Abstract
Nonhemolytic streptococci (NHS) cause serious infections, such as endocarditis and septicemia. Many conventional phenotypic methods are insufficient for the identification of bacteria in this group to the species level. Genetic analysis has revealed that single-gene analysis is insufficient for the identification of all species in this group of bacteria. The aim of the present study was to establish a method based on sequence analysis of the 16S-23S intergenic spacer (ITS) region and the partial gdh gene to identify clinical relevant NHS to the species level. Sequence analysis of the ITS region was performed with 57 NHS reference or clinical strains. Satisfactory identification to the species level was achieved for 14/19 NHS species included in this study on the basis of sequence analysis of the ITS region. Streptococcus salivarius and Streptococcus vestibularis obtained the expected taxon as the best taxon match, but there was a short maximum score distance to the next best match (distance, <10). Streptococcus mitis, Streptococcus oralis, and Streptococcus pneumoniae could not be unambiguously discriminated by sequence analysis of the ITS region, as was also proven by phylogenetic analysis. These five species could be identified to the group level only by ITS sequence analysis. Partial gdh sequence analysis was applied to the 11 S. oralis strains, the 11 S. mitis strains, and the 17 S. pneumoniae strains. All except one strain achieved a satisfactory identification to the species level. A phylogenetic algorithm based on the analysis of partial gdh gene sequences revealed three distinct clusters. We suggest that sequence analysis of the combination of the ITS region and the partial gdh gene can be used in the reference laboratory for the species-level identification of NHS.
Streptococci are a heterogeneous group of bacteria consisting of more than 50 species. In addition to the traditional pathogenic pneumococci and hemolytic streptococci (HS), many species of non-HS (NHS), which are part of the commensal microbiota in the human body, are known to be opportunistic pathogens that cause serious systemic and local infections. These infections include subacute infective endocarditis (24), bacteremia in immunocompromised patients (19, 44), brain abscesses (34), meningitis (35), and pneumonia (5).
There are reports describing associations between some specific species and clinical manifestations. Streptococcus gallolyticus and Streptococcus lutetiensis in the bovis group are reported to have a strong association with colon cancer (31-33). Streptococcus anginosus and Streptococcus intermedius in the anginosus group are associated with abscess formation (9, 26), and Streptococcus mitis and other normal oral commensals have a strong association with infective endocarditis (6, 10, 22). The precise species-level identification of NHS from relevant clinical specimens is crucial to making the right diagnosis and understanding the pathogenesis of the infection.
The conventional phenotypic tests do not always allow accurate identification. The automated systems, such as the Rapid ID 32 Strep and the Vitek 2 GP systems (BioMerieux, France), which are based on phenotypic tests, are widely applied in the clinical microbiology laboratory. The large number of species relative to the number of biochemical traits examined, the variability of several traits within species, the poor reproducibility of some tests, and the lack of sufficient phenotypic data for more recently described species in the underlying databases often result in shortcomings with regard to the exact species designations (14, 23, 29, 41). In a study by Hoshino et al. (23) with 148 strains consisting of 115 clinical isolates and 33 reference strains, the rate of correct identification by commercial kits was below 50% but varied significantly between species. The most significant problems were observed with S. mitis, S. oralis, and the 11 Streptococcus species that have been described since 1991. These inherent problems call for alternative means of identification.
Early and effective antimicrobial treatment can result in negative cultures with important clinical specimens, e.g., heart valve tissue or brain abscess material. This stresses the need for the possibility of performing non-culture-based molecular biology examinations (43).
Gene sequence analysis has been applied in an attempt to make an accurate species-level identification of streptococci. The target sequences have included genes encoding functional RNA (the 16S rRNA gene [3], rnpB [45]), protein-coding genes (sodA [23], tuf [37], groESL [42], rpoB [11], gdh and ddl [12]), and noncoding spacer regions like the intergenic spacer (ITS) region (7). However, it seems that the analysis of no single gene is sufficient for this very heterogeneous group (23, 40).
Alternatively, the performance of multilocus sequence analysis, as suggested by some studies (23), is not always realistic in the local clinical microbiology laboratory or even in reference laboratories.
The ITS region has been reported to be used for the strain typing of staphylococci and Clostridium difficile (15, 16). It is also suggested to be a good candidate for species identification (17, 20, 36). According to Chen et al., the intraspecies similarity scores of 11 viridans group streptococcal species were high and ranged from 0.97 to 1.0, and pairwise comparison of two species of viridans group streptococci revealed a lower level of sequence similarity between their ITS regions than between their 16S rRNA gene sequences (7). These characteristics indicate that the ITS region might constitute a more discriminative target sequence than the 16S rRNA gene for the differentiation of closely related species of NHS.
The gdh gene, which is about 1,500 bp in length, encodes a 45-kDa glutamate-6-phosphate dehydrogenase. It is used together with six other housekeeping genes for multilocus sequence typing of pneumococci (23). The gdh gene is reportedly highly conserved, as it exhibits an extremely small number of point mutations relative to the numbers in other genes (30). It has been shown that partial gdh sequences could be used to unambiguously differentiate S. pneumoniae from S. mitis and S. oralis (25, 30).
