Abstract
Following a long-distance outbreak of Legionnaires' disease from an industrial air scrubber in Norway in 2005, a seroepidemiological study measuring levels of immunoglobulin G (IgG) and IgM antibodies to Legionella pneumophila was performed with a polyvalent enzyme-linked immunosorbent assay. One year after the outbreak, IgG levels in employees (n = 213) at the industrial plant harboring the scrubber and in blood donors (n = 398) from the outbreak county were low but significantly higher (P ≤ 0.002) than those in blood donors (n = 406) from a nonexposed county. No differences in IgM levels among the three groups were found after adjustment for gender and age. Home addresses of the seroresponders in the exposed county clustered to the city of the outbreak, in contrast to the scattering of addresses of the seroresponding donors in the nonexposed county. Factory employees who operated at an open biological treatment plant had significantly higher IgG and IgM levels (P ≤ 0.034) than those working >200 m away. Most of the healthy seroresponders among the factory employees worked near this exposure source. Immunoblotting showed that IgG and IgM antibodies in 82.1% of all seroresponders were directed to the lipopolysaccharide of the L. pneumophila serogroup 1 outbreak strain. In conclusion, 1 year after the long-distance industrial outbreak a small increase in IgG levels of the exposed population was observed. The open biological treatment plant within the industrial premises, however, constituted a short-distance exposure source of L. pneumophila for factory employees working nearby.
In May 2005, an outbreak of Legionella pneumophila serogroup 1 occurred from an air scrubber located at a large wood-based-chemical factory in the southeastern part of Norway. Legionnaires' disease (LD) was diagnosed in 56 patients, of whom 10 died (35). Fifty-one of the patients lived in the county where the industrial plant is located. The bacteria spread more than 10 km away from the scrubber, probably due to the high emission velocity of the aerosols at the top of the scrubber and the prevailing weather conditions (35). Recently, the number of patients with LD from this outbreak was adjusted to 103, based on antibody responses in patients with pneumonia from the region at the time of the outbreak (34). It is the largest outbreak of LD in Norway so far; only one outbreak, in 2001 (with 28 cases of LD), was reported previously (5). In between these two outbreaks, there were yearly about 25 LD cases, about half of which were imported, corresponding to an incidence of 0.6/100,000 (34).
The prevalence of antibodies to L. pneumophila in healthy individuals following larger outbreaks has been described in several studies (7, 8, 13, 14, 19, 30, 36). The aim of our study was to determine whether the long-distance outbreak from the air scrubber in 2005 (35) had resulted in increased levels of antibody to L. pneumophila in healthy blood donors residing in the exposed county compared with levels in blood donors in a nonexposed county. Employees at the chemical factory were also included to investigate whether they had been subjected to a higher exposure to the outbreak strain than blood donors in the same county. Antibody levels were measured by an enzyme-linked immunosorbent assay (ELISA) with a pool of L. pneumophila serogroups 1 to 7 as the antigen, and risk factors for LD, such as gender, age, smoking, and chronic lung diseases (16), were assessed from a questionnaire. In addition, the specificity of the antibody responses in participants who showed increased antibody levels by the ELISA was examined by immunoblotting with the serogroup 1 outbreak strain.
(Parts of this work were presented at the 22nd and 23rd Meetings of the European Working Group for Legionella Infections [42, 43].)
MATERIALS AND METHODS
Blood samples and questionnaires.
Blood samples were collected 11 to 13 months after the industrial outbreak, which took place in Østfold County in the southeastern part of Norway. A total of 1,017 volunteers were recruited: (i) 213 healthy factory employees working at different sites on the industrial premises, (ii) 398 blood donors from the exposed county, and (iii) 406 blood donors from Oslo, Norway, 80 km away, serving as a nonexposed group. After informed consent, each of the volunteers supplied a 10-ml blood sample. The participants answered an anonymous questionnaire about gender, age, address in the form of postal codes, occupation, chronic lung diseases, and smoking habits to assess risk factors for LD (16). They were also asked whether they had experienced respiratory infections, such as dry coughing, bronchitis, or flulike disease with muscle pain, during the outbreak period in May 2005, as a possible indication of Legionella infection. In addition, factory employees were questioned about where on the premises they worked. The study was planned with a power of 0.8 based on a 1.5-fold difference in antibody levels between exposed and nonexposed groups, as shown in a previous outbreak (7). The study was approved by the Regional Committee for Medical Research Ethics.
ELISA.
Sera were collected by centrifugation of the blood samples and stored in aliquots at −20°C until analyses. Levels of immunoglobulin G (IgG) and IgM antibodies to a pool of L. pneumophila serogroups 1 to 7 as the antigen were determined separately with a commercial quantitative ELISA (40) by use of an ELISA robot (DSX automated system; Dynex Technologies, Inc., VA). Rheumatoid factors were adsorbed before the IgM analyses. This ELISA performed well regarding sensitivity and false-positive rates for LD compared with other commercial ELISAs (15) and had been used in previous outbreak studies (7, 8, 13, 22). According to the kit's manufacturer, the negative, borderline, and positive values for L. pneumophila infection were <50 U/ml, 50 to 70 U/ml, and >70 U/ml for IgG, respectively, with corresponding values of <120 U/ml, 120 to 140 U/ml, and >140 U/ml for IgM (40). Single serum samples were analyzed twice on different dates, except for those of the factory employees, which were analyzed as duplicates the first time. Arithmetic means of these results were used as antibody levels. Neither the IgG nor the IgM levels of the duplicates showed significant differences, indicating good reproducibility of the ELISA. The coefficients of variation for four standard samples per plate throughout this study were 6.9% for IgG (range, 2.4 to 18.9%) and 4.1% for IgM (range, 1.0 to 7.4%), compared with the maximal interserial coefficient of 16% given by the manufacturer. Individuals with levels of IgG or IgM to L. pneumophila in the positive and borderline ranges were defined in our study as seroresponders.
