Abstract
The suitability and accuracy of using simple human immunodeficiency virus (HIV) rapid (SR) tests in community-based clinics in northwest Tanzania were determined to assess eligibility for participation in clinical trials. The HIV rapid and ELISA test results for 789 women aged 16 to 54 who were screened for two clinical trials of HIV prevention were compared. Women were offered voluntary HIV counseling and testing (VCT) at screening; those who accepted were tested with the Abbott Determine and Trinity Biotech Capillus SR tests in parallel. The results were confirmed by two parallel HIV enzyme-linked immunosorbent assay (ELISA) tests (Abbott Murex HIV Ag/Ab combination and Vironostika Uniform II HIV Ag/Ab) to determine eligibility. Positive samples for any of the four assays were confirmed by a line immunoassay and p24 testing. The parallel SR tests had high concordance (96.2%) with the parallel ELISA algorithm. The sensitivities of the SR tests were 98.6% for Capillus (95% confidence interval [CI], 95.1 to 99.8%), 99.3% for Determine (95% CI, 96.2 to 100%), and 98.6% for the parallel SR (95% CI, 95.1 to 99.8%). The specificities were 99.7% for Capillus (95% CI, 98.9 to 100%), 99.7% for Determine (95% CI, 98.9 to 100%), and 100% for the parallel SR (95% CI, 99.4 to 100%). SR tests are suitable for use in community-based clinical research settings to assess eligibility both for trial participation and for the provision of on-site VCT services.
Antibody testing for human immunodeficiency virus (HIV) began with the introduction of enzyme-linked immunosorbent assays (ELISA) in the 1980s. Those tests, together with Western blotting, formed the cornerstone of HIV testing algorithms until 1992, when the Global Programme on AIDS of the World Health Organization (WHO) first recommended the use of simple rapid (SR) tests (14). SR tests have been recommended for use in blood safety screening, in surveillance, and in diagnosis in prenatal or voluntary counseling and testing (VCT) centers (15). SR tests have been widely used throughout Africa and the developing world.
By the end of 2007, an estimated 33.2 million people were living with HIV/AIDS, with 2.5 million people being newly infected and 2.1 million dying during 2007 (9). More than 60% of those infected were in sub-Saharan Africa (9). Even in circumstances where treatment is widely available, the incidence remains high, and identifying new interventions for the prevention of HIV must therefore remain a research priority (11).
HIV prevention trials frequently use community-based clinics for screening, recruitment, and distributing trial products to participants. These clinics are often located far from a centralized laboratory. In most HIV prevention trials, HIV screening is an essential procedure to assess eligibility for the trial, for which participants must be HIV negative to be eligible for enrollment. In these settings, SR tests offer many advantages over laboratory-based testing. SR tests are simple to use, require little or no equipment, are cheaper than laboratory-based HIV antibody tests, can be stored at room temperature, and are easy to read. They allow for real-time, on-site HIV testing so that both VCT, if offered as part of the clinical care package provided by the trial, and trial screening can be offered in one visit. Individuals can receive their test results immediately, which is of personal benefit and likely to increase the uptake of VCT as well as being a more efficient way to screen volunteers for HIV prevention research. There is no need for a second visit to collect negative results, although positive SR results may have to be confirmed by a laboratory-based assay according to trial-specific procedures.
SR tests are particularly suited for use in sub-Saharan Africa, where up to 2 weeks or more may be needed for laboratory results to become available (8). Indeed, studies have shown that SR tests can greatly enhance VCT services, especially in rural areas (5, 7, 13). In contrast, the use of a centralized laboratory may result in reporting delays, which may slow trial recruitment or affect follow-up rates during the trial, if participants must return for a second visit to get their test results. Data from a number of evaluations suggest that the sensitivity and specificity of SR tests are similar to those of ELISA- and Western blotting-based algorithms (6, 16). However, more recent studies have reported lower-than-expected sensitivities and specificities of SR tests (1, 4). Therefore, for use in clinical trials, SR tests should be validated locally and undergo external quality assessment. We report a validation exercise carried out for two clinical trials in northwest Tanzania, comparing SR test results from the field with double ELISA tests conducted in our main testing laboratory in Mwanza, Tanzania.
MATERIALS AND METHODS
Study design.
Subjects for our validation study were participating in two large community-based clinical trials in northwest Tanzania, one in Mwanza City and the other in 19 sites in the Lake Victoria region.
