Abstract
The incidence of blood donors seropositive for Trypanosoma cruzi in North America has increased with population migration and more rigorous surveillance. The United States, considered nonendemic for T. cruzi, could therefore be at risk to exposure to parasite transmission through blood or organ donations. Current tests show variable reactivity, especially with Central American sera. Here we describe the development of a lateral flow immunoassay for the rapid detection of T. cruzi infection that has a strong correlation to the radioimmunoprecipitation assay (RIPA) “gold standard” in the United States. Such a test could have utility in small blood banks for prescreening donors, as well as in cardiac transplantation evaluation. T. cruzi consensus and/or RIPA-positive sera from Central and South America were evaluated in enzyme immunoassays (EIAs). These included commercial panels from Boston Biomedica, Inc. (BBI) (n = 14), and HemaBio (n = 21). Other sources included RIPA-positive sera from the American Red Cross (ARC) (n = 42), as well as from Chile. Sera were tested with the multiepitope recombinant TcF. All but one of the BBI samples were positive and 7 of 21 HemaBio samples and 6 of 42 ARC samples were low positive or negative. This observation indicated the need for additional antigens. To complement TcF reactivity, we tested the sera with peptides 30, 36, SAPA, and 1.1, 1.2, and 1.3 His fragments of 85-kDa trans-sialidase. We identified a promising combination of the tested antigens and constructed a single recombinant protein, ITC6, that enhanced the relative sensitivity in U.S. blood donor sera compared to that of TcF. The data on its evaluation using RIPA-confirmed positive sera in EIA and lateral flow immunoassay studies are presented, along with an additional recombinant protein, ITC8.2, with two additional sequences for peptide 1 and Kmp-11. The latter, when evaluated in a dipstick assay with consensus positive sera, had a sensitivity of 99.2% and a specificity of 99.1%.
Trypanosoma cruzi infection is endemic in Latin America and is the causative agent of Chagas' disease. The parasite is transmitted to humans via direct contact with feces from infected the reduviid bug, congenitally or via blood transfusion (24, 31, 32, 39). The latter has become the most prevalent route of infection and in some countries up to 10% of the blood supply is affected. After infection an acute phase of disease occurs for 1 to 2 months, after which the disease frequently resolves, and individuals become asymptomatic for long periods of time (years). During this phase individuals have low levels of detectable parasite and measurable antibody titers. Up to a quarter of this group will progress to chronic disease, resulting frequently in cardiac failure and death. There is growing evidence that with increased migration of populations, people in countries such as the United States, considered nonendemic for T. cruzi, could be at risk for exposure to parasite transmission through blood donations (20). Any test developed also needs to be capable of detecting different clones of T. cruzi that are evident in Central America as opposed to most of South America (27). It is also evident from other studies with various recombinant proteins and sera from Central and South America that wide geographical differences in reactivity are observed (36, 37).
Several methods for the diagnosis of T. cruzi infection are available but not applicable for field testing. These include the enzyme-linked immunosorbent assay (ELISA), the immunofluorescence antibody test (IFAT), or the indirect hemagglutination test (4, 7, 19, 34). Hemoculture and xenodiagnosis are frequently used as reference standards of parasite presence, but they suffer from variability in sensitivity and are not recommended for routine diagnosis (30). Other researchers are evaluating dipstick assays with other sets of antigens than those discussed here, but there are still issues of sensitivity and specificity over a broad geographical area (26, 29). More recently, radioimmunoprecipitation assay (RIPA) has been used in the United States (19) as the “gold standard. Although these tests are sensitive and specific, there is a need for a rapid, sensitive, and specific diagnostic test for screening surveys or use in small rural clinics or in cardiac transplantation situations. Such a test needs to maintain a high level of sensitivity and specificity irrespective of geographical location.
TcF is a multiepitope recombinant protein containing four immunodominant repeating peptide epitopes, and its reactivity and that of related peptides with Chagas' serum has been described by various groups in the literature (2, 6, 9-13, 28). It is reactive in enzyme immunoassay (EIA) with T. cruzi-positive sera with a high level of sensitivity and specificity, particularly with South American sera, e.g., in sera from Brazil, which were used in initially identifying many of the epitopes. On closer inspection, TcF was found to vary in sensitivity or intensity of signal when tested against T. cruzi-positive sera from the U.S. and Central American blood donors or patients. This might indicate their infection with a different T. cruzi clone (27). This prompted the search for additional epitopes that would complement TcF and that subsequently could be incorporated into a next-generation multiepitope recombinant protein. The data presented here describe (i) the selection of antigens complementary to TcF; (ii) the development of a novel multiepitope recombinant protein, ITC6, and, subsequently, ITC8.2, incorporating complementary sequences; and (iii) the development of a prototype lateral flow assay for the detection of T. cruzi antibodies in serum.
This novel assay demonstrated increased sensitivity and signal in T. cruzi-positive U.S. blood donors and Central American sera than were previously seen with TcF alone.
MATERIALS AND METHODS
TcF or ITC6 or ITC8.2 recombinant EIA.
