Abstract
Approximately 8% of Rift Valley fever (RVF) cases develop severe disease, leading to hemorrhage, hepatitis, and/or encephalitis and resulting in up to 50% of deaths. A major obstacle in the management of RVF and other viral hemorrhagic fever cases in outbreaks that occur in rural settings is the inability to rapidly identify such cases, with poor prognosis early enough to allow for more-aggressive therapies. During an RVF outbreak in Kenya in 2006 to 2007, we evaluated whether quantitative real-time reverse transcription-PCR (qRT-PCR) could be used in the field to rapidly identify viremic RVF cases with risk of death. In 52 of 430 RVF cases analyzed by qRT-PCR and virus culture, 18 died (case fatality rate [CFR] = 34.6%). Levels of viremia in fatal cases were significantly higher than those in nonfatal cases (mean of 105.2 versus 102.9 per ml; P < 0.005). A negative correlation between the levels of infectious virus particles and the qRT-PCR crossover threshold (CT) values allowed the use of qRT-PCR to assess prognosis. The CFR was 50.0% among cases with CT values of <27.0 (corresponding to 2.1 × 104 viral RNA particles/ml of serum) and 4.5% among cases with CT values of ≥27.0. This cutoff yielded 93.8% sensitivity and a 95.5% negative predictive value; the specificity and positive predictive value were 58% and 50%, respectively. This study shows a correlation between high viremia and fatality and indicates that qRT-PCR testing can identify nearly all fatal RVF cases.
Rift Valley fever (RVF) virus is a zoonotic mosquito-borne member of the Phlebovirus genus in the Bunyaviridae family of viruses that was first isolated in Kenya in the 1930s (4, 6). In livestock, the virus is associated with abortions and high levels of mortality in young animals (11, 17). Humans acquire RVF virus through bites from infected mosquitoes or through exposure to the blood, body fluids, or tissue of infected animals or other humans (29). Periodic epidemics of RVF involving both livestock and humans occur following heavy rainfalls, primarily in eastern Africa (Kenya, Somalia, and Tanzania) but also in other African countries (3, 9, 16, 22, 31). In 2000, the virus was introduced to the Arabian Peninsula through the importation of infected livestock from East Africa, causing a severe outbreak in Saudi Arabia and Yemen (1, 2, 15).
At least 80% of human RVF cases are asymptomatic, and less than 8% develop into severe disease characterized by generalized hemorrhagic syndromes, acute hepatitis manifested by jaundice, encephalitis, and retinitis (14, 20). The overall human case fatality rate (CFR) for RVF virus infection has been estimated at 1 to 3%, but the rate can be as high as 50% among cases with severe disease (18, 20). Risk factors and symptoms associated with the development of severe RVF are not clearly elucidated. In the Saudi Arabia outbreak, bleeding abnormalities, neurological symptoms, and jaundice were independently associated with high mortality (2). Jaundice is believed to be the result of acute hepatocellular failure due to virus-induced damage to hepatocytes, while the pathogeneses of meningoencephalitis and retinitis are not understood (14). As is the case with other viral hemorrhagic fevers, the hemorrhagic syndrome from RVF virus is likely the result of injury to the microvasculature and increased endothelial permeability, leading to leakage of blood into tissue and mucosal surfaces (23, 26).
A major obstacle in the management of viral hemorrhagic fever patients is the inability to identify cases with poor prognosis early enough to allow for more-aggressive supportive therapy and possibly the administration of experimental chemotherapeutic drugs. Some studies have suggested a correlation between infectious viral load and the development of severe disease; however, standard laboratory methods for determining infectious viral levels are time-consuming and, for hemorrhagic fever viruses, require laboratories with high-biosafety systems (27). On the other hand, studies have shown that quantitative real-time reverse transcription-PCR (qRT-PCR) is potentially useful for estimating levels of infectious hemorrhagic fever viruses (8, 24, 28). An RVF outbreak occurred in Kenya from December 2006 through March 2007, resulting in more than 700 suspected cases and approximately 150 fatalities, a higher CFR (21.4%) than the historical figure of less that 8%, possibly due to the inability to trace all the RVF cases in the country (5, 30). A risk assessment study found an association between animal contact and the development of severe disease, perhaps because animal exposure resulted in infection with a higher viral load compared to that of the mosquito (S. Amwayi, personal communication). We use laboratory and fatality outcomes from this outbreak to determine the association between the level of viremia and fatality and the usefulness of field qRT-PCR testing to rapidly identify highly viremic RVF cases at elevated risk of death.
