Abstract
Studies were undertaken to determine whether anti-ovine progressive pneumonia virus (OPPV) antibody responses in serum or OPP provirus levels in peripheral blood associate with the degree of histologically measured tissue lesions in naturally OPPV-infected sheep. Sections of formalin-fixed, paraffin-embedded, and hematoxylin- and eosin-stained lung, mammary gland, carpal synovial membrane, and brain tissues from 11 OPPV-infected ewes (mean age of 8.6 years) and 5 OPPV-uninfected ewes (mean age of 6 years) were evaluated for lesion severity. Ovine progressive pneumonia (OPP) provirus levels and anti-OPPV antibody titers in peripheral blood and serum samples, respectively, were measured upon euthanasia and 3 years prior to euthanasia. Both mean peripheral OPP provirus levels and mean serum anti-surface envelope glycoprotein (anti-SU) antibody titers at the time of euthanasia were significantly higher in ewes with moderate to severe histological lesions than in ewes with no to mild histological lesions. However, although mean peripheral blood OPP provirus levels at euthanasia and 3 years prior to euthanasia significantly correlated with the highest histological lesion score for any affected tissue (two-tailed P values, 0.03 and 0.02), mean serum anti-SU antibody titers, anti-capsid antibody titers, and anti-transmembrane 90 antibody titers at euthanasia did not show a significant correlation with the highest histological lesion score for any tissue (two-tailed P values, 0.32, 0.97, and 0.18, respectively). These data are the first to show that OPP provirus levels predict and correlate with the extent of OPPV-related histological lesions in various OPPV-affected tissues. These findings suggest that peripheral OPP provirus levels quantitatively contribute more to the development of histological lesions than the systemic anti-SU antibody host immune response.
Ovine progressive pneumonia virus (OPPV), maedi-visna virus (MVV), and caprine arthritis-encephalitis virus (CAEV) are small-ruminant lentiviruses (SRLV) of the family Retroviridae which are horizontally transmitted and cause infections that can slowly manifest as one or a combination of clinical signs including arthritis, mastitis, cachexia, dyspnea, and ataxia. The diagnosis of SRLV infection is based upon the detection of anti-SRLV antibodies in adult sheep by serological diagnostic tests such as enzyme-linked immunosorbent assays (ELISAs) and the agar gel immunodiffusion assay or the presence of provirus in peripheral blood cells (6, 12). The appearance of clinical signs in SRLV-infected animals can be variable, and therefore, histological assessment is considered the reference standard for determining the extent of disease in the OPPV-affected tissues: lungs, mammary glands, carpal synovial membranes, and the central nervous system (7). In CAEV-infected goats, serum and synovial fluid anti-surface envelope glycoprotein (anti-SU) antibody titers associate with and predict the severity of carpal synovial membrane lesions found in CAEV-seropositive goats (15). In OPPV- or MVV-infected sheep, it is unknown whether serum anti-SU antibody titers associate with and predict the severity of histological lesions in any one of the affected tissues.
OPPV persists in monocytes and macrophages by reverse transcribing its RNA into cDNA and integrating into the host genome as provirus (8, 9). A previous study showed that the presence of ovine progressive pneumonia (OPP) provirus in alveolar macrophages significantly associates with the presence of moderate and severe lung lesions (2). Furthermore, another study showed that the mean maedi-visna provirus levels in alveolar macrophages in six MVV-seropositive sheep with mild to severe lung lesions (6.2 × 105 copies of pol/500 ng of DNA) were higher than those in six seropositive sheep without lung lesions (6.5 × 104 copies of pol/500 ng of DNA) (19). In this same study, a mean provirus level of 3.6 × 104 pol copies/500 ng of DNA was found in adherent peripheral mononuclear cells in 6 of 12 OPPV-seropositive sheep; however, it was not stated whether these 6 sheep had mild to severe lung lesions. We recently developed a real-time OPPV quantitative PCR (qPCR) assay for determining provirus levels in peripheral blood (12), and currently, it is unknown whether peripheral OPP provirus levels correlate with and predict histological lesions in lungs, mammary glands, carpal synovial membranes, or brain tissues. In this study, we tested whether peripheral OPP provirus levels or serum anti-SU antibody titers correlated with the degrees of histopathology in OPPV-affected tissues of 11 OPPV-infected ewes.