The TcF, ITC6, and 8.2 and other recombinant protein and peptide EIAs were performed as follows. Microtiter plates (Immulon-2; flat bottom, high binding) were coated with recombinant proteins at 100 ng/well overnight at 4°C in a carbonate/bicarbonate buffer (pH 9.6). After three washes with phosphate-buffered saline containing 0.05% Tween 20 (PBST), the plates were blocked with PBS containing 1% bovine serum albumin and 0.05% Tween 20 for 1 h. Serum samples were then added at a 1/50 dilution and incubated for 30 min at 37°C, followed by six washes with PBST. Goat anti-human immunoglobulin G (IgG)-horseradish peroxidase (1/50,000) was then added to the plate, followed by incubation for 30 min at 37°C. After six washes in PBST, the plate was developed with TMB (3,3′,5,5′-tetramethylbenzidine) substrate for 15 min at ambient temperature and then stopped with 1 N H2SO4. The plates were immediately read at an optical density at 450 nm (OD450). The cutoff was calculated as the mean of the negative population plus three standard deviations.
ITC lateral flow immunoassay.
A lateral flow assay was performed with the ITC recombinants as the solid-phase antigen in the test line. An affinity-purified goat anti-human IgG antibody was labeled with colloidal gold and used as the mobile phase. The control line comprised recombinant protein A.
ITC recombinant proteins were coated on the membrane at a concentration of 0.35 mg/ml as the test line. Colloidal gold conjugate was prepared by using goat anti-human IgG and adding gold salt. After incubation, bovine serum albumin was added as a blocking reagent. The gold was diluted to the appropriate OD at 520 to 540 nm using gold suspension buffer at an appropriate concentration. The antibody gold conjugate was then sprayed onto the conjugate pad. The control line was recombinant protein A sprayed at a concentration of 1 mg/ml. Human sera (25 μl) were applied to the sample pad, followed by 3 drops of chase buffer. In recent studies, the intensity of the rapid test line has been compared to the intensity of lines of a dilution panel with a scale of 0 to 14 based on intensity. A score of 14 is the highest intensity and would be similar to that seen in the control line. A photograph of the dilution panel was used as a reference. Any line faint or otherwise was considered a positive result. Also, it is recommended that the tests should be read by someone other than the one who runs the samples to remove any subjectivity. Dipsticks are read at 15 min but are stable if air dried and maintained dry and free of exposure to moisture.
ITC recombinant proteins.
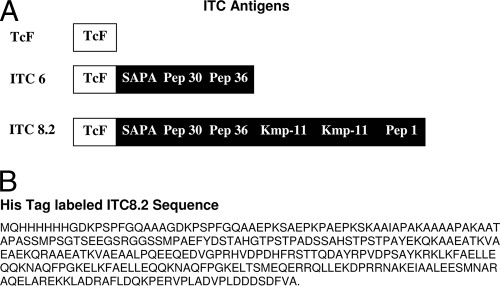
TcF was expressed in E. coli with His6 tags and purified by nickel affinity chromatography as previously described (9-13). ITC6 and ITC8.2 were also initially expressed with a His6 tag. Subsequently, ITC8.2 was expressed with an appropriate tag as described below to facilitate secretion and yield. The components of the ITC6 and ITC8.2 recombinants compared to TcF are outlined in Fig. 1.
FIG. 1.
(A) Schematic of structure of ITC6 and ITC8.2 compared to TcF. ITC6 contains TcF with SAPA and peptides 30 and 36. ITC8.2 contains, in addition, two copies of the Kmp-11 peptide and peptide 1. (B) Amino acid sequence of ITC8.2 labeled with the His6 tag.
His-ITC8.2.
The ITC8.2 insert was cloned between the NdeI and XhoI sites of a modified pET28 vector lacking the N-terminal His6 and T7 tags present in the commercial vector (Novagen, La Jolla, CA). An N-terminal His tag was added to the ITC8.2 sequence via PCR amplification prior to subcloning. The sequence-verified pET28/Itc8.2 DNA was transformed into the Rosetta2 (DE3)/pLysS expression strain (Novagen), and transformants were maintained on LB-kanamycin (50 μg/ml)-chloramphenicol (34 μg/ml). For ITC8.2 protein expression, an overnight culture of Rosetta2 (DE3)/pLysS/pET28Itc8.2 cells was subcultured at a ratio of 1:80 (vol/vol) in selective medium, and the new culture was grown at 37°C to an OD560 of 0.6 to 0.7. Expression was induced after the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to 1 mM, and cells were harvested at 3 h postinduction by centrifugation at 6,000 rpm for 10 min. Purification was performed on a nickel affinity columns by using an imidazole buffer gradient.
SUMO-ITC8.2.
The ITC8.2 insert was amplified via PCR using the primer pair GGTGATAAGCCTAGCCCATTTGGT (sense) and CAATTGCTCGAGTTACGCGACAAAATCGCT (antisense) and an annealing temperature of 68°C. The PCR product was gel purified and TA cloned into pETSUMO by using a Champion pETSUMO protein expression kit (Invitrogen) according to the manufacturer's directions. Transformants were screened by PCR for the presence of insert in the correct orientation, and DNA was extracted from one positive clone and confirmed to be correct by sequencing. The sequence-verified plasmid was transformed into the BL21(DE3) strain for expression and maintained on Luria broth with kanamycin (50 μg/ml) and glucose. For SUMO/Itc8.2 protein expression, an overnight culture of BL21(DE3)/pETSUMOItc8.2 cells was subcultured in selective media and expression was induced after the addition of 1 M IPTG. Cells were harvested at 3 h postinduction by centrifugation at 6,000 rpm for 10 min. Purification was performed on a nickel affinity columns using an imidazole buffer gradient after protease removal of the SUMO.
