% for r-GRA2, 15.5% for r-ROP1, and 7.5% for r-MAG1) (14). The results indicate also that r-GRA2, r-ROP1, and r-MAG1 proteins better differentiated specific anti-T. gondii IgG antibodies from acute and chronic serum samples than r-GRA7 (69% and 31%) (24), r-GRA6 (93.9% and 63.1%), and r-P35 (87.9% and 54.5%) (13). In summary, we can conclude that the T. gondii r-GRA2 and r-ROP1 antigens should be included in the group of molecular markers successfully increasing the sensitivity and specificity of IgG ELISAs for detection of the early phase of toxoplasmosis.
Acknowledgments
This work was supported by the Polpharma Foundation for Development of Polish Pharmacy and Medicine.
We thank Graham William Kay for editorial help.
Footnotes
Published ahead of print on 18 February 2009.
REFERENCES
- 1.Altcheh, J., N. S. Diaz, C. M. Pepe, V. Martin, M. Nigro, H. Freilija, and S. O. Angelc. 2006. Kinetic analysis of the humoral immune response against 3 Toxoplasma gondii-recombinant proteins in infants with suspected congenital toxoplasmosis. Diagn. Microbiol. Infect. Dis. 56161-165. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, D., G. T. Maine, I. Villena, J. C. Hunt, L. Howard, M. Sheu, S. Brojanac, L. E. Chovan, S. F. Nowlan, and J. M. Pinon. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 381144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastien, P. 2002. Molecular diagnosis of toxoplasmosis. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1)S205-S215. [DOI] [PubMed] [Google Scholar]
- 4.Beghetto, E., W. Buffolano, A. Spadoni, M. Del Pezzo, M. Di Cristina, O. Minenkova, E. Petersen, F. Felici, and N. Gargano. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 415414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73114-123. [PubMed] [Google Scholar]
- 6.Cesbron-Delauw, M. F., L. Lecordier, and C. Mercier. 1996. Role of secretory dense granule organelles in the pathogenesis of toxoplasmosis. Curr. Top. Microbiol. Immunol. 21959-65. [DOI] [PubMed] [Google Scholar]
- 7.Contini, C., S. Seraceni, R. Cultrera, C. Incorvaia, A. Sebastiani, and S. Picot. 2005. Evaluation of a real-time PCR-based assay using the LightCycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int. J. Parasitol. 35275-283. [DOI] [PubMed] [Google Scholar]
- 8.Cozon, G. J., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 1732-36. [DOI] [PubMed] [Google Scholar]
- 9.Ferrandiz, J., C. Mercier, M. Wallon, S. Picot, M.-F. Cesbron-Delauw, and F. Peyron. 2004. Limited value of assays using detection of immunoglobulin G antibodies to the two recombinant dense granule antigens, GRA1 and GRA6 Nt of Toxoplasma gondii, for distinguishing between acute and chronic infections in pregnant women. Clin. Diagn. Lab. Immunol. 111016-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golkar, M., S. Rafati, M. S. Abdel-Latif, M. P. Brenier-Pinchart, H. Fricker-Hidalgo, B. K. Sima, J. Babaie, H. Pelloux, M. F. Cesbron-Delauw, and C. Mercier. 2007a. The dense granule protein GRA2, a new marker for the serodiagnosis of acute Toxoplasma infection: comparison of sera collected in both France and Iran from pregnant women. Diagn. Microbiol. Infect. Dis. 58419-426. [DOI] [PubMed] [Google Scholar]
- 11.Golkar, M., M. A. Shokrgozar, S. Rafati, K. Musset, M. Assmar, R. Sadaie, M. F. Cesbron-Delauw, and C. Mercier. 2007b. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine 254301-4311. [DOI] [PubMed] [Google Scholar]
- 12.Hiszczyńska-Sawicka, E., A. Brillowska-Dąbrowska, S. Dąbrowski, H. Pietkiewicz, P. Myjak, and J. Kur. 2003. High yield expression and single-step purification of Toxoplasma gondii SAG1, GRA1, and GRA7 antigens in Escherichia coli. Protein Expr. Purif. 27150-157. [DOI] [PubMed] [Google Scholar]
