Abstract
Emmonsia crescens is a saprophytic fungus that is distributed worldwide, causing diseases mostly in rodents. It has also been described, though rarely, as an etiologic agent of pulmonary pathology in humans, potentially leading to death. A case of pulmonary adiaspiromycosis is reported in a 30-year-old immunocompetent man. The patient presented with a history of several weeks of weakness, cough, fever, and weight loss of 10 kg. Clinical and radiographic findings showed pulmonary lesions consistent with tuberculosis or histoplasmosis, but no pathogen was found with classical microbiological procedures. The diagnosis of adiaspiromycosis due to Emmonsia crescens was initially made using molecular biology techniques. Histological observations subsequently confirmed the presence of adiaspores in granulomas. To our knowledge, this is the first case of adiaspiromycosis diagnosed by PCR and sequencing. The patient was treated with itraconazole and was seen at 1 month with symptomatic improvement. Here we will discuss this rare fungal infection and its difficult treatment and diagnosis. As represented in this case, molecular biology is a powerful method to optimize diagnostic tests and therefore improve the care of the infected patient.
CASE REPORT
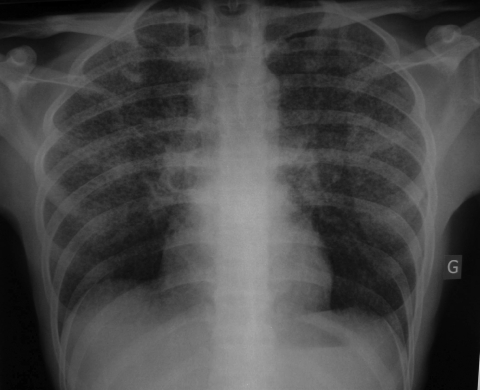
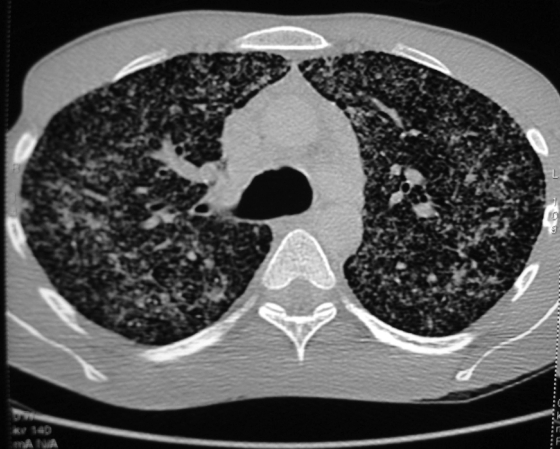
A 30-year-old man was admitted to the Hospital of Metz on 21 May 2008 with a 2-week history of unproductive cough, fever at 39°C, progressive dyspnea, thoracic pain, generalized weakness, and weight loss of 10 kg in 1 month. The chest radiography revealed diffuse bilateral interstitial pneumonia with a micronodular pattern (Fig. 1). Computerized tomography of the chest (high resolution) showed disseminated pulmonary nodules (Fig. 2). The peripheral blood leukocyte count was 12 g/liter with 76% neutrophils and 14% lymphocytes. Bronchoalveolar lavage (BAL) showed 13,330 leukocytes/μl with 1,333 lymphocytes (CD4/CD8 = 1.25). The CRP was increased to 287 mg/liter, but no other abnormalities were found.
FIG. 1.
Chest radiography. The chest radiography revealed a miliary condition suggestive of tuberculosis. G, gauche (i.e., left side of patient).
FIG. 2.
High-resolution computerized tomography of the thorax. A diffuse interstitial pneumonia is shown with a micronodulary infiltrate in the whole lung.
Based on the clinical symptoms and radiological features, the main differential diagnosis was tuberculosis or histoplasmosis as the patient had traveled several months and years earlier in areas where these diseases were endemic. Other noninfectious hypotheses, such as lymphoma or sarcoidosis, were also suspected based on the clinical and biological features with an important alteration of the patient status associated with polyadenopathies. All of these hypotheses were then excluded by corresponding analyses.
Diagnostic procedures.
Direct examination and bacterial and mycological cultures of a BAL sample were negative. Serological tests for Histoplasma capsulatum were performed at the Pasteur Institute of Paris and revealed negative immunodiffusion. The BAL sample was sent to the Mycology Unit of the Nancy Hospital for further investigation.