RIPA.
RIPAs were performed by David Leiby at the ARC, Rockville, MD (19), using three negative and three positive control samples. Briefly, the assay detects two T. cruzi-specific glycoproteins of 72 and 90 kDa identified in radioautographs after radioimmunoprecipitation with T. cruzi-positive sera (17, 18). Where possible, the radioautographs were graded by their intensity.
EIA and IFAT Chile.
The samples from Chile were evaluated by Myriam Lorca, University of Chile, using immunofluorescence titration (i.e., the IFAT) and confirmed by using EIA. The IFAT was performed according to the Camargo methodology (4), as modified by the Lorca group (24). Epimastigotes of T. cruzi from Diamond cultures were used. After three washes, the cultures were fixed with 2% formol in PBS. The serum samples were diluted in PBS (pH 7.2; 1/20 or serial dilutions) and incubated with the antigen at 37°C for 45 min. After two washes the slides were incubated with the second antibody (fluorescein isothiocyanate-labeled anti-human IgG) plus Evans blue as a contrast (25). The slides were read in UV microscope (Olympus PS-2D) at a magnification of ×40. The EIA was performed according to the routine methodology (7) using whole extract of epimastigotes of T. cruzi (2 μg/ml). Plates were blocked with nonfat dried milk diluted in PBS. Serum dilutions were made in PBS-nonfat dried milk.
Serum samples.
Several panels were available for testing. Forty-two samples were received from the ARC that had tested RIPA positive. These included several donors of Central American origin. A panel of 15 sera primarily from Venezuela, Nicaragua, Honduras, and Argentina (14 positive and 1 negative) was available from Boston Biomedica, Inc., West Bridgewater, MA. A panel of 21 sera was available from HemaBio (formerly Teragenix), and these included sera from Central and South America. Myriam Lorca also provided us with a panel of 25 positive sera by epimastigote ELISA and IFAT that included known peptide 1, peptide 2, and SAPA (shed acute-phase antigen) reactive sera. She also performed initial evaluations in Chile of an ITC8.2 dipstick with an extensive panel of sera shown to be consensus positive by the IFAT and/or EIA. This panel included Chagas' disease patients (n = 118) that were chronic asymptomatic (n = 112), cardiopathy (n = 4), or digestive symptomatic (n = 2) samples, as well as nonendemic controls and samples with other parasitic infections, e.g., toxoplasmosis, leishmaniasis, and nonparasitic diseases (e.g., syphilis) and rheumatoid factor (n = 106). Nonendemic controls for the present study were from Southern Chile, and all were shown to be IFAT and EIA negative, as were samples from other parasitic and nonparasitic infections. All of the panels of sera evaluated at InBios were tested by D. Leiby in a RIPA to confirm their status. Where possible, the radioautographs were graded based on intensity. Control sera were also obtained from Seracare. Serum samples were collected by venipuncture, separated by centrifugation, and stored at −20°C until used.
For the serum samples used for cross-reactivity studies with visceral leishmaniasis (VL), the Chagas positive, controls, and VL samples were tested with the Abbott Chagas ELISA and with the rK39 dipstick assay for VL (3). All VL sera were from Salvador, Brazil.
RESULTS
Several recombinant antigens and peptides were evaluated to determine their ability to complement TcF in detecting T. cruzi-positive sera. These included peptides 30, 36, and SAPA (22, 23, 38). Other recombinant antigens included but were not restricted to 1.1, 1.2, and 1.3 His. 1.1 His is a 30-kDa fragment of a family member of the abundant 85-kDa trans-sialidase membrane protein, 1.2 His is a 48-kDa fragment of a second family member, and 1.3 His is a 30-kDa fragment of a third family member (14-16). Table 1 summarizes the example of complementation of the key antigens with TcF low or nonreactive sera that were determined to be RIPA positive. These were sera from U.S. blood donors primarily of Central American origin, as well as four consensus-positive sera from Brazil.
TABLE 1.