The purpose of this study was to establish a method based on ITS and partial gdh sequence analysis that is capable of unambiguously identifying clinically relevant NHS to the species level. At the same time, the method should be able to be easily applied in a reference laboratory.
MATERIALS AND METHODS
Bacterial strains.
The 68 streptococcal strains used in our study are listed in Table 1. There were 57 strains representing 19 species of NHS and 11 strains representing 5 species of HS. Twenty-three of the strains were obtained from the American Type Culture Collection (ATCC; Manassas, VA), the National Collection of Type Strains (NCTC; London, United Kingdom), or the Culture Collection of the University of Göteborg (CCUG; Göteborg Sweden). The strains with SSI numbers were reference strains from the Neisseria and Streptococcus Reference Laboratory at the Statens Serum Institut (SSI; Copenhagen, Denmark). The strains with SK numbers were obtained from M. Kilian (Institute of Medical Microbiology and Immunology, University of Aarhus, Aarhus, Denmark). All the strains used in the study were well characterized by conventional phenotypic methods, including microscopy; the evaluation of growth characteristics; performance of the catalase test; evaluation with the Rapid ID32 Strep system; determination of the production of pyrrolidonyl aminopeptidase, leucine aminopeptidase, β-galactosidase, β-N-acetylglucosaminidase, β-glucosidase, α-galactosidase, alkaline phosphatase, arginine decarboxylase, urease, extracellular polysaccharide (dextran and levan), and esculetin from esculin; performance of the peroxide test; and the detection of acid production from inulin, salicin, raffinose, amygdalin, and glycogen. The reference strains from SSI, ATCC, NCTC, and CCUG were also identified by 16S rRNA gene sequence analysis at SSI (8). Most of the SK strains were also well identified by phylogenetic analysis of the nucleotide sequences of four housekeeping genes, ddl, sodA, gdh, and rpoB, at the Institute of Medical Microbiology and Immunology, University of Aarhus (23).
TABLE 1.
The 68 strains of the genus Streptococcus used in the study
| Group | Taxon | Strain identifier | GenBank accession no.
|
|
|---|---|---|---|---|
| ITS region | Partial gdh gene | |||
| Hemolytic group | HS group A | ATCC 51500 | EU860331a | |
| HS group A | ATCC 700294 | AE004092c | ||
| HS group A | SSI-SF 130 | EU860332a | ||
| HS group B | Group B III, M781 | EU860333a | ||
| HS group B | SSI 848 | EU860334a | ||
| HS group C | 74 Lancefield | EU860335a | ||
| HS group C | SSI 329/04 (S. zooepidemicus) | EU860336a | ||
| HS group G | ATCC 2394 D166B | EU860339a | ||
| HS group L | NCTC 10238 SHC | EU860340a | ||
| Streptococcus dysgalactiae | NCTC 4335 | EU860341a | ||
| Streptococcus uberis | NCTC 3858 | EU860355a | ||
| Anginosus group | Streptococcus anginosus | SSI 1353/99 | EU860342a | |
| Streptococcus constellatus | NCTC 11325T | EU860343a | ||
| Streptococcus intermedius | NCTC 11324T | EU860344a | ||
| Sanguinis group | Streptococcus gordonii | NCTC 3165 | EU860337a | |
| NCTC 7865T | EU860346a | |||
| Streptococcus sanguinis | SSI 1655/99 | EU860347a | ||
| Streptococcus parasanguinis | SSI 16/03 | EU860350a | ||
| Mitis group | Streptococcus mitis | SSI 992/99 | EU860348a | EU850792a |
| Streptococcus mitis | SK320 | EU860298a | EU850784a | |
| Streptococcus mitis | SK599 | EU860300a | EU850785a | |
| Streptococcus mitis | SK612 | EU860301a | EU850786a | |
| Streptococcus mitis | SK614 | EU860302a | EU850787a | |
| Streptococcus mitis | SK632 | EU860303a | EU850788a | |
| Streptococcus mitis | SK648 | EU860304a | EU850789a | |
| Streptococcus mitis | SK661 | EU860305a | EU850790a | |
| Streptococcus mitis | SK677 | EU860306a | EU850791a | |
| Streptococcus mitis | SK572 | EU860299a | AB199460b | |
| Streptococcus mitis | CCUG31611T | AY347550b | EU850793a | |
| Streptococcus oralis | SK100 | EU860322a | AB199466b | |
| Streptococcus oralis | SK152 | EU860323a | AB199487b | |
| Streptococcus oralis | SK394 | EU860325a | AB199488b | |
| Streptococcus oralis | SK555 | EU860326a | AB199490b | |
| Streptococcus oralis | SK570 | EU860327a | AB199470b | |
| Streptococcus oralis | SK573 | EU860328a | AB199471b | |
| Streptococcus oralis | SK580 | EU860329a | AB199472b | |
| Streptococcus oralis | SSI 220/02 | EU860349a | EU850795a | |
| Streptococcus oralis | SK155 | EU860324a | EU850796a | |
| Streptococcus oralis | SK610 | EU860330a | EU850797a | |
| Streptococcus oralis | CCUG 24891T | AY347551b | AB199448b | |
