Abstract
We report the first nosocomial outbreak of human metapneumovirus (hMPV) infection in a pediatric patient population. Among 15 pediatric hMPV infections from March to May 2007, there was a nosocomial outbreak involving two patients sharing a room in a hemato-oncology ward with a patient with a community-acquired case of hMPV infection. The estimated incubation period was 7 to 9 days for a symptomatic nosocomial case. Sequencing of fusion genes of 15 isolates revealed two clusters belonging to subgroup A2 and one cluster belonging to subgroup B2. Three isolates from the outbreak had sequences identical to those of samples in the A2 cluster. There was also a separate nosocomial case represented by the B2 cluster.
Human metapneumovirus (hMPV), a recently discovered virus and a member of the subfamily Pneumovirinae, is a major cause of hospitalization of infants suffering from respiratory tract infection (14). Transmission is likely to occur via direct contact and droplet, as with respiratory syncytial virus (RSV) transmission (12, 16). Although there have been a few reports of nosocomial hMPV infections involving infants (2, 6, 7), there have been no studies of outbreaks of hMPV infection in pediatric patient populations in acute care hospitals. Most outbreaks of hMPV infection involve elderly patients in long-term care facilities and neuropsychiatry wards (1, 3, 9, 11, 12). The objective of this study was to characterize the epidemiology of a nosocomial outbreak of hMPV infection in a pediatric patient population.
We retrospectively investigated 15 pediatric patients in a 2,200-bed tertiary-care hospital whose nasopharyngeal aspirates tested positive for hMPV by reverse transcriptase PCR (RT-PCR) from March to May 2007. hMPV RT-PCR was performed using the primer set of a Seeplex RV detection kit (Seegene, Seoul, Korea), as described previously (15). All patients were negative for RSV, influenza virus, parainfluenza virus, and adenovirus, as determined by direct antigen test, shell vial culture, and RT-PCR. Sequences (460 bp) of the fusion (F) protein gene were aligned with those of prototype hMPV strains by use of the ClustalW2 algorithm (www.ebi.ac.uk/Tools/clustalw/). Phylogenetic trees were generated by the neighbor-joining method and the Kimura 2-parameter substitution model using MEGA software (version 4.0).
The median age of the 15 patients was 1.6 years (range, 0.3 to 7 years), and there were 11 males and 4 females. The clinical diagnoses attributed to hMPV infection included 10 cases of pneumonia and 4 cases of bronchiolitis. Three patients had hospital-acquired hMPV infections (HA-hMPV), designated Asan-HA01, Asan-HA02, and Asan-HA03, and the other 12 patients had community-acquired hMPV infections (CA-hMPV).
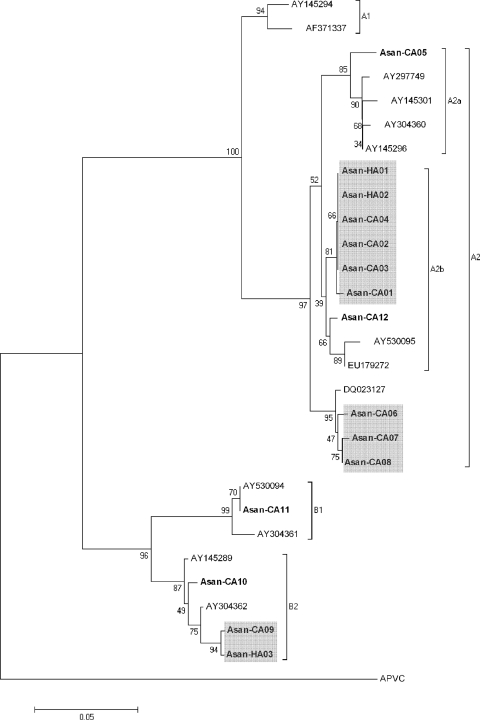
The sequences of the F protein genes of hMPVs in samples from our patients had 96 to 99% homology to those of the type strain published in the NCBI database. Four major hMPV lineages (A1, A2, B1, and B2) have been previously described (17). Phylogenetic analysis classified 11 isolates into subgroup A2, 1 isolate into subgroup B1, and 3 isolates into subgroup B2 (Fig. 1). The sequences of the 11 strains of subgroup A2 had nucleotide similarity of 94.8 to 100%. There were two closely related clusters in subgroup A2: cluster 1, consisting of six isolates with homology of 99 to 100%, and cluster 2, consisting of three isolates with homology of 98 to 99%. There was also a cluster of two isolates found in subgroup B2. It is useful to analyze molecular lineages of the isolates for a certain period to provide more information about the epidemiologic relationship of hMPV.
FIG. 1.
Phylogenetic analysis of F gene fragments of hMPV from 15 cases positive for hMPV by RT-PCR. The tree was constructed by the neighbor-joining method using the Kimura two-parameter model, and bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 20 nucleotides. Shaded boxes denote clusters: two clusters of subgroup A2 and one cluster of subgroup B2.