Results of complementation studiesa
| Serum | Complementation (S/CO) of TcF with other peptides
|
||||||
|---|---|---|---|---|---|---|---|
| TcF | 1.1 His | 1.2 His | 1.3 His | SAPA | Peptide 30 | Peptide 36 | |
| 349-003A | 1.891 | 0.221 | 6.562 | 0.358 | 2.719 | 2.116 | 2.682 |
| 351-003A | 1.160 | 0.628 | 0.396 | 0.402 | 4.393 | 1.541 | 5.364 |
| 360-003A | 4.288 | 0.256 | 0.184 | 0.225 | 0.315 | 0.315 | 5.795 |
| 375-003A | 0.282 | 0.339 | 0.332 | 0.338 | 0.230 | 1.103 | 0.477 |
| RR04 | 2.885 | 0.201 | 0.237 | 0.250 | 0.326 | 0.288 | 0.670 |
| RR26 | 0.417 | 0.369 | 0.240 | 0.275 | 0.253 | 12.623 | 0.489 |
| RR34 | 0.962 | 0.410 | 0.989 | 0.480 | 0.309 | 6.110 | 0.852 |
| RR52 | 0.788 | 0.209 | 0.191 | 0.225 | 5.843 | 0.418 | 1.023 |
| RR57 | 0.737 | 2.018 | 6.357 | 0.338 | 0.708 | 0.384 | 4.602 |
| RR71 | 3.295 | 0.201 | 0.587 | 0.270 | 0.562 | 0.452 | 0.818 |
| RR75 | 4.827 | 0.151 | 0.622 | 0.314 | 0.399 | 0.959 | 0.591 |
| RR78 | 2.712 | 0.219 | 0.459 | 0.299 | 0.461 | 0.425 | 0.534 |
| RR38 | 3.846 | 1.053 | 5.664 | 0.995 | 3.112 | 20.870 | 9.727 |
| RR86 | 0.994 | 0.166 | 0.237 | 0.289 | 0.573 | 0.322 | 3.864 |
| RR94 | 0.628 | 0.314 | 0.163 | 0.407 | 0.573 | 1.795 | 3.636 |
| RR99 | 2.705 | 0.183 | 0.350 | 0.279 | 0.438 | 0.295 | 1.205 |
| RR66 | 0.776 | 0.402 | 0.816 | 0.333 | 0.388 | 1.596 | 0.864 |
| RR85 | 2.673 | 0.359 | 0.459 | 0.588 | 0.652 | 0.774 | 1.250 |
| Cutoff | 0.156 | 0.398 | 0.283 | 0.204 | 0.178 | 0.146 | 0.088 |
The results demonstrate the complementation of different peptides and recombinant proteins with TcF in EIAs of TcF-negative or low-positive sera. Values are expressed as S/CO to facilitate comparison. The cutoff OD for each antigen is shown and represents the mean plus three standard deviations of negatives. Samples are considered positive if the S/CO value is >1. Boldface values indicate positivity with a particular peptide or recombinant protein.
The data, expressed as signal/cutoff (S/CO) ratios to enable comparison of the reactivities, indicate that peptides 30, 36, and SAPA all contributed to complementing the reactivity of TcF. For example, in Table 1 the TcF-negative sera RR26 and RR34 were complemented with peptide 30, and RR57 and RR86 were complemented with peptide 36. SAPA also further enhanced activity, as seen in sera, e.g., RR52. The His proteins showed some reactivity with the sera but were always positive by SAPA, peptide 30, or peptide 36 and did not appear to improve overall reactivity. Based on these complementation studies and other similar evaluations, a new multiepitope antigen ITC6 was constructed and expressed as a recombinant protein in an E. coli expression system as described in Materials and Methods. This protein included peptide 30, peptide 36, and SAPA in conjunction with the four epitopes of TcF (11-13). The comparison of ITC6 reactivity versus TcF on RIPA-positive donor samples from the ARC, as well as the BBI panel which was confirmed positive with RIPA, are illustrated in Table 2. Significant improvements in reactivity were observed in problematic sera, particularly in many of the low-reactive or TcF-negative ARC sera. For instance, the TcF-negative sera or equivocal samples in Table 1 (RR26, RR34, RR52, RR57, RR86, RR94, and RR66) were all positive with ITC6, with S/CO values ranging from 1.695 to 12.206. Comparison of TcF and ITC6 in the ARC and BBI panels indicated an increased sensitivity (49/56 to 56/56) and significant increases in the S/CO, as indicated by the mean S/CO and 99th percentile. This finding of improved reactivity with ITC6 was further evident in a comparative study of RIPA with TcF EIA and ITC6 EIA and ITC6 dipstick in the serum panels, as outlined in Table 3, where TcF was reactive with 93 of 102 RIPA-positive sera compared to 101 of 102 sera for the ITC6 EIA or dipstick. One sample in the Hemabio panel that was equivocal by RIPA (63225) was negative by both TcF EIA and the ITC6 rapid test.
TABLE 2.
Reactivity of TcF and ITC6 recombinant proteins in EIA with RIPA-confirmed positive seraa
| Serum panel | No. of sera | TcF EIA
|
ITC6 EIA
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. positive | Mean S/CO | SD | 99th percentile | No. positive | Mean S/CO | SD | 99th percentile | ||
| ARC | 42 | 36 | 3.575 | 3.2 | 0.306-11.171 | 42 | 7.545 | 4.877 | 1.387-16.077 |
| BBI | 14 | 13 | 5.485 | 3.084 | 0.368-10.490 | 14 | 10.164 | 3.651 | 4.654-16.402 |
| Controls | 4 | 0 | 0.429 | 0.19 | 0.224-0.644 | 0 | 0.694 | 0.102 | 0.551-0.776 |
Data for both antigens are expressed as the mean S/CO and include the 99th percentiles to facilitate comparison of the data. RIPA-confirmed positive sera from the ARC (n = 42) and BBI panel sera confirmed positive by RIPA (n = 14), and normal human serum-negative controls (n = 4) are included. The data indicate improved S/CO and assay sensitivity due to the incorporation of peptides 30, 36, and SAPA.
TABLE 3.
Relationship of tests to RIPAa
| Serum panel | No. of sera | No. of positive samples
|
||||
|---|---|---|---|---|---|---|
| RIPA | TcF EIA | ITC6 EIA | ITC6 dipstick | ITC8.2 dipstick | ||
| HemaBio | 21 | 21 | 19 | 20 | 20 | 20 |
| ARC | 42 | 42 | 36 | 42 | 42 | 42 |
| BBI | 14 | 14 | 13 | 14 | 14 | 14 |
| Chile | 25 | 25 | 25 | 25 | 25 | 25 |
| Total | 102 | 102 | 93 | 101 | 101 | 101 |
The serum panels that were confirmed positive by RIPA were tested in TcF EIA, ITC6 EIA, and ITC6 and ITC8.2 dipsticks. Both the ITC6 and the ITC8.2 dipsticks showed improved sensitivity compared to RIPA than the TcF EIA.