MATERIALS AND METHODS
Animals.
Eleven ewes of the Suffolk, Polypay, Rambouillet, and Columbia breeds at 7 to 9 years of age (mean age ± standard deviation, 8.6 ± 0.8 years) were defined as OPPV infected due to the presence of (i) anti-SU antibodies as measured by a competitive ELISA (cELISA; VMRD, Inc., Pullman, WA) (10) and (ii) OPP provirus in peripheral blood mononuclear cells as detected by a real-time OPPV qPCR assay (12, 13). As determined by a previous validation study (10), a positive cELISA result for sheep is defined as a percent inhibition of greater than 20.9%. At 6 years of age, one ewe developed mastitis in the form of a hard bag on one side of her udder, and at 7 years of age, she was euthanized due to severe cachexia. Another ewe was experiencing severe lameness, but not swollen joints or arthritis, at the time of euthanasia at 6 years of age. Five 6-year old ewes of the Suffolk, Columbia, and Rambouillet breeds were defined as OPPV-uninfected controls due to the absence of anti-OPPV antibodies detectable by cELISA in their sera and undetectable OPP provirus in peripheral blood. These five OPPV-seronegative sheep were tested by cELISA every 2 weeks for at least 3 years prior to euthanasia at 6 years of age. The five OPPV-uninfected ewes had been naturally exposed to OPPV from birth through 8 months of age but remained uninfected during their lifetimes (6 years). Ten of the OPPV-infected ewes (LMH11, LMH12, LMH13, LMH14, LMH15, LMH16, LMH17, LMH18, LMH19, and LMH20) were naturally exposed to OPPV at birth in an Idaho flock in which OPPV was endemic, first tested serologically positive (by cELISA) for OPPV at 3 years of age, and remained persistently infected during their lifetimes (7 or 9 years). One 2-year-old OPPV-uninfected ewe (112-45) was naturally exposed to 9 of the 10 above-mentioned OPPV-infected ewes, and this animal first tested serologically positive (by cELISA) at 5 years of age and remained persistently infected during the rest of her lifetime (6 years). The animals in this study were housed on Washington State University grounds and utilized under a protocol approved by the International Animal Care and Use Committee at Washington State University.
Measurement of serum anti-SU, anti-CA, and anti-TM90 antibody titers.
Anti-capsid (anti-CA) and anti-transmembrane 90 (anti-TM90) antibody titers were determined by a previously described Western blotting analysis using serum dilutions (end point method) on OPPV WLC1 lysate according to the methods described previously (16). The preparation of OPPV WLC1 lysate was as reported previously, except that this lysate was not radiolabeled and was quantitated using the bicinchoninic acid protein assay (Pierce) (10).
OPPV qPCR assay.
One microgram of genomic DNA from peripheral blood mononuclear cells was utilized in the previously described real-time OPPV qPCR assay, which targets the transmembrane regions of envelope protein (12, 13). At least three independent OPPV qPCR assays were performed for each ewe.
Tissue processing and histological assessment.
Sections of the right and left caudal lung lobes, right and left cranial lobes, middle lung lobe, right and left mammary glands, right and left carpal synovial membranes, and choroid plexus were collected within 2 h postmortem and immediately fixed in neutral buffered 10% formalin for at least 24 h. Fixed tissues were trimmed, placed into cassettes, and embedded in paraffin. Sequential 3-μm-thick paraffin sections were individually placed onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and heated overnight at 37°C in a dry oven. Thereafter, the slides were deparaffinized in a series of alcohols and stained with hematoxylin and eosin (H&E) for standard histological evaluation.