MATERIALS AND METHODS
Patient selection.
Medical providers at health care facilities throughout Kenya collected sera from 430 of approximately 700 suspected cases. The specimens were sent to the nearest of two laboratories for testing, as follows: a field laboratory established at Garissa Provincial Hospital in the Northeastern Province, which was the early epicenter of the outbreak, or the Kenya Medical Research Institute/CDC reference laboratory in Nairobi that also received specimens from other countries in the East African region. Epidemiology staff from the Kenya Ministry of Health reviewed medical charts and interviewed patients, family members, and medical staff to ascertain clinical symptoms, onset timeline, and final disposition (alive or died) of the patient. The study, including specimen collection and testing, was approved and supervised by the Kenya Ministry of Health.
IgG and IgM ELISA.
Patient sera were tested for the presence of RVF virus immunoglobulin M (IgM) using the capture enzyme-linked immunosorbent assay (ELISA) method, as described previously (19). Briefly, goat antiserum against the human μ chain of IgM (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was diluted at 1:500 and used to coat plates overnight. After being washed and blocked, test and control sera diluted at 1:400 in diluent buffer were added to wells, and plates were incubated at 37°C for 1 h. After being washed, RVFV antigens diluted at 1:400 were added to two wells and mock antigen to the other two wells of each sample on the plate. After incubation at 37°C for 1 h and being washed, mouse anti-RVFV antibodies, diluted at 1:2,000, were added to each well, followed by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) diluted at 1:10,000 (heavy plus light chain; Kirkegaard & Perry Laboratories, Gaithersburg, MD). Immunoreactivity was detected using the 2,2′-azino-diethyl-benzothiazoline-sulfonic acid as peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at room temperature, and optical density (OD) at 405 nm was read. The mean OD readings were converted into percentages of high-positive control serum (PP) values using the following equation: (mean net OD of test sample/mean net OD of high-positive control) × 100.
For IgG antibodies, diluted patient sera were added to plates coated with mouse anti-RVFV antibody, and immunoreactivity was detected using horseradish peroxidase-conjugated goat anti-mouse IgG, followed by peroxidase substrate (Kirkegaard & Perry Laboratories). The mean OD readings were again converted into PP values, and specimens producing PP values of ≥18 were considered positive.
Antigen capture ELISA.
The RVF antigen detection assay uses polyclonal mouse ascitic fluid raised against RVF virus (strain Zagzig 501) as a capture antibody, as described previously (13). Rabbit hyperimmune serum raised against RVF virus was used as a detector antibody.
qRT-PCR.
The one-step qRT-PCR developed by Drosten et al. (7) was performed using the portable LightCycler 2.0 system (Roche Molecular Diagnostics, Mannheim, Germany). Briefly, the RT-PCR test used the AmpliTaq Gold Taq polymerase (Applied Biosystems, Foster City, CA) in 5′ nuclease assays. The primers used were 5′-AAAGGAACAATGGACTCTGGTCA-3′ (nucleotide [nt] positions 349 to 371; GenBank accession no. AF134508), forward, and 5′-CACTTCTTACTACCATGTCCTCCAAT-3′ (nt positions 443 to 417), reverse, which amplified a 94-nt fragment from the G2 gene of the virus. The 5′ nuclease probe used was 5′-AAAGCTTTGATATCTCTCAGTGCCCCAA-3′ (nt positions 388 to 416), labeled with 6-carboxyfluorescein at the 5′ end and with 6-carboxy-N,N,N,N-tetramethylrhodamine at the 3′ end.
The cycling profile included a reverse transcription step performed at 50°C for 30 min, followed by preincubation at 95°C for 15 min. Then, 45 cycles were run at 95°C for 5 s and annealing and extension at 57°C for 35 s. Fluorescence was read at the combined annealing-extension step at 57°C.
A standard curve was generated using procedures described previously (7). A selection of samples was amplified in parallel with an in vitro-transcribed RVF RNA standard in a reference laboratory. In the field, an external standard curve was used to transform crossover threshold (CT) values into viral RNA copies. Cases with CT values between 37.1 and 40.0 were classified as indeterminate, requiring a second sample for final determination. The qRT-PCR test did not discriminate between viral mRNA and genomic RNA.