Though ITC6 improved sensitivity versus TcF, it was still considered prudent to add additional peptides. M. Lorca and coworkers have strongly indicated the need to include peptide 1 in T. cruzi assays (22, 38). These researchers provided a panel of 25 sera positive by epimastigote EIA or IFAT. The sera were also tested with peptide 1, peptide 2, and SAPA.
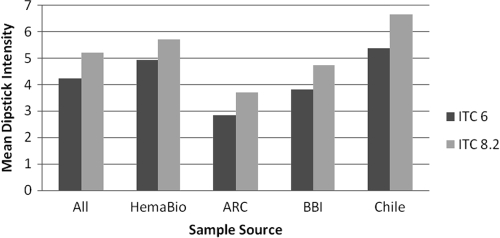
Within this panel three sera were only reactive with peptide 1 out of the peptide antigens tested by M. Lorca, so it was decided to include this antigen in a new recombinant ITC8.2 along with the Kmp-11 peptide. The latter epitope has been shown to react specifically with >50% of T. cruzi sera when chemically immobilized on a 96-well plate (33, 35). By including the Kmp-11 sequence in the multiepitope recombinant, it was not subject to the need for such immobilization. The new recombinant ITC8.2 was initially made with a His6 tag but was subsequently made with a SUMO tag, which was then cleaved as described in Materials and Methods. The sequence of the His6-tagged ITC8.2 is shown in Fig. 1B. As is evident from Table 3, this recombinant antigen reacted with all of the peptide 1-positive sera in EIAs, although to date no serum reactive with only peptide 1 or Kmp-11 has been identified. To confirm the reactivity of a peptide 1-reactive serum with ITC8.2, a peptide 1-reactive serum was affinity purified on a peptide 1 affinity column. This serum was then shown to react in an EIA with peptide 1 and ITC8.2 but not with ITC6, which lacks the peptide 1 sequence. ITC8.2 dipsticks were prepared and compared to the reactivity of TcF and ITC6 EIA, RIPA, and ITC6 dipsticks with the serum panels. Table 3 shows the ITC8.2 dipstick reactivity, along with the ITC6 dipstick reactivity, compared to RIPA. In both cases 101 of 102 sera were positive, and both showed improved reactivity over TcF. In addition, Fig. 2 shows a comparison of the intensities of dipstick reactivity for ITC6 and ITC8.2 and indicates that, in general, increased intensity is seen in ITC8.2 dipsticks versus ITC6 dipsticks without affecting the background. Whether this is due to the Kmp-11 or peptide 1 reactivity inclusion is uncertain at this time due to the lack of a Kmp-11- or peptide 1-only reactive serum. ITC8.2 was then chosen for further studies, and a more extensive evaluation of the ITC8.2 dipstick was performed in Chile. Table 4 shows the reactivity of the ITC8.2 dipstick test with serum samples from 118 IFAT-positive Chagas' sera, as well as 106 control sera that include sera with rheumatoid factor, toxoplasmosis, syphilis, nonparasitic diseases, and cancers. The ITC8.2 dipstick was positive in 116 of the IFAT-positive sera. Of the two sera that were discrepant, one was negative by EIA (epimastigotes) and the other was positive. The single false-positive result in the dipstick assay was confirmed to be ELISA negative. The initial positive concordance was 98.3%, and the negative concordance was 99.1%, with a total concordance of 98.7%. If IFAT and EIA (epimastigotes) are used in combination (consensus positive), the positive concordance was 99.2%, and the negative concordance was 99.1%. The total concordance increased to 99.1%. The discrepant samples could indicate the absence of antibodies to the ITC8.2 epitopes, inaccessibility of epitopes in the recombinant protein to serum antibodies, or an extremely low antibody titer.
FIG. 2.
Reactivity of ITC6 and ITC8.2 dipsticks with RIPA-confirmed positive sera from several sources. The vertical axis represents the mean dipstick intensity compared to a reference chart showing intensity scale from 0 to 14 (see Materials and Methods). In both cases, the antigen was sprayed on the dipstick at the desired concentration.
TABLE 4.
ITC8.2 dipstick reactivity with Chilean positive and negative sera compared to IFAT and IFAT/EIA results with epimastigotesa
| ITC8.2 dipstick result | No. of sera evaluated by:
|
|||
|---|---|---|---|---|
| IFAT
|
IFAT/EIA
|
|||
| Positive | Negative | Positive | Negative | |
| Positive | 116 | 1 | 117 | 1 |
| Negative | 2 | 105 | 1 | 105 |
Chagasic sera (n = 118) and control sera (n = 106) were evaluated in the ITC8.2 dipstick assay. Versus IFAT, the positive and negative concordances were 98.3% and 99.1%, respectively. Compared to IFAT and EIA, the positive and negative concordances were 99.2% and 99.1%, respectively.