The grading of lesion severity in the lung was determined using a previously published grading system with slight modifications, whereby lung lesion severity was characterized as no lesions or mild, moderate, or severe lesions by using a numeric score with two components (1). The first component represented the mean number of prominent peribronchiolar, perivascular, or parenchymal lymphoid follicles per five random microscopic fields of view at a total magnification of ×100 and included three categories: 1, one to two follicles; 2, three to five follicles; and 3, six or more follicles. If an animal had no lymphoid follicles detected, it was given a numerical value of 1. The second component was a numeric score deduced by assessing the thickening of the alveolar septa by the interstitial accumulation of inflammatory cells and exudate and included three categories: 1, multifocal interstitial leukocytes without protein exudate; 2, multifocal and intermittent confluent areas of leukocyte infiltration, with or without interstitial protein exudate; and 3, confluent areas of alveolar septal thickening with leukocytes and protein exudate. The numbers of the highest-numbered categories observed for component 1 and component 2 for any of the five lung lobes were added together and divided by two for a total numeric score of the lung histological lesions: no lesions (0 to 0.4) and mild (0.5 to 1.4), moderate (1.5 to 2.4), and severe (>2.4) lesions (Table 1).
TABLE 1.
OPPV-associated total histological scores for and descriptions of histopathology in the lung, mammary gland, carpal synovium, and choroid plexus tissues
| Tissuea | Total scoreb | Description |
|---|---|---|
| L | 0-0.4 | No histological lesions based upon total numeric score |
| 0.5-1.4 | Mild histological lesions based upon total numeric score | |
| 1.5-2.4 | Moderate histological lesions based upon total numeric score | |
| >2.4 | Severe histological lesions based upon total numeric score | |
| MG | 0 | No histological lesions |
| 1 | Mild lesions: small numbers of lymphocytes or plasma cells surrounding alveoli and ducts | |
| 2 | Mild lesions: medium numbers of mononuclear cells disrupting occasional alveoli | |
| 3 | Moderate lesions: periductal follicle formation and disruption of alveoli by mononuclear cells | |
| 4 | Severe lesions: extensive fibrosis with or without the above-described lesions | |
| CS | 0 | No histological lesions |
| 1 | Mild lesions: synovial membrane hyperplasia with no to small numbers of lymphocytes | |
| 2 | Moderate lesions: hyperplasia of synoviocytes plus perivascular and subintimal inflammation with villous hypertrophy | |
| 2+ | Moderate lesions: more severe inflammation with large and more numerous perivascular cuffs than in grade 2 lesions | |
| 3 | Severe lesions: lesions of grade 2+ but also including synovial villous hypertrophy with diffuse mononuclear infiltration of subsynovial connective tissue and coalescing foci of inflammatory cells in the underlying periarticular tissue | |
| 4 | Severe lesions: lesions associated with grades 1 to 3 with the addition of necrosis of the synovial lining | |
| CP | 0 | No lesions |
| 1 | Mild lesions: few lymphocytes and histiocytes in the choroid plexus | |
| 2 | Moderate lesions: multifocal, small to medium clusters of lymphocytes and plasma cells in combination with lymphoid nodules |
L, lung; MG, mammary gland; CS, carpal synovium; and CP, choroid plexus.
For lung tissue, the total numeric score was calculated as follows: (component 1 category + component 2 category)/2. (See Materials and Methods.)
The grading of lesion severity in the carpal synovial and mammary gland tissues was determined using previous established criteria, with minor changes (3, 4) (Table 1). Photomicrographs were taken using a Zeiss Axioskop2 Plus microscope with AxioVision 3.0 software.
Statistical analyses.
Fisher's exact test (two sided) was utilized to test for significant associations between the numbers of animals with at least one moderate to severe histological lesion in any of the tissues and detectable OPP provirus levels or positive cELISA results by using InStat version 3.0b (GraphPad Software Inc.). An unpaired, two-tailed t test with Welch correction was employed to compare mean peripheral OPP provirus levels and mean serum anti-SU antibody titers between ewes with no to mild histological lesions and ewes with moderate to severe histological lesions by using InStat version 3.0b (GraphPad Software Inc.). This test does not assume equal variances. Graphs were made using Prism 4, version 4.0b (GraphPad Software Inc.). In addition, a two-tailed Pearson correlation, which assumes that data were obtained from Gaussian populations, was utilized to determine whether there was a correlation between the highest histological score for any tissue and the mean peripheral OPP provirus level or the mean serum anti-SU antibody titer by using GraphPad software.