Infectious virus titer.
Virus titration was performed as described previously (12). Briefly, individual patient sera were diluted 10-fold in Eagle's minimum essential medium (BioWhittaker) containing 100 IU penicillin, 100 μg streptomycin, and 0.25 μg amphotericin B (BioWhittaker). Four replicates of 100 μl per dilution (from 10−1 to 10−7) were transferred into flat-bottom 96-well cell culture microplates (Nunc), and equal volumes of Vero cell suspension in Eagle's minimum essential medium containing 2 × 105 cells/ml, 8% fetal bovine serum/ml (Gibco), and standard concentrations of antibiotics were added. The inoculated microplates were incubated at 37°C in a CO2 incubator and observed microscopically for cytopathic effects for 10 days postinoculation. Virus concentrations were expressed as 50% tissue culture infective doses (TCID50)/ml of serum. The limit of detection was 1 particle of infectious virus particles per 10 μl of serum, which corresponds to 100 infectious particles per ml of serum.
Assessing sensitivity and specificity of qRT-PCR.
To assess the sensitivity of the qRT-PCR test as a measure of RVF virus infection, 61 of the 430 serum specimens were randomly selected and tested by both qRT-PCR and antigen capture ELISA, routinely used for RVF diagnosis in previous outbreaks. Infectious virus titration was conducted at the reference laboratory on 143 of the 272 cases to determine the positive predictive value of the qRT-PCR test. To test whether qRT-PCR could be used to determine severity of disease, CT values were examined for the 52 cases for which the final disposition was known. We compared these values with viral load, which is the standard test for disease severity. For various cutoff levels of CT, we calculated the CFR, sensitivity, specificity, positive and negative predictive values, and concordance for delineation of fatal versus nonfatal cases.
RESULTS
Sensitivity of qRT-PCR for diagnosis of RVF.
The qRT-PCR test was used as a substitute for the viral antigen detection test (Table 1), alongside tests detecting virus-specific IgM antibodies to confirm RVF cases during the outbreak. Of the 272 confirmed cases from various regions of Kenya, the qRT-PCR test detected 172 cases (63.2%), whereas the IgM test detected 162 cases (59.6%). A total of 62 cases (38%) were positive by both tests (Table 1). To establish the sensitivity of the qRT-PCR test as a substitute for the antigen ELISA, a small subset of 61 specimens were tested by both qRT-PCR and the antigen capture ELISA. A total of 28 of the 61 specimens were positive by either test. More importantly, all the 28 positive specimens were positive by qRT-PCR and virus isolation, indicating a sensitivity for the qRT-PCR test of 100%, whereas only 14 of the 28 specimens (50%) were positive by antigen capture ELISA. Overall, 81 RVF specimens from confirmed cases had adequate volumes and were subjected to virus isolation, and virus was isolated from 39 of them (47.5%). These 39 specimens with successful virus isolation were all negative for virus-specific IgM and IgG antibodies and tested positive by qRT-PCR.
TABLE 1.
Test results for confirmed RVF cases
| qRT-PCR result | No. of confirmed cases (n = 272) (% of total) | No. of cases with indicated test result (% of total)
|
||
|---|---|---|---|---|
| IgM negative (n = 110) | IgM positive (n = 162) | IgM and IgG positive (n = 51) | ||
| Positive | 172 (63) | 110 (100) | 62 (38) | 23 (45) |
| Negative | 100 (37) | 0 (0) | 100 (62) | 28 (55) |
The qRT-PCR detected all of the 110 confirmed RVF cases that were negative for virus-specific antibodies. In contrast, the test detected only 62 of 162 (38%) RVF cases that were positive for IgM and 23 of 51 (45.1%) cases in which both IgM and IgG antibodies were present. When we plotted qRT-PCR-positive RVF cases according to their CT values, two clusters emerged, as follows: those with CT values of <27.0 (strong positives) and those with CT values of ≥27.0 (weak positives) (Fig. 1A). These two clusters persisted when plots were limited to RVF cases lacking detectable circulating antiviral antibodies (IgM or IgG) (Fig. 1B) but disappeared when plots were limited to cases with detectable RVF antibodies (Fig. 1C). Most RVF cases with detectable antiviral antibodies were only weakly positive by qRT-PCR (CT, ≥27.0), suggesting a better chance of survival for them than for cases without antibodies.