IFA.
Sera from the seroresponders were also tested by an immunofluorescence assay (IFA), with a pool of L. pneumophila serogroups 1 to 6 as the antigen (Meridian Bioscience Europe, Milan, Italy) and conjugated anti-human Ig as the secondary antibody.
Strain characterization.
L. pneumophila strains were grown on buffered charcoal yeast extract agar plates at 35°C for 2 days, followed by confluent growth for 24 h. Cells were harvested in phosphate-buffered saline (PBS), pH 7.2, with 0.02% Na-azide, and inactivated by being heated to 60°C for 1 h. The whole-cell suspensions were used as antigens for dot blotting and immunoblotting. Serogrouping of L. pneumophila strains was performed by dot blotting (46) with the Dresden panel of monoclonal antibodies (MAbs) directed to lipopolysaccharides (LPS) (20, 21). Two microliters of the whole-cell suspensions (0.5 mg/ml) was dotted on nitrocellulose strips and incubated overnight with the MAbs diluted in PBS with 3% bovine serum albumin. Antibody binding was detected with rabbit anti-mouse Ig conjugated to peroxidase (1:1,000) (Dako AS, Denmark).
Immunoblotting.
Whole cells (50 μg protein per gel) were boiled for 5 min in sample buffer with sodium dodecyl sulfate and mercaptoethanol (28), separated in 12% acrylamide gels, and electrotransferred to nitrocellulose filters (7 by 9.5 cm; pore size, 0.45 μm) as described previously (41, 45). Each blot was cut into about 25 strips, which were incubated overnight at room temperature with human sera diluted 1:200 in PBS with 3% bovine serum albumin. Levels of IgG and IgM binding, respectively, were detected with 1:500 dilutions of rabbit anti-human IgG and rabbit anti-human IgM conjugated to horseradish peroxidase (Dako AS, Denmark). Blotting was also performed with whole cells treated with proteinase K as the antigen (Qiagen GmbH, Hilden, Germany) (23).
Statistical analyses.
Data were analyzed with SPSS 14.0, Stata 10.0, GraphPad Prism 4.03, and SigmaStat 3.1 programs. P values of <0.05 were considered significant. Proportions were compared with Fisher's test instead of the chi-square test for numbers of individuals <5. Arithmetic means of the IgG and IgM antibody levels were not normally distributed, nor were they after log transformation, except for IgM levels of factory employees and nonexposed blood donors. Nonparametric methods (Mann-Whitney rank sum test for differences between groups and Spearman rank order test for correlations) were therefore used for statistical analyses. Only variables that gave significant results in univariate analyses were included in multivariate regression analyses.
RESULTS
Description of study participants.
Table 1 shows the characteristics of the three groups of participants in the study, i.e., the employees at the factory where the outbreak source was located, blood donors from the exposed county, and blood donors from a nonexposed county, based on information from the questionnaires. Response rates for the various questions ranged from 95% to 100%. Several significant differences (P = 0.0001 to 0.031) among the groups were observed. There were nearly twice as many men among the factory employees as among the two blood donor groups and also more men among the nonexposed blood donors than among the exposed donors. The last group was a few years older than the two other groups. Proportions of smokers among the factory employees and the exposed blood donors were twofold higher than those in the nonexposed group. A daily consumption of >10 cigarettes was reported by two to three times more factory employees than members of the blood donor groups. Similar differences were also observed for chronic lung diseases and airway infections during the outbreak period in May 2005.
TABLE 1.
Characteristics of the study participants
| Characteristic | Resulta for:
|
|||
|---|---|---|---|---|
| Factory employees (n = 213) | Exposed blood donors (n = 398) | Nonexposed blood donors (n = 406) | All participants (n = 1,017) | |
| Gender | ||||
| Male | 192 (90.1)b | 188 (47.2)c | 226 (55.7) | 606 (59.6) |
| Female | 21 (9.9) | 210 (52.8) | 180 (44.3) | 411 (40.4) |
| Age (yr) | ||||
| Median | 44 | 46d | 43 | 44 |
| Range | 20-62 | 18-69 | 19-68 | 18-69 |
| Smoker | ||||
| Yes | 73 (34.3) | 113 (28.4) | 64 (15.8)e,f | 250 (24.6) |
| 0-10 cigarettes/day | 20 (27.4) | 75 (66.4) | 49 (77.8) | 144 (57.8) |
| >10 cigarettes/day | 53 (72.6)b | 38 (33.6) | 14 (22.2) | 105 (42.2) |
| No | 140 (65.7) | 285 (71.6) | 340 (84.2) | 765 (75.4) |
| Chronic lung diseases | ||||
| Yes | 14 (6.6)b | 10 (2.5) | 9 (2.2) | 33 (3.3) |
| No | 199 (93.4) | 385 (97.5) | 395 (97.8) | 979 (96.7) |
| Airway infections in May 2005 | ||||
| Yes | 30 (14.1)b | 28 (7.2) | 17 (4.3) | 75 (7.5) |
| No | 171 (80.3) | 323 (82.6) | 344 (86.6) | 838 (83.7) |
| Uncertain | 12 (5.6) | 40 (10.2) | 36 (9.1) | 88 (8.8) |
Data represent the numbers (percentages) of participants, unless otherwise indicated.