| Streptococcus pneumoniae | SSISP1/4 | EU860357a | EU850782a | |
| Streptococcus pneumoniae | SSISP 3/6 | EU860358a | EU850783a | |
| Streptococcus pneumoniae | SSI6A | EU860319a | EU850779a | |
| Streptococcus pneumoniae | SSI6B | EU860318a | EU850780a | |
| Streptococcus pneumoniae | SSI14 | EU860320a | EU850770a | |
| Streptococcus pneumoniae | SSI18A | EU860317a | EU850771a | |
| Streptococcus pneumoniae | SSI18B | EU860316a | EU850772a | |
| Streptococcus pneumoniae | SSI18C | EU860315a | EU850773a | |
| Streptococcus pneumoniae | SSI18F | EU860314a | EU850774a | |
| Streptococcus pneumoniae | SSI 19A | EU860313a | EU850775a | |
| Streptococcus pneumoniae | SSI 19B | EU860312a | EU850776a | |
| Streptococcus pneumoniae | SSI 19C | EU860311a | EU850777a | |
| Streptococcus pneumoniae | SSI 19F | EU860310a | EU850778a | |
| Streptococcus pneumoniae | ATCC 49619 | EU860321a | EU850781a | |
| Streptococcus pneumoniae | SSI R6 | AE008485c | AE008485c | |
| Streptococcus pneumoniae | TIGR4 | AE005672c | AE005672c | |
| Streptococcus pneumoniae | CCUG28588T | AY347557b | EU860362a | |
| Salivarius group | Streptococcus salivarius | ATCC 9759 | EU860351a | |
| Streptococcus vestibularis | NCTC 12166T | EU860352a | ||
| Streptococcus thermophilus | ATCC BAA-250 | EU860353a | ||
| Mutans group | Streptococcus sobrinus | NCTC 10921 | DQ204559b | |
| Streptococcus mutans | Type C 10449 | EU860356a | ||
| Bovis group | Streptococcus gallolyticus subsp. gallolyticus | CCUG 35224T | EU860360a | |
| Streptococcus infantarius subsp. infantarius | CCUG 43820T | EU860359a | ||
| Streptococcus lutetiensis | CCUG 46149T | EU860361a | ||
| Streptococcus equinus | ATCC 15351d | EU860345a | ||
| Streptococcus equinus | NCTC 10389 | EU860338a | ||
| Single species | Streptococcus suis | R735 | EU860354a | |
Sequences obtained from our study.
Sequences already published in GenBank.
Published whole genome.
This strain was named S. bovis earlier.
DNA extraction.
The genomic DNA of 35 strains was extracted from the cultures by using a QIAmp DNA minikit (Qiagen, Hilden, Germany), according to the manufacturer's specifications. The genomic DNA of 33 strains was extracted by boiling the culture: one to three colonies of each strain were boiled for 10 min at 95°C in 100 μl PCR-grade water.
PCR primers.
To amplify the ITS region, we designed a forward primer, primer Strep16S-1471F (5′-GTG GGA TAG ATG ATT GGG GTG AAG T-3′), the 5′ end of which is located at position 1471 of the 16S rRNA gene (Escherichia coli numbering). Reverse primer 6R-IGS (5′-GGG TTC CCC CAT TCG GAH AT-3′) was adapted and improved from the reverse primer of Chen et al. (7). The 5′ end of primer 6R-IGS is located at position 108 downstream of the 5′ end of the 23S rRNA gene (E. coli numbering).
To amplify the partial gdh gene, we used two primers, primer Strep-gdhF (5′-ATGGACAAACCAGCNAGYTT-3′) and primer Strep-gdhR (5′-GCT TGA GGT CCC ATR CTN CC-3′), which amplify a 660-bp amplicon.
PCR analysis of ITS region and partial gdh gene.
PCR of the ITS region and subsequent sequence analysis were performed with all 68 streptococcal strains. PCR of the partial gdh gene and subsequent sequence analysis were performed only with the 39 strains belonging to the mitis group (11 S. oralis strains, 11 S. mitis strains, and 17 S. pneumoniae strains).
The PCR was performed with 50-μl reaction volumes consisting of 1× HotStarTaq master mix (containing final concentrations of 2.5 U HotStarTaq DNA polymerase, 1× PCR buffer, 200 μM each deoxynucleoside triphosphate; Qiagen) and 0.4 μM (final concentration) each primer. The conditions of the PCR with the primers for the ITS region (primers Strep16S-1471F and 6R-IGS) were as follows: 95°C for 15 min and 40 cycles of 94°C for 30 s, 61°C for 30 s, and 72°C for 30 s. The conditions of the PCR with the primers for the partial gdh region were as follows: 95°C for 15 min and 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Both PCRs were performed with a DNA Engine Dyad cycler (Bio-Rad). The PCR products were analyzed by 2% gel electrophoresis after staining of the gels with ethidium bromide. The PCR products were purified with spin columns (Microcon YM-100 filter units; Millipore, Billerica, MA).
Sequencing of PCR products. (i) sequencing of ITS region and sequence editing.
Both DNA strands of the amplicons were sequenced on an ABI Prism 3100 Avant genetic analyzer (Applied Biosystems, Foster City, CA) with a BigDye (version 3.1) kit (Applied Biosystems). Primers Strep16S-1471F and 6R-IGS were used as sequencing primers.