The BAL was microscopically reexamined with chlorazole black, and any fungal elements were visualized. Cultures of the sample on Sabouraud agar containing cycloheximide (0.5 mg/ml) at 30°C and 37°C yielded no growth.
Molecular biology studies.
In order to exclude or assess the diagnosis of histoplasmosis, a panfungal PCR amplification was performed on the BAL by using previously designed primers His3 and His4. Genomic DNA was first extracted from the BAL by using the High Pure PCR template preparation kit according to the manufacturer's instructions (Roche Diagnostics, France). The primer His3 (5′-GTCGTAACAAGGTTTCCGTAG-3′) was located at the end of 18S rRNA at position 9 to 29 according to the sequence of Histoplasma capsulatum U18363, whereas His4 (5′-AGCGGGTATCCCTACCTGAT-3′) was located at the beginning of 28S at position 601 to 620 according to the same reference.
The PCR assay was performed in a 50-μl reaction volume containing 2 U of FastStart Taq DNA polymerase (Roche Diagnostics, France), 5 μl of 10× PCR buffer-MgCl2 (20 mM), 20 pmol of each primer, 200 μM of dATP, dGTP, dTTP, and dCTP, and 5 μl of DNA. The PCR was run in an iCycler IQ thermal cycler (Bio-Rad, France) under the following conditions: initial denaturation of DNA at 94°C for 3 min, followed by 30 cycles at 94°C for 1 min, 58°C for 40 s, and 72°C for 1 min. These steps were followed by an additional 5-min extension step at 72°C. Five microliters of PCR product was electrophoresed in a 2% agarose gel in the presence of ethidium bromide and visualized under UV light. The expected PCR product size was 613 bp. As the band obtained was extremely faint, a reamplification of the product was performed by using the same primers in order to improve the signal. The BigDye Terminator v1.1 cycle sequencing kit was then used to sequence the two strands of the obtained amplified DNA with the PCR primers according to the manufacturer's instructions (Applied Biosystems, France). Products of sequencing were analyzed on an automated sequencer ABI Prism 3100 genetic analyzer. The BLAST analysis revealed that the sequences corresponded to the fungus Emmonsia crescens with 100% identity on 510 nucleotides. The internal transcribed spacer 1, 5.8S rRNA, and internal transcribed spacer 2 resulting partial sequences appear in GenBank under the accession number FJ214160.
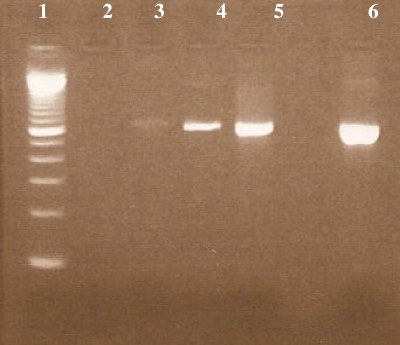
A transbronchial biopsy and tracheal aspiration were then performed in order to confirm the diagnosis of adiaspiromycosis due to Emmonsia crescens. DNA of the biological samples was extracted as previously described and successfully amplified. Sequence analysis confirmed the presence of the DNA of Emmonsia crescens in both samples. Three types of pulmonary samples were examined for this patient: BAL, a tracheal aspiration, and a transbronchial biopsy. Specific amplifications were successful with all of these samples with an increase in the intensity of the amplicons (Fig. 3).
FIG. 3.
Amplification patterns of DNA extracted from pulmonary samples of a patient suffering from adiaspiromycosis. Lane 1, 100-bp ladder; lane 2, negative control (water); lane 3, BAL sample; lane 4, tracheal aspiration; lane 5, transbronchial biopsy; lane 6, positive control (DNA of Histoplasma capsulatum).
Histopathological findings.
