Abstract
Diagnosis of tuberculosis (TB) in children is difficult because symptoms are often nonspecific or absent in infected children, diagnostic specimens are difficult to obtain from younger children, and >50% have negative TB cultures. Thus, there is an urgent need for improved diagnosis of pediatric TB. This study aimed to evaluate the diagnostic value of a new serological method, the ALS (antibodies in lymphocyte supernatant) assay, for the diagnosis of active TB in children with clinically identified TB. The ALS test is based on the concept that antigen-specific plasma cells are present in the circulation only at times of acute infection and not in latency. A cross-sectional study of pediatric patients (age range, 11 to 167 months) who were clinically identified as TB (n = 58) or non-TB (n = 16) patients was conducted, and they were monitored for 6 months. Healthy children (n = 58) were enrolled as controls. Spontaneous release of TB antigen-specific antibodies by in vitro-cultured, unstimulated peripheral blood mononuclear cells was assessed by an enzyme-linked immunosorbent assay using Mycobacterium bovis bacillus Calmette-Guérin (BCG) as the detecting antigen. Of the patients clinically diagnosed with TB, 15% had culture-confirmed TB, 64% were positive for TB by clinically established scoring charts (K. Edwards, P. N. G. Med. J. 30: 169-178, 1987; G. Stegen, K. Jones, and P. Kaplan, Pediatrics 43: 260-263, 1969; and stop TB Partnership, Childhood TB subgroup, World Health Organization, Int. J. Tuberc. Lung Dis. 10: 1091-1097, 2006), and 91% were TB positive by the ALS method. All TB patients had significantly higher BCG-specific ALS titers at enrollment (optical density [OD], 1.06 ± 0.32) than healthy-control children (OD, 0.18 ± 0.06) and non-TB children (OD, 0.21 ± 0.10) (P = 0.001). The ALS titers declined in children with active disease from enrollment through 6 months following anti-TB therapy (P = 0.001). The ALS assay is a novel diagnostic method with potential applications in the diagnosis of pediatric TB and in subsequent monitoring of treatment effectiveness.
According to World Health Organization (WHO) estimates, about 1 million children annually develop tuberculosis (TB) worldwide, accounting for about 11% of all TB cases (25, 42, 48). Bangladesh ranks 6th among the 22 high-TB-burden countries in the world; about 45% of its population is tuberculin positive at the age of 14 years (22, 48). Childhood TB is often not considered a priority by national TB control programs, because children acquire Mycobacterium tuberculosis infections from adults and do not contribute to disease transmission. However, failure to identify and treat TB in children can lead to death in a majority of <3-year-old children (14, 23).
TB-infected children are often asymptomatic, and bacteriologic confirmation is rare, due to the difficulty of obtaining specimens (8, 31). Most pediatric TB cases are diagnosed using a combination of clinical and epidemiological features, which include characteristic chest radiographs (18), reactive tuberculin skin tests (TST), and a history of contact with active TB cases (31, 34); computed tomography and bronchoscopy are performed in industrialized countries (19). In areas where TB is endemic, most of the population acquires TB infection during childhood, and transmission is not restricted to the household (32, 46). This situation limits the diagnostic contribution of documented household exposure and positive TST (10, 47). Interpretation of TST reactivity is further complicated by the high prevalences of Mycobacterium bovis BCG vaccination and malnutrition. Radiographic changes in children can be quite variable (18). Serodiagnostic methods are generally low in sensitivity and specificity, especially for children, and seroreactivity data for pediatric TB are also limited (3, 4, 12, 20, 26, 41). Recent studies evaluating multiple antigens by an enzyme-linked immunosorbent assay (ELISA) or a multiantigen print immunoassay for the serodetection of M. tuberculosis infection appear to have potential but have not been tested on humans (13, 15, 16). Thus, in developing countries, heavy reliance is placed on clinical and epidemiological features for the diagnosis of pulmonary TB in children, and a scoring system is generally adopted in order to arrive at a diagnosis. Many scoring systems based on combinations of these features have been developed to detect childhood TB (21, 24, 37, 45).
We developed a novel method, called the ALS (antibodies in lymphocyte secretions) assay (29), for diagnosing active TB disease. It is based on the hypothesis that M. tuberculosis antigen-specific B cells, or plasmablasts, are short-lived and are present in the circulation only during active disease, not during latency or previously acquired of immunity (17, 43). Performance of the test with adult patients indicated that the method was highly sensitive and specific in diagnosing active TB (29). Here we aimed to evaluate the diagnostic value of this method for clinically identified pediatric TB patients, since no valid gold-standard method was available. Our secondary objective was to compare the ALS method with the existing scoring charts by using the clinical diagnosis as the reference. Furthermore, the patients were monitored for 6 months in order to determine how therapeutic response affected the ALS assay.
MATERIALS AND METHODS
Pediatric subjects.
The study was conducted with pediatric TB suspects who were either inpatients in the pulmonology ward or outpatients seen in the pediatric unit of the Bangabandhu Sheikh Mujib Medical University Hospital, a referral hospital in Dhaka, or who were seen at the Dhaka Hospital of the International Centre for Diarrheal Diseases Research, Bangladesh (ICDDR,B). The Dhaka Hospital runs a program for the diagnosis, treatment, and follow-up of children with TB. About two-thirds of the children who undergo nutritional rehabilitation for severe malnutrition at the ICDDR,B are diagnosed as having TB (1). All pediatric patients referred for evaluation of suspected TB were recruited consecutively. Seventy-seven pediatric patients (age range, 11 to 167 months) presenting with clinical features suggestive of TB with a mean duration of illness of 14 weeks were initially screened at one of the two hospitals.
Healthy children (age range, 30 to 120 months; n = 58) from the neighborhood of the ICDDR,B, some of whom were children of ICDDR,B staff, were enrolled as healthy controls. The health status of the control children was examined by the study clinician. The criteria for selection were that none of the children had any known exposure to active TB cases and that they were otherwise healthy, with no concurrent illness. Informed consent was obtained from a parent or guardian of each participant. The study was approved by ICDDR,B's Ethical Review Committee.