FIG. 1.
Scatter plots to demonstrate the distribution patterns of acute RVF cases (n = 90), with their CT values obtained from rapid qRT-PCR testing. (A) The distribution pattern of all cases (n = 90) against the cutoff CT value of 27.0, which was indicative of prognosis. (B) The distribution among the subset of these cases (n = 51) that did not have detectable RVF antibodies (IgM or IgG). (C) The distribution among cases (n = 39) that were positive for RVF IgM (blue), IgG (purple), or both IgM and IgG (yellow).
Fatal versus nonfatal RVF cases.
Of the 52 RVF cases whose final disposition was known and whose sera were analyzed by both qRT-PCR and virus culture, 18 died (CFR = 34.6%) while the rest survived the infection. The fatal cases had a mean of 8.6 × 106 viral RNA copies/ml of serum (range, 2.5 × 103 to 54.0 × 107) and 105.2 infectious virus particles/ml of serum (range, 101.3 to 107.8). Levels of infectious virus were significantly lower (mean of 102.9 per ml) in nonfatal cases (P < 0.005); viral RNA levels were also lower (2.4 × 106 copies/ml) in nonfatal cases, but this difference was not statistically significant (P = 0.25). Because the levels of viral RNA and infectious virus can be influenced by the timeline of specimen collection relative to the onset of disease, we used a questionnaire administered to all suspected RVF cases to determine the time of specimen collection in relation to disease onset. Both fatal and nonfatal RVF cases sought medical attention around day 3 postonset (ranges, 1 to 7 days for fatal cases and 0 to 11 days for surviving cases), indicating no difference in the timeline of specimen collection between fatal and nonfatal cases. The use of a portable real-time thermocycler in a field laboratory made it possible to obtain the qRT-PCR results within 3 h of the patients' arrival at the hospital. Because most patients were not available for follow-up, we were unable to systematically correlate levels of viremia with the development of severe clinical disease characterized by severe jaundice, encephalitis, and bleeding. However, bleeding symptoms were recorded in 11 of 14 (78.6%) fatal cases and associated with poor prognosis. For example, in the two major epicenters of the outbreak, Northeastern and Coast provinces, 50 of 63 (79.4%) RVF cases that died reported bleeding manifestations compared with 95 of 213 (44.6%) of surviving cases reporting those (relative risk, 3.74; 95% confidence interval, 2.3 to 6.58; P value of <0.0001). In contrast, severe jaundice, encephalitis, and retinitis were rarely observed in the fatal cases.
Use of CT value to identify cases at risk of death.
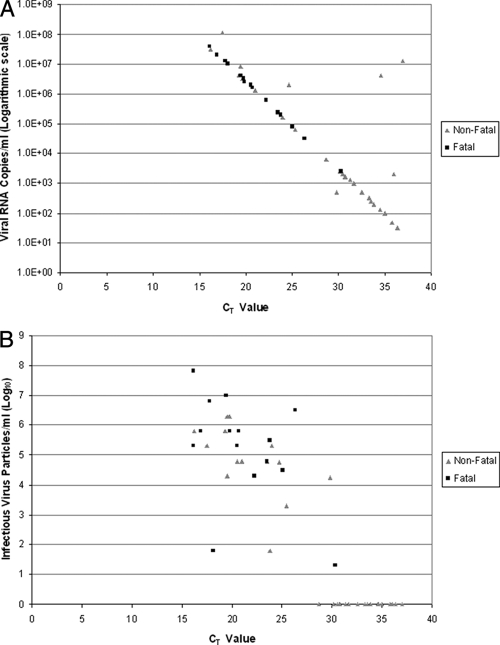
There was a negative correlation between the CT values and levels of infectious virus particles or viral RNA (Fig. 2A and B). Fatal cases had significantly more infectious virus particles/ml of serum than nonfatal cases (mean of 105.2 versus 102.9; P value of <0.005). Fatal cases also tended to have more viral RNA copies/ml of serum than nonfatal cases (mean of 8.6 × 106 copies/ml versus 2.4 × 106 copies/ml, respectively); however, the difference was not statistically significant. No infectious virus was detected in 20 of the 36 nonfatal cases, all of which had CT values of >27.0, whereas infectious virus titers ranging from 101.3 to 107.8 particles/ml of serum were detected in all 18 fatal cases.