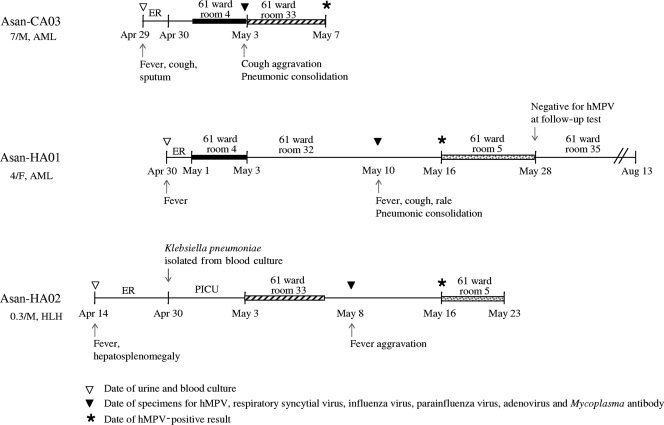
Cluster 1 included two HA-hMPV isolates and four CA-hMPV isolates. Among the patients whose samples were in cluster 1, two HA-hMPV patients shared a room with a CA-hMPV patient for 3 to 5 days before hMPV was detected during their hospital stays (Fig. 2). The sequences of F genes of all three isolates were identical, indicating that this CA-hMPV patient represented the index case. Even though the index CA-hMPV patient had fever, cough, and sputum on admission, a pneumonic consolidation was found on hospital day 4. Standard precautions were taken during the hospital stay but not those appropriate for preventing droplet transmissions. Considering the overlapping periods of their stays, the incubation period was 7 days at minimum and 9 days at maximum for a symptomatic nosocomial case. They were all hemato-oncology patients: two were receiving treatment for acute myeloid leukemia and one for hemophagocytic lymphohistiocytosis. All of these patients exhibited fever symptoms, and hospital personnel took blood and urine cultures at admission but grew no microorganisms. Mycoplasma antibody tests performed at the time of sampling for hMPV were all negative. After the index case patient was diagnosed as positive for hMPV infection, he was immediately discharged and the two patients exhibiting signs of nosocomial infection who had shared a room with him received hMPV tests and were isolated together in same room. All four CA-hMPV strains of cluster 1 were isolated for a 1-month period in April and May 2007 from patients who lived in the neighborhood of our hospital. In Korea, the peak season for hMPV is spring, rather than late winter (4, 10, 18). The first report that described the lineage of hMPV isolates in Korea also showed a predominance of the A2 lineage among the isolates obtained from 2003 to 2005 at an acute care hospital in Seoul (5). A2 seemed to be a cause of community outbreak at that time as well as at this time. These findings suggest that the increase of CA-hMPV during the peak season is associated with the risk of an outbreak in a hospital. All three patients whose samples belonged to cluster 2 had CA-hMPV infections (Fig. 1). The results obtained with one patient with a case of HA-hMPV, Asan-HA03, whose sample belonged to cluster 3 (Fig. 1), were epidemiologically separate from those of a patient with a case of CA-hMPV, Asan-CA09, whose sample belonged to the same cluster, and from those of all other patients in this study.
FIG. 2.
Chronological illustration of hospitalization and clinical and laboratory findings for three patients involved in the nosocomial outbreak. Solid bars and striped bars indicate the overlap periods of hospital stays between each of two HA-hMPV patients and the index CA-hMPV patient, respectively. Dotted bars indicate the isolation of two nosocomial cases together in the same room. Respiratory symptoms of Asan-HA01 commenced at 9 days after the start of the overlap periods. AML, acute myeloid leukemia; HLH, hemophagocytic lymphohistiocytosis; ER, emergency room; PICU, pediatric intensive care unit. At the left side of the figure, numerals followed by a slash and the letter “M” or “F” represent patient age (in years) and patient sex (M, male; F, female), respectively.
This is the first report of a nosocomial outbreak of hMPV infection among pediatric patients. The first confirmed nosocomial case seen with pediatric patients was that of an infant who never left the hospital after birth and was among 19 patients diagnosed in a single winter season at a tertiary referral hospital (6). Another previous report described 3 HA-hMPV cases among a total of 37 hMPV-infected patients during a 1-year period at a large tertiary-care pediatric center (2). However, all were separate cases without evidence of proven transmission. In this study, a nosocomial oubreak occurred among the patients sharing a room for several days. Therefore, contact seemed to be needed to acquire hMPV infection. Nosocomial outbreaks of RSV infection in pediatric wards, including neonatal intensive care units, have been previously reported (8, 13), but nosocomial outbreaks of hMPV are rarely reported at acute care facilities. There are several reports of outbreaks of hMPV infection among elderly patients institutionalized in long-term care facilities (1, 3, 11, 12). One previous study reported a nosocomial outbreak of hMPV infection among elderly patients in a psychiatric ward (3). These patients had attack rates of 56%, but only seven symptomatic patients showed RT-PCR confirmation of infection, and all seven of these isolates were of the same genotype. Another study of a large outbreak at a long-term care facility reported an attack rate of 72%, as measured by the frequency of respiratory symptoms, but only six hMPV isolates were sequenced and classified into two clusters (1). Considering the high attack rate of hMPV infections at institutional environments, emergence of a nosocomial outbreak in pediatric wards with patients of a susceptible age is not surprising.