The sample size required for diagnostic accuracy was determined on the basis of the receiver-operating-characteristic curve index using NCSS and PASS software (Number Cruncher Statistical Systems, Kaysville, UT). Based on the results of the ALS assay for adult patients with TB (29), the required sample size was 58.
Screening, assessment, and scoring charts.
To classify the referred patients, each patient underwent an assessment for specific symptoms, including a persistent, nonremitting cough of >2 weeks' duration; anorexia; unexplained weight loss (>5%) or failure to gain weight over 2 to 3 months with intermittent fever; acute febrile illness with pleural pain; abdominal swelling with ascites; a hard, painless abdominal mass; painless firm swelling of the superficial lymph nodes in the neck; spinal deformity; a history of contact with active TB cases; and nonresponsiveness to conventional antibiotic therapy. A TST was performed after blood was collected; an induration with a diameter of >10 mm after 72 h was regarded as a positive TST result. Chest radiographs were examined independently by two physicians for confirmation of TB in the absence of bacteriologic confirmation. Clinicians formulated a final diagnosis. A diagnosis of TB was reached through a panel decision on the basis of suggestive clinical and radiological signs (in the absence of evidence for alternative infectious or noninfectious diseases) with or without an induration of >10 mm after the TST and was reevaluated after assessment of the response to anti-TB chemotherapy. Since bacteriologic confirmation was not possible in the majority of the cases, the clinical consensus diagnosis was used as a reference standard. Good clinical response was defined as complete resolution of symptoms with a regression/resolution of at least two-thirds of the original lesion found by X-ray, and/or a reduction in the size of the lymph node and a weight gain of ≥10% of the body weight at diagnosis, after 2 months of assigned anti-TB treatment.
The group of non-TB patients included children for whom TB was considered as part of the differential diagnosis of their illnesses. The illnesses included Hodgkin's lymphoma, intestinal cancer, nonspecific lymphadenitis, lower respiratory tract infection, and pneumonitis.
Two scoring systems that have some degree of overlap with the clinical diagnosis were used to diagnose TB: the modified Kenneth Jones scoring chart (KJSC), which is currently used in the ICDDR,B hospital, and the WHO scoring chart (WHO-SC) (9, 37, 42). In the WHO-SC, a score of ≥7 indicates that the individual is to be treated for TB. In the modified KJ scoring system, ≥7 points indicate unquestionable TB; 5 to 6 points indicate probable TB, such that therapy may be justified; and 3 to 4 points indicate that further investigations are needed.
Specimens.
Gastric lavage specimens were collected from children below the age of 6 years for an acid-fast bacillus (AFB) smear and mycobacterial culture. Blood (3.5 ml) was collected from each child at enrollment before the TST was performed and 2 months and 6 months after enrollment. The weight and height of each child were measured at enrollment and at two follow-up intervals (2 and 6 months) in order to assess the age- and sex-specific body mass index (BMI) for children above the age of 5 years (according to the CDC BMI-for-age growth charts for boys and girls [http://www.cdc.gov/growthcharts/]), and Z-scores were calculated for children below the age of 5 years according to the Child Growth Standards of the WHO by using the WHO Anthro 2005 software and macros (http://www.who.int/childgrowth/software/en/). Blood was also obtained from healthy-control children, and anthropometric measurements were recorded.
Management.
Standard anti-TB therapy was given according to the WHO guidelines for the management of pediatric TB. The standard treatment regimen for the first 2 months included daily doses of rifampin (rifampicin) at 10 mg, isoniazid at 5 mg, and pyrazinamide at 25 mg per kg of body weight per day, followed by rifampin at 10 mg and isoniazid at 5 mg for an additional 4 months. For multidrug-resistant TB cases, a modified regimen that included 15 mg of streptomycin, 15 mg of ethambutol, and 20 mg of ciprofloxacin per kg body of weight per day was considered for a further 3 to 6 months.
Mycobacterial culture.
Sputum specimens were collected from older children (n = 22) who could produce sputum and were processed for mycobacterial culture in solid Lowenstein-Jensen medium and liquid MGIT medium using standard procedures. Some of the children with lymphoadenopathy (n = 12) underwent a fine-needle aspiration procedure to collect pus cells, which were processed for cytology and mycobacterial culture on Lowenstein-Jensen medium by means of standard culture techniques. Gastric lavage specimens were collected from children younger than 6 years for AFB smears and culture; only 25 specimens could be used for culture. All cultures were continued up to 8 weeks. Positive cultures were inoculated for drug sensitivity testing.
The ALS assay.
The ALS assay was performed as described previously (29) except that the in vitro cell cultures were performed at two additional cell densities. Peripheral blood mononuclear cells (PBMCs) were separated from blood on Ficoll-Paque by differential centrifugation. PBMCs at three concentrations (1 × 106, 2.5 × 106, and 5 × 106 cells/ml) were suspended in 48-well tissue culture plates (Costar, Cambridge, MA) in tissue culture medium. Cells were incubated at 37°C under 5% CO2, and the supernatant was collected 72 h later and stored at −70°C until it was used for the measurement of immunoglobulin G (IgG) titers. The BCG vaccine (freeze-dried, glutamate-BCG vaccine for intradermal use; lot 1861; Japan BCG Laboratories, Japan) that is used in the Extended Program on Immunization for the vaccination of infants and children in Bangladesh was used as the coating antigen for this ELISA. Polystyrene microtiter plates (MaxiSorp; Nunc) were coated with BCG vaccine (1 μg/well) in carbonate buffer (0.1 M sodium bicarbonate and 5 mM magnesium chloride [pH 9.8]) and incubated overnight at 4°C. After a wash, the plates were incubated first with 10% fetal bovine serum in phosphate-buffered saline (pH 7.2) and then with lymphocyte supernatants, each for 2 h at 37°C, with intermittent washing. Horseradish peroxidase-conjugated rabbit anti-human IgG was added and incubated for 2 h at room temperature. Plates were developed with the substrate o-phenylenediamine, and the optical density (OD) was measured at 492 nm after the enzyme reaction was stopped. Pooled sera from M. tuberculosis culture-positive patients were used as positive controls (OD, >1.0). Antigen-specific responses were expressed as relative titers.
