Abstract
Substance P (SP) is a member of the tachykinin family and has an important role in immune responses. SP is detectable in plasma in a free and bound state. Simple modification of a commercially available SP enzyme-linked immunosorbent assay allows the dissociation and capture of plasma SP without solid-phase extraction.
The undecapeptide substance P (SP) is a member of the tachykinin family. Its role as a neurotransmitter (21, 22, 23) includes regulation of immune responses (19). Elevated levels of serum or plasma SP have been associated with disorders such as inflammatory bowel disease (16), sickle cell crisis (20), depression and anxiety (2, 11, 14, 19, 26), rheumatologic diseases (1, 17), infectious diseases (28), including human immunodeficiency virus (7, 8, 13), and cancer (24, 27). SP exerts its influence on immune responses through the induction of regulatory cytokines (3, 18).
Considerable variability has been reported for SP levels in similar biological fluids from healthy subjects and patients with diverse disease processes (5, 7, 8, 9, 11, 12). Corbally et al. (6) reported that endogenous human plasma-derived SP was reversibly and nonspecifically bound to both high-molecular-mass proteins (>400,000 Da) and intermediate-molecular-mass proteins (58,000 Da). SP was readily dissociated from proteins by repeat gel filtration. These observations suggested that SP binding to plasma proteins was mediated by weak hydrogen bonding and led to the development of methods for the extraction of SP from a variety of biological fluids (10, 25). Such extractions should be considered carefully since most are designed for the enrichment of low-molecular-mass peptides that can potentially exclude peptides nonspecifically bound to high-molecular-mass plasma or serum proteins. Such procedures may lead to underestimates of the total quantity of SP (4, 6).
This study investigated the influence of hydrogen ion concentrations on nonspecific SP-plasma protein dissociations that allow for the optimal binding of dissociated SP by capturing antibody.
Human plasma.
Whole-blood-derived EDTA anticoagulated plasmas obtained from 28 healthy adult control subjects were stored at −70°C until evaluated for SP levels.
SP EIA.
A commercially available SP antigen competition enzyme immunoassay (EIA; Cayman Chemical Company, Ann Arbor, MI) was modified to investigate the influence of hydrogen ion concentration on the dissociation and subsequent capture of SP in pooled normal adult human plasma. The SP EIA uses microtiter wells coated with monoclonal mouse anti-rabbit immunoglobulin G, an SP-specific polyclonal rabbit capture antibody, and an acetylcholinesterase-conjugated SP tracer. The substrate for acetylcholinesterase consists of acetylthiocholine and 5,5-dithio-bis-(2-nitrobenzoic acid). The colored end product is 5-thio-2-nitrobenzoic acid with absorbance read at 412 nm. A phosphate-citrate (PC) buffer was selected as the SP dissociation buffer with the evaluation of buffers having pH values of 7.3 to 2.2. Pooled normal adult human plasma was first diluted 1:8 in Cayman buffer (pH 7.3) followed by a 1:2 dilution in the various PC buffers yielding pH values of 7.3 to 3.6 for the treated plasma. SP standards used for construction of the standard curve were initially prepared in Cayman buffer (pH 7.3), yielding final SP concentrations of 3.9 to 125 pg/ml. After identification of the optimal-pH PC buffer that yielded maximum recovery of plasma-derived SP, SP standards were first diluted in Cayman buffer yielding SP concentrations of 250 to 7.8 pg/ml. This was followed by a 1:2 dilution in the optimal-pH PC buffer to yield SP concentrations of 125 to 3.9 pg/ml. After acidification, 100 μl of the treated plasma described above and SP standards were added in quadruplicate to the appropriate microtiter wells. Then, 50 μl of SP-specific polyclonal rabbit capture antibody and 50 μl of the acetylcholinesterase-conjugated SP tracer were added to the mixture. The capture antibody and SP tracer were prepared with Cayman buffer (pH 7.3) according to the manufacturer's instructions. After an overnight incubation at 4°C, the plates were washed with Cayman buffer followed by the addition of 200 μl of reconstituted enzyme substrate. The plates were allowed to develop until the absorbance readings at 412 nm for Bo (maximum binding) wells were between 0.3 and 1.0 absorbance unit, using a PowerWave XS plate reader (Bio-Tek Instruments, Inc., Winooski, VT). Absorbance data were downloaded and analyzed using Bio-Tek KC4 v3.4 software.