The first is a six-center, randomized, double-blind, placebo-controlled trial of a candidate vaginal microbicide (PRO2000/5) for HIV prevention, being conducted in four countries in sub-Saharan Africa (10). The Mwanza City site is enrolling women aged 16 years and older, working in bars, guesthouses, and other food and recreational facilities. After informed consent, women are offered VCT, interviewed at community-based reproductive health clinics, asked to provide specimens for HIV/sexually transmitted infection (STI) and pregnancy testing, and given their HIV results by trained counselors. Those that are HIV negative are invited to participate in enrollment within 6 weeks and are then followed up every 4 weeks for 52 weeks. At the screening visit, in addition to parallel SR tests in the field, all samples are tested for HIV by ELISA.
The second trial, which has recently been completed, was a randomized, double-blind, placebo-controlled trial of acyclovir, 400 mg twice daily, in women who were herpes simplex virus type 2 (HSV-2) seropositive (12). The trial investigated whether suppressive therapy for herpes could reduce either HIV incidence in women who were HIV negative or HSV-2/HIV genital viral shedding in dually infected women. In total, 2,735 female food and recreational facility workers aged 16 to 35 years were screened for antibodies to HIV and HSV-2, and 1,305 HSV-2 seropositive women (821 HIV negative and 484 HIV positive) were enrolled from 19 communities at mobile health clinics. After informed consent, women were interviewed and examined, provided specimens for HIV/STI and pregnancy testing, and were asked to follow up every 3 months for 12 to 30 months, depending on the enrollment date. Participants were offered free VCT by a trained counselor at each visit. In addition, all samples were tested in Mwanza for HIV by ELISA at screening, enrollment, and the end of the follow-up period to determine HIV seroconversion for participants who were initially HIV negative.
Whole blood and serum specimens were used for this validation study. It includes data from all women screened for the microbicide trial from January to June 2006 (n = 257). In the HSV suppressive treatment trial, serial SR tests were initially used at screening and parallel SR tests at enrollment. This validation study, therefore, uses enrollment specimens (n = 382) and screening specimens after June 2004 (n = 150) when parallel testing was introduced at screening.
HIV assays.
Whole blood was tested using the Determine HIV-1/-2 SR test (Abbott Laboratories, United Kingdom) and the Capillus HIV-1/-2 SR test (Trinity Biotech, Ireland) in parallel. All SR tests were performed on-site in the community-based clinics by trained counselors and pre- and posttest counseling was provided at the same visit. Participants found to be HIV positive were referred to the nearest centers providing HIV care and support services.
Serum was tested using the Murex HIV Ag/Ab combination ELISA (Murex Biotech, Dartford, United Kingdom) and the Vironostika Uniform II HIV Ag/Ab ELISA (BioMérieux, France) in a parallel testing strategy. All ELISAs were performed at the National Institute for Medical Research (NIMR) Mwanza Centre STI Laboratory. The laboratory personnel were blinded to the clinic SR test results.
In Mwanza, our experience with samples from adolescents in these communities has shown that the majority of the weak positives from ELISA are actually negative (2). Therefore, the Mwanza STI Reference Laboratory classifies samples as indeterminate if the optical density/cutoff ratios (OD/CO) lie in the range of 1.00 to 1.99 for the Murex or Uniform II ELISA. The samples with different results (positive, indeterminate, or negative) for the Murex and Uniform II ELISA (e.g., Murex positive/Uniform II negative) are classified as discordant.
The samples that were negative for both ELISA and both SR tests were defined as HIV seronegative. All other samples, including samples positive for all four assays, were sent to the Institute of Tropical Medicine, Antwerp, Belgium, for testing by the Inno-LIA HIV I/II line immunoassay (LIAInnogenetics, Ghent, Belgium). If a sample was determined to be positive, this was taken as the final HIV result. The samples that were negative for Inno-LIA were tested by an HIV-1 p24 Ag enzyme immunoassay (Bio-Rad Genetic Systems, CA). If a sample was negative for both p24 and Inno-LIA, this was taken as the final HIV result.
Statistical analysis.
Data were double entered into dBase IV or an SQL server database and analyzed in STATA version 9 (Statacorp, TX). The sensitivity, specificity, and predictive values of the SR assay and ELISA were calculated. A true positive was defined as positive for Inno-LIA or positive for p24. A true negative was defined as negative for Inno-LIA and p24 or negative for Murex, Uniform, Capillus, and Determine. A test positive for the single assays (Murex, Uniform, Capillus, or Determine) was defined as positive for that assay; a test negative was defined as negative for that assay or as negative or indeterminate for the ELISA. For the parallel SR or ELISA strategies, a test positive was defined as positive for both assays. A test negative was defined as negative for either assay or, for the ELISA, as negative or indeterminate for either assay (i.e., a negative or discordant overall result for the SR strategy or a negative, indeterminate, or discordant overall result for the ELISA strategy).