Statistical analysis.
Comparisons between the treatment days were made by one-way analysis of variance. Two-way analysis of variance was performed to compare the groups. In the absence of the gold-standard method (mycobacterial culture confirmation), concordance between the ALS test and clinical diagnosis or the scoring charts was assessed by using Cohen's kappa coefficient to determine the diagnostic agreement between the methods. McNemar's χ2 test was applied to compare the proportions of negative and positive results among the different diagnostic methods. Statistical analyses were performed with SPSS statistical software for Windows (SPSS, version 12; SPSS Inc., Chicago, IL).
RESULTS
Subjects.
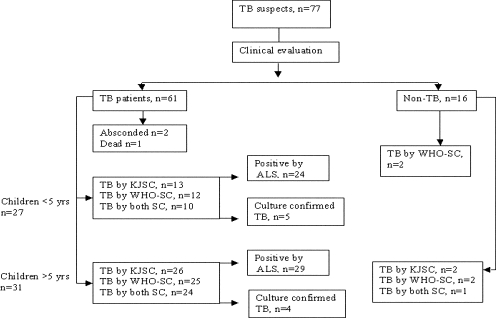
Sixty-one of 77 patients were finally classified clinically as active TB cases (Fig. 1). The remaining 16 patients were categorized as non-TB patients and were later diagnosed with other diseases (Table 1). Among those classified as TB patients, one died before treatment could be initiated and two absconded from the hospital. Twenty-eight of the remaining 58 TB patients (3 did not have the Mantoux test done) and 11 of 48 healthy children had indurations with diameters of ≥10 mm. All of these healthy-control children were >5 years old. Ten of the healthy children did not undergo the TST test. All patients who were given BCG vaccinations received them within 1 month after birth, as required by the Extended Program on Immunization schedule. The interval between immunization with BCG and the collection of blood for ALS testing was >10 months. All patients were followed up as long as 4 months after inclusion in the study; however, 12 TB patients and 5 non-TB patients were lost to follow-up at the 6-month time point. Mycobacterium tuberculosis was isolated from 9 of 58 children; of these, four isolates were obtained from sputum culture (older children), two from lymph node aspirate culture, and three from gastric lavage culture.
FIG. 1.
Profile showing the number of TB suspects initially screened for inclusion in the study and the numbers of TB patients clinically identified, diagnosed by the Kenneth Jones or WHO score chart or the ALS assay, and confirmed by culture.
TABLE 1.
Features of pediatric patients with and without TB and of healthy controls
| Feature | Value for:
|
||
|---|---|---|---|
| TB patients (n = 58) | Non-TB patients (n = 16) | Healthy controls (n = 58) | |
| Median (range) age (mos) | 72 (24-120) | 84 (69-126) | 60 (18.5-102) |
| Males/females | 32/26 | 7/9 | 30/28 |
| No. (%) with: | |||
| Malnutritiona | 49 (84.4) | 9 (56) | 13 (22) |
| BCG vaccination given | 48 (83) | 15 (94) | 58 (100) |
| History of TB contact | 39 (67) | 7 (44) | 0 |
| Positive TST, induration of ≥10 mmb | 28 (51) | 3 (19) | 11 (23) |
| Abnormal chest radiographc | 49 (87.5) | 11 (69) | ND |
| Duration (wks) of symptoms at enrollment (mean ± SD) | 14.1 ± 18 | 5.9 ± 7.2 | |
| No. (%) with the following type of disease: | |||
| Pulmonary TB | 41 (71) | ||
| Glandular TB | 9 (15.5) | ||
| Intestinal TB | 3 (5) | ||
| Spinal TB | 3 (5) | ||
| TB meningitis | 2 (3.4) | ||
| Intestinal cancer, Hodgkin's lymphoma | 2 (12) | ||
| Nonspecific lymphadenitis | 13 (21) | ||
| Pneumonitis | 1 (6) | ||
| Lower respiratory tract infection | 1 (7) | ||
| No. (%) lost to follow-up | 12 (20) | 5 (31) | |
Weight-for-age Z-scores were used for children below the age of 5 years, while BMI was used for children >5 years old.
The TST was not performed on 3 TB patients and 10 healthy children.
The chest X-ray was not performed for two TB patients or any healthy controls (ND, not done).
Scoring charts.
We categorized 58 TB patients into two age groups: 27 children below the age of 5 years and 31 children above the age of 5 years (Fig. 1, flow chart). According to the scoring charts, 42/58 children (72%), including 15 children (48%) ≤5 years old and 27 (87%) children >5 years old, were classified as having TB (Fig. 1). Sixteen of the 58 children, 12 below and 4 above the age of 5 years, were classified as TB negative by the scoring charts. These included 2 children diagnosed with intestinal TB, 2 with glandular TB, and 12 with pulmonary TB. All these children diagnosed with TB but negative by the score charts were moderately to severely malnourished; all had TST indurations with diameters between 0 and 4 mm; 7 (44%) had a history of contact with a TB patient; and 12 (75%) had parahilar or paratracheal lymphadenopathy on their chest radiographs. Fifteen (94%) had been vaccinated with BCG in infancy.
Among the 16 clinically diagnosed non-TB cases, 2 in the <5-year group were positive by the WHO-SC and 3 in the >5-year group were positive by at least one scoring chart (Fig. 1).
The ALS test is based on detection of BCG-specific antibodies released by plasma cells.