Influence of pH on recovery of human plasma-derived SP.
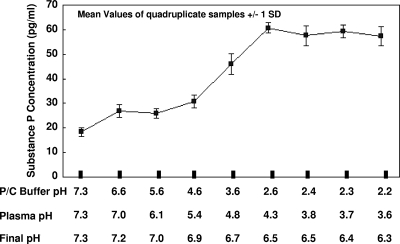
Figure 1 illustrates the effect of pretreatment of human plasma with PC buffers of decreasing pH on the quantitation of SP in plasma. Maximal SP recovery occurred using a PC buffer (pH 2.6) that yielded a plasma pH of 4.3 and a final pH of 6.5. The human plasma pool yielded an SP value of 18 pg/ml when evaluated under a physiologic pH of 7.3 and a value of 61 pg/ml upon exposure to pH 2.6 conditions. This indicated that approximately 70% of the plasma SP is present in a reversibly bound state.
FIG. 1.
Influence of pH on recovery of SP from unextracted human plasma. Plasma samples were diluted 1:8 in Cayman buffer (pH 7.3), followed by a 1:2 dilution in the indicated PC buffers of various pHs. The pHs of the buffers, buffer-treated plasma, and buffer-treated plasma after addition of enzyme-labeled SP tracer and capture anti-SP antibody are listed on the x axis with resulting SP values on the y axis. Each data point represents the mean ± 1 standard deviation.
Influence of pH on SP standard curve.
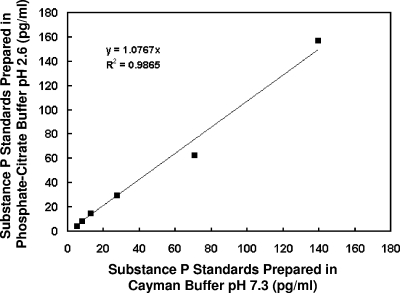
In order to exclude the possibility that the observed increase in plasma SP levels after exposure to PC buffers of decreasing pH may be attributable to artifacts introduced by the altered pH of the modified assay, SP standards (3.9 to 125pg/ml) were prepared in parallel using both the manufacturer's recommended Cayman buffer (pH 7.3) and the modified procedure that employed the PC buffer (pH 2.6). Figure 2 illustrates a regression analysis of SP standards assayed by both methods, yielding a correlation coefficient of 0.9865. These data indicate that the observed increase in plasma SP levels using the modified assay was not attributable to alteration of the standard curve.
FIG. 2.
Linear regression analysis of SP standards prepared in Cayman buffer (pH 7.3) and PC buffer (pH 2.6). SP standards of 3.9 to 125 pg/ml were prepared in Cayman buffer only. The same SP standard preparation was diluted to yield 7.81 to 250 pg/ml, followed by a 1:2 dilution in PC buffer (pH 2.6) to yield 3.9 to 125 pg/ml. These treatments yielded a correlation coefficient of 0.9865 for the two curves.
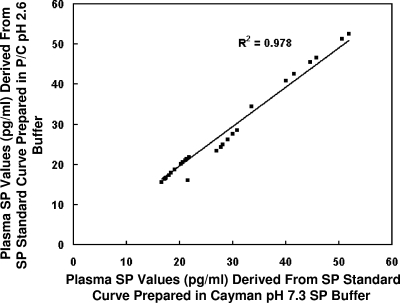
Correlation of individual plasma SP levels derived from SP standard curves prepared in Cayman buffer (pH 7.3) or PC buffer (pH 2.6).
Although the modified procedure had little effect on the quantitative measurement of SP standards, individual plasma samples (n = 28) were evaluated using SP standard curves prepared as described in the legend to Fig. 2. As shown in Fig. 3, linear regression analysis revealed excellent correlations of SP values derived from either standard curve, yielding an R2 of 0.978.
FIG. 3.
Linear regression analysis of individual plasma (n = 28) SP values derived from standard curves prepared in Cayman buffer (pH 7.3) and PC buffer (pH 2.6). The SP values yielded a correlation coefficient of 0.978.