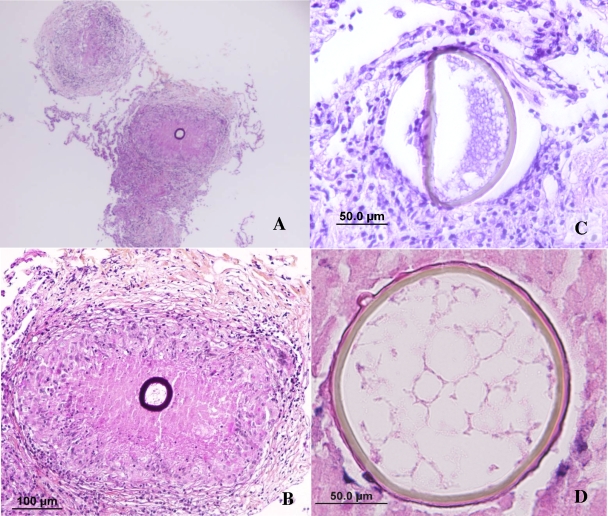
Some transbronchial biopsy samples were fixed in buffered formalin and included in paraffin. Sections of 4 μm were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Gomori-Grocott methamine-silver. Microscopically, empty granulomas were seen in some of the H&E and PAS sections. Multiple deeper sections were needed to reveal the organism. Finally, adiaspores ranging in size from about 50 μm to 100 μm with a thick wall that was highly PAS positive were observed within some granulomas (Fig. 4A and B). Most frequently the fungus was observed in the center of the granuloma. The shapes of the adiaspores were generally round or ovoid, but in some cases the walls of the spherules collapsed, forming elliptical shapes (Fig. 4C). Some of the sections showed granulovacuolar material contained in the adiaspores (Fig. 4D).
FIG. 4.
Histopathology of adiaspiromycosis. (A and B) Pulmonary fibrocellular and necrotizing granuloma containing one thick-walled adiaspore of Emmonsia crescens (PAS). (C) Degenerated adiaspore (H&E). (D) An adiaspore with the “honeycomb-like” appearance of cytoplasm (H&E).
Treatment and outcome.
After confirmation of the diagnosis of adiaspiromycosis, an antifungal treatment with oral itraconazole (200 mg/day) was started on 10 June 2008. The patient was discharged on 20 June and was seen 1 month later; his respiratory status had improved, and a new chest radiography showed stability of the pulmonary lesions.
Discussion.
Adiaspiromycosis is a rare pulmonary infection caused by dimorphic fungi from the genus Emmonsia. Among species in this genus, Emmonsia crescens (recently renamed Chrysosporium parvum var. crescens) is widespread in Europe, whereas Emmonsia parva (recently renamed Chrysosporium parvum var. parva) is found mainly in some xerothermic regions, including parts of the Americas, Central Asia, and Africa (2). These saprophytic fungi are most commonly isolated from soil but also from several small mammals, such as rodents (2).
The first report of human adiaspiromycosis due to Emmonsia crescens was made in France by Doby-Dubois et al. in 1964 (3). Symptomatic cases were subsequently reported, but this fungus has most frequently been found incidentally during autopsy or in biopsy specimens (4, 14, 20). The severity of the disease depends on the amount of inhaled dust-borne spores. For E. crescens, these adiaspores of 2 μm to 4 μm in size reach the alveoli but fail to germinate in the host and progressively increase to become giant adiaspores with a diameter of 50 μm to 500 μm. In a limited inoculum, the patients may be asymptomatic, or the disease may remain very localized, but in heavy inocula the fungus affects both lungs, and a reticulonodular infiltrate is present (22). In tissues, the fungus will induce a granulomatous inflammatory reaction and may lead to pneumonia or fatal respiratory failure (2). Clinically, the infection most commonly regresses spontaneously but may persist and require surgical intervention; the role of antifungals is controversial (1). Until now, only a few fatal cases of disseminated pulmonary infection have been documented, the first one reported in 1989 in a farm worker in Brazil with no prior medical history (12, 15).
Diagnosis of adiaspiromycosis is difficult because the fungus is not easily cultured and there are not yet any reliable serological tests. The gold standard remains histological examination of a biopsy. Pathologists must then recognize large spherules with a trilamellar wall that can be surrounded by granulomas with or without necrosis or fibrosis. In latter chronic stages, the fungus may take on different sizes, thereby resembling other fungi or inhaled pollen grains or even helminths, according to some authors (4).
We present a case of disseminated bilateral pulmonary infection in a 30-year-old immunocompetent man caused by Emmonsia crescens. Even if the pathology was clinically symptomatic, classical mycological examination for the first histological observations was negative. Diagnosis of adiaspiromycosis was first suggested from results of molecular techniques.