RESULTS
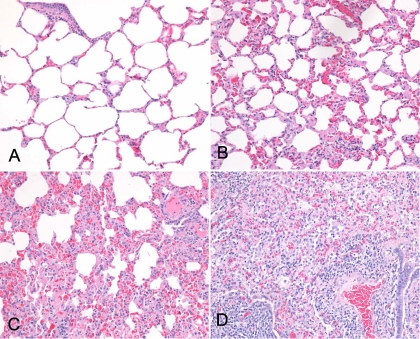
As a first step, a histological assessment was performed on sections of right and left cranial lung lobe, right and left caudal lung lobe, middle lung lobe, right and left mammary gland, right and left carpal synovial membrane, and choroid plexus tissues of 16 ewes naturally exposed to OPPV, including 11 OPPV-infected ewes (mean age, 8.6 years) and 5 OPPV-uninfected ewes (mean age, 6 years). Of the 11 OPPV-infected ewes, 10, or 91%, exhibited moderate to severe lesions consistent with OPPV infection in one of the lung tissues, mammary glands, or carpal synovial membranes and 1 seropositive ewe (LMH12) exhibited mild lesions consistent with OPPV infection. Seven of the 10 ewes with moderate to severe lesions in one tissue also showed moderate to severe lesions in two or more tissues, and only 1 ewe (LMH11) had clinical signs consistent with OPPV infection at the time of euthanasia (Table 2). All five of the OPPV-uninfected ewes had no or mild histological lesions in the tissues. Figure 1 shows histological examples of lung tissues defined as having no lesions and mild, moderate, and severe histological lesions. In addition, none of the 16 sheep, either infected or uninfected, had moderate to severe lesions in the choroid plexus. However, two OPPV-infected ewes had multifocal small to medium clusters of plasma cells and lymphocytes in the choroid plexus, but lymphoid nodules were absent.
TABLE 2.
Breeds of and clinical signs and histopathology in 11 OPPV-infected sheep
| Animal | Breed | Clinical sign(s) of OPPV infection | Tissue(s) with moderate to severe lesionsa |
|---|---|---|---|
| LMH11 | Polypay | Mastitis, cachexia | L, MG, CS |
| LMH12 | Polypay | None | None |
| LMH13 | Rambouillet | None | MG, CS |
| LMH14 | Rambouillet | None | L |
| LMH15 | Columbia | None | MG |
| LMH16 | Rambouillet | None | L, MG, CS |
| LMH17 | Columbia | None | L, MG, CS |
| LMH18 | Columbia | None | L, MG, CS |
| LMH19 | Rambouillet | None | L, MG, CS |
| LMH20 | Rambouillet | None | L, CS |
| 112-45 | Suffolk | None | L |
L, lung; MG, mammary gland; CS, carpal synovial membrane.
FIG. 1.
(A) Example of a formalin-fixed, paraffin-embedded, H&E-stained lung tissue section from an uninfected ewe exhibiting no histological lesions. Total original magnification, ×400. (B) Example of a formalin-fixed, paraffin-embedded, H&E-stained lung tissue section from an OPPV-infected ewe exhibiting mild histological lesions. Total original magnification, ×400. (C) Example of a formalin-fixed, paraffin-embedded, H&E-stained lung tissue section from an OPPV-infected ewe exhibiting moderate histological lesions. Total original magnification, ×400. (D) Example of a formalin-fixed, paraffin-embedded, H&E-stained lung tissue section from an OPPV-infected ewe exhibiting severe histological lesions. Total original magnification, ×400.