After sequencing of the PCR product, the sequence had to be edited, as described by Chen et al. (7). Portions of the 16S and 23S rRNA gene regions were removed to obtain the full sequences of the ITS region with CTAAGG at the 5′ ends and AATAA at the 3′ ends of the sequences of the ITS region.
(ii) Sequencing of partial gdh sequence and sequence editing.
Primers Strep-gdhF and Strep-gdhR were used for the sequencing PCR. The sequences were manually edited as described by M. Kilian (Institute of Medical Microbiology and Immunology, University of Aarhus) so that the sequences were more comparable in the NCBI BLAST search engine. For all the strains evaluated in this study, the 5′ ends of the gdh sequences were TTTAAAAACCT, whereas the 3′ ends of the gdh sequences were cut just before the sequence AGA ACC ATA C, so that the 3′ end of the edited sequence was TGC TTC/A TCC.
The edited sequences of the ITS region and the partial gdh gene were compared to sequences deposited in the NCBI database by using the BLAST search engine and by taking into consideration the percentage and number of identities, the maximum score, and E values for the best and the next best taxon matches.
Phylogenetic analysis.
DNA sequences were aligned by using the ClustalW program built into the MEGA (version 4.0) program package. Phylogenetic analysis on the basis of the sequences of the ITS region and the partial gdh gene for the 39 strains belonging to the mitis group were performed by the neighbor-joining and minimal evolution methods in the MEGA (version 4.0) program package (downloaded from http://www.megasoftware.net). The distance between the sequences was calculated by using the Kimura two-parameter model.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the sequences of the ITS region and the partial gdh gene obtained from our study are listed in Table 1.
RESULTS
PCR amplification and determination of sequences of ITS region and partial gdh gene.
PCR of the ITS region yielded a single band for all strains, and the sizes of the bands varied from 550 bp to 650 bp. The DNA fragment encompassed a small portion of the 16S rRNA gene region, the ITS region, and a small portion of the 23S rRNA gene region.
PCR of the partial gdh gene yielded a single band at 660 bp, with the size being constant independent of the species.
Identification of Streptococcus strains (HS and NHS) on the basis of sequence analysis of ITS region.
Sequence analysis of the ITS region was performed with all 68 streptococcal strains. The sizes of the edited ITS region sequences varied from 248 to 498 bp. All species except for the S. mitis and S. pneumoniae strains achieved the expected taxon as the best taxon match. S. anginosus had only a 96% sequence identity. Low maximum score differences from the second best taxon match (difference, <10) were obtained for the S. salivarius and S. vestibularis strains, although the percent sequence identities were high (99 to 100%), and the next best taxon matches belonged to the salivarius group. Among the 39 strains in the mitis group, all 11 S. oralis strains achieved the expected taxon as the best taxon match, although maximum score differences to the next best taxon match (S. mitis or S. pneumoniae) were relatively low (differences, 7 to 17). Four of the 11 strains of S. mitis (strains SK612, SK614, SK648, and SK661) had S. pneumoniae as the best taxon match and S. mitis as the second best taxon match. However, the maximum score differences between the two taxons were very low (differences, 5 to 6). Six of the 11 S. mitis strains and the 17 S. pneumoniae strains had the expected taxon as the best taxon match, and the next best taxon matches were S. pneumoniae and S. mitis, respectively. The maximum score differences between the two best taxon matches were low (differences, 0 to 11) (Table 2).
TABLE 2.
Identification of HS and NHS strains on the basis of ITS sequence dataa
| Taxonomic groups and species | No. of strains | Best taxon match | ITS size (bp) | Identity
|
Maximum score | Maximum score difference from next best taxon match | Identification level | |
|---|---|---|---|---|---|---|---|---|
| No. of base pairs | % | |||||||
| Hemolytic group | 11 | 284-496 | 97-100 | 563-971 | 18-422 | Species | ||
| S. pyogenes | 3 | S. pyogenes | 364-391 | 391 | 100 | 775 | 365-381 | Species |
| S. agalactiae | 2 | S. agalactiae | 295-372 | 295-372 | 100 | 585-735 | 48-49 | Species |
| S. dysgalactiae | 4 | S. dysgalactiae | 301 | 285-369 | 97-100 | 565-648 | 58-79 | Species |