FIG. 2.
(A) Correlation of CT values with viral RNA copies among fatal (n = 18) and nonfatal (n = 36) cases of RVF. The RVF RNA concentration in serum was calculated using a standard curve developed using a qRT-PCR as described in Materials and Methods. The ≥95% chance limit of detection as determined by probit regression analysis by Drosten et al. (7) was 2,835 RNA copies/ml (95% confidence interval, 2,143 to 4,525) of serum. The CT values were determined from a one-step real-time RT-PCR, with fluorescence read at the combined annealing-extension step at 57°C. Using a cutoff CT value of 27.0, cases registering CT values of ≤27.0 were associated with a CFR of ≥50.0, whereas those with CT values of >27.0 had a CFR of 4.5%, a sensitivity of 93.8%, and a negative predictive value of 95.5%. (B) Correlation of levels of infectious RVF virus in serum (viremia), with CT values among fatal (n = 18) and nonfatal (n = 36) cases of RVF. Viremia was determined by inoculating patient sera in Vero cells and expressed as TCID50/ml of serum. The limit of detection was 100 infectious RVF virus particles per ml of serum. The mean infectious virus levels were fourfold higher in fatal cases than those in nonfatal cases of RVF. No infectious virus was detected in 20 of the 36 nonfatal cases, all of which had CT values of >27.0, whereas infectious virus titers ranging from 101.3 to 107.8 TCID50 were detected in all the fatal cases.
Statistical measures of association were evaluated to determine an optimal CT cut point for the identification of cases that have a high risk of death (Table 2). In general, as the cut point increases and more cases were screened positive, the CFR among them decreases, positive predictive value and specificity decrease, and negative predictive value and sensitivity increase. We selected a CT cut point of 27.0 (corresponding to approximately 2.1 × 104 RNA particles/ml of serum) as the optimal balance between these factors, at which 58% of cases would be treated, including 17 of 18 fatal cases (94.4%) and 15 of 36 nonfatal cases (42%), with CFRs of 50.0% among those screened positive and 4.5% among those screened negative. This cut point yielded a sensitivity of 94% and negative predictive value of 96%, ensuring that nearly all at-risk cases would have received treatment. Specificity and positive predictive value were lower, at 58% and 50%, respectively.
TABLE 2.
Use of CT values to predict death from RVF in Kenya, 2006 to 2007a
| CT cut point | Fatal cases (no.) | Nonfatal cases (no.) | Case fatality (%) | PPV (%)b | NPV (%)c | Sensitivity (%) | Specificity (%) | Correct (%) | Treated (%) |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 18 | 36 | 33.3 | ||||||
| <19 | 5 | 2 | 71.4 | 71.4 | 75.6 | 31.3 | 94.4 | 75.0 | 13.5 |
| ≥19 | 11 | 34 | 24.4 | ||||||
| <20 | 8 | 8 | 50.0 | 50.0 | 77.8 | 50.0 | 77.8 | 69.2 | 30.8 |
| ≥20 | 8 | 28 | 22.2 | ||||||
| <21 | 10 | 9 | 52.6 | 52.6 | 81.8 | 62.5 | 75.0 | 71.2 | 36.5 |
| ≥21 | 6 | 27 | 18.2 | ||||||
| <23 | 11 | 10 | 52.4 | 52.4 | 83.9 | 68.8 | 72.2 | 71.2 | 40.4 |
| ≥23 | 5 | 26 | 16.1 | ||||||
| <24 | 13 | 12 | 52.0 | 52.0 | 88.9 | 81.3 | 66.7 | 71.2 | 48.1 |
| ≥24 | 3 | 24 | 11.1 | ||||||
| <25 | 13 | 14 | 48.1 | 48.1 | 88.0 | 81.3 | 61.1 | 67.3 | 51.9 |
| ≥25 | 3 | 22 | 12.0 | ||||||
| <26 | 14 | 15 | 48.3 | 48.3 | 91.3 | 87.5 | 58.3 | 67.3 | 55.8 |
| ≥26 | 2 | 21 | 8.7 | ||||||
| <27 | 15 | 15 | 50.0 | 50.0 | 95.5 | 93.8 | 58.3 | 69.2 | 57.7 |
| ≥27 | 1 | 21 | 4.5 | ||||||
| <31 | 16 | 22 | 42.1 | 42.1 | 100.0 | 100.0 | 38.9 | 57.7 | 73.1 |
| ≥31 | 0 | 14 | 0.0 |
Boldface type indicates predictor values at the cutoff CT of 27.0
PPV, positive predictive value.