RESULTS
The validation study population comprised 789 female food and recreational facility workers aged 16 to 54 years (mean, 29.0 years; standard deviation, 6.8 years) in northwest Tanzania. Based on parallel SR testing with the Determine and Capillus assays, 143 (18.1%) samples were classified as HIV positive, 641 (81.2%) were HIV negative, and 5 samples (0.6%) were discordant (Table 1). Based on parallel ELISA testing with the Murex and Uniform II assays, 146 (18.5%) samples were HIV positive, 621 (78.7%) were HIV negative, and 22 (2.8%) were indeterminate or discordant (Table 1).
TABLE 1.
Comparison of the results of each assay with those of the gold standard
| Test strategy and result | Total no. (%) of samples | No. of samples with the indicated gold standard resulta
|
|
|---|---|---|---|
| Positive | Negative | ||
| Parallel SR | |||
| Negative | 641 (81.2) | 1 | 640 |
| Discordant | 5 (0.6%) | 1 | 4 |
| Positive | 143 (18.1%) | 143 | 0 |
| Capillus | |||
| Negative | 644 (81.6%) | 2 | 642 |
| Positive | 145 (18.4%) | 143 | 2 |
| Determine | |||
| Negative | 643 (81.5%) | 1 | 642 |
| Positive | 146 (18.5%) | 144 | 2 |
| Parallel ELISA | |||
| Negative | 621 (78.7%) | 0 | 621 |
| Discordant/indeterminate | 22 (2.8%) | 1 | 21 |
| Positive | 146 (18.5%) | 144 | 2 |
| Murex | |||
| Negative | 632 (80.1%) | 0 | 632 |
| Indeterminate | 7 (0.9%) | 0 | 7 |
| Positive | 150 (19.0%) | 145 | 5 |
| Uniform | |||
| Negative | 631 (80.0%) | 1 | 630 |
| Indeterminate | 10 (1.3%) | 0 | 10 |
| Positive | 148 (18.8%) | 144 | 4 |
| Total | 789 (100%) | 145 | 644 |
Samples that were negative for all four assays (Murex, Uniform, Capillus, and Determine) were defined as HIV seronegative. All other samples were tested by InnoLIA and p24. A true positive was defined as positive for InnoLIA or p24. A true negative was defined as negative for InnoLIA and p24 or negative for all four assays.
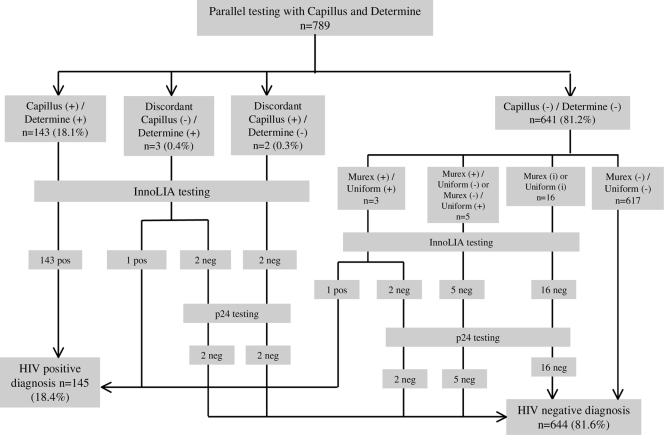
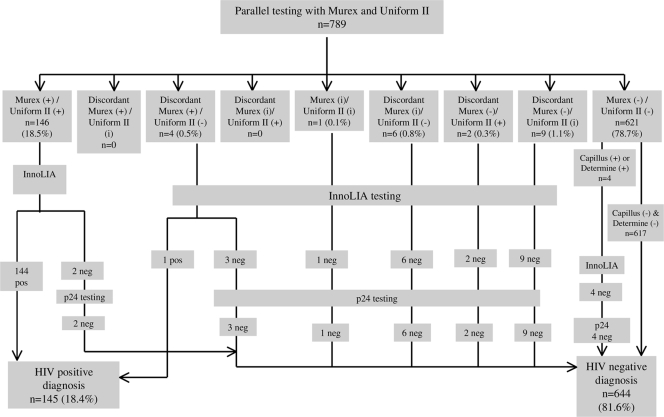
The test algorithms for the validation study are detailed in Fig. 1 and 2. Overall, 172 (21.8%) samples were positive or indeterminate for at least one SR test or ELISA and went for InnoLIA testing for confirmation. Among those, 145 (18.4%) were confirmed positive. A total of 142 (18.0%) samples were HIV positive for both SR tests and both ELISAs, and 617 (78.2%) were HIV negative for all four tests (Table 2). Overall concordance, defined as the proportion of samples that were positive for all four tests or negative for all four tests, between the parallel ELISA and parallel SR tests was 96.2% (759/789; 95% CI, 94.6% to 97.4%).