Significantly higher (P ≤ 0.015) than for the other two groups.
Significantly lower (P = 0.0168) than for nonexposed blood donors.
Significantly higher (P ≤ 0.031) than for the other two groups.
Significantly lower (P = 0.0001) than for the other two groups.
One smoker did not specify cigarette consumption.
Description of seroresponders.
Levels of IgG and IgM antibodies to L. pneumophila as measured by the polyvalent ELISA showed that the numbers of seroresponders among the factory employees, the exposed blood donors, and the nonexposed blood donors were 9 (4.2%), 9 (2.3%), and 10 (2.4%), respectively, but the differences among the groups were not significant. Only one individual in each group had elevated IgM levels. About 60% of the seroresponders in the two blood donor groups were women, while all in the factory group were men. No significant differences in age, airway infection, or smoking habit among the responders in the three groups were observed.
Of the nine seroresponders in the factory group, five worked at an open biological treatment plant shown to emit aerosols with L. pneumophila and other Legionella species (3), two worked at the outbreak air scrubber 200 m away, and one worked at a combustion plant 125 m from the biodegradation plant. The remaining individual worked 2 km from the main premises. Of the 213 employees recruited, 63 worked at the treatment plant, the air scrubber, or the combustion plant and 150 worked at other sites on the industrial premises. There was a highly significant association (P = 0.0003) between being a seroresponder and having a workplace at or near the biodegradation plant.
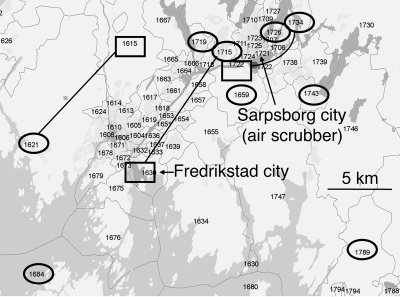
About 1/3 of the 398 exposed blood donors had home addresses within a radius of 5 km from the city where the outbreak occurred. Six of the nine seroresponders in this group resided within this range (Fig. 1). The remaining 2/3 of the exposed donors resided either in a larger city about 15 km southwest or elsewhere in the county. One of the remaining three seroresponders worked 10 km away from the outbreak city, while two resided farther away. A clustering of addresses to the city of the outbreak was thus observed for the seroresponders in the group of exposed blood donors. In contrast, home addresses of the 10 seroresponders among the nonexposed blood donors were scattered all over the region (data not shown).
FIG. 1.
Addresses, shown as postal codes, for the nine seroresponders to L. pneumophila among the 398 blood donors in the exposed county. Home and workplace addresses are shown within ovals and rectangles, respectively. Seven of the nine seroresponders resided or worked within a range of about 10 km from the city where the outbreak took place (Sarpsborg, Norway), although 2/3 of the exposed blood donors lived in a larger neighbor city (Fredrikstad, Norway) or elsewhere in the county. Different home and workplace addresses for three of the seroresponders are shown by connecting lines. Seroresponders were defined as individuals with positive or borderline IgG and IgM levels by the polyvalent ELISA. The map was created with the ArcGIS 9.2 commercial software program, supplied by Geodata AS (Oslo, Norway).
Comparison of antibody levels of the seroresponders by ELISA and IFA.
When sera from all seroresponders were analyzed by IFA with a pool of L. pneumophila serogroups 1 to 6, the median titer was 1:128 (range, 1:32 to 1:1,024), thus supporting the increased levels obtained by ELISA. According to the manufacturer of the IFA, a titer of ≥1:128 provides evidence of a recent L. pneumophila infection. Significant correlations between IFA titers and IgG levels by ELISA (Spearman rank order correlation coefficient, 0.489; P = 0.006) or between IFA titers and sums of IgG and IgM levels (Spearman rank order correlation coefficient, 0.495; P = 0.006) were obtained.
IgG and IgM antibody levels of exposed and nonexposed groups by ELISA.
Low median levels of antibody to L. pneumophila were found in all three groups (Table 2). Although the differences were small, with a statistical power in the lower range (0.63), factory employees and exposed blood donors had significantly higher IgG levels (P ≤ 0.002) than nonexposed blood donors. The median IgM antibody levels of the two groups of blood donors were similar and significantly higher (P ≤ 0.005) than those for the factory group. As reported below, this difference was mainly due to a gender difference associated with IgM levels.
TABLE 2.
Levels of IgG and IgM antibodies to L. pneumophila in exposed and nonexposed study groups, as determined by ELISA
| Group | Median IgG value in U/ml (range) | Median IgM value in U/ml (range) |
|---|---|---|
| Factory employees | 12 (3-372) | 12b (1-238) |
| Exposed blood donors | 13 (1-109) | 16 (1-124) |
| Nonexposed blood donors | 11a (1-120) | 16 (1-124) |
Significantly lower than for factory employees (P = 0.002) and exposed blood donors (P = 0.001).
Significantly lower than for exposed (P = 0.005) and nonexposed (P = 0.001) blood donors.
Association of antibody levels by ELISA and risk factors for LD.
Levels of IgG to L. pneumophila of all participants remained constant over the age range of 18 to 69 years. In contrast, IgM levels showed a weak but significant decrease with age (Spearman rank order correlation coefficient, −0.177; P = 0.0001).
Men and women had similar median IgG levels (11 U/ml). In line with the high IgG levels of the factory group and the group of exposed blood donors, shown in Table 2, men in these two groups also had higher IgG levels (12 and 13 U/ml, respectively) than men in the nonexposed group (11 U/ml) (P ≤ 0.009). Exposed female blood donors also had significantly higher IgG levels than nonexposed females (12 versus 11 U/ml, respectively; P = 0.014).