NPV, negative predictive value.
DISCUSSION
This study demonstrates the use of rapid qRT-PCR testing to detect high viremia levels in RVF virus cases and to show a strong correlation between high viremia and fatality. These findings have considerable practical relevance because the qRT-PCR test can be performed quickly in a field-based laboratory setting, potentially enabling health officials to target those with poor prognosis for special intensive clinical management. Presently, the antiviral drug ribavirin is listed as the drug of choice for RVF by the World Health Organization because if has been shown to suppress viremia in RVF virus-infected rhesus monkeys and to suppress RVF virus yields in cell cultures (21). However, ribavirin has not yet been approved by the U.S. Food and Drug Administration, in part because it can cause serious side effects, including bronchospasms, anemia, neurotoxicity, and gastrointestinal effects (10, 25). Hyperimmune serum and interferon-stimulating drugs have also been suggested for use in acute severe RVF cases, but they are expensive and/or not readily available (21). Because of these drawbacks, identifying cases with high risk of death is critical for ensuring appropriate case management.
The choice of the CT cut point is subjective and requires balancing the competing priorities of identifying patients likely to die and reducing the risk and cost associated with treating patients who would have recovered without treatment. By using a CT cut point of 27.0, we were able to successfully distinguish between cases at high and low risk of death, with 94% sensitivity and a 96% negative predictive value, indicating that a vast majority of cases with poor prognosis would be identified for possible treatment. Furthermore, half of all patients within this level resulted in fatalities, ensuring that treatment based on this cut point would be reserved for those truly at high risk of death.
We also found that qRT-PCR had superior sensitivity for case confirmation compared to standard antigen detection, identifying 100% of cases rather than 50% of cases for antigen capture ELISA among cases tested by both methods. These results are similar to the Saudi Arabia outbreak, in which antigen capture ELISA was able to confirm only one-third of the cases, whereas qRT-PCR detected two-thirds (15). Furthermore, it is likely that the qRT-PCR may be better able to detect early RVF cases than antigen detection or IgM antibody assays; however, we were not able to test this hypothesis, as most cases sought medical attention within 3 days after the onset of symptoms (approximately 4 to 6 days postexposure), when virus levels in serum were typically greater than 102.3 infectious virus particles/ml of serum. While the qRT-PCR test had 100% sensitivity in detecting acute RVF cases before the onset of antiviral antibodies, it was less effective once antibodies were developed; thus, it is best used in settings where patients are likely to seek treatment soon after initial symptoms develop.
The qRT-PCR results during this outbreak were available within 3 h of the patients' arrival at the hospital, enabling rapid diagnosis, case confinement, and case management. Furthermore, the ability to conduct qRT-PCR with a portable thermocycler makes this test ideal for use in remote areas where outbreaks often occur. The field laboratory established for this outbreak was located in a rural area with little infrastructure; however, a variety of critical steps were implemented to ensure that the tests were carried out safely and did not endanger laboratory staff or create risks in the surrounding environment. These precautions included the use of proper personal protective equipment, immediate inactivation of specimens before testing, and on-site incineration of all biological waste. In addition, most of the laboratory staff were vaccinated with the inactivated RVF virus vaccine donated by the U.S. Army.
While the proposed CT cut point successfully identified nearly all fatal cases in this outbreak, it also positively screened a substantial number of nonfatal cases. In addition to laboratory test results, clinical symptoms and other factors have been shown to be associated with poor prognosis, including coinfections, nonimmune status, poor nutritional status, and delay in receiving medical attention. Furthermore, the CT cut point derived from this study was based on a relatively small number of cases for whom fatality information was known. Thus, larger studies that collect more-detailed patient information may be warranted to validate these results, evaluate additional factors associated with fatality, and refine the treatment decision algorithm proposed here.
Acknowledgments
Funding for this work was provided by the U.S. government through the Centers for Diseases Control and Prevention.