The clinical information or the diagnostic reports of the patients were not blinded; however, laboratory personnel were not aware of the diagnosis during the performance of the ALS test. Unstimulated in vitro-cultured PBMCs (72 h) were used to determine the levels of BCG antigen-specific IgG antibodies produced and secreted by circulating plasma cells. A higher concentration of PBMCs gave higher BCG-specific IgG titers (Fig. 2) at a given time point. For TB patients, the presence of plasma cells generated BCG-specific IgG titers that were significantly higher in the supernatants of 2.5 × 106 PBMCs and 5 × 106 PBMCs than in those of 1 × 106 PBMCs (P < 0.05), in contrast to the lack of such plasma cells in healthy children, who had low anti-TB IgG titers that were similar with all three PBMC concentrations (Fig. 2).
FIG. 2.
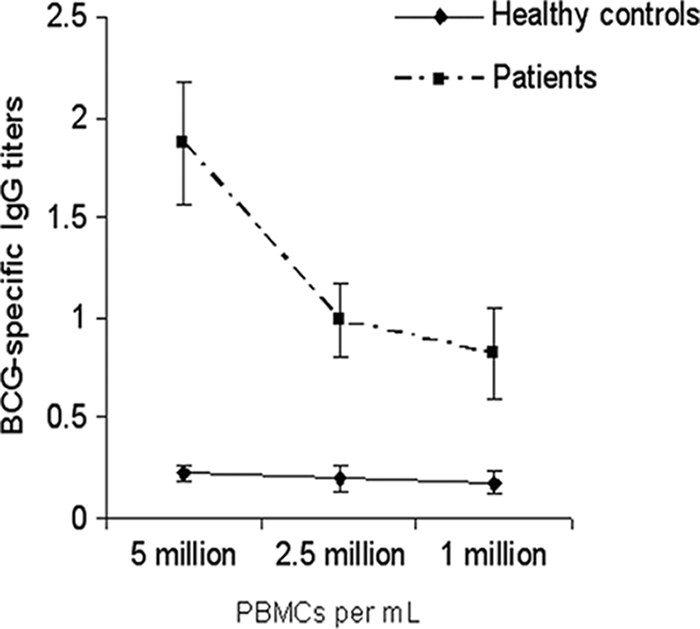
Comparison of BCG-specific IgG responses (relative titers) in lymphocyte secretions at various PBMC concentrations for patients with TB (▪) and healthy-control subjects (♦). Error bars represent SD. Significantly higher BCG-specific IgG titers were obtained at higher PBMC concentrations (2.5 × 106 and 5 × 106 PBMCs/ml) than at 1 × 106 PBMCs/ml (P < 0.04). Healthy-control subjects had consistently low BCG-specific IgG titers at all PBMC concentrations.
The average ALS titer for healthy-control children plus 3 standard deviations (SD) was considered the cutoff for positive ALS titers in patients. The cutoff for <5-year-old children was 0.350 OD unit, and for >5-year-old children, it was 0.356 OD unit. Thus, children with antibody titers higher than 0.35 OD unit were considered positive by the ALS method. Children clinically diagnosed with TB (mean OD ± SD, 1.06 ± 0.32) had significantly higher ALS titers than children clinically diagnosed as non-TB patients (OD, 0.21 ± 0.10) and healthy controls (OD, 0.18 ± 0.06) (P < 0.001) (Fig. 3). No differences in the ALS titers were found between non-TB and control children (P = 0.4), between BCG-vaccinated (OD, 0.98 ± 0.39) and nonvaccinated (OD, 0.97 ± 0.46) TB patients (P = 0.73), and between female (OD, 1.13 ± 0.49) and male (OD, 0.86 ± 0.36) patients (P = 0.3). Comparison of ALS titers between culture-positive (OD, 1.4 ± 0.5) and culture-negative (OD, 0.94 ± 0.37) TB patients also failed to show any significant difference (P = 0.37). The levels of ALS titers in healthy-control children with TST indurations of >10 mm were similar to those in children with TST indurations of <10 mm (P = 0.57).
FIG. 3.
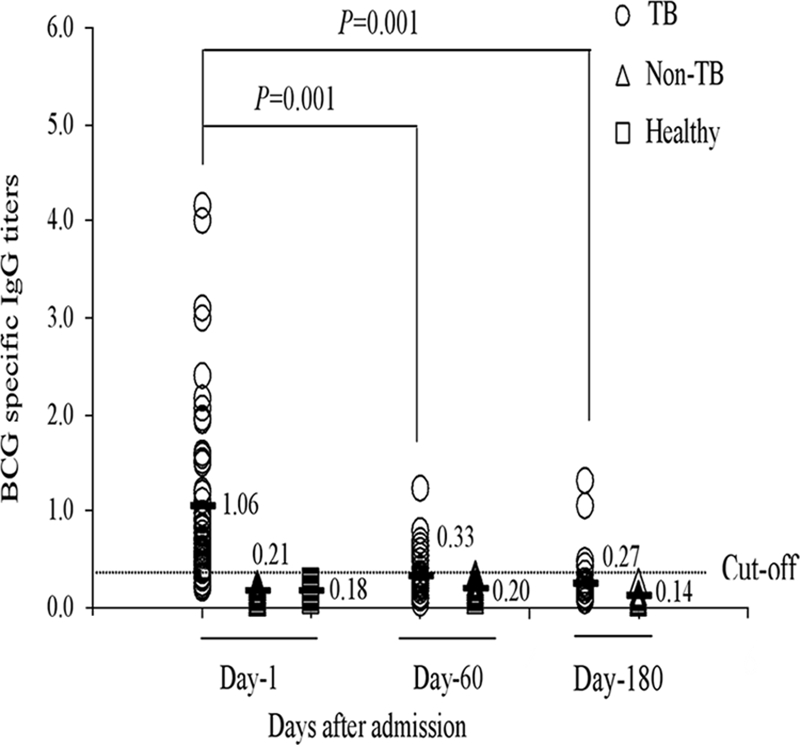
Comparison of BCG-specific IgG titers in TB and non-TB patients at different time points after enrollment and in healthy controls. The specific ALS responses (IgG titers) at days 60 and 180 were significantly lower than those on day 1 (P = 0.001). The specific ALS responses of TB patients on day 1 were significantly higher than those of non-TB patients and healthy controls. The straight horizontal line represents the cutoff at 0.35 OD unit. Horizontal bars indicate means.