A gender difference associated with IgM was observed, as the median IgM levels in women were 1.5-fold higher than those in men (19 versus 13 U/ml, respectively; P = 0.0001). The high IgM levels of the groups of blood donors compared with those of the factory employees, as shown in Table 2, were explained by the large proportion of women among the donors (Table 1) and the age-dependent fall in IgM levels reported above. Multivariate regression analyses, adjusted for age and gender, showed no significant differences in IgM levels among the groups.
Airway infections during the outbreak period, high levels of cigarette consumption, and chronic lung diseases were reported by more factory employees than blood donors (Table 1), but no association between these variables and levels of IgG or IgM to L. pneumophila was demonstrated.
Antibody levels of male factory employees according to workplace at the industrial premises are shown in Table 3. Those who worked at the air scrubber, the biological treatment plant, or the combustion plant had higher median IgG and IgM levels than those working at other sites on the main premises or 2 km away, but significant differences (P = 0.013 to 0.046) were found only for employees at the treatment plant. Women were not included in this analysis, as all but 1 of the 21 female workers were employed at other sites on the main premises.
TABLE 3.
Median levels of IgG and IgM to L. pneumophila in male employees working at different sites on the industrial premises, as determined by ELISA
| Workplace | No. of employees | Median IgG value (U/ml) | Median IgM value (U/ml) |
|---|---|---|---|
| Air scrubber | 34 | 12.5 | 15.5 |
| Biological treatment plant | 21 | 14a | 17b |
| Combustion plant | 7 | 14 | 17 |
| Other sites at main premises | 108 | 11 | 11 |
| Plant 2 km away | 22 | 10.5 | 10.5 |
| All workplaces | 192 | 12 | 12 |
Significantly higher than for those working at other sites on the main premises (P = 0.034) or 2 km away (P = 0.028).
Significantly higher than for those working at other sites on the main premises (P = 0.013) or 2 km away (P = 0.046).
Antibody specificities of the seroresponders by immunoblotting.
The ELISA measurements were performed with a pool of L. pneumophila serogroups 1 to 7 as the antigen. To analyze whether the antibody responses of the seroresponders were directed to the serogroup 1 outbreak strain (35), the sera were immunoblotted with this strain as the antigen. An L. pneumophila serogroup 4 strain, which had been isolated in air samples near the biological treatment plant just after blood sampling of the volunteers in our study (3), was used as a comparative antigen. Dot blotting with LPS-specific MAbs demonstrated that the serogroup 1 and 4 strains belonged to subgroups Benidorm and Portland, respectively.
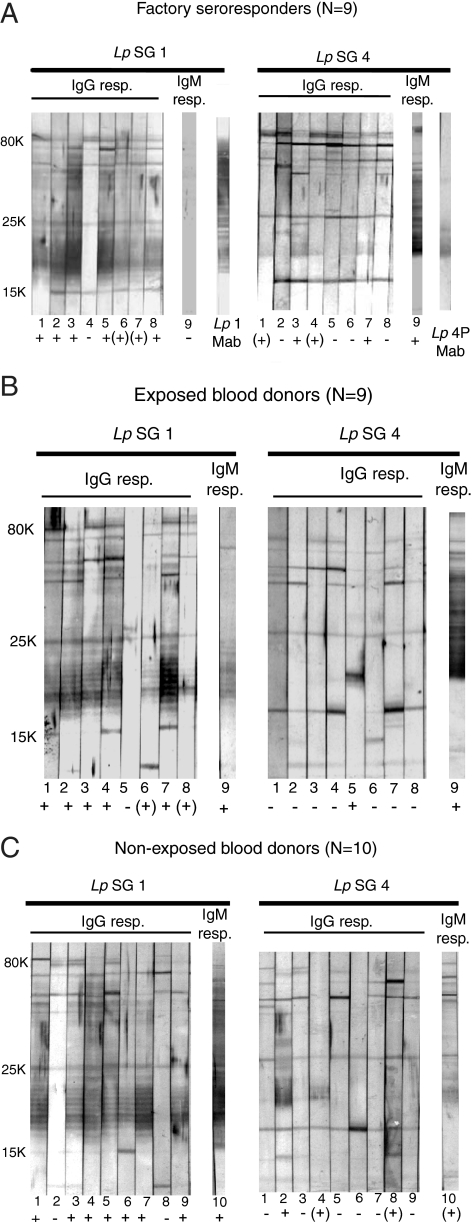
The general immunoblotting pattern of all 28 seroresponders with the serogroup 1 strain (Fig. 2A to C) consisted of distinct IgG antibody binding to several protein bands in the molecular mass range of 80 to 15 kDa and background staining including a strong-to-weak, ladderlike pattern in the lower range that corresponded to the one seen with the serogroup 1 LPS-specific MAb (Fig. 2A). Most individual sera bound with roughly the same IgG binding intensities to the proteins of the serogroup 4 strain as to those of the serogroup 1 strain (Fig. 2A to C). Few sera reacted with the serogroup 4 LPS of a molecular mass of about 20 kDa, as seen by the binding of the subgroup 4 Portland MAb (Fig. 2A). The position of this band and the ladderlike pattern of the serogroup 1 strain in the 25- to 15-kDa molecular mass range matched the LPS patterns seen with silver-stained sodium dodecyl sulfate gels (39) after proteinase K treatment of the two strains (not shown). LPS is considered to represent the serogroup-specific antigen in patients with LD (9, 18). As seen in Fig. 2A to C, all IgG and IgM seroresponders, except for one individual in the group of exposed blood donors and two in each of the other groups, showed the ladderlike LPS pattern with the serogroup 1 strain. In contrast, a total of 11 individuals in all groups had IgG and IgM antibodies to serogroup 4 LPS; 6 of these reacted with LPS of both strains and the remaining 5 with serogroup 4 LPS only. Thus, all seroresponders were found to have LPS-specific antibodies to one or both strains.