| S. equi | 1 | S. equi | 498 | 496 | 99 | 971 | 767 | Species |
| S. uberis | 1 | S. uberis | 431 | 331 | 99 | 630 | 422 | Species |
| Anginosus group | 3 | 222-319 | 96-100 | 389-632 | 38-394 | Species | ||
| S. anginosus | 1 | S. anginosus | 292 | 222 | 96 | 389 | 38 | Species |
| S. constellatus subsp. constellatus | 1 | S. constellatus | 396 | 319 | 100 | 632 | 394 | Species |
| S. intermedius | 1 | S. intermedius | 347 | 270 | 100 | 535 | 143 | Species |
| Salivarius group | 3 | 272-350 | 99-100 | 533-694 | 8-36 | Species | ||
| S. salivarius | 1 | S. salivarius | 273 | 272 | 99 | 533 | 8 | Group |
| S. vestibularis | 1 | S. vestibularis | 350 | 350 | 100 | 694 | 8 | Group |
| S. thermophilus | 1 | S. thermophilus | 365 | 365 | 100 | 659 | 38 | Species |
| Mutans group | 2 | 229-388 | 99-100 | 454-755 | 232-543 | Species | ||
| S. mutans | 1 | S. mutans | 389 | 388 | 99 | 755 | 543 | Species |
| S. sobrinus | 1 | S. sobrinus | 407 | 229 | 100 | 454 | 232 | Species |
| Bovis group | 5 | 273-274 | 100 | 493-495 | 6-47 | Species | ||
| S. gallolyticus subsp. gallolyticus | 1 | S. gallolyticus subsp. gallolyticus | 274 | 274 | 100 | 495 | 6 | Species |
| S. infantarius subsp. infantarius | 1 | S. infantarius subsp. infantarius | 273 | 273 | 100 | 493 | 47 | Species |
| S. lutetiensis | 1 | S. lutetiensis | 273 | 273 | 100 | 493 | 14 | Species |
| S. equinus | 2 | S. equinus | 364 | 284 | 100 | 563 | 18 | Species |
| Mitis group | 39 | 194-248 | 99-100 | 351-492 | 0-17 | Group | ||
| S. mitis | 7 | S. mitis | 248-249 | 248-249 | 99-100 | 444-448 | 1-11 | Group |
| S. mitis | 4 | S. pneumoniae | 248-249 | 239-248 | 100 | 432-448 | 5-6 | |
| S. oralis | 11 | S. oralis | 246 | 194-246 | 99-100 | 351-472 | 7-17 | Group |
| S. pneumoniae | 17 | S. pneumoniae | 248 | 247-248 | 99-100 | 443-492 | 0-5 | Group |
| Sanguinis group | 4 | 246-336 | 98-100 | 476-628 | 127-397 | Species | ||
| S. gordonii | 2 | S. gordonii | 323-324 | 246-247 | 99-100 | 476-488 | 127-133 | Species |
| S. sanguinis | 1 | S. sanguinis | 341 | 294 | 100 | 583 | 397 | Species |
| S. parasanguinis | 1 | S. parasanguinis | 341 | 336 | 98 | 628 | 253 | Species |
| S. suis | 1 | S. suis | 419 | 419 | 100 | 831 | 617 | Species |
Data are compiled for strains belonging to the same species.
Identification of strains belonging to the mitis group on the basis of sequence analysis of partial gdh gene.
Sequence analysis of the partial gdh gene was performed with the 39 strains belonging to the mitis group. All of the edited sequences of the partial gdh gene were 431 bp in length (Table 3). Among these strains, 38 strains achieved satisfactory identification to the expected taxon with a long maximum score distance to the next best taxon (difference, 18 to 195). Only one S. mitis strain (strain SK611) could not be allocated to a single species on the basis of partial gdh gene sequence analysis, as the maximum scores for S. mitis and S. pseudopneumoniae in both cases were 710. Three other S. mitis strains (strains SK612, SK614, and SK648) that could not be allocated to the expected taxon on the basis of sequence analysis of the ITS region achieved the correct identification on the basis of the subsequent sequence analysis of the partial gdh gene.
TABLE 3.
Identifications of the 39 strains of S. mitis, S. pneumoniae, and S. oralis on the basis of the partial gdh sequencesa
| Taxonomic species | No. of strains | Best taxon match | Next best taxon match | Identity
|
Maximum score | Maximum score difference from next best taxon match | |
|---|---|---|---|---|---|---|---|
| No. of base pairs | % | ||||||
| S. mitis | 10 | S. mitis | S. pneumoniae | 410-419 | 98.5-100 | 708-722 | 18-27 |
| S. mitis | 1 | S. mitis | S. pseudopneumoniae | 410 | 97 | 702 | 0 |
| S. oralisb | 11 | S. oralis | S. mitis | 430-431 | 99-100 | 778 | 36-195 |
| S. pneumoniae | 17 | S. pneumoniae | S. mitis | 423-434 | 99-100 | 764-780 | 67-91 |
Data are compiled for strains belonging to the same species. The partial gdh gene sequences are all 431 bp in length.
The gdh sequences of strains SSI 220/202, SK155, and SK610 and type strain CCUG24891 were achieved in our laboratory. The gdh sequences of strains SK100, SK152, SK555, SK394, SK570, SK573, and SK580 were downloaded from M. Kilian's website (www.immi.au.dk/service/download/kilian).
No misidentification was observed on the basis of sequence analysis of the ITS region and the partial gdh gene.
Phylogenetic analysis on the basis of the sequences of the ITS region and partial gdh gene.