Mean ALS titers for TB-infected children were at peak at enrollment and subsequently declined by day 60 and day 180 (P = 0.001) after treatment (Fig. 3) but still remained higher than those for control children throughout the study period, although the difference was no longer significant at day 180 (P < 0.1).
Evaluation of the sensitivity and specificity of the ALS test by comparison to the scoring charts.
When scoring charts and the ALS method were compared for the patients clinically diagnosed with TB, 92% were positive for TB by the ALS method as opposed to 64% and 67% by the WHO-SC and KJSC, respectively. Among the >5-year-old children, 77% were considered positive by both the scoring charts, while of the <5-year-old children, only 44% and 48% were positive by the WHO-SC and KJSC, respectively. The specificity of the ALS method and the KJSC was 87.5%, while that of the WHO-SC was 75%. The sensitivity and specificity of the ALS test were assessed against three different definitions of TB (clinical diagnosis, KJSC, and WHO-SC) (Table 2). In the older group of children, the ALS results matched with 86% of KJSC and 85% of WHO-SC results, while in the younger group, the ALS results matched with 77% of KJSC and 64% of WHO-SC results (Table 2). However, the specificity, or proportion of negative cases, by the ALS method was lower against scoring charts than against clinical diagnosis. The positive predictive value and negative predictive value of the ALS method were also higher when clinical diagnosis was used as the standard than when the scoring charts were used (Table 2).
TABLE 2.
Sensitivity and specificity of the ALS method in comparison with clinical diagnosis, the Kenneth Jones scoring chart, and the WHO scoring chart
| Reference method and patient group | ALS method parametera (%)
|
|||
|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |
| Clinical diagnosis | ||||
| >5 yr | 94 | 89 | 97 | 80 |
| <5 yr | 89 | 86 | 96 | 67 |
| All children | 91 | 87 | 96 | 74 |
| KJSC | ||||
| >5 yr | 86 | 50 | 80 | 60 |
| <5 yr | 77 | 29 | 40 | 67 |
| All children | 83 | 36 | 62 | 63 |
| WHO-SC | ||||
| >5 yr | 85 | 46 | 77 | 60 |
| <5 yr | 64 | 20 | 36 | 44 |
| All children | 78 | 30 | 58 | 53 |
PPV, positive predictive value, NPV, negative predictive value.
Concordance between the ALS test and the three different diagnostic tests for TB.
Table 3 shows the number of patients with concordent results for each group of patients and the agreement between the tests. The overall agreement between the ALS assay and clinical diagnosis was 72% (53/74), with a κ of 0.74, which was very good (P = 0.45). The agreement was better for the older group of children than for the younger group (κ, 0.79 and 0.67, respectively) (Table 3). The overall concordance between ALS and the scoring charts was lower, ∼43 to 46%, with a κ in the range of 0.21 to 0.24 (P < 0.03). The agreement was fair for the older group of children (κ, 0.37 and 0.33); however, among children below the age of 5 years, there was disagreement between the scoring charts and the ALS method (P ≤ 0.03) (Table 3).
TABLE 3.
Concordance and agreement between the ALS assay, clinical diagnosis, KJSC, and WHO-SC results for different groups of patients
| Reference methoda and patient group | Concordanceb | Agreementc | P value (McNemar's) |
|---|---|---|---|
| Clinical diagnosis | |||
| >5 yr | 29/40 (72.50) | 0.79 (0.11) | 1.00 |
| <5 yr | 24/34 (70.58) | 0.67 (0.15) | 0.62 |
| Total | 53/74 (71.62) | 0.74 (0.09) | 0.45 |
| KJSC | |||
| >5 yr | 24/40 (60.00) | 0.37 (0.16) | 0.75 |
| <5 yr | 10/34 (29.41) | 0.05 (0.13) | 0.01 |
| Total | 34/74 (45.94) | 0.21 (0.11) | 0.01 |
| WHO-SC | |||
| >5 yr | 23/4 (57.50) | 0.33 (0.16) | 0.55 |
| <5 yr | 9/34 (26.47) | 0.14 (0.14) | 0.03 |
| Total | 32/74 (43.24) | 0.24 (0.11) | 0.02 |
The ALS assay was compared with each reference method in turn.
Expressed as the number of patients with concordant results/total number of patients (percent).
Expressed as Cohen's κ coefficient (standard error).
DISCUSSION
In this study we report the application of the ALS assay for the diagnosis of TB in children. An active infection continuously exposes the immune system to the antigens, thereby stimulating antigen-specific antibody-producing plasmablasts in the peripheral circulation that are short-lived (17, 43). The ALS method is based on culturing and identifying these plasmablasts without any in vitro restimulation for 2 to 3 days; spontaneous secretion of anti-TB antibodies from these cells in the culture supernatant is detected and assessed by ELISA (29). ALS are different from stable antibodies in serum provided by long-lived, nondividing plasma cells that survive in limited survival niches present in bone marrow to maintain humoral antibody memory (43). Anti-TB chemotherapy for 6 months led to a significant decline in ALS titers in all children with TB, as shown previously for adult patients (28, 29, 33), suggesting the usefulness of the assay in monitoring therapeutic responses in pediatric TB patients.