FIG. 2.
Immunoblots demonstrating IgG and IgM binding of sera from all 28 seroresponders to L. pneumophila among the exposed factory employees (A), exposed blood donors (B), and nonexposed blood donors (C). Only one individual in each group was an IgM responder (resp.). The individual seroresponders are identified by the numbers below the strips. Antigens for immunoblotting were whole cells of L. pneumophila serogroup 1 (Lp SG 1) of subgroup Benidorm and serogroup 4 (Lp SG 4) of subgroup Portland, corresponding to 50 μg protein per blot. Binding patterns of the specific serogroup 1 (Lp 1 MAb) and serogroup 4 Portland (Lp 4P MAb) MAbs are shown in panel A. IgG and IgM binding intensities of each serum to LPS of molecular masses from 25 to 15 kDa are rated as + (strong), (+) (weak), and − (none) and are the results of five experiments of which representative blots are shown. All strips, incubated with the human sera, were stained for 10 min with peroxidase substrate. Adobe Photoshop CS4 was used for the figure preparation.
Additional blotting experiments were performed to study the specificity of the antibody activity (not shown). When cells of the serogroup 1 and 4 strains were treated with proteinase K before immunoblotting, sera from the responders showed the same LPS binding patterns as with untreated cells. The seroresponders demonstrated weak or no IgG binding to LPS of the serogroup 1 subgroup Philadelphia reference strain (ATCC 33152), indicating low levels of cross-reactive antibodies. Thirty randomly selected IgG-negative sera (10 from each study group) generally displayed the same IgG binding intensities to the proteins of the two strains as those seen for the seroresponders in Fig. 2A to C. Only one serum with an IgG level near the borderline range (42 U/ml) reacted with the serogroup 1 LPS, and a negative serum (26 U/ml) reacted with the serogroup 4 LPS. The blotting reagents did not contain Tween 20, because this detergent may abolish antibody binding to LPS (2), as has been demonstrated previously for meningococcal LPS (44). In our hands, no IgG binding to the serogroup 1 LPS was observed on blots with Tween 20 concentrations greater than 0.1%. Taken together, the blotting results demonstrated that 23 of the 28 seroresponders (82.1%) in all three study groups had IgG or IgM antibodies that reacted with LPS of the serogroup 1 outbreak strain. This reaction was not observed with sera having antibody levels in the lower negative range, as measured by the polyvalent ELISA.
DISCUSSION
In this study we measured levels of antibody to L. pneumophila in exposed and nonexposed groups following a 10-km long-distance outbreak of LD from an industrial air scrubber in Norway (35). Healthy employees at the industrial plant which harbored the scrubber and blood donors from the same county had low IgG levels but significantly higher IgG levels than blood donors from a nonexposed region, despite the fact that the statistical power of the study was in the low range. Although it cannot be excluded that the small differences may be due to cross-reactive antibodies to other bacteria (15, 16), our results suggested a higher level of exposure to L. pneumophila in the county of the outbreak than in the nonexposed region. IgG and IgM antibody levels were determined with a polyvalent ELISA for LD used in a previous large-outbreak study (7, 8). Interestingly, in that study the recognition of small boosting effects of L. pneumophila in seroepidemiological outbreak studies was emphasized (6, 7). The similar IgG levels among the factory group and the exposed blood donors were supported by the fact that no cases of LD among the factory employees were reported during the outbreak. This observation was explained by both the height of the air scrubber and the high velocity of the emitted aerosols, leading to low bacterial concentrations on the industrial premises (35).
The blood samples were collected about 1 year after the outbreak, and higher IgG antibody levels might have been detected if the sampling had been performed closer to the outbreak period. IgG levels decrease with time, although levels in exposed healthy individuals may persist for some years after outbreaks (12, 30). The higher levels of IgM to L. pneumophila in the two groups of blood donors than in the factory group were caused by the age-dependent decrease in IgM antibodies and the higher proportions of women among the blood donors than among the factory group, as no significant differences among the groups were found after adjustment for gender and age. Age-dependent IgM decreases in healthy exposed individuals and in patients with LD have been reported previously (8, 37), as have gender-dependent IgM differences, e.g., healthy women exposed to L. pneumophila tended to have higher IgM levels than men tested with the same ELISA (8), and female blood donors had higher titers than men as determined by a microagglutination assay which measures IgM (17). The antibody levels in our study were in the lower negative range obtained by others with this ELISA (7, 8, 22).
Proportions of seroresponders, i.e., about 4% among the factory employees and about 2% among the blood donors, were within the seroprevalence range of 1 to 16% for healthy individuals (10). Our seroprevalence results corresponded to those determined in Sweden (12), and both are lower than those determined in Denmark (38), suggesting different levels of exposure to L. pneumophila within the Scandinavian countries.