Difficulty in the accurate measurement of SP in plasma is attributed, in part, to reversible nonspecific binding of SP to plasma proteins (4, 6). This study was undertaken to develop a gentle nonchromatographic, liquid-phase procedure that permits the dissociation of SP from plasma proteins though limited hydrogen ion pressure, allowing for the capture of SP by a specific antibody of higher avidity after a return to more physiologic conditions. Exposure of human plasma to PC buffers of decreasing pH led to an enhanced release of protein-bound SP with optimal dissociation occurring at a plasma pH of 4.3 produced by a 1:2 dilution of plasma (diluted 1:8 in Cayman buffer [pH 7.3]) with PC buffer (pH 2.6). A final pH of 6.5 was achieved after the addition of the capture antibody and SP tracer, both of which were prepared in Cayman buffer (pH 7.3). These data indicate that a significant proportion of SP in human plasma exists in a bound state.
Possible alteration of the SP standards and/or other reactive components in the assay as a result of the introduction of the PC buffer (pH 2.6) was investigated by the parallel evaluation of the SP standard curve, formulated as recommended in Cayman buffer (pH 7.3), and the low-pH-modified procedure. Linear regression analysis of standard SP values obtained with both buffer systems yielded an R2 of 0.9865. These data indicate insignificant differences between the two buffer systems regarding assay performance. An evaluation of human plasma (n = 28) using SP standard curves formulated by both methods also revealed insignificant differences in SP values upon linear regression analysis (R2 = 0.978).
This study describes a simple liquid-phase method for the recovery of a significant proportion of SP bound to human plasma proteins. The modified EIA described here should allow for more accurate estimates of total SP present in human plasma.
Acknowledgments
This study was supported by NIH grants P01 MH076388 and R01 MH49981 to S.D.D.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Anichini, M., S. Cesaretti, M. Lepori, S. Maddali Bongi, M. Maresca, and M. Zoppi. 1997. Substance P in the serum of patients with rheumatoid arthritis. Rev. Rhum. Engl. Ed. 6418-21. [PubMed] [Google Scholar]
- 2.Bondy, B., T. C. Baghai, C. Minov, C. Schule, J. M. Schwarz, P. Zwanzger, R. Rupprecht, and J.-H. Moller. 2003. Substance P serum levels are increased in major depression: preliminary results. Biol. Psychiatry 53538-542. [DOI] [PubMed] [Google Scholar]
- 3.Bost, K. L. 2004. Tachykinin-mediated modulation of the immune response. Front. Biosci. 93331-3332. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, D. E., N. Raftery, R. Tustin III, N. B. Tustin, M. L. DeSilvio, A. Cnaan, P. P. Aye, A. A. Lackner, and S. D. Douglas. 2006. Measurement of plasma-derived substance P: biological, methodological, and statistical considerations. Clin. Vaccine Immunol. 131197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark, J. W., P. D. Senanayake, G. D. Solomon, and C. Gallagher. 1994. Substance P: correlation of CSF and plasma levels. Headache 34261-264. [DOI] [PubMed] [Google Scholar]
- 6.Corbally, N., D. Powell, and K. F. Tipton. 1990. The binding of endogenous and exogenous substance-P in human plasma. Biochem. Pharmacol. 391161-1166. [DOI] [PubMed] [Google Scholar]
- 7.Douglas, S. D., A. Cnaan, K. G. Lynch, T. Benton, H. Zhao, D. R. Gettes, and D. L. Evans. 2008. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res. Hum. Retrovir. 24375-378. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, S. D., W.-Z. Ho, D. R. Gettes, A. Cnaan, H. Zhao, J. Leserman, J. M. Petitto, R. N. Golden, and D. L. Evans. 2001. Elevated substance P levels in HIV-infected men. AIDS 152043-2045. [DOI] [PubMed] [Google Scholar]
- 9.Faulhaber, H. D., R. Rathsack, G. Rostock, V. Homuth, D. Pfeiffer, E. Naumann, W. Hartrodt, R. C. Gorne, and P. Oehme. 1983. Evidence of decreased plasma substance P levels in human essential hypertension and influence of prazosin treatment. Biomed. Biochim. Acta 421019-1025. [PubMed] [Google Scholar]