The coating antigen used in the ELISA was BCG. In a recently completed study to validate the ALS method with adult patients with culture-confirmed pulmonary TB, we utilized a number of TB-specific antigens, including those from region of difference 1 (RD1) of the M. tuberculosis genome (unpublished data). The sensitivity and specificity of the ALS assay for the different antigens were close to those for BCG vaccine. The same vaccine lot was used as the antigen source in the current study. Serological tests based on detecting serum antibodies specific to mycobacterial antigens have found little place in routine clinical practice due to preexisting M. tuberculosis-specific antibodies originating from long-term memory in settings where TB is endemic, resulting in the inability to distinguish acute infection from TB latency by such a method (4, 41), and to the wide clinical disease spectrum (26). The antigen composition of tubercle bacilli differs with different stages of TB infection (2); use of such purified antigens in an ELISA may improve the sensitivity and the specificity of the method. Two recent systematic reviews assessed the accuracy of commercial antibody detection tests for the diagnosis of pulmonary and extrapulmonary TB (40, 41). The reviews revealed that all commercial tests provided highly variable estimates of sensitivity and specificity and did not have adequate sensitivity, specificity, or reproducibility under various clinical conditions to be useful for diagnosing TB in adults or in children. Another meta-analysis carried out to assess the immunodiagnostic potential of antigens evaluated in research laboratories (in-house) for the serodiagnosis of pulmonary TB showed that combinations of select antigens (median sensitivity, 76%) provide higher sensitivity than single antigens (median sensitivity, 53%) (39). Data on seroreactivity to antigens in sputum smear-negative or pediatric patients were insufficient, and none of the antigens achieved sufficient sensitivity to replace sputum smear microscopy. The use of a case-control design with healthy controls for the majority of studies was a limitation. In the present study, a non-TB control group consisting of children with illnesses in which TB was part of the differential diagnosis was included.
The fact that humoral responses to mycobacterial antigens are lower in children than in adults makes the interpretation of serological tests difficult (14, 30). In this study, the use of higher in vitro cell numbers yielded higher ALS IgG titers, thereby reducing the chances of missing a positive case, since uninfected individuals showed no increase in antibody titers at higher cell concentrations (Fig. 2). This step gives the ALS method an advantage over serum-based ELISAs. We did not see a significant difference in ALS titers between pediatric and adult TB patients (P = 0.39 [data not shown]), although the titers were somewhat lower in children, and thus the cutoff value (OD, 0.35) was lower than that for adult patients (OD, 0.42) (29). The T-cell based gamma interferon release assay (IGRA) used for adults is highly sensitive in the diagnosis of latent TB but has suboptimal sensitivity for active TB, comparable to that of the TST (6, 27). Studies evaluating the IGRA for children with latent TB are promising (5, 7, 44), since the assay is less affected by BCG vaccination than is the TST, and it can reduce the frequency of false diagnosis of M. tuberculosis infection for children with nontuberculous mycobacterial disease, but reports on its application in prospective studies of children with active TB are not available. Moreover, discordances between IGRAs are more evident in children below the age of 5 years (11). We saw no differences (P = 0.6) in the ALS titers between children below and above the age of 5 years, as opposed to the scoring charts, which showed lower sensitivity for the <5-year-old children.
Approximately 10% of immunocompetent normal children with culture-confirmed TB do not show a positive response to TST initially (36), but later during treatment, these children show reactive skin test results, suggesting that TB disease contributed to immunosuppression (35, 38). In the present study, only 15.5% of the TB patients had optimal nutritional status at the time of enrollment, and 51% of the clinically diagnosed TB patients did not show a positive TST result, although they had higher than normal ALS titers. From these findings, we may speculate that T-cell function may be hampered during active TB disease; however, B-cell function may remain less affected. Thus, the ALS test may be helpful for patients with immune-suppressed T cells, e.g., for human immunodeficiency virus-infected patients.
The AFB stain in sputum is positive for as many as 75% of adults with pulmonary TB, while <20% of children with TB have a positive AFB smear of sputum or gastric aspirates (49). In the current study, none of the children showed positive AFB smears of gastric aspirates; only 15% (3/20) of children had cultures positive for M. tuberculosis; and there were no significant differences in ALS titers between culture-negative and culture-positive patients. To establish the accuracy of diagnosis of active TB in patients, an appropriate reference test is needed to avoid major sources of bias; mycobacterial culture is the gold standard. However, such a reference test is mostly absent in the evaluation of pediatric TB, especially for children under the age of 5 years. The WHO-SC was evaluated by van Beekhuizen; a sensitivity of 62% and a specificity of 95% were reported (45). The modified Kenneth Jones scoring system was validated in the Dhaka Hospital of ICDDR,B with about 400 children (1). In the present study, we compared the ALS test with clinical diagnosis and the Kenneth Jones and WHO TB scoring systems. The agreement between ALS and clinical diagnosis was good for children; however, agreement between ALS and the scoring systems was fair or absent.
The limitations of the study include the small number of children with microbiologically confirmed disease and the lack of a true gold-standard test to allow comparison and assessment of the sensitivity and specificity of the ALS assay. Thus, the ALS assay was compared with clinical diagnosis and scoring charts in order to determine the levels of diagnostic agreement between various tests in the absence of a true gold standard and to evaluate which method agrees most with the possibility of true TB disease based on the clinical and epidemiological characteristics of patients.
In summary, the ALS assay is a novel diagnostic method that may supplement the clinical diagnosis of pediatric TB and other forms of TB when bacteriologic confirmation is not possible, due to difficulties in obtaining specimens and the paucibacillary nature of the disease, and may be useful in monitoring treatment effectiveness. Further studies are needed to further evaluate the ALS assay using purified RD1 antigens singly and in combinations with a larger number of microbiologically confirmed pediatric TB patients. Currently, the ALS method is being evaluated with a larger number of children in a hospital setting in Bangladesh and with adult TB patients with and without human immunodeficiency virus coinfection in Africa.
Acknowledgments
We are grateful to the guardians of all the children who participated in the study.
The research study was conducted at the ICDDR,B with support from the Academy of Sciences for the Developing World (TWAS; grant GR-00508), the Swedish Agency for Research Cooperation with Developing Countries (Sida/SAREC; Agreement support to R.R. and J.A., grant 2002-2004), and ICDDR,B. ICDDR,B acknowledges with gratitude the commitment of TWAS and Sida/SAREC to the Centre's research efforts. J. Andersson was funded by the Swedish Heart and Lung Foundation. ICDDR,B also gratefully acknowledges the following donors, which provide unrestricted support for the Centre's research efforts: the Australian Agency for International Development (AusAID), the Government of the People's Republic of Bangladesh, the Canadian International Development Agency (CIDA), the Embassy of the Kingdom of The Netherlands (EKN), the Swedish International Development Cooperation Agency (Sida), the Swiss Agency for Development and Cooperation (SDC), and the Department for International Development, United Kingdom (DFID).