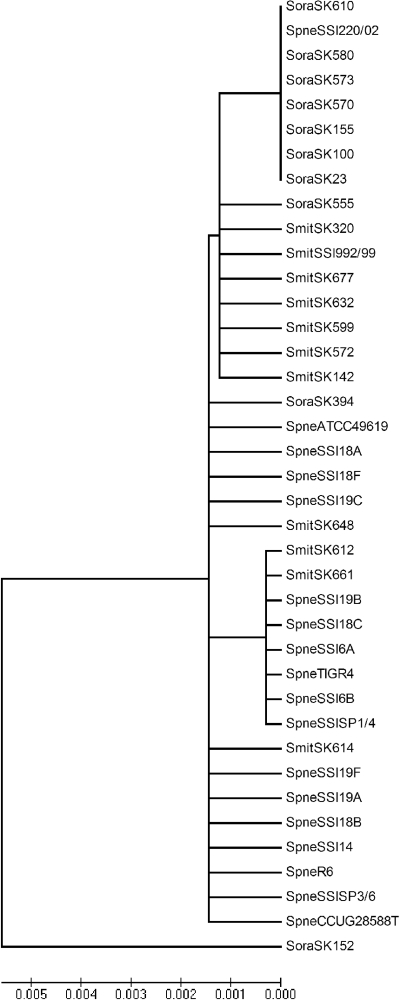
The phylogenetic relationship of S. mitis, S. oralis, and S. pneumoniae derived from the sequences of the ITS region is presented in Fig. 1. The 39 strains failed to form any distinct clusters according to the taxon. The evolutionary distances for 38 strains were within 0.002. The distance between the last S. oralis strain (strain SK152) to the other strains was only 0.005. Therefore, sequence analysis of the ITS region was insufficient for discrimination of the three species S. oralis, S. pneumoniae, and S. mitis from each other.
FIG. 1.
Phylogenetic tree determined on the basis of the sequences of the ITS regions of 11. S. mitis (Smit) strains, 11 S. oralis (Soral) strains, and 17 S. pneumoniae (Spneu) strains obtained by the unrooted neighbor-joining method in the MEGA (version 4.0) program package. The scale bar indicates the evolutionary distance between the sequences determined by calculation of the percent sequence divergence. It clearly demonstrates that S. mitis, S. pneumoniae, and S. oralis are genetically closely related species and cannot be discriminated from each other on the basis of the sequences of their ITS regions.
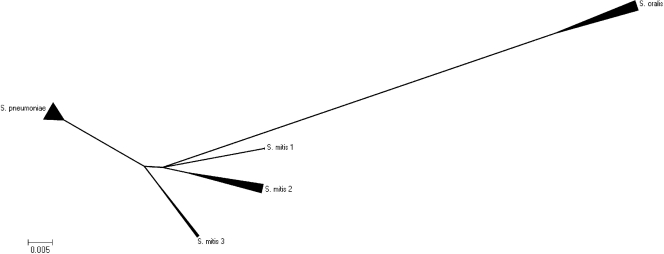
The phylogenetic relationship of S. mitis, S. oralis, and S. pneumoniae on the basis of the sequences of the partial gdh genes of the 39 strains are presented in Fig. 2. S. oralis, S. pneumoniae, and S. mitis were separated as three distinct clusters, with the type strains represented in each of the distinct clusters. The S. pneumoniae and the S. mitis clusters had a shorter distance to each other than to the S. oralis cluster. The S. mitis cluster was much more scattered than the S. pneumoniae cluster. The 11 S. mitis strains formed three subclusters within the S. mitis cluster.
FIG. 2.
Minimal evolution algorithm (suppressed) obtained by using the MEGA (version 4.0) program and based on the partial gdh gene sequences of 11 S. oralis strains, 17 S. pneumoniae strains, and 13 S. mitis strains. It shows that the three species form three distinct clusters. The S. oralis cluster has a longer distance to the two other clusters, indicating that S. pneumoniae and S. mitis are genetically more closely related on the basis of gdh gene evolution. There are three subclusters within the S. mitis cluster, indicating that the species S. mitis contains a heterogeneous group of strains.
DISCUSSION
We describe a method for species-level identification by combining sequence analysis of the ITS region and sequence analysis of the partial gdh gene that is capable of identifying 24 clinically relevant streptococcal species (19 NHS, 5 HS).
In our study, sequence analysis of the ITS region was used as a first-line tool for the identification of species in the genus Streptococcus. All 11 strains (100%) belonging to the five HS species achieved the correct species as the best taxon match on the basis of sequence analysis of the ITS region. The maximum score differences from the next best taxon match varied from 18 to 422. These differences are large enough for the differentiation of species. Of the strains belonging to the 19 NHS species, 53/57 strains (94%) achieved the correct species designation as the best taxon match.
Members of the mutans, sanguinis, and anginosus groups achieved unambiguous identifications with high identity scores, and the differences in the maximum scores from the best to the next best taxon match were significant (232 to 543, 127 to 397, and 38 to 394, respectively).
A high degree of heterogeneity within the S. anginosus species has been reported previously (27). It was suggested that the species contains several subspecies or new species. In our study, we included only one strain of S. anginosus. Therefore, the low percent identity (96%) probably reflects the heterogeneity in this species. Further molecular taxonomic studies are needed to explore this heterogeneity within strains belonging to this species.