In conclusion, hMPV may be responsible for nosocomial outbreaks among pediatric patients during peak seasons of CA-hMPV infection. To prevent a nosocomial outbreak of hMPV infection, early diagnosis and prompt isolation are required for pediatric patients complaining of respiratory symptoms, especially in a season of community outbreak.
Acknowledgments
We do not have any conflict of interest relating to this article.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Boivin, G., G. De Serres, M. E. Hamelin, S. Cote, M. Argouin, G. Tremblay, R. Maranda-Aubut, C. Sauvageau, M. Ouakki, N. Boulianne, and C. Couture. 2007. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin. Infect. Dis. 441152-1158. [DOI] [PubMed] [Google Scholar]
- 2.Chano, F., C. Rousseau, C. Laferriere, M. Couillard, and H. Charest. 2005. Epidemiological survey of human metapneumovirus infection in a large pediatric tertiary care center. J. Clin. Microbiol. 435520-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, V. C. C., A. K. L. Wu, C. H. Y. Cheung, S. K. P. Lau, P. C. Y. Woo, K. H. Chan, K. S. M. Li, I. K. S. Ip, E. L. W. Dunn, R. A. Lee, L. Y. C. Yam, and K. Y. Yuen. 2007. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J. Hosp. Infect. 67336-343. [DOI] [PubMed] [Google Scholar]
- 4.Choi, E. H., H. J. Lee, S. J. Kim, B. W. Eun, N. H. Kim, J. A. Lee, J. H. Lee, E. K. Song, S. H. Kim, J. Y. Park, and J. Y. Sung. 2006. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin. Infect. Dis. 43585-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, J. Y., T. H. Han, B. E. Kim, C. K. Kim, S. W. Kim, and E. S. Hwang. 2006. Human metapneumovirus infection in hospitalized children with acute respiratory disease in Korea. J. Korean Med. Sci. 21838-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esper, F., D. Boucher, C. Weibel, R. A. Martinello, and J. S. Kahn. 2003. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 1111407-1410. [DOI] [PubMed] [Google Scholar]
- 7.Evashuk, K., S. Forgie, S. Gilmour, H. Huynh, B. Lee, and J. Robinson. 2008. Respiratory failure associated with human metapneumovirus infection in an infant posthepatic transplant. Am. J. Transplant. 81567-1569. [DOI] [PubMed] [Google Scholar]
- 8.Halasa, N. B., J. V. Williams, G. J. Wilson, W. F. Walsh, W. Schaffner, and P. F. Wright. 2005. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 241040-1044. [DOI] [PubMed] [Google Scholar]
- 9.Honda, H., J. Iwahashi, T. Kashiwagi, Y. Imamura, N. Hamada, T. Anraku, S. Ueda, T. Kanda, T. Takahashi, and S. Morimoto. 2006. Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J. Am. Geriatr. Soc. 54177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, Y. K., and H. J. Lee. 2005. Human metapneumovirus-associated lower respiratory tract infections in Korean infants and young children. Pediatr. Infect. Dis. J. 241111-1112. [DOI] [PubMed] [Google Scholar]
- 11.Louie, J. K., D. P. Schnurr, C. Y. Pan, D. Kiang, C. Carter, S. Tougaw, J. Ventura, A. Norman, V. Belmusto, J. Rosenberg, and G. Trochet. 2007. A summer outbreak of human metapneumovirus infection in a long-term-care facility. J. Infect. Dis. 196705-708. [DOI] [PubMed] [Google Scholar]
- 12.Lysyshyn, M., R. L. Rodin, P. Huston, and S. Aziz. 2008. Human metapneumovirus: an emerging issue in Canada. Canada Communicable Disease Report, vol. 1, issue 13. http://www.phac-aspc.gc.ca/ccdrw-rmtch/2008/r1308-2-eng.php.
- 13.Mlinaric-Galinovic, G., and D. Varda-Brkic. 2000. Nosocomial respiratory syncytial virus infections in children's wards. Diagn. Microbiol. Infect. Dis. 37237-246. [DOI] [PubMed] [Google Scholar]
- 14.Osterhaus, A., and R. Fouchier. 2003. Human metapneumovirus in the community. Lancet 361890-891. [DOI] [PubMed] [Google Scholar]
- 15.Sung, H., S. J. Park, Y. D. Woo, B. H. Choi, and M. N. Kim. 2008. Evaluation of Seeplex RV detection kit for detecting rhinovirus, human metapneumovirus, and coronavirus. Korean J. Lab. Med. 28109-117. [DOI] [PubMed] [Google Scholar]
- 16.Tang, Y. W., and J. E. Crowe. 2007. Respiratory syncytial virus and human metapneumovirus, p. 1361-1377. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed., vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 17.van den Hoogen, B. G., S. Herfst, L. Sprong, P. A. Cane, E. Forleo-Neto, R. L. de Swart, A. D. Osterhaus, and R. A. Fouchier. 2004. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 10658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo, S. J., E. Y. Kuak, and B. M. Shin. 2007. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system. Korean J. Lab. Med. 27420-427. [DOI] [PubMed] [Google Scholar]