To determine the potential cross-reactivity with VL, sera from 20 individuals with rK39-positive sera were tested in the ITC8.2 Chagas dipstick along with 10 Chagas positive sera and 10 control sera. The data shown in Table 5 indicate no cross-reactivity with VL-positive sera. This finding is in contrast to some commercial ELISAs that show high cross-reactivity with VL-positive sera (10).
TABLE 5.
Cross-reactivity studiesa
| Serum type | No. of sera
|
||
|---|---|---|---|
| Total | ITC8.2 dipstick positive | rK39 dipstick positive | |
| Chagas sera | 10 | 10 | 0 |
| Visceral leishmaniasis sera | 20 | 0 | 20 |
| Control sera | 10 | 0 | 0 |
ITC8.2 Chagas dipsticks were evaluated for cross-reactivity with visceral leishmaniasis sera (n = 20). The sera were evaluated for ITC8.2 reactivity and rK39 dipstick assay for evidence of visceral leishmaniasis. Chagasic sera (n = 10) were consensus positive. Control sera from Seracare (n = 10) were negative for both rK39 and ITC8.2.
DISCUSSION
EIA was used to identify additional epitopes that complemented the multiepitope recombinant protein TcF and improved detection of T. cruzi-positive sera from Central America. From these studies peptides 1, 30, 36, and SAPA were identified as complementary in detecting antibodies to T. cruzi in serum samples. Based on these studies, multiepitope recombinant proteins (ITC6 and ITC8.2) have been synthesized containing the components of TcF and some or all of the additional epitopes described. ITC6 incorporated peptides 30, 36, and SAPA, and ITC8.2 further included peptides 1 and Kmp-11. Peptide 1 has been described as being reactive with a large percentage of symptomatic and asymptomatic patients with Chagas' disease (22, 23). Kmp-11 is a cytoskeletal associated protein that is a major component of the cell membrane, and a C terminus peptide has been shown to be reactive with >50% of chagasic sera (33, 35). These newly developed recombinant proteins, in particular ITC8.2, have been used in the development of a prototype lateral flow assay for the detection of T. cruzi antibodies in serum. The ITC8.2 assay was shown to have a high correlation with the confirmatory RIPA used in the United States and demonstrates improved reactivity with T. cruzi-positive sera from Central American and U.S. blood donors compared to TcF. The dipstick test had a specificity of 99.1% and a sensitivity of 98.3% compared to IFAT, and 99.1 and 99.2%, respectively, compared to IFAT in combination with EIA (epimastigotes) (Table 4). In the studies described here, the ITC8.2 dipstick also showed good correlation with RIPA. It was positive with 25 of 25 RIPA-positive sera from Chile, 14 of 14 BBI sera, and 20 of 21 sera from the HemaBio panel (Table 3). Serum 63225 in the latter panel was equivocal by RIPA and was borderline positive by the Hemagen EIA as provided in the HemaBio specifications. The EIA data indicate that both ITC6 and ITC8.2 have improved reactivity over TcF and that ITC8.2 is the antigen of choice in developing the dipstick assay. These assays could become the first choice for screening purposes in disease surveillance or intervention programs and could have utility in cardiac and other organ transplantations (1, 21). The assay is designed for point of care, epidemiological settings, and remote diagnostic laboratories and for testing on an individual basis. Although it could potentially be used for pretesting blood donors, removing an individual testing positive from a blood donor pool should first be considered for retesting with a commercially available ELISA.
Serological expression cloning with TcF-negative or TcF low-reactive sera that were identified while evaluating the additional sequences has also been performed, and additional, potentially complementary antigens to TcF have been identified. Discussion of these findings, however, will be the subject of a separate publication. In addition, studies are currently under way to evaluate the use of whole-blood samples in the ITC8.2 dipstick assay. The studies presented here show the utility of the ITC6 and ITC8.2 antigens in the detection of human Chagas' disease. Other investigators have also demonstrated the utility of the ITC6 and 8.2 antigens in detecting T. cruzi antibodies in canines (5) and in a case of autochthonous transmission of T. cruzi in Louisiana (8).
Acknowledgments
We thank Raodoh Mohamath at the Infectious Disease Research Institute, Seattle, WA, for assisting in developing recombinant proteins and performing the serological expression cloning. We also thank our collaborators at the ARC for samples and for performing RIPA assays.
This study was supported by NIH grant R44 AI0522683-02 (S.R.).