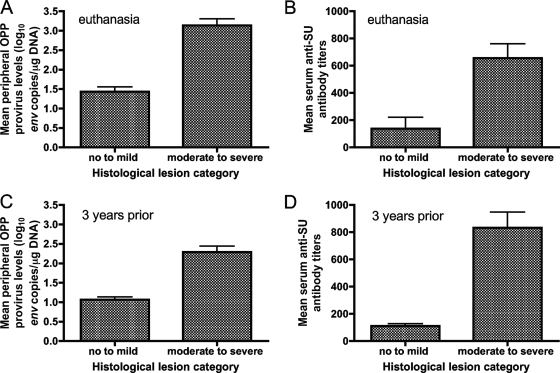
We addressed whether there was an association between the numbers of ewes with at least one moderate to severe lesion in any affected tissue and detectable OPP provirus in peripheral blood or a positive cELISA result at euthanasia. The number of ewes with at least one moderate to severe lesion in a given affected tissue significantly associated with the number of ewes with detectable peripheral OPP provirus (two-sided P value, 0.0014; Fisher's exact test). In addition, the number of ewes with at least one moderate to severe lesion in a given affected tissue significantly associated with the number of ewes with a positive cELISA result (two-sided P value, 0.0014; Fisher's exact test). Furthermore, we checked whether there was a statistical difference in mean peripheral OPP provirus levels or mean serum anti-SU antibody titers between ewes with no to mild histological lesions and ewes with moderate to severe histological lesions. Ewes were categorized by histological lesions based upon the highest histological lesion scores for any of the tissues examined. Mean peripheral OPP provirus levels at euthanasia differed significantly between the ewes with no to mild histological lesions (mean ± standard error [SE], 1.43 ± 0.12 log10 env copies/μg of DNA) and the ewes with moderate to severe histological lesions (3.14 ± 0.17 log10 env copies/μg of DNA) (two-tailed P value, <0.0001; unpaired t test) (Fig. 2A). In addition, mean serum anti-SU antibody titers were significantly lower in ewes with no to mild histological lesions than in ewes with moderate to severe histological lesions (two-tailed P value, 0.0008; unpaired t test) (Fig. 2B). We also examined whether mean peripheral OPP provirus levels or mean serum anti-OPPV SU antibody titers 3 years prior to euthanasia differed significantly between the resulting histological lesion categories. Both mean peripheral OPP provirus levels and mean serum anti-SU antibody titers 3 years prior to euthanasia differed significantly between the two histological lesion categories (two-tailed P value, 0.0001 for both; unpaired t test) (Fig. 2C and D).
FIG. 2.
(A) Mean peripheral OPP provirus levels (log10 env copies per microgram of DNA) ± SEs at euthanasia for ewes with no to mild histological lesions and ewes with moderate to severe histological lesions. (B) Mean serum anti-SU antibody titers ± SEs at euthanasia for ewes with no to mild histological lesions and ewes with moderate to severe histological lesions. (C) Mean peripheral OPP provirus levels (log10 env copies per microgram of DNA) ± SEs 3 years prior to euthanasia for ewes with no to mild histological lesions and ewes with moderate to severe histological lesions. (D) Mean serum anti-SU antibody titers ± SEs 3 years prior to euthanasia for ewes with no to mild histological lesions and ewes with moderate to severe histological lesions. Samples from LMH12, LMH13, LMH14, LMH15, LMH16, LMH17, LMH18, LMH19, and LMH20 were assessed for both peripheral OPP provirus levels and serum anti-SU antibody titers 3 years prior to euthanasia, whereas samples from LMH11 and 112-45 were assessed 75 and 39 weeks, respectively, prior to euthanasia because these animals had to be euthanized for health reasons.
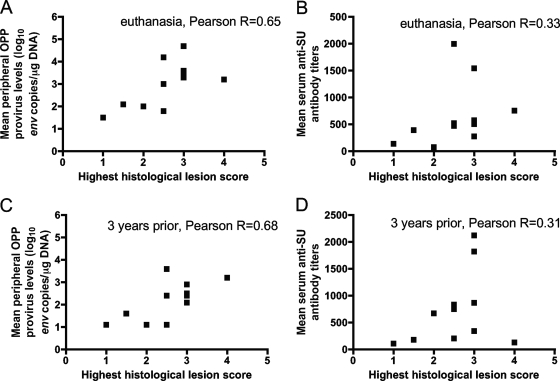
Additionally, to evaluate the basis of the histological lesion category, we tested whether mean peripheral OPP provirus levels or mean serum anti-SU antibody titers correlated with the highest histological lesion score for any affected tissue. Mean peripheral OPP provirus levels at euthanasia (Fig. 3A) significantly correlated with the highest histological lesion score for any affected tissue (two-tailed P value, 0.03) (Fig. 3A). In contrast, mean serum anti-SU antibody titers at euthanasia did not show a significant correlation with the highest histological lesion score for any tissue (two-tailed P value, 0.32) (Fig. 3B). When mean peripheral OPP provirus levels and mean serum anti-SU antibody titers 3 years prior to euthanasia were evaluated for associations with the highest histological lesion score, mean peripheral OPP provirus levels and not mean serum anti-SU antibody titers significantly associated with the highest histological lesion score (two-tailed P values, 0.02 and 0.36, respectively) (Fig. 3C and D).