Our study pointed to the biological treatment plant as another exposure source at the industrial premises, as eight of the nine seroresponders among the factory employees worked at or within a distance of 200 m from this plant. This plant contains two large open aeration ponds, shown to disperse aerosols with L. pneumophila and other Legionella species up to 200 m downwind from the ponds (3). Such ponds thus represent a potential occupational risk for Legionella infections (1), and similar transmission distances from biological wastewater systems have been reported previously (26, 31, 32). The spread of airborne L. pneumophila from these ponds provided an explanation for the significant association of being a seroresponder and working at or near the biological treatment plant, as well as the higher IgG and IgM levels in employees at this plant than in those working farther away on the industrial premises. These findings corresponded with a preliminary study showing high IFA titers among workers at such plants (27). It is possible that airborne Legionella species from the aeration ponds originally had infected the air scrubber (35).
While the outbreak strain of L. pneumophila serogroup 1 (subgroup Benidorm) was also isolated from or near the ponds closer to the outbreak period (4, 35), an L. pneumophila serogroup 4 strain (subgroup Portland) and various Legionella species predominated in aerosols from this site closer to the blood sampling period in our study (3, 4). The antibody levels measured by ELISA, based on a pool of L. pneumophila serogroups 1 to 7, may thus result from exposure to several Legionella strains and possibly to other cross-reacting bacteria (15, 16). Immunoblotting was therefore used to study the reactions of sera from the seroresponders with the serogroup 1 and 4 strains. Nearly all seroresponders had IgG or IgM antibodies that bound to the serogroup-specific LPS antigen of the outbreak strain. Some of these sera also bound to serogroup 4 LPS, but few reacted with this LPS only, and none was without any LPS response. The demonstration of serogroup 1 LPS-specific antibodies in sera with elevated antibody levels by ELISA was supported by another study showing that this ELISA detected a high number of LD patients with serogroup 1 disease (15). However, the predominant LPS antibody reaction with the outbreak strain did not imply that that all responders had been subclinically infected with this strain. Several subgroups within serogroup 1 present the same ladderlike LPS profile in silver-stained gels and in immunoblots (24, 33). Infection with such strains might have induced corresponding LPS binding patterns, but the seroresponders gave only negligible antibody reactions with LPS of the serogroup 1 subgroup Philadelphia strain. Induction of cross-reactive LPS antibodies by other serogroups of L. pneumophila is disputed (11, 24, 33), and few other Legionella species have the same ladderlike LPS profile (25). The LPS response was probably not caused by exposure to other gram-negative bacteria, as their LPS are different from Legionella LPS (29).
The characteristics of the seroresponders in the three study groups may reveal the impacts of the various long- and short-distance infection sources. The scattering of the home addresses of the seroresponders among the nonexposed blood donors suggested different domestic or workplace exposure sources. Such sources may also have contributed to the seroresponders among the exposed blood donors. However, their addresses clustered around the city of the outbreak, despite the fact that the majority of the exposed donors lived or worked in the neighboring city. The distribution of the seroresponders' addresses was roughly similar to that of the patients with LD in the outbreak (35), indicating the air scrubber as the exposure source. The short transmission distance of airborne Legionella strains from the aeration ponds (3) is less likely to account for this clustering, as are potentially unfavorable weather conditions leading to longer travel of the aerosols from the ponds, aerosols from the nearby river from which the outbreak strain was also identified (35), and later accidental unrecognized emissions of L. pneumophila from the air scrubber.
In conclusion, the long-distance outbreak of LD was reflected 1 year later in a small but significant increase in IgG levels of the exposed study groups. Within the industrial premises, a biological treatment plant was found to represent a short-distance infection source for factory employees who worked nearby. Most of the seroresponders in all three study groups had IgG and IgM antibodies directed to the serogroup-specific LPS antigen of the outbreak strain, but the possible contribution of cross-reactive antibodies from exposure to other L. pneumophila strains cannot be excluded.
Acknowledgments
We thank the personnel at Borregaard Ind. Ltd. Health Service, Ullevaal University Hospital, and Østfold Hospital Trust, who distributed information material and collected the blood samples, and Eva Holten Lindberg, Østfold Hospital Trust, for performing the IFA. We also thank Berit Bakken, Berit Heier, Per Kristian Svendsen, and Grace Lubega, Norwegian Institute of Public Health, for registration of the data in SPSS, for supplying the postal code maps, for final preparation of the figures, and for support with the references, respectively.
The study was funded by the Norwegian Institute of Public Health.
Footnotes
Published ahead of print on 18 February 2009.
REFERENCES
- 1.Allestam, G., B. de Jong, and J. Langmark. 2006. Biological treatment of industrial wastewater: a possible source of Legionella infections, p. 493-496. In N. P. Cianciotto (ed.), Legionella. State of the art 30 years after its recognition. ASM Press, Washington, DC.
- 2.Bangsborg, J. M., G. Shand, E. Pearlman, and N. Høiby. 1991. Cross-reactive Legionella antigens and the antibody response during infection. APMIS 99854-865. [PubMed] [Google Scholar]
- 3.Blatny, J. M., B. A. P. Reif, G. Skogan, Ø. Andreassen, E. A. Høiby, E. Ask, V. Waagen, D. Aanonsen, I. S. Aaberge, and D. A. Caugant. 2008. Tracking airborne Legionella and Legionella pneumophila at a biological treatment plant. Environ. Sci. Technol. 427360-7367. [DOI] [PubMed] [Google Scholar]
- 4.Blatny, J. M., G. Skogan, B. A. P. Reif, Ø. Andreassen, G. M. B. Thomassen, T. Aarskaug, E. M. Fykse, and J. S. Olsen. 2007. Sampling and identification of Legionella spp. at Borregaard Ind. Ltd. FFI report 2007/00643. Norwegian Defence Research Establishment (FFI), Kjeller, Norway.