FIG. 1.
Results of simple rapid HIV assays for the diagnosis of HIV infection. +, positive (ELISA OD/CO, >2); i, indeterminate (ELISA OD/CO, 1 to 1.99); −, negative (ELISA OD/CO, 0 to 0.99).
FIG. 2.
Results of the parallel serology testing using ELISA for the diagnosis of HIV infection. +, positive (ELISA OD/CO, >2); i, indeterminate (ELISA OD/CO, 1 to 1.99); −, negative (ELISA OD/CO, 0 to 0.99).
TABLE 2.
Comparison of parallel ELISA (Murex and Uniform II) results with parallel SR (Determine and Capillus) results
| Parallel ELISA result | No. (%) of samples with indicated parallel SR result
|
|||
|---|---|---|---|---|
| Negative | Positive | Discordanta | Total | |
| Negative | 617 (78.2) | 0 | 4 (0.5) | 621 (78.7) |
| Positive | 3 (0.4) | 142 (18.0) | 1 (0.1) | 146 (18.5) |
| Indeterminateb | 1 (0.1) | 0 | 0 | 1 (0.1) |
| Discordantc | 20 (2.5) | 1 (0.1) | 0 | 21 (2.7) |
| Total | 641 (81.2) | 143 (18.1) | 5 (0.6) | 789 (100) |
Discordant SR results: positive (P)/negative (N) or N/P.
OD/CO between 1.0 and 1.99.
Discordant ELISA results: P/intermediate (I), I/P, P/N, N/P, I/N, or N/I.
The sensitivity and specificity of parallel ELISA testing were 99.3% (95% CI, 96.2 to 100%) and 99.7% (95% CI, 98.9 to 100%) (Table 3), respectively, compared to the gold standard. The positive and negative predictive values were 98.6% (95% CI, 95.1 to 99.8%) and 99.8% (95% CI, 99.1 to 100%), respectively. The sensitivity and specificity of the parallel SR testing were 98.6% (95% CI, 95.1 to 99.8%) and 100% (95% CI, 99.4 to 100%) (Table 3), respectively, compared to the gold standard. The positive and negative predictive values were 100% (95% CI, 97.5 to 100%) and 99.7% (95% CI, 98.9 to 100%), respectively.
TABLE 3.
Sensitivity and specificity of rapid assays and ELISA for HIV
| Assay | % (95% CI) for:
|
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Capillus | 98.6 (95.1-99.8) | 99.7 (98.9-100) | 98.6 (95.1-99.8) | 99.7 (98.9-100) |
| Determine | 99.3 (96.2-100) | 99.7 (98.9-100) | 98.6 (95.1-99.8) | 99.8 (99.1-100) |
| Parallel SRa | 98.6 (95.1-99.8) | 100 (99.4-100) | 100 (97.5-100) | 99.7 (98.9-100) |
| Murexb | 100 (97.5-100) | 99.2 (98.2-99.7) | 96.7 (92.4-98.9) | 100 (99.4-100) |
| Uniformb | 99.3 (96.2-100) | 99.4 (98.4-99.8) | 97.3 (93.2-99.3) | 99.8 (99.1-100) |
| Parallel ELISAc | 99.3 (96.2-100) | 99.7 (98.9-100) | 98.6 (95.1-99.8) | 99.8 (99.1-100) |
Test positive defined as positive for both the Capillus and Determine assays; test negative defined as negative for at least one of the assays.
Test positive defined as positive for the assay; test negative defined as negative or indeterminate for the assay.
Test positive defined as positive for both the Murex and Uniform assays; test negative defined as negative or indeterminate for at least one of the assays.