FIG. 3.
(A) Mean peripheral OPP provirus levels (log10 env copies per microgram DNA) at euthanasia show a significant correlation (two-tailed P value, 0.03) with the highest histological lesion scores for 11 OPPV-infected sheep. (B) Mean serum anti-SU antibody titers at euthanasia do not show a correlation (two-tailed P value, 0.32) with the highest histological lesion scores for 11 OPPV-infected sheep. (C) Mean peripheral OPP provirus levels (log10 env copies per microgram of DNA) 3 years prior to euthanasia show a significant correlation (two-tailed P value, 0.02) with the highest histological lesion scores for 11 OPPV-infected sheep. (D) Mean serum anti-SU antibody titers 3 years prior to euthanasia do not show a correlation (two-tailed P value, 0.36) with the highest histological lesion scores for 11 OPPV-infected sheep.
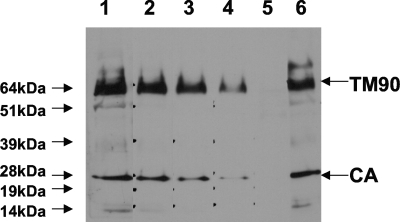
Although SU is B-cell immunodominant in OPPV-infected sheep by radioimmunoprecipitation (11), CA and/or the transmembrane is immunodominant in MVV- and OPPV-infected sheep by Western blot analysis (14, 16). To test whether serum anti-CA and antitransmembrane antibody titers correlated with the histological lesion score for any tissue, serum anti-CA and anti-TM90 antibody titers were determined by serum dilution on OPPV WLC1 lysate by using Western blot analysis (Fig. 4). Like serum anti-SU antibody titers evaluated by cELISA, the serum anti-CA or anti-TM90 antibody end point titers determined by Western blot analysis did not significantly correlate with the extent of histopathology in any affected tissue (data not shown) (Pearson's r, 0.01 and 0.44; two-tailed P values, 0.97 and 0.18, respectively).
FIG. 4.
Representative Western blot analysis of a serum titration on OPPV WLC1 lysate to determine anti-TM90 and anti-CA antibody titers. Lanes 1 through 4 show results for threefold-decreasing amounts of serum from an OPPV-infected sheep, with dilutions starting at 1:20 and ending at 1:540. Lane 5 shows the lack of reactivity of a pool of uninfected sheep sera diluted 1:20, and lane 6 shows the reactivity of a pool of OPPV-infected sheep sera diluted 1:20. Apparent molecular mass markers are shown to the left with arrows, and serum reactivity to OPPV TM90 and CA is shown to the right with arrows.
DISCUSSION
Sheep are defined as infected or not infected with OPPV based upon positive serological tests or detectable peripheral blood OPPV provirus levels in live adult animals, but the extent of disease is determined by the histological assessment of tissues postmortem. In general, it is unknown how OPPV causes histological changes to the lung, mammary gland, carpal synovial membrane, and brain tissues. The bulk of research into SRLV suggests that these viruses are immunopathological, meaning that mononuclear cells infiltrate at tissue sites, resulting in inflammation and the thickening of the tissues (5). It is unknown whether virus or host responses to the virus contribute more to the development of histological lesions in the affected tissues of persistently OPPV-infected sheep. However, our results for naturally OPPV-infected sheep are the first to show that peripheral OPP provirus levels statistically correlate (P < 0.05) with the highest histological lesion scores and that systemic responses with unbound anti-OPPV antibody do not statistically correlate (P > 0.05). This initial finding strongly suggests that circulating provirus quantitatively contributes more to the development of histological lesions than circulating host antibody responses.