The type strain of S. gallolyticus subsp. gallolyticus achieved the expected taxon as the best taxon match, although the maximum score difference from the next best taxon match, S. macedonicus, was only 6. The taxonomy of the bovis group has undergone dynamic changes in the last two decades. Recent studies based on DNA-DNA hybridization, 16S rRNA gene sequencing, and sodA gene sequencing revealed that the S. bovis/S. equinus complex consists of five clusters. S. gallolyticus and S. macedonicus belong to one cluster. The DNA-DNA hybridization data from the same study revealed that the genomes of S. macedonicus and S. gallolyticus display >70% homology, which supports the hypothesis that S. macedonicus and S. gallolyticus are a single species (38). The four other strains belonging to the bovis group included in this study, S. infantarius subsp. infantarius, S. lutetiensis, and S. equinus, achieved unambiguous species identification on the basis of sequence analysis of the ITS region. Strain ATCC 15351 was previously named S. bovis. The high degrees of similarity by both DNA-DNA hybridization and 16S rRNA gene sequencing brought the conclusion that the species S. equinus and S. bovis belong to a single species. The name S. equinus has nomenclatural priority. Therefore, S. bovis is no longer a recognized taxon (39).
Among the members of the salivarius group, only S. salivarius is commonly identified from a variety of human infections (4, 13, 21). S. thermophilus has been isolated only from dairy products. S. vestibularis was identified from the human oral cavity, and its association with human infections has not been confirmed. In our study, both S. salivarius and S. vestibularis achieved the correct taxon as the best taxon match, although the maximum score distance to the next taxon was only 8. The second best taxon for S. salivarius was S. vestibularis and vice versa. The failure of S. salivarius to produce extracellular polysaccharides on sucrose-containing agar is helpful in securing a correct distinction between these two species.
The differences in maximum scores between the best and the next best taxon matches were very small for the strains belonging to the mitis group (range of differences, 0 to 17), often making it impossible to allocate the strain examined to a specific species. Four strains of S. mitis had S. pneumoniae as the best taxon match, followed very closely by S. mitis (maximum score differences, 5 to 6). The phylogenetic analysis of the results of sequence analysis of the ITS region revealed that S. oralis did not form a distinct cluster in relation to S. mitis and S. pneumoniae. It is well known that members of the mitis group are also closely related on the basis of their 16S rRNA gene sequences (18, 28).
On the basis of these results, we concluded that the ITS region can be used for the species-level identification of strains belonging to the hemolytic, anginosus, mutans, and sanguinis groups. Strains belonging to the salivarius group and the mitis group can be identified only to the group level.
Sequence analysis of the partial gdh gene proved to be useful in separating S. mitis, S. oralis, and S. pneumoniae, which are otherwise hard to differentiate from each other. In this study, 17/17 S. pneumoniae strains (100%), 11/11 S. oralis strains (100%), and 10/11 S. mitis strain (90.9%) were correctly identified. Only one S. mitis strain could not be discriminated from S. pseudopneumoniae. The maximum score difference between these two taxons was 0. This is probably because of the taxonomic changes that have been made in recent years. S. pseudopneumoniae is a relatively new taxon that was first described in 2004 and that is closely related to S. mitis and S. pneumoniae (1). Phylogenetic analysis based on the sodA gene sequence showed that the strains assigned to the species S. pseudopneumoniae were more closely aligned with S. mitis than with S. pneumoniae (1). The phylogenetic analysis based on concatenated partial sequences of the ddl, gdh, rpoB, and sodA genes showed that S. pseudopneumoniae is included within the pneumoniae-mitis-pseudopneumoniae cluster (29). This species was, however, not included in our study.
The three distinct clusters of S. mitis, S. pneumoniae, and S. oralis in the phylogenetic algorithm based on partial gdh sequences proved that gdh sequence analysis is capable of discriminating these three genetically closely related species from each other. As a result, we have a tool for the species-level identification of these species.
The phylogenetic algorithm also gave us information about the genetic relationship between the three species. There was a much longer distance from the S. oralis cluster to the S. mitis and S. pneumoniae clusters than between the S. mitis and S. pneumoniae clusters. This suggests that S. mitis and S. pneumoniae are genetically more closely related to each other than they are to S. oralis. The S. mitis cluster was much more scattered than the clusters of the other two species, and it formed several subclusters. This suggests that S. mitis is genetically more heterogeneous. This is in accordance with the recent observations of Bek-Thomsen et al., who observed that the range of interstrain gdh sequence distances was significantly larger for S. mitis than for what was found among S. pneumoniae strains (2).
In this study, we present a reliable method for the identification of clinically relevant streptococci, with a focus on NHS, to the species level. The method is easy to perform in a laboratory that has sequencing facilities. By sequence analysis of the ITS region, all HS strains and most NHS strains could be identified to the species level. S. mitis, S. pneumoniae, and S. oralis could not be unambiguously discriminated from each other by sequence analysis of the ITS region. A second sequence analysis based on the partial gdh gene distinguished these three species from each other. Only one S. mitis strain could not be unambiguously discriminated from S. pseudopneumoniae, which is probably because of the new nomenclature change. The phylogenetic tree based on the gdh gene sequences clearly shows that S. oralis, S. mitis, and S. pneumoniae form three distinct clusters. If colonies are available, sequencing of the ITS region and the partial gdh gene can both be completed within 24 to 72 h.
On the basis of the results from this study, we conclude that the combination of sequence analysis of the ITS region and sequence analysis of the partial gdh gene is a potential tool for the identification of clinically relevant streptococci in a clinical microbiology reference laboratory.