DISCUSSION
HIV testing is a key component of HIV prevention trials, for assessing eligibility, for evaluating the effectiveness of trial interventions, and as part of the VCT services offered to trial participants. Although SR tests, on their own, are not sufficient for the confirmation of seroconversion as a primary trial outcome, they can play a central role in the service component of any HIV prevention trial. In community-based trials, SR tests can allow trial eligibility to be assessed in the field. Furthermore, they can provide an indication of seroconversion during trial follow-up and alert the investigator to the need for confirmatory tests.
Our validation study found that, in field-based settings in Tanzania, parallel SR tests were slightly more specific and only slightly less sensitive than ELISA-based algorithms. Although ELISA-based tests are highly accurate, they are impractical for field conditions in developing countries because of the high cost, the need for trained personnel and specialized equipment, and the long turnaround time.
Our results demonstrate that the parallel SR strategy can provide an ideal screening test to assess eligibility for HIV prevention trials. Its sensitivity against the gold standard was 98.6% with one true HIV-positive participant having negative results on both SR tests and one having discordant results on the two tests. In practice, the latter would undergo further evaluation, and so only one true positive would have been missed completely by this algorithm, corresponding to a sensitivity of 99.3%. In a clinical trial, the few HIV-positive cases missed by rapid testing would be detected through subsequent HIV testing during the trial, at which time back testing could be done using more sensitive tests to see whether the participant was HIV positive at entry. In contrast with the parallel ELISA algorithm, the parallel SR tests gave only a few discordant results, reducing the need for further testing to determine trial eligibility. Finally, the parallel SR algorithm was highly specific, 100% in our study population, suggesting that participants with positive results can be referred to HIV care and treatment centers without the delay of further confirmatory testing.
The Determine SR test has been reported to have sensitivities and specificities of 100% and 99 to 100%, respectively (3, 16). Reported sensitivities and specificities of the Capillus SR test are 99 to 100% (3, 16). We found the specificities of Determine and Capillus to be within the reported range. However, we found the sensitivities of both tests to be slightly lower than reported and the sensitivity of Capillus to be lower than that of Determine (98.6% and 99.3%, respectively). Poor sensitivity has been reported elsewhere in sub-Saharan Africa (1, 4). In contrast, we found the sensitivities of the Murex and Uniform II ELISA tests to be 100% and 99.3%, respectively.
The sensitivity of the parallel SR algorithm was 98.6%, compared with 99.3% for the parallel ELISA algorithm. The ELISAs used in our validation study incorporate a p24 detection system, which increases the sensitivity of these tests. However, the specificity of the parallel SR was higher than that of the parallel ELISA (100% versus 99.7%). The lower specificity of the parallel ELISA may be a result of cross-reactivity with other infections that are endemic in this region of Tanzania.
In summary, our results show that in this study population, with an HIV prevalence of 18%, the parallel SR algorithm had both high sensitivity and specificity, comparable to the sensitivity and specificity of ELISA-based algorithms. Parallel SR tests may provide an efficient and accurate way of assessing eligibility for HIV prevention trials, particularly in resource-poor settings. Furthermore, such tests may play a valuable role in the VCT service component of a trial, by allowing HIV-positive participants to be referred for care and treatment without delay.
Acknowledgments
We thank the Ministry of Health, Tanzania, the National AIDS Control Programme, Tanzania, and the Director General of the National Institute for Medical Research (NIMR), Tanzania, for permission to conduct and publish the studies. We also thank Greet Beelaert and Eddy van Dyck of the Institute of Tropical Medicine, Antwerp, Belgium, for confirming the HIV test results for InnoLIA. We are grateful to our colleagues in the MDP301 and HSV suppressive treatment trials for their dedicated work. We thank the administrative staff in the African Medical & Research Foundation (AMREF), the London School of Hygiene & Tropical Medicine (LSHTM), and the NIMR/AMREF/LSHTM collaborative projects office for their support. We received advice from many other colleagues in the NIMR, AMREF, LSHTM, and the Clinical Trials Unit of the Medical Research Council (MRC). We are very grateful to all the women who participated in this study.
Funding was provided by the Department for International Development, Medical Research Council, United Kingdom, and the Wellcome Trust, United Kingdom. The Microbicides Development Programme (MDP) is funded by the United Kingdom Department for International Development (DfID) and the MRC. The HSV suppressive treatment trial is funded by the Wellcome Trust, DfID, and the MRC.