Although titers of systemic, unbound anti-OPPV antibody did not significantly correlate with the highest histological lesion score for any affected tissue, mean serum anti-SU antibody titers differed significantly between the two categorical assignments for histological lesions (none to mild and moderate to severe). This finding suggests that circulating anti-OPPV SU antibody responses may be related qualitatively, but not quantitatively, to histopathology in the tissue. Our results differ from a previous result for orally CAEV-infected goat kids, in which anti-CAEV SU antibody titers in sera and synovial fluids significantly associated with the severity of arthritic lesions in the carpal synovial membranes of the goats (15). One reason for the lack of anti-OPPV antibody titers correlating with the highest histopathological lesion score is that more tissues (lungs, joints, and mammary glands) are affected in naturally OPPV-infected sheep than in CAEV-63/CAEV-Co-infected goats, in which predominantly joints and, in some animals, mammary glands are affected (4). Since primarily joints in CAEV-infected goats are affected, the majority of antibody production in response to viral variants within the joint would be derived from lymphoid follicles in and around the synovial membranes and draining lymph node of the joint and would contribute maximally to circulating antibody. In the case of naturally OPPV-infected sheep, the increased number of tissue sites producing antibody to specific viral variants may be diluting the effect of any specific circulating anti-SU, anti-CA, or anti-TM90 antibody that may correlate with histological lesions. Furthermore, although not examined in this study, tissue-specific production of anti-SU, anti-CA, or anti-TM90 may negatively or positively correlate with virus replication in the tissue. Future studies are planned to analyze whether lung-specific, mammary gland-specific, or synovial fluid-specific OPPV antibodies correlate with histological lesions and/or virus replication in the lungs, mammary glands, or synovial membranes, respectively.
In human immunodeficiency virus-infected humans, provirus loads or levels significantly correlate with the progression of human immunodeficiency virus infection, especially when retroviral therapies are given (17, 18). One future direction may be to establish whether peripheral OPP provirus levels in the early stages of infection in younger ewes also correlate with and predict the extent of histological lesions in the tissues. If early peripheral OPP provirus levels predict the extent of histological lesions in younger animals, this knowledge would provide a useful tool for sheep producers to eliminate those younger animals with a given OPP provirus level that is indicative of moderate to severe histological tissue lesions. Regardless, our study has shown that, for most sheep, the presence of a detectable provirus load predicts that the sheep will develop moderate to severe histological lung lesions if it lives to 9 years of age. In addition, higher OPP provirus levels are indicative of more severe lesions in any one of the OPPV-affected tissues.
Acknowledgments
We thank Nicholas Durfee and Liam Broughton for technical assistance. We also thank Emma Karel, Lori Fuller, and Duane Chandler for animal handling.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Brodie, S. J., K. A. Marcom, L. D. Pearson, B. C. Anderson, A. de la Concha-Bermejillo, J. A. Ellis, and J. C. DeMartini. 1992. Effects of virus load in the pathogenesis of lentivirus-induced lymphoid interstitial pneumonia. J. Infect. Dis. 166531-541. [DOI] [PubMed] [Google Scholar]
- 2.Brodie, S. J., L. D. Pearson, M. C. Zink, H. M. Bickle, B. C. Anderson, K. A. Marcom, and J. C. DeMartini. 1995. Ovine lentivirus expression and disease virus replication, but not entry, is restricted to macrophages of specific tissues. Am. J. Pathol. 146250-263. [PMC free article] [PubMed] [Google Scholar]
- 3.Cheevers, W. P., K. R. Snekvik, J. D. Trujillo, N. M. Kumpula-McWhirter, K. J. Pretty On Top, and D. P. Knowles. 2003. Prime-boost vaccination with plasmid DNA encoding caprine arthritis-encephalitis lentivirus env and viral SU suppresses challenge virus and development of arthritis. Virology 306116-125. [DOI] [PubMed] [Google Scholar]