We thank the entire RVF response team, which included staff from Kenya Ministry of Health, Kenya Ministry of Livestock and Fisheries Development, Kenya Medical Research Institute World Health organization, Centers for Disease Control and Prevention (CDC), Health Canada, National Institute of Communicable Diseases—South Africa, U.S. Naval Medical Research Unit 3, and Médecins Sans Frontières. The Special Pathogens Branch in the Division of Viral and Rickettsial Diseases at the CDC, Atlanta, provided reagents for immunodiagnosis. A special thanks goes to Heather Burke for providing administrative support and to Rosemary Sang, Solomon Gikundi, Cyrus Wachira, Newton Wamola, Sylvia Omulo, Victor Otieno, Samson Limbaso, and Leonard Nderitu, who were involved in testing the RVF cases. We also thank Allen Hightower for assistance with statistical analysis.
The findings and conclusions of this work are ours and should not be construed to represent the CDC's determination or policy.
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Al-Afaleq, A. I., E. M. Abu Elzein, S. M. Mousa, and A. M. Abbas. 2003. A retrospective study of Rift Valley fever in Saudi Arabia. Rev. Sci. Tech. 22867-871. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hazmi, M., E. A. Ayoola, M. Abdurahman, S. Banzal, J. Ashraf, A. El-Bushra, A. Hazmi, M. Abdullah, H. Abbo, A. Elamin, T. Al-Sammani, M. Gadour, C. Monon, M. Hamza, I. Rahim, M. Hafez, M. Jambavalikar, H. Arishi, and A. Aqeel. 2003. Epidemic Rift Valley fever in Saudi Arabia: a clinical study of severe illness in humans. Clin. Infect. Dis. 36245-252. [DOI] [PubMed] [Google Scholar]
- 3.Anyamba, A., K. J. Linthicum, and C. J. Tucker. 2001. Climate-disease connections: Rift Valley fever in Kenya. Cad. Saude Publica 17133-140. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, D. H., C. H. Calisher, J. Casals, M. P. Chumakov, S. Y. Gaidamovich, C. Hannoun, D. K. Lvov, I. D. Marshall, N. Oker-Blom, R. F. Pettersson, J. S. Porterfield, P. K. Russell, R. E Shope, and E. G. Westaway. 1980. Bunyaviridae. Intervirology 14125-143. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2007. Rift Valley fever outbreak—Kenya, November 2006-January 2007. MMWR Morb. Mortal. Wkly. Rep. 5673-76. [PubMed] [Google Scholar]
- 6.Daubney, R., J. R. Hudson, and P. C. Granham. 1931. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J. Pathol. Bacteriol. 34545-579. [Google Scholar]
- 7.Drosten, C., S. Gottig, S. Schiling, M. Asper, M. Panning, H. Schmitz, and S. Gunther. 2002. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 402323-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duh, D., A. Sakida, M. Petrovec, S. Ahmeti, I. Dedushaj, M. Panning, C. Drosten, and T. Avsic-Zupanc. 2007. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg. Infect. Dis. 131769-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Akkad, A. M. 1978. Rift Valley fever outbreak in Egypt, October-December 1977. J. Egypt. Public Health Assoc. 53123-128. [PubMed] [Google Scholar]
- 10.Inuoye, R. T., L. A. Panther, C. M. Hay, and S. M. Hammer. 2002. Antiviral agents, p. 171-242. In D. D. Richman, R. J. Whitley, and F. G. Hayden (ed.), Clinical virology, 2nd ed. ASM Press, Washington, DC.