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Altclas, J. D., L. Barcan, C. Nagel, R. Lattes, and A. Riarte. 2007. Organ transplantation and Chagas disease. JAMA 2982171-2181. [DOI] [PubMed] [Google Scholar]
- 2.Betonico, G. N., E. O. Miranda, D. A. O. Silva, R. Houghton, S. G. Reed, A. Campos-Neto, and J. R. Mineo. 1999. Evaluation of a synthetic tripeptide as antigen for detection of IgM and IgG antibodies to Trypanosoma cruzi in serum samples from patients with Chagas' disease or viral diseases. Trans. R. Soc. Trop. Med. Hyg. 93603-606. [DOI] [PubMed] [Google Scholar]
- 3.Boelaert, M., S. El-Safi, A. Hailu, M. Mukhtar, S. Rijal, S. Sundar, M. Wasunna, A. Aseffa, J. Mbui, J. Menten, P. Desjeux, and R. W. Peeling. 2008. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Trans. R. Soc. Trop. Med. Hyg. 10232-40. [DOI] [PubMed] [Google Scholar]
- 4.Camargo, M., E. Segura, I. Kagan, J. Souza, J. Carvalheiro, J. Yanovsky, and M. Guimaraes. 1986. Three years collaboration on the standardization of Chagas disease serodiagnosis in the Americas: an appraisal. Bull. PAHO 20233-244. [PubMed] [Google Scholar]
- 5.Cardinal, M. V., R. Reithinger, and R. E. Gürtler. 2006. Use of an immunochromatographic dipstick test for rapid detection of Trypanosoma cruzi in sera from animal reservoir hosts. J. Clin. Microbiol. 443005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chico, M., C. Sandoval, A. Guevara, M. Calvopina, P. J. Cooper, S. G. Reed, and R. H. Guderian. 1997. Chagas' disease in Ecuador: evidence for disease transmission in an indigenous population in the Amazon region. Mem. Inst. Oswaldo Cruz 92317-320. [DOI] [PubMed] [Google Scholar]
- 7.Contreras, M. C., P. Salinas, L. Sandoval, and F. A. Solis y Rojas. 1992. Usefulness of the ELISA IgG test in sera and filter paper blood eluates in the Chagas diagnosis. Bol. Chil. Parasitol. 4776-81. [PubMed] [Google Scholar]
- 8.Dorn, P. L., L. Perniciaro, M. J. Yabsley, D. M. Roellig, G. Balsamo, J. Diaz, and D. Wesson. 2007. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg. Infect. Dis. 13605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira, A. W., Z. R. Belem, E. A. Lemos, S. G. Reed, and A. Campos-Neto. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 394390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlin, J., S. Rossmann, G. Robertson, F. Stallone, N. Hirschler, K. A. Nguyen, R. Gilcher, H. Fernandes, S Alvey, P. Ajongwen, P. Contestable, and H. Warren. 2008. Evaluation of a new Trypanosoma cruzi antibody assay for blood donor screening. Transfusion 48531-540. [DOI] [PubMed] [Google Scholar]
- 11.Houghton, R. L., D. R. Benson, L. Reynolds, P. McNeill, P. Sleath, M. Lodes, Y. A. W. Skeiky, D. Leiby, R. Badaro, and S. G. Reed. 1999. A multiepitope peptide ELISA for the detection of antibodies to Trypanosoma cruzi in RIPA confirmed and consensus positive sera. J. Infect. Dis. 1791226-1234. [DOI] [PubMed] [Google Scholar]
- 12.Houghton, R. L., D. R. Benson, L. Reynolds, P. McNeill, P. Sleath, M. Lodes, Y. A. W. Skeiky, R. Badaro, A. U. Krettlie, and S. G. Reed. 2000. Multiepitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in patients with treated or untreated Chagas' disease. J. Infect. Dis. 181325-330. [DOI] [PubMed] [Google Scholar]
- 13.Houghton, R. L., Y. Y. Stevens, J. Guderian, M. Okamoto, M. Kabir, P. Arauz-Ruiz, K. Visona, S. G. Reed, D. A. Leiby, W. J. W. Morrow, and S. Raychaudhuri. 2006. Geographically robust lateral flow immunoassay for diagnosis of T. cruzi infection with high correlation to radio-immunoprecipitation assay (RIPA) Am. J. Trop. Med. Hyg. 75146. (Abstr.) [Google Scholar]
- 14.Kahn, S., M. Kahn, W. C. van Voorhis, A. Goshorn, A. Strand, N. Hoagland, H. Eisen, and S. Pennathur. 1993. SA85-1 proteins of Trypanosoma cruzi lack sialidase activity. Mol. Biochem. Parasitol. 60149-152. [DOI] [PubMed] [Google Scholar]
- 15.Kahn, S., T. G. Colbert, J. C. Wallace, N. A. Hoagland, and H. Eisen. 1991. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc. Natl. Acad. Sci. USA 884481-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn, S., W. C. van Voorhis, and H. Eisen. 1990. The major 85-kDa surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J. Exp. Med. 172589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchhoff, L. V., A. A. Gam, R. de Gusmao, R. S. Goldsmith, J. M. Rezende, and A. Rassi. 1987. Increased specificity of serodiagnosis of Chagas' disease by detection of antibody to the 72- and 90-kilodalton glycoproteins of Trypanosoma cruzi. J. Infect. Dis. 155561-564. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhoff, L. V. 1990. Trypanosoma species (American trypanosomiasis, Chagas' disease): biology of trypanosomes, p. 2077. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, NY.