- 5.Blystad, H., E. Bjorlow, P. Aavitsland, and J. Holm. 2001. Outbreak of legionellosis in Stavanger, Norway—final report. Eurosurveill. Wkly. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2059.
- 6.Boshuizen, H. C., N. J. Nagelkerke, J. W. Den Boer, H. de Melker, J. F. Schellekens, M. F. Peeters, H. van Vliet, and M. A. Conyn-van Spaendonck. 2006. Estimation of minimum infection rates with Legionella pneumophila in an exposed population. Epidemiol. Infect. 134579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshuizen, H. C., S. E. Neppelenbroek, H. van Vliet, J. F. Schellekens, J. W. Den Boer, M. F. Peeters, and M. A. Conyn-van Spaendonck. 2001. Subclinical Legionella infection in workers near the source of a large outbreak of Legionnaires disease. J. Infect. Dis. 184515-518. [DOI] [PubMed] [Google Scholar]
- 8.Boshuizen, H. C., S. E. Neppelenbroek, J. A. van Vliet, J. F. P. Schellekens, J. W. Den Boer, M. F. Peeters, H. Verbakel, M. L. A. Heijnen, and M. A. E. Conyn-van Spaendonck. 2000. Serological findings and health complaints in exhibitors working on the 1999 Westfriese Flora in Bovenkarspel. Report 213690.006. Dutch National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
- 9.Brown, A., M. W. Lema, and M. S. Brown-Schlumpf. 2008. Antigenic specificity of the antibody response in humans during legionellosis. Infection 14108-113. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1997. Guidelines for prevention of nosocomial pneumonia. Morb. Mortal. Wkly. Rep. 461-79. [Google Scholar]
- 11.Ciesielski, C. A., M. J. Blaser, and W. L. Wang. 1986. Serogroup specificity of Legionella pneumophila is related to lipopolysaccharide characteristics. Infect. Immun. 51397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darelid, J., S. Lofgren, B. E. Malmvall, M. A. Olinder-Nielsen, G. Briheim, and H. Hallander. 2003. Legionella pneumophila serogroup 1 antibody kinetics in patients with Legionnaires' disease: implications for serological diagnosis. Scand. J. Infect. Dis. 3515-20. [DOI] [PubMed] [Google Scholar]
- 13.Den Boer, J. W., E. P. F. Yzerman, J. Schellekens, K. D. Lettinga, H. C. Boshuizen, J. E. Van Steenbergen, A. Bosman, S. Van den Hof, H. A. Van Vliet, M. F. Peeters, R. J. Van Ketel, P. Speelman, J. L. Kool, and M. A. E. Conyn-van Spaendonck. 2002. A large outbreak of Legionnaires' disease at a flower show, the Netherlands, 1999. Emerg. Infect. Dis. 837-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Ory, F., J. M. Echevarria, C. Pelaz, A. Tellez, M. A. Mateo, and J. Lopez. 2000. Detection of specific IgM antibody in the investigation of an outbreak of pneumonia due to Legionella pneumophila serogroup 1. Clin. Microbiol. Infect. 664-69. [DOI] [PubMed] [Google Scholar]
- 15.Elverdal, P., C. S. Jorgensen, and S. A. Uldum. 2008. Comparison and evaluation of four commercial kits relative to an in-house immunofluorescence test for detection of antibodies against Legionella pneumophila. Eur. J. Clin. Microbiol. Infect. Dis. 27149-152. [DOI] [PubMed] [Google Scholar]
- 16.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzin, L., and F. Scramuzza. 1995. Prevalence of Legionella pneumophila serogroup 1 antibodies in blood donors. Eur. J. Epidemiol. 11475-478. [DOI] [PubMed] [Google Scholar]
- 18.Gabay, J. E., and M. A. Horwitz. 1985. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J. Exp. Med. 161409-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haley, C. E., M. L. Cohen, J. Halter, and R. D. Meyer. 1979. Nosocomial Legionnaires' disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann. Intern. Med. 90583-586. [DOI] [PubMed] [Google Scholar]
- 20.Helbig, J. H., S. Bernander, P. M. Castellani, J. Etienne, V. Gaia, S. Lauwers, D. Lindsay, P. C. Luck, T. Marques, S. Mentula, M. F. Peeters, C. Pelaz, M. Struelens, S. A. Uldum, G. Wewalka, and T. G. Harrison. 2002. Pan-European study on culture-proven Legionnaires' disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur. J. Clin. Microbiol. Infect. Dis. 21710-716. [DOI] [PubMed] [Google Scholar]
- 21.Helbig, J. H., J. B. Kurtz, M. C. Pastoris, C. Pelaz, and P. C. Luck. 1997. Antigenic lipopolysaccharide components of Legionella pneumophila recognized by monoclonal antibodies: possibilities and limitations for division of the species into serogroups. J. Clin. Microbiol. 352841-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heudorf, U., W. Hentschel, M. Hoffmann, C. Luck, and R. Schubert. 2001. Legionellas in domestic warm water—effects on the health of residents. Gesundheitswesen 63326-334. (In German.) [DOI] [PubMed] [Google Scholar]
- 23.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jürgens, D., and F. J. Fehrenbach. 1995. Cross-reacting lipopolysaccharide antigens in Legionella pneumophila serogroups 1 to 14. Infect. Immun. 632180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jürgens, D., and F. J. Fehrenbach. 1997. Identification of Legionella species by lipopolysaccharide antigen pattern. J. Clin. Microbiol. 353054-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusnetsov, J., K.-P. Martimo, J. Aho, P. Jappinen, A. Lehtniemi, M. Lehtola, J. Mutru, T. T. J. Putus, J. Taipale, M. Toivola, E. Torvinen, and T. M. N. Nguyen. 2007. Finnish forest industry employees' exposure to Legionella and other respiratory pathogens (FEEL study). Project 105304. The Finnish Work Environment Fund, Helsinki, Finland.