- 11.Gerdes, G. H. 2004. Rift Valley fever. Rev. Sci. Tech. 23613-623. [DOI] [PubMed] [Google Scholar]
- 12.Kärber, G. 1931. Beitrag zur kollectiven Behandlung pharmakologischer Reihensversuche. Arch. Exp. Pathol. Pharmacol. 162480-483. [Google Scholar]
- 13.Ksiazek, T. G., P. E. Rollin, A. J. Williams, D. S. Bressler, M. L. Martin, R. Swanepoel, F. J. Burt, P. A. Leman, A. S. Khan, A. K. Rowe, R. Mukunu, A. Sanchez, and C. J. Peters. 1999. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 179S177-S187. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin, L. W., J. M. Meegan, L. J. Strausbaugh, D. M. Morens, and R. H. Watten. 1979. Epidemic Rift Valley fever in Egypt: observations on the spectrum of human illness. Trans. R. Soc. Trop. Med. Hyg. 73630-633. [DOI] [PubMed] [Google Scholar]
- 15.Madani, T. A., Y. Y. Al-Mazrou, M. H. Al-Jeffri, A. A. Mishkhas, A. M. Al-Rabeah, A. M. Turkistani, M. O. Al-Sayed, A. A. Abodahish, A. S. Khan, T. G. Ksiazek, and O. Shobokshi. 2003. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 371084-1092. [DOI] [PubMed] [Google Scholar]
- 16.Meegan, J. M. 1981. Rift Valley fever in Egypt: an overview of epizootics in 1977 and 1978. Contrib. Epidemiol. Biostat. 3100-113. [Google Scholar]
- 17.Meegan, J. M., and C. H. Bailey. 1988. Rift Valley fever, p. 51-76. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology, vol. 4. CRC Press, Boca Raton, FL. [Google Scholar]
- 18.Nichol, S. T. 2001. Bunyavirus, p. 1603-1633. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 19.Paweska, J. T., F. J. Burt, and R. Swanepoel. 2005. Validation of IgG-sandwich and IgM-capture ELISA for the detection of antibody to Rift Valley fever virus in humans. J. Virol. Methods 124173-181. [DOI] [PubMed] [Google Scholar]
- 20.Peters, C. J. 2000. California encephalitis, hantavirus pulmonary syndrome, and Bunyaviridae hemorrhagic fevers, p. 1849-1855. In G. I. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 21.Peters, C. J., J. A. Reynolds, T. W. Slone, D. E. Jones, and E. L. Stephen. 1986. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and macrophage activator. Antivir. Res. 6285-297. [DOI] [PubMed] [Google Scholar]
- 22.Saluzzo, J. F., J. P. Digoutte, C. Chartier, D. Martinez, and R. Bada. 1987. Focus of Rift Valley fever transmission in southern Mauritania. Lancet i504. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez, A., A. S. Khan, S. R. Zaki, G. J. Nabel, T. G. Ksiazek, and C. J. Peters. 2001. Filoviridae: Marburg and Ebola viruses, p. 1279-1304. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 24.Sanchez, A., M. Lukwiya, D. Bausch, S. Mahanty, A. J. Sanchez, K. D. Wagoner, and P. E. Rollin. 2004. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J. Virol. 7810370-10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidwell, R. W., and D. F. Smeel. 2003. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antivir. Res. 57101-111. [DOI] [PubMed] [Google Scholar]
- 26.Srikiatkhachorn, A., C. Ajariyakhajorn, T. P. Endy, S. Kalayanarooj, D. H. Libraty, S. Green, F. A. Ennis, and A. L. Rothman. 2007. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J. Virol. 811592-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storch, G. A. 2001. Bunyaviruses, p. 493-531. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 28.Towner, J. S., P. E. Rollin, D. G. Bausch, A. Sanchez, S. M. Crary, M. Vincent, W. F. Lee, C. F. Spiropoulou, T. G. Ksiazek, M. Lukwiya, F. Kaducu, R. Downing, and S. T. Nichol. 2004. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J. Virol. 784330-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods, C. W., A. M. Karpati, T. Grein, N. McCarthy, P. Gaturuku, E. Muchiri, L. Dunster, A. Henderson, A. S. Khan, R. Swanepoel, I. Bonmarin, L. Martin, P. Mann, B. L. Smoak, M. Ryan, T. G. Ksiazek, R. R. Arthur, A. Ndikuyeze, N. N. Agata, C. J. Peters, and World Health Organization Hemorrhagic Fever Task Force. 2002. An outbreak of Rift Valley fever in northeastern Kenya, 1997-98. Emerg. Infect. Dis. 8138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 2007. Outbreak of Rift Valley fever in Kenya, Tanzania, and Somalia, December 2006-April 2007. Wkly. Epidemiol. Rec. 82169-178. [PubMed] [Google Scholar]
- 31.Zeller, H. G., D. Fontenille, M. Traore-Lamizana, Y. Thiongane, and J. P. Digoutte. 1997. Enzootic activity of Rift Valley fever virus in Senegal. Am. J. Trop. Med. Hyg. 56265-272. [DOI] [PubMed] [Google Scholar]