- 19.Leiby, D. A., S. Wendel, D. T. Takaoka, R. M. Fachini, L. C. Oliveira, and M. A. Tibbals. 2000. Serologic testing for Trypanosoma cruzi: comparison of radioimmunoprecipitation assay with commercially available indirect immunofluorescence assay, indirect hemagglutination assay and enzyme-linked immunosorbent assay kits. J. Clin. Microbiol. 38639-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiby, D. A., E. J. Read, B. A. Lenes, A. J. Yund, R. J. Stumpf, L. V. Kirchhoff, and R. Y. Dodd. 1997. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas' disease in US blood donors. J. Infect. Dis. 1761047-1052. [DOI] [PubMed] [Google Scholar]
- 21.Leiby, D. A., F. J. Rentas, K. E. Nelson, V. A. Stambolis, P. M. Ness, C. Parnis, H. A. McAllister, D. H. Yawn, R. J. Stumpf, and L. V. Kirchhoff. 2000. Evidence of Trypanosoma cruzi infection (Chagas' disease) among patients undergoing cardiac surgery. Circulation 1022978-2982. [DOI] [PubMed] [Google Scholar]
- 22.Lorca, M., A. Gonzalez, V. Reyes, C. Veloso, U. Vergara, and C. Frasch. 1993. The diagnosis of chronic Chagas' disease using recombinant antigens of Trypanosoma cruzi. Rev. Med. Chile 121363-368. [PubMed] [Google Scholar]
- 23.Lorca, M., A. Gonzalez, C. Veloso, V. Reyes, and U. Vergara. 1992. Immunodetection of antibodies in sera from symptomatic and asymptomatic Chilean Chagas' disease patients with Trypanosoma cruzi recombinant antigens. Am. J. Trop. Med. 4644-49. [DOI] [PubMed] [Google Scholar]
- 24.Lorca, M., and E. Thiermann. 1994. Congenital Chagas disease and its serological diagnosis through conventional serology and methods of molecular biology, p. 160-166. In R. Ehrlich and A. Nieto (ed.), Biology of parasitism. Trilce, Montevideo, Uruguay.
- 25.Luquetti, A., and A. Rassi. 2000. Diagnóstico laboratorial da infeccion pelo Trypanosoma cruzi, p. 344-378. In Z. Brenner, Z. Andrade, and M. Barral-Neto (ed.), Trypanosoma cruzi e doença de Chagas, 2nd ed. Guanabara Koogan, Rio de Janeiro, Brazil.
- 26.Luquetti, A. O., C. Ponce, E. Ponce, J. Esfandiari, A. Schijman, S. Revollo, N. Anez, B. Zingales, R. Rangel-Aldao, A. Gonzales, M. J. Levin, E. S. Umezawa, and J. F. da Silveira. 2003. Chagas' disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn. Microbiol. Infect. Dis. 46265-271. [DOI] [PubMed] [Google Scholar]
- 27.Machadi, C. A., and F. J. Ayala. 2001. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA 987396-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peralta, J. M., M. G. Teixera, W. G. Shreffler, J. B. Pereira, J. M. Burns, Jr., P. R. Sleath, and S. G. Reed. 1994. Serodiagnosis of Chagas' disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J. Clin. Microbiol. 32971-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponce, C., E. Ponce, E. Vinelli, A. Montoya, V. de Aguilar, A. Gonzales, B. Zingales, R. Rangel-Aldao, M. J. Levin, J. Esfandiari, E. S. Umezawa, A. O. Luquetti, and J. F. da Silveira. 2005. Validation of a rapid and reliable test for the diagnosis of Chagas' disease by detection of Trypanosoma cruzi-specific antibodies in the blood of donors and patients in Central America. Clin. Microbiol. 435065-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portela-Lindoso, A. A., and M. A. Shikanai-Yasuda. 2003. Chronic Chagas's disease: from xenodiagnosis and hemoculture to polymerase chain reaction. Cad. Saude Publica 37107-115. [DOI] [PubMed] [Google Scholar]
- 31.Schmunis, G. A. 1991. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and non-endemic countries. Transfusion 31547. [DOI] [PubMed] [Google Scholar]
- 32.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, M. C., M. V. Longobardo, E. Carmelo, C. Maranon, L. Planelles, M. E. Patarroyo, C. Alonso, and M. C. Lopez. 2001. Mapping of the antigenic determinant of the Trypanosoma cruzi kinetoplastid membrane protein-11. Identification of a linear epitope specifically recognized by human chagasic sera. Clin. Exp. Immunol. 123465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobler, L. H., P. Contestable, L. Pitina, H. Groth, S. Shaffer, G. R. Blackburn, H. Warren, S. R. Lee, and M. P. Busch. 2007. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in U.S. blood donors. Transfusion 4790-96. [DOI] [PubMed] [Google Scholar]
- 35.Trujillo, C., R. Ramirez, I. D. Velez, and C. Berberich. 1999. The humoral response to the kinetoplastid membrane protein 11 in patients with American leishmaniasis and Chagas' disease: prevalence of IgG subclasses and mapping of epitopes. Immunol. Lett. 70203-209. [DOI] [PubMed] [Google Scholar]
- 36.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. F. da Silveira. 1999. Evaluation of recombinant antigens for the serodiagnosis of Chagas disease in South and Central America. J. Clin. Microbiol. 371554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umezawa, E. S., A. O. Luquetti, G. Levitus, C. Ponce, E. Ponce, D. Henriquez, S. Revollo, B. Espinoza, O. Sousa, B. Khan, and J. F. da Silveira. 2004. Serodiagnosis of chronic and acute Chagas' disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J. Clin. Microbiol. 42449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergara, U., C. Veloso, A. Gonzalez, and M. Lorca. 1992. Evaluation of an enzyme-linked immunoabsorbent assay for the diagnosis of Chagas' disease using synthetic peptides. Am. J. Trop. Med. Hyg. 4639-43. [DOI] [PubMed] [Google Scholar]
- 39.Wendel, S., and A. L. Gonzaga. 1993. Chagas' disease and blood transfusion: a New World problem? Vox Sang 641-12. [DOI] [PubMed] [Google Scholar]