We have no financial conflicts of interest.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Ahmed, T., F. Sobhan, S. A. M. Ahmed, S. Banu, A. M. Mahmood, K. A. Hyder, M. J. Chisti, K. Abdullah, M. Mahfuz, and M. A. Salam. 2008. Childhood tuberculosis: a review of epidemiology, diagnosis and management. Infect. Dis. J. Pakistan 1752-60. [Google Scholar]
- 2.Davidow, A., G. V. Kanaujia, L. Shi, J. Kaviar, X. Guo, N. Sung, G. Kaplan, D. Menzies, and M. L. Gennaro. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 736846-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dayal, R., G. Sirohi, M. K. Singh, P. P. Mathur, B. M. Agarwal, V. M. Katoch, B. Joshi, P. Singh, and H. B. Singh. 2006. Diagnostic value of ELISA serological tests in childhood tuberculosis. J. Trop. Pediatr. 52433-437. [DOI] [PubMed] [Google Scholar]
- 4.Demkow, U., J. Ziolkowski, B. Bialas-Chromiec, M. Filewska, T. Zielonka, M. Wasik, and E. Rowinska-Zakrzewska. 2006. Humoral immune response against mycobacterial antigens in children with tuberculosis. J. Physiol. Pharmacol. 57(Suppl. 4)63-73. [PubMed] [Google Scholar]
- 5.Detjen, A. K., T. Keil, S. Roll, B. Hauer, H. Mauch, U. Wahn, and K. Magdorf. 2007. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin. Infect. Dis. 45322-328. [DOI] [PubMed] [Google Scholar]
- 6.Dewan, P. K., J. Grinsdale, and L. M. Kawamura. 2007. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin. Infect. Dis. 4469-73. [DOI] [PubMed] [Google Scholar]
- 7.Dogra, S., P. Narang, D. K. Mendiratta, P. Chaturvedi, A. L. Reingold, J. M. Colford, Jr., L. W. Riley, and M. Pai. 2007. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J. Infect. 54267-276. [DOI] [PubMed] [Google Scholar]
- 8.Eamranond, P., and E. Jaramillo. 2001. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int. J. Tuberc. Lung Dis. 5594-603. [PubMed] [Google Scholar]
- 9.Edwards, K. 1987. The diagnosis of childhood tuberculosis. P. N. G. Med. J. 30169-178. [PubMed] [Google Scholar]
- 10.Enarson, P. M., D. A. Enarson, and R. Gie. 2005. Management of tuberculosis in children in low-income countries. Int. J. Tuberc. Lung Dis. 91299-1304. [PubMed] [Google Scholar]
- 11.Ferrara, G., M. Losi, R. D'Amico, P. Roversi, R. Piro, M. Meacci, B. Meccugni, I. M. Dori, A. Andreani, B. M. Bergamini, C. Mussini, F. Rumpianesi, L. M. Fabbri, and L. Richeldi. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 3671328-1334. [DOI] [PubMed] [Google Scholar]
- 12.Gennaro, M. L. 2000. Immunologic diagnosis of tuberculosis. Clin. Infect. Dis. 30(Suppl. 3)S243-S246. [DOI] [PubMed] [Google Scholar]
- 13.Khan, I. H., R. Ravindran, J. Yee, M. Ziman, D. M. Lewinsohn, M. L. Gennaro, J. L. Flynn, C. W. Goulding, K. DeRiemer, N. W. Lerche, and P. A. Luciw. 2008. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin. Vaccine Immunol. 15433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewinsohn, D. A., M. L. Gennaro, L. Scholvinck, and D. M. Lewinsohn. 2004. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int. J. Tuberc. Lung Dis. 8658-674. [PubMed] [Google Scholar]
- 15.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, D. Greenwald, C. A. Nacy, S. Gibson, P. J. Didier, M. Washington, P. Szczerba, S. Motzel, L. Handt, J. M. Pollock, J. McNair, P. Andersen, J. A. Langermans, F. Verreck, S. Ervin, F. Ervin, and C. McCombs. 2007. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin. Vaccine Immunol. 141158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 24291-100. [DOI] [PubMed] [Google Scholar]
- 17.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23367-386. [DOI] [PubMed] [Google Scholar]
- 18.Marais, B. J., P. R. Donald, R. P. Gie, H. S. Schaaf, and N. Beyers. 2005. Diversity of disease in childhood pulmonary tuberculosis. Ann. Trop. Paediatr. 2579-86. [DOI] [PubMed] [Google Scholar]
- 19.Marais, B. J., R. P. Gie, H. S. Schaaf, J. R. Starke, A. C. Hesseling, P. R. Donald, and N. Beyers. 2004. A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr. Radiol. 34886-894. [DOI] [PubMed] [Google Scholar]
- 20.Marais, B. J., and M. Pai. 2007. Recent advances in the diagnosis of childhood tuberculosis. Arch. Dis. Child. 92446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur, H. C., S. Saxena, and R. M. Bhardwaj. 1974. Evaluation of Kenneth Jones' criteria for diagnosis of childhood tuberculosis. Indian J. Pediatr. 41349-355. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health and Family Welfare. 2001. National TB control programme of Bangladesh: review and strategic plan 2001-2005. Ministry of Health and Family Welfare, Government of Bangladesh, Dhaka, Bangladesh.