- 27.Kusnetsov, J., K. P. Martimo, J. Aho, A. Lehtniemi, M. Lehtola, J. Mutru, T. Putus, J. Taipale, M. Toivola, E. Torvinen, N. N. Tran Minh, and P. Jappinen. 2007. Exposure to legionella and mycobacteria at industrial waste water treatment plants, abstr. Or21, p. 47. Twenty-Second Meet. Euro. Working Group Legionella Infect., Stockholm, Sweden.
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lambert, M. A., and C. W. Moss. 1989. Cellular fatty acid compositions and isoprenoid quinone contents of 23 Legionella species. J. Clin. Microbiol. 27465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattimer, G. L., L. V. I. Rhodes, J. S. Salventi, J. P. Galgon, V. Stonebraker, S. Boley, and G. Haas. 1979. The Philadelphia epidemic of Legionnaire's disease: clinical, pulmonary, and serologic findings two years later. Ann. Intern. Med. 90522-526. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu, L., E. Robine, M. Deloge-Abarkan, S. Ritoux, D. Pauly, P. Hartemann, and D. Zmirou-Navier. 2006. Legionella bacteria in aerosols: sampling and analytical approaches used during the Legionnaires disease outbreak in Pas-de-Calais. J. Infect. Dis. 1931333-1335. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, T. M. N., D. Ilef, S. Jarraud, L. Rouil, C. Campese, D. Che, S. Haeghebaert, F. O. Ganiayre, F. Marcel, J. Etienne, and J. C. Desenclos. 2006. A community-wide outbreak of Legionnaires disease linked to industrial cooling towers—how far can contaminated aerosols spread? J. Infect. Dis. 193102-111. [DOI] [PubMed] [Google Scholar]
- 33.Nolte, F. S., C. A. Conlin, and M. A. Motley. 1986. Electrophoretic and serological characterization of the lipopolysaccharides of Legionella pneumophila. Infect. Immun. 52676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norwegian Institute of Public Health. 23 January 2009, accession date. Statistics from the Norwegian Surveillance System for Communicable Diseases. MSIS. http://www.msis.no.
- 35.Nygard, K., O. Werner-Johansen, S. Ronsen, D. A. Caugant, O. Simonsen, A. Kanestrom, E. Ask, J. Ringstad, R. Odegard, T. Jensen, T. Krogh, E. A. Hoiby, E. Ragnhildstveit, I. S. Aaberge, and P. Aavitsland. 2008. An outbreak of Legionnaires disease caused by long-distance spread from an industrial air scrubber in Sarpsborg, Norway. Clin. Infect. Dis. 4661-69. [DOI] [PubMed] [Google Scholar]
- 36.O'Mahony, M. C., R. E. Stanwell-Smith, H. E. Tillett, D. Harper, J. G. Hutchison, I. D. Farrell, D. N. Hutchinson, J. V. Lee, P. J. Dennis, H. V. Duggal, J. A. Scully, and C. Denne. 1990. The Stafford outbreak of Legionnaires' disease. Epidemiol. Infect. 104361-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojas, A., M. D. Navarro, F. E. Fornes, E. Serra, E. Simarro, J. Rojas, and J. Ruiz. 2005. Value of serological testing for diagnosis of legionellosis in outbreak patients. J. Clin. Microbiol. 434022-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudbeck, M., K. Molbak, and S. Uldum. 2008. High prevalence of antibodies to Legionella spp. in Danish blood donors. A study in areas with high and average incidence of Legionnaires' disease. Epidemiol. Infect. 136257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119115-119. [DOI] [PubMed] [Google Scholar]
- 40.Virion\Serion. 2004. Serion ELISA classic. Legionella pneumophila 1-7 IgG/IgM (quant). Instructions. Institut Virion\Serion GmbH, Wurzburg, Germany.
- 41.Wedege, E. 2001. Immunoblot analysis of sera from patients and vaccinees, p. 275-288. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press Inc., Totowa, NJ. [DOI] [PubMed]
- 42.Wedege, E., T. Bergdal, K. Bolstad, D. A. Caugant, J. Efskind, H. E. Heier, A. Kanestrøm, B. H. Strand, and I. S. Aaberge. 2007. A seroepidemiological study following an industrial outbreak of Legionnaires' disease in Norway in May 2005, abstr. Or34, p. 54. Twenty-Second Meet. Euro. Working Group Legionella Infect., Stockholm, Sweden.
- 43.Wedege, E., K. Bolstad, and A. Kanestrom. 2008. Antigen specificities of seroresponders in a seroepidemiological Legionella pneumophila outbreak study, abstr. CO2.5, p. 19. Twenty-Third Meet. Euro. Working Group Legionella Infect., Madrid, Spain.
- 44.Wedege, E., K. Bryn, and L. O. Frøholm. 1988. Restoration of antibody binding to blotted meningococcal outer membrane proteins using various detergents. J. Immunol. Methods 11351-59. [DOI] [PubMed] [Google Scholar]
- 45.Wedege, E., E. A. Hoiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 663223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wedege, E., E. A. Hoiby, E. Rosenqvist, and L. O. Froholm. 1990. Serotyping and subtyping of Neisseria meningitidis isolates by co-agglutination, dot-blotting and ELISA. J. Med. Microbiol. 31195-201. [DOI] [PubMed] [Google Scholar]