Emmonsia crescens is a saprophytic dimorphic fungus that has been reported worldwide in several mammalian species, most commonly small rodents but also otters (19), ground squirrels (10), goats (8), dogs (9), hedgehogs (17), raccoons (6), horses (16), beavers (13), and other species. Despite its occurrence in animals throughout the world, this fungus has received little attention because it rarely affects humans. Nevertheless, Emmonsia crescens has been found to cause pulmonary infections and disseminated diseases leading to death (12, 15). We report a case of disseminated adiaspiromycosis with a severe clinical manifestation. The identification of the pathogen Emmonsia crescens was first hypothesized using molecular techniques. To our knowledge, this is the first human case of disseminated adiaspiromycosis diagnosed by molecular biology. We report this case in the hope of improving the diagnosis of such rare infections caused by unusual microorganisms. Diagnosis of adiaspiromycosis relies on the identification of the fungus in a pulmonary specimen by histological studies. Nevertheless, since this mycosis is rare and often unknown by clinicians, biopsy is not systematically being performed. In our case, direct examination and mycological cultures of biological samples were negative. The preliminary diagnosis of adiaspiromycosis was made on the basis of molecular biology results after sequencing of an amplicon obtained from the BAL specimen. A transbronchial biopsy was then performed in order to assess this hypothesis, but the first histological features revealed only granulomas. This observation would not have been linked to a fungal infection if the nucleotidic sequence analyses had not clearly indicated the presence of Emmonsia crescens. Subsequently, pathologists detected adiaspores in granulomas in serial sections. Cultures of biological specimens were kept on mycological medium for more than 1 month but yielded no growth. This case emphasizes the importance of using molecular techniques to identify pathogens that can be misdiagnosed with classic methods.
Some of the patients reported in the literature had diverse pulmonary disorders, including emphysema, tuberculosis, or aspergillosis, or were immunocompromised, while others were healthy (3-5). In the case presented here, the patient had previously been in excellent health with no pulmonary symptoms and was a nonsmoker. Many of the reported cases were related to outdoor activities and patients were therefore at risk of exposure to dust containing spores (1, 14). The patient's history revealed that he became sick a few weeks after having played in the surroundings of an animal burrow, which may have played the role of reservoir for Emmonsia crescens. In this environment he could have inhaled a large amount of dust containing fungal spores. Further investigations would have been necessary to confirm this source of the contamination.
A therapeutic strategy for adiaspiromycosis has not been yet clearly established (11, 14, 18, 21). Most of the time, patients with disseminated adiaspiromycosis have recovered without treatment (4). As adiaspiromycosis has been reported to be fatal and our patient showed severe diffuse and bilateral pulmonary infiltration, he was treated with itraconazole. Improvement in the patient's condition was observed after 1 month, but we cannot assess if it was due to the antifungal treatment.
The ability to control such rare infections is largely dependent on the diagnosis of the etiological agents. In the present report, classical techniques used in microbiology failed to detect the fungus. After the first identification of the pathogen by molecular techniques in BAL, a tracheal aspiration and a transbronchial biopsy were performed and analyzed. The intensity of the amplicons was higher with the deepest sample. This emphasizes the importance of obtaining the deepest sample to improve diagnosis when any microorganism has previously been found, despite acute and noisy symptoms. Molecular biology has the potential to optimize the way in which diagnostic tests are carried out and therefore improve the care of the infected patient.
Acknowledgments
We thank Olivier Lortholary for his precious help in the choice of therapy and Françoise Dromer and Michel Huerre at the Pasteur Institute in Paris, France, for helpful discussion on this case. We thank Tiffany Dunham for editorial assistance.
All authors have no potential conflicts of interest.
Footnotes
Published ahead of print on 25 February 2009.