- 23.Mubarik, M., B. Nabi, G. M. Ladakhi, and A. S. Sethi. 2000. Childhood tuberculosis (Part I). Epidemiology, pathogenesis, clinical profile. JK Pract. 712-15. [PubMed] [Google Scholar]
- 24.Narayan, S., S. Mahadevan, and V. T. Serane. 2003. Keith Edwards score for diagnosis of tuberculosis. Indian J. Pediatr. 70467-469. [DOI] [PubMed] [Google Scholar]
- 25.Nelson, L. J., and C. D. Wells. 2004. Global epidemiology of childhood tuberculosis. Int. J. Tuberc. Lung Dis. 8636-647. [PubMed] [Google Scholar]
- 26.Pai, M., S. Kalantri, and K. Dheda. 2006. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev. Mol. Diagn. 6423-432. [DOI] [PubMed] [Google Scholar]
- 27.Pai, M., and D. Menzies. 2007. Interferon-gamma release assays: what is their role in the diagnosis of active tuberculosis? Clin. Infect. Dis. 4474-77. [DOI] [PubMed] [Google Scholar]
- 28.Raqib, R., S. M. Kamal, M. J. Rahman, Z. Rahim, S. Banu, P. K. Bardhan, F. Chowdhury, G. Ara, K. Zaman, R. F. Breiman, J. Andersson, and D. A. Sack. 2004. Use of antibodies in lymphocyte secretions for detection of subclinical tuberculosis infection in asymptomatic contacts. Clin. Diagn. Lab. Immunol. 111022-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raqib, R., J. Rahman, A. K. Kamaluddin, S. M. Kamal, F. A. Banu, S. Ahmed, Z. Rahim, P. K. Bardhan, J. Andersson, and D. A. Sack. 2003. Rapid diagnosis of active tuberculosis by detecting antibodies from lymphocyte secretions. J. Infect. Dis. 188364-370. [DOI] [PubMed] [Google Scholar]
- 30.Rosen, E. U. 1990. The diagnostic value of an enzyme-linked immune sorbent assay using adsorbed mycobacterial sonicates in children. Tubercle 71127-130. [DOI] [PubMed] [Google Scholar]
- 31.Salazar, G. E., T. L. Schmitz, R. Cama, P. Sheen, L. M. Franchi, G. Centeno, C. Valera, M. Leyva, S. Montenegro-James, R. Oberhelman, R. H. Gilman, and M. J. Thompson. 2001. Pulmonary tuberculosis in children in a developing country. Pediatrics 108448-453. [DOI] [PubMed] [Google Scholar]
- 32.Schaaf, H. S., I. A. Michaelis, M. Richardson, C. N. Booysen, R. P. Gie, R. Warren, P. D. van Helden, and N. Beyers. 2003. Adult-to-child transmission of tuberculosis: household or community contact? Int. J. Tuberc. Lung Dis. 7426-431. [PubMed] [Google Scholar]
- 33.Sousa, A. O., A. Wargnier, Y. Poinsignon, N. Simonney, F. Gerber, F. Lavergne, J. L. Herrmann, and P. H. Lagrange. 2000. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tuber. Lung Dis. 8027-33. [DOI] [PubMed] [Google Scholar]
- 34.Starke, J. R. 2000. Diagnosis of tuberculosis in children. Pediatr. Infect. Dis. J. 191095-1096. [DOI] [PubMed] [Google Scholar]
- 35.Starke, J. R. 1989. Prevention of tuberculosis. Semin. Respir. Infect. 4318-325. [PubMed] [Google Scholar]
- 36.Starke, J. R., and K. T. Taylor-Watts. 1989. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics 8428-35. [PubMed] [Google Scholar]
- 37.Stegen, G., K. Jones, and P. Kaplan. 1969. Criteria for guidance in the diagnosis of tuberculosis. Pediatrics 43260-263. [PubMed] [Google Scholar]
- 38.Steiner, P., M. Rao, M. S. Victoria, H. Jabbar, and M. Steiner. 1980. Persistently negative tuberculin reactions: their presence among children with culture positive for Mycobacterium tuberculosis (tuberculin-negative tuberculosis). Am. J. Dis. Child. 134747-750. [DOI] [PubMed] [Google Scholar]
- 39.Steingart, K. R., N. Dendukuri, M. Henry, I. Schiller, P. Nahid, P. C. Hopewell, A. Ramsay, M. Pai, and S. Laal. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16260-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax 62911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stop TB Partnership, Childhood TB Subgroup, World Health Organization. 2006. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int. J. Tuberc. Lung Dis. 101091-1097. [PubMed] [Google Scholar]
- 43.Tarlinton, D., A. Radbruch, F. Hiepe, and T. Dorner. 2008. Plasma cell differentiation and survival. Curr. Opin. Immunol. 20162-169. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, R. E., A. J. Cant, and J. E. Clark. 2008. Potential impact of NICE tuberculosis guidelines on paediatric tuberculosis screening. Arch. Dis. Child. 93200-203. [DOI] [PubMed] [Google Scholar]
- 45.van Beekhuizen, H. J. 1998. Tuberculosis score chart in children in Aitape, Papua New Guinea. Trop. Doct. 28155-160. [DOI] [PubMed] [Google Scholar]
- 46.Verver, S., R. M. Warren, Z. Munch, M. Richardson, G. D. van der Spuy, M. W. Borgdorff, M. A. Behr, N. Beyers, and P. D. van Helden. 2004. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet 363212-214. [DOI] [PubMed] [Google Scholar]
- 47.Weismuller, M. M., S. M. Graham, N. J. Claessens, S. Meijnen, F. M. Salaniponi, and A. D. Harries. 2002. Diagnosis of childhood tuberculosis in Malawi: an audit of hospital practice. Int. J. Tuberc. Lung Dis. 6432-438. [PubMed] [Google Scholar]
- 48.World Health Organization. 2006. Global tuberculosis control: surveillance, planning, financing. WHO report 2006. WHO/HTM/TB/2006.362. World Health Organization, Geneva, Switzerland.
- 49.Zar, H. J., D. Hanslo, P. Apolles, G. Swingler, and G. Hussey. 2005. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 365130-134. [DOI] [PubMed] [Google Scholar]