- 13.Hiszczyńska-Sawicka, E., J. Kur, H. Pietkiewicz, L. Holec, A. Gąsior, and P. Myjak. 2005. Efficient production of the Toxoplasma gondii GRA6, p35 and SAG2 recombinant antigens and their applications in the serodiagnosis of toxoplasmosis. Acta Parasitol. 50249-254. [Google Scholar]
- 14.Holec, L., E. Hiszczyńska-Sawicka, A. Gasior, A. Brillowska-Dabrowska, and J. Kur. 2007. Use of MAG1 recombinant antigen for diagnosis of Toxoplasma gondii infection in humans. Clin. Vaccine Immunol. 14220-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongert, E., S. de Craeye, J. Dewit, and K. Huygen. 2007. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol. 29445-453. [DOI] [PubMed] [Google Scholar]
- 16.Li, S., G. Galvan, F. G. Araujo, Y. Suzuki, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7781-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liesenfeld, O., J. G. Montoya, N. J. Tathineni, M. Davis, B. W. Brown, Jr., K. L. Cobb, J. Parsonnet, and J. S. Remington. 2001. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 184140-145. [DOI] [PubMed] [Google Scholar]
- 18.Marcolino, P. T., D. A. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin. Diagn. Lab. Immunol. 7384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercier, C., D. K. Howe, D. Mordue, M. Lingnau, and L. D. Sibley. 1998. Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect. Immun. 664176-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray, A., C. Mercier, A. Decoster, L. Lecordier, A. Capron, and M. F. Cesbron-Delauw. 1993. Multiple B-cell epitopes in a recombinant GRA2 secreted antigen of Toxoplasma gondii. Appl. Parasitol. 34235-244. [PubMed] [Google Scholar]
- 21.Nguyen, T. D., M. de Kesel, G. Bigaignon, P. Hoet, G. Pazzaglia, M. Lammens, and M. Delmee. 1996. Detection of Toxoplasma gondii tachyzoites and bradyzoites in blood, urine, and brains of infected mice. Clin. Diagn. Lab. Immunol. 3635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigro, M., A. Gutierrez, A. M. Hoffer, M. Clemene, F. Kaufer, L. Carral, V. Martin, E. A. Guarnera, and S. O. Angel. 2003. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47609-613. [DOI] [PubMed] [Google Scholar]
- 23.Pfrepper, K. I., G. Enders, M. Gohl, D. Krczal, H. Hlobil, D. Wassenberg, and E. Soutschek. 2005. Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin. Diagn. Lab. Immunol. 12977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietkiewicz, H., E. Hiszczyńska-Sawicka, J. Kur, E. Petersen, H. V. Nielsen, M. Stankiewicz, I. Andrzejewska, and P. Myjak. 2004. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 421779-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistoia, V., P. Facchetti, F. Ghiotto, M. F. Cesbron-Delauw, and I. Prigione. 1996. Characterization of human T cell clones specific for Toxoplasma gondii. Curr. Top. Microbiol. Immunol. 219165-173. [DOI] [PubMed] [Google Scholar]
- 26.Saffer, L. D., O. Mercereau-Puijalon, J. F. Dubremetz, and J. D. Schwartzman. 1992. Localization of a Toxoplasma gondii rhoptry protein by immunoelectron microscopy during and after host cell penetration. J. Protozool. 39526-530. [DOI] [PubMed] [Google Scholar]
- 27.Sam-Yellowe, T. Y. 1996. Rhoptry organelles of the Apicomplexa: their role in host cell invasion and intracellular survival. Parasitol. Today 12308-316. [DOI] [PubMed] [Google Scholar]
- 28.Sensini, A., S. Pascoli, D. Marchetti, R. Castronari, M. Marangi, G. Sbaraglia, C. Cimmino, A. Favero, M. Castelletto, and A. Mottola. 1996. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin. Microbiol. Infect. 225-29. [DOI] [PubMed] [Google Scholar]