- 10.Fehder, W. P., W.-Z. Ho, D. E. Campbell, W. W. Tourtellotte, L. Michaels, J. R. Cutilli, M. Uvaydova, and S. D. Douglas. 1998. Development and evaluation of a chromatographic procedure for partial purification of substance P with quantitation by an enzyme immunoassay. Clin. Diagn. Lab. Immunol. 5303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehder, W. P., J. Sachs, M. Uvaydova, and S. D. Douglas. 1997. Substance P as an immune modulator of anxiety. Neuroimmunomodulation 442-48. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Rodriguez, C. M., J. Prieto, J. Quiroga, J. M. Zozoya, A. Andrade, M. Nunez, B. Sangro, and J. Penas. 1995. Plasma levels of substance P in liver cirrhosis: relationship to the activation of vasopressor systems and urinary sodium excretion. Hepatology 2135-40. [DOI] [PubMed] [Google Scholar]
- 13.Ho, W.-Z., and S. D. Douglas. 2004. Substance P and neurokinin-1 receptor modulation of HIV. Neuroimmunology 15748-55. [DOI] [PubMed] [Google Scholar]
- 14.Kramer, M. S., N. Cutler, J. Feighner, R. Shrivastava, J. Carman, J. J. Sramek, S. A. Reines, G. Liu, D. Snavely, E. Wyatt-Knowles, J. J. Hale, S. G. Mills, M. MacCoss, C. J. Swain, T. Harrison, R. G. Hill, F. Hefti, E. M. Scolnick, M. A. Cascieri, G. G. Chicchi, S. Sadowski, A. R. Williams, L. Hewson, D. Smith, E. J. Carlson, R. J. Hargreaves, and N. M. J. Rupniak. 1998. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 2811640-1645. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Mantyh, C. R., T. S. Gates, R. P. Zimmerman, M. L. Welton, E. P. Passaro, Jr., S. R. Vigna, J. E. Maggio, L. Kruger, and L. W. Mantyh. 1988. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc. Natl. Acad. Sci. USA 853235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall, K. W., B. Chiu, and R. D. Inman. 1990. Substance P and arthritis: analysis of plasma and synovial fluid. Arthritis Rheum. 3387-90. [DOI] [PubMed] [Google Scholar]
- 18.McGillis, J. P., M. Mitsuhashi, and D. G. Payan. 1990. Immunomodulation by tachykinin neuropeptides. Ann. N. Y. Acad. Sci. 59485-94. [DOI] [PubMed] [Google Scholar]
- 19.McLean, S. 2005. Do substance P and the NK1 receptor have a role in depression and anxiety? Curr. Pharm. Des. 111529-1547. [DOI] [PubMed] [Google Scholar]
- 20.Michaels, L. A., K. Ohene-Frempong, H. Zhao, and S. D. Douglas. 1998. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood 923148-3151. [PubMed] [Google Scholar]
- 21.Nicoll, R. A., C. Schenker, and S. E. Leeman. 1980. Substance P as a neurotransmitter candidate. Annu. Rev. Neurosci. 3227-268. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka, M., and T. Takahashi. 1977. Putative peptide neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 17425-439. [DOI] [PubMed] [Google Scholar]
- 23.Otsuka, M., and K. Yoshioka. 1993. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 73229-308. [DOI] [PubMed] [Google Scholar]
- 24.Palma, C., and C. A. Maggi. 2000. The role of tachykinins via NK1 receptors in progression of human gliomas. Life Sci. 67985-1001. [DOI] [PubMed] [Google Scholar]
- 25.Rissler, K. 1995. Sample preparation, high-performance liquid chromatographic separation and determination of substance P-related peptides. J. Chromatogr. B 665233-270. [DOI] [PubMed] [Google Scholar]
- 26.Rupniak, N. M. J. 2002. New insights into the antidepressant actions of substance P (NK-1 receptor) antagonists. Can. J. Physiol. Pharmacol. 80489-494. [DOI] [PubMed] [Google Scholar]
- 27.Singh, D., D. D. Joshi, M. Hameed, J. Qian, P. Gascon, B. P. Maloof, A. Mosenthal, and P. Rameshwar. 2000. Increased expression of preprotachykinin-1 and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proc. Natl. Acad. Sci. USA 97388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripp, R. A., A. Barskey, L. Goss, and L. J. Anderson. 2002. Substance P receptor expression on lymphocytes is associated with the immune response to respiratory syncytial virus infection. J. Neuroimmunol. 129141-153. [DOI] [PubMed] [Google Scholar]