- 4.Cheevers, W. P., D. P. Knowles, T. C. McGuire, D. R. Cunningham, D. S. Adams, and J. R. Gorham. 1988. Chronic disease in goats orally infected with two isolates of the caprine arthritis-encephalitis virus. Lab. Investig. 58510-517. [PubMed] [Google Scholar]
- 5.Cheevers, W. P., and T. C. McGuire. 1988. The lentiviruses: maedi/visna, caprine arthritis-encephalitis, and equine infectious anemia. Adv. Virus Res. 34189-215. [DOI] [PubMed] [Google Scholar]
- 6.de Andres, D., D. Klein, N. J. Watt, E. Berriatua, S. Torsteinsdottir, B. A. Blacklaws, and G. D. Harkiss. 2005. Diagnostic tests for small ruminant lentiviruses. Vet. Microbiol. 10749-62. [DOI] [PubMed] [Google Scholar]
- 7.DeMartini, J. C., S. J. Brodie, A. de la Concha-Bermejillo, J. A. Ellis, and M. D. Lairmore. 1983. Pathogenesis of lymphoid interstitial pneumonia in natural and experimental ovine lentivirus infection. Clin. Infect. Dis. 17(Suppl. 1)S236-S242. [DOI] [PubMed] [Google Scholar]
- 8.Gendelman, H. E., O. Narayan, S. Kennedy-Stoskopf, P. G. Kennedy, Z. Ghotbi, J. E. Clements, J. Stanley, and G. Pezeshkpour. 1986. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J. Virol. 5867-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorrell, M. D., M. R. Brandon, D. Sheffer, R. J. Adams, and O. Narayan. 1992. Ovine lentivirus is macrophage tropic and does not replicate productively in T lymphocytes. J. Virol. 662679-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann, L. M., W. P. Cheevers, K. L. Marshall, T. C. McGuire, M. M. Hutton, G. S. Lewis, and D. P. Knowles. 2003. Detection of serum antibodies to ovine progressive pneumonia virus in sheep by using a caprine arthritis-encephalitis virus competitive-inhibition enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 10862-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann, L. M., T. C. McGuire, I. Hötzel, G. S. Lewis, and D. P. Knowles. 2005. Surface envelope glycoprotein is B-lymphocyte immunodominant in sheep naturally infected with ovine progressive pneumonia virus. Clin. Diagn. Lab. Immunol. 12797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann-Hoesing, L. M., G. H. Palmer, and D. P. Knowles. 2007. Evidence of proviral clearance following postpartum transmission of an ovine lentivirus. Virology 362226-234. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann-Hoesing, L. M., S. N. White, G. S. Lewis, M. R. Mousel, and D. P. Knowles. 2007. Development and validation of an ovine progressive pneumonia virus quantitative PCR. Clin. Vaccine Immunol. 141274-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houwers, D. J., and I. M. Nauta. 1989. Immunoblot analysis of the antibody response to ovine lentivirus infections. Vet. Microbiol. 19127-139. [DOI] [PubMed] [Google Scholar]
- 15.Knowles, D., Jr., W. Cheevers, T. McGuire, T. Stem, and J. Gorham. 1990. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J. Virol. 642396-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers-Evert, D. K., and L. M. Herrmann-Hoesing. 2006. Ovine progressive pneumonia virus capsid is B-cell immunodominant using Western blot analysis: a comparison of sensitivity between Western blot analysis and immunoprecipitation. J. Virol. Methods 137339-342. [DOI] [PubMed] [Google Scholar]
- 17.Verhofstede, C., S. Reniers, F. Van Wanzeele, and J. Plum. 1994. Evaluation of proviral copy number and plasma RNA level as early indicators of progression in HIV-1 infection: correlation with virological and immunological markers of disease. AIDS 81421-1427. [DOI] [PubMed] [Google Scholar]
- 18.Vitone, F., D. Gibellini, P. Schiavone, and M. C. Re. 2005. Quantitative DNA proviral detection in HIV-1 patients treated with antiretroviral therapy. J. Clin. Virol. 33194-200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Z., N. J. Watt, J. Hopkins, G. Harkiss, and C. J. Woodall. 2000. Quantitative analysis of maedi-visna virus DNA load in peripheral blood monocytes and alveolar macrophages. J. Virol. Methods 8613-20. [DOI] [PubMed] [Google Scholar]