REFERENCES
- 1.Barbas Filho, J. V., M. B. Amato, D. Deheinzelin, P. H. Saldiva, and C. R. de Carvalho. 1990. Respiratory failure caused by adiaspiromycosis. Chest 971171-1175. [DOI] [PubMed] [Google Scholar]
- 2.Chantrey, J. C., A. M. Borman, E. M. Johnson, and A. Kipar. 2006. Emmonsia crescens infection in a British water vole (Arvicola terrestris). Med. Mycol. 44375-378. [DOI] [PubMed] [Google Scholar]
- 3.Doby-Dubois, M., M. L. Chevrel, J. M. Doby, and M. Louvet. 1964. 1st human case of adiaspiromycosis, caused by Emmonsia Crescens, Emmons and Jellison, 1960. Bull. Soc. Pathol. Exot. Filiales 57240-244. (In French.) [PubMed] [Google Scholar]
- 4.England, D. M., and L. Hochholzer. 1993. Adiaspiromycosis: an unusual fungal infection of the lung. Report of 11 cases. Am. J. Surg. Pathol. 17876-886. [PubMed] [Google Scholar]
- 5.Fingerland, A. 1973. Pathomorphology of adiaspiromycosis in man. Arkh. Patol. 3565-69. (In Russian.) [PubMed] [Google Scholar]
- 6.Hamir, A. N. 1999. Pulmonary adiaspiromycosis in raccoons (Procyon lotor) from Oregon. J. Vet. Diagn. Investig. 11565-567. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Koller, L. D., and D. H. Helfer. 1978. Adiaspiromycosis in the lungs of a goat. J. Am. Vet. Med. Assoc. 17380-81. [PubMed] [Google Scholar]
- 9.Koller, L. D., N. M. Patton, and D. K. Whitsett. 1976. Adiaspiromycosis in the lungs of a dog. J. Am. Vet. Med. Assoc. 1691316-1317. [PubMed] [Google Scholar]
- 10.Leighton, F. A., and G. Wobeser. 1978. The prevalence of adiaspiromycosis in three sympatric species of ground squirrels. J. Wildl. Dis. 14362-365. [DOI] [PubMed] [Google Scholar]
- 11.Martins, R. L., C. G. Santos, F. R. Franca, and M. A. Moraes. 1997. Human adiaspiromycosis. A report of a case treated with ketoconazole. Rev. Soc. Bras. Med. Trop. 30507-509. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 12.Moraes, M. A., M. C. de Almeida, and A. N. Raick. 1989. A fatal case of human pulmonary adiaspiromycosis. Rev. Inst. Med. Trop. Sao Paulo 31188-194. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 13.Mörner, T., A. Avenas, and R. Mattsson. 1999. Adiaspiromycosis in a European beaver from Sweden. J. Wildl. Dis. 35367-370. [DOI] [PubMed] [Google Scholar]
- 14.Nuorva, K., R. Pitkanen, J. Issakainen, N. P. Huttunen, and M. Juhola. 1997. Pulmonary adiaspiromycosis in a two year old girl. J. Clin. Pathol. 5082-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peres, L. C., F. Figueiredo, M. Peinado, and F. A. Soares. 1992. Fulminant disseminated pulmonary adiaspiromycosis in humans. Am. J. Trop. Med. Hyg. 46146-150. [DOI] [PubMed] [Google Scholar]
- 16.Pusterla, N., P. A. Pesavento, C. M. Leutenegger, J. Hay, L. J. Lowenstine, M. M. Durando, and K. G. Magdesian. 2002. Disseminated pulmonary adiaspiromycosis caused by Emmonsia crescens in a horse. Equine Vet. J. 34749-752. [DOI] [PubMed] [Google Scholar]
- 17.Seixas, F., P. Travassos, M. L. Pinto, I. Pires, and M. A. Pires. 2006. Pulmonary adiaspiromycosis in a European hedgehog (Erinaceus europaeus) in Portugal. Vet. Rec. 158274-275. [DOI] [PubMed] [Google Scholar]
- 18.Severo, L. C., G. R. Geyer, J. J. Camargo, and N. S. Porto. 1989. Adiaspiromycosis treated successfully with ketoconazole. J. Med. Vet. Mycol. 27265-268. [DOI] [PubMed] [Google Scholar]
- 19.Simpson, V. R., and D. Gavier-Widen. 2000. Fatal adiaspiromycosis in a wild Eurasian otter (Lutra lutra). Vet. Rec. 147239-241. [DOI] [PubMed] [Google Scholar]
- 20.Thomas De Montpréville, V., M. Huerre, and E. Dulmet. 1999. Adiaspiromycosis, 2 cases of incidental finding. Ann. Pathol. 19513-515. (In French.) [PubMed] [Google Scholar]
- 21.Vojtek, V., R. Kod'ousek, A. Fingerland, V. Vortel, Z. Sery, K. Kucera, V. Hajek, and I. Berkova. 1972. Clinical aspects and therapy of lung adiaspiromycosis in an 11-year-old boy. Prax. Pneumol. 2699-105. (In German.) [PubMed] [Google Scholar]
- 22.Watts, J. C., and F. W. Chandler. 1990. Adiaspiromycosis. An uncommon disease caused by an unusual pathogen. Chest 971030-1031. [DOI] [PubMed] [Google Scholar]