D.B.E. prepared the first draft of the manuscript. He is guarantor. K.B. carried out the analyses. A.V. and D.W.-J. were the site principal investigators in Mwanza, Tanzania, for the microbicide and HSV suppressive treatment trials, respectively. D.B.E. and J.C. were responsible for the supervision of the laboratory work involved in the study. C.C. prepared the data sets for the microbicide trial. L.K. prepared the data sets for the HSV trial. K.M. supervised field work for the HSV trial. U.J. provided guidance on the interpretation of the assay results and quality control for the laboratory work. S.M. and C.L. are the principal investigators for the microbicide trial. R.J.H. and D.A.R. are investigators on both the microbicide and HSV suppressive treatment trials. R.J.H., D.A.R. and J.C. are the coinvestigators for the HSV suppressive treatment trial. All coauthors read and commented on drafts of the paper.
Both trials received ethical clearance from the Tanzanian Medical Research Coordinating Committee and the ethics committee of the London School of Hygiene and Tropical Medicine, United Kingdom.
Footnotes
Published ahead of print on 25 February 2009.
REFERENCES
- 1.Claassen, M., G. U. van Zyl, S. N. Korsman, L. Smit, M. F. Cotton, and W. Preiser. 2006. Pitfalls with rapid HIV antibody testing in HIV-infected children in the Western Cape, South Africa. J. Clin. Virol. 3768-71. [DOI] [PubMed] [Google Scholar]
- 2.Everett, D. B., H. A. Weiss, J. Changalucha, A. Anemona, T. Chirwa, D. A. Ross, et al. 2007. Low specificity of the Murex fourth-generation HIV enzyme immunoassay in Tanzanian adolescents. Trop. Med. Int. Health 121323-1326. [DOI] [PubMed] [Google Scholar]
- 3.Granade, T. C., B. S. Parekh, P. M. Tih, T. Welty, E. Welty, M. Bulterys, et al. 2005. Evaluation of rapid prenatal human immunodeficiency virus testing in rural Cameroon. Clin. Diagn. Lab Immunol. 12855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray, R. H., F. Makumbi, D. Serwadda, T. Lutalo, F. Nalugoda, P. Opendi, et al. 2007. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. Br. Med. J. 335188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson, P. L., and X. Mahlalela. 2006. Utilization of voluntary counseling and testing services in the Eastern Cape, South Africa. AIDS Care 18446-455. [DOI] [PubMed] [Google Scholar]
- 6.Lien, T. X., N. T. Tien, G. F. Chanpong, C. T. Cuc, V. T. Yen, R. Soderquist, et al. 2000. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 62301-309. [DOI] [PubMed] [Google Scholar]
- 7.McKenna, S. L., G. K. Muyinda, D. Roth, M. Mwali, N. Ng'andu, A. Myrick, et al. 1997. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS 11(Suppl. 1)S103-S110. [PubMed] [Google Scholar]
- 8.Respess, R. A., M. A. Rayfield, and T. J. Dondero. 2001. Laboratory testing and rapid HIV assays: applications for HIV surveillance in hard-to-reach populations. AIDS 15(Suppl. 3)S49-S59. [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS. 2007. AIDS epidemic update. UNAIDS, Geneva, Switzerland.
- 10.Vallely, A., S. Kasindi, I. R. Hambleton, L. Knight, T. Chirwa, R. Balira, et al. 2007. Microbicides development program, Tanzania—baseline characteristics of an occupational cohort and reattendance at 3 months. Sex. Transm. Dis. 34638-643. [DOI] [PubMed] [Google Scholar]
- 11.van de Wijgert, J. H., and R. J. Shattock. 2007. Vaginal microbicides: moving ahead after an unexpected setback. AIDS 212369-2376. [DOI] [PubMed] [Google Scholar]
- 12.Watson-Jones, D., H. A. Weiss, M. Rusizoka, J. Changalucha, K. Baisley, K. Mugeye, et al. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 3581560-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson, D., N. Wilkinson, C. Lombard, D. Martin, A. Smith, K. Floyd, et al. 1997. On-site HIV testing in resource-poor settings: is one rapid test enough? AIDS 11377-381. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. 1992. Global programme on AIDS. Recommendations for the selection and use of HIV antibody tests. Wkly. Epidemiol. Rec. 67145-149. [PubMed] [Google Scholar]
- 15.World Health Organization. 1998. The importance of simple/rapid assays in HIV testing. Wkly. Epidemiol. Rec. 73321-326. [PubMed] [Google Scholar]
- 16.World Health Organization. 2002. HIV assays: operational characteristics. Report 12: simple/rapid tests, whole blood specimens. World Health Organization, Geneva, Switzerland.