Acknowledgments
We thank Mogens Kilian, Institute of Medical Microbiology and Immunology, University of Aarhus, for his kind advice in choosing the housekeeping gene gdh for sequencing and for supplying us with reference and clinical strains.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Arbique, J. C., C. Poyart, P. Trieu-Cuot, G. Quesne, M. G. Carvalho, A. G. Steigerwalt, R. E. Morey, D. Jackson, R. J. Davidson, and R. R. Facklam. 2004. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 424686-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bek-Thomsen, M., H. Tettelin, I. Hance, K. E. Nelson, and M. Kilian. 2008. Population diversity and dynamics of Streptococcus mitis, Streptococcus oralis, and Streptococcus infantis in the upper respiratory tracts of adults, determined by a nonculture strategy. Infect. Immun. 761889-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosshard, P. P., S. Abels, M. Altwegg, E. C. Bottger, and R. Zbinden. 2004. Comparison of conventional and molecular methods for identification of aerobic catalase-negative gram-positive cocci in the clinical laboratory. J. Clin. Microbiol. 422065-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carley, N. H. 1992. Streptococcus salivarius bacteremia and meningitis following upper gastrointestinal endoscopy and cauterization for gastric bleeding. Clin. Infect. Dis. 14947-948. [DOI] [PubMed] [Google Scholar]
- 5.Carratala, J., B. Roson, A. Fernandez-Sevilla, F. Alcaide, and F. Gudiol. 1998. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch. Intern. Med. 158868-872. [DOI] [PubMed] [Google Scholar]
- 6.Catto, B. A., M. R. Jacobs, and D. M. Shlaes. 1987. Streptococcus mitis. A cause of serious infection in adults. Arch. Intern. Med. 147885-888. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. C., L. J. Teng, and T. C. Chang. 2004. Identification of clinically relevant viridans group streptococci by sequence analysis of the 16S-23S ribosomal DNA spacer region. J. Clin. Microbiol. 422651-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, J. J., R. Dargis, M. S. Kaltoft, K. Andresen, and M. Kemp. 2006. Ribosomal DNA sequencing of streptococci: usefulness in species identification? Int. Congr. Ser. 1289155-158. [Google Scholar]
- 9.Claridge, J. E., III, S. Attorri, D. M. Musher, J. Hebert, and S. Dunbar. 2001. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin. Infect. Dis. 321511-1515. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, C. W., J. Heath, K. K. Hampton, and F. E. Preston. 1993. Identity of viridans streptococci isolated from cases of infective endocarditis. J. Med. Microbiol. 39179-182. [DOI] [PubMed] [Google Scholar]
- 11.Drancourt, M., V. Roux, P. E. Fournier, and D. Raoult. 2004. rpoB gene sequence-based identification of aerobic gram-positive cocci of the genera Streptococcus, Enterococcus, Gemella, Abiotrophia, and Granulicatella. J. Clin. Microbiol. 42497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnier, F., G. Gerbaud, P. Courvalin, and M. Galimand. 1997. Identification of clinically relevant viridans group streptococci to the species level by PCR. J. Clin. Microbiol. 352337-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam, M., K. B. Chopra, D. D. Douglas, R. A. Stewart, and S. Kusne. 2007. Streptococcus salivarius bacteremia and spontaneous bacterial peritonitis in liver transplantation candidates. Liver Transplant. 131582-1588. [DOI] [PubMed] [Google Scholar]
- 14.Gorm, J. T., K. H. Bossen, and B. Bruun. 1999. Evaluation of the Rapid ID 32 Strep system. Clin. Microbiol. Infect. 5417-423. [DOI] [PubMed] [Google Scholar]
- 15.Gurtler, V. 1993. Typing of Clostridium difficile strains by PCR-amplification of variable length 16S-23S rDNA spacer regions. J. Gen. Microbiol. 1393089-3097. [DOI] [PubMed] [Google Scholar]
- 16.Gurtler, V., and H. D. Barrie. 1995. Typing of Staphylococcus aureus strains by PCR-amplification of variable-length 16S-23S rDNA spacer regions: characterization of spacer sequences. Microbiology 141(Pt 5)1255-1265. [DOI] [PubMed] [Google Scholar]
- 17.Gurtler, V., and V. A. Stanisich. 1996. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 142(Pt 1)3-16. [DOI] [PubMed] [Google Scholar]
- 18.Haanpera, M., J. Jalava, P. Huovinen, O. Meurman, and K. Rantakokko-Jalava. 2007. Identification of alpha-hemolytic streptococci by pyrosequencing the 16S rRNA gene and by use of VITEK 2. J. Clin. Microbiol. 45762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, X. Y., M. Kamana, and K. V. Rolston. 2006. Viridans streptococci isolated by culture from blood of cancer patients: clinical and microbiologic analysis of 50 cases. J. Clin. Microbiol. 44160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry, T., P. C. Iwen, and S. H. Hinrichs. 2000. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 381510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoecker, J. L., L. K. Pickering, D. Groschel, and S. Kohl. 1978. Streptococcus salivarius sepsis in children with malignancies. J. Pediatr. 92337-338. [DOI] [PubMed] [Google Scholar]
- 22.Horaud, T., and F. Delbos. 1984. Viridans streptococci in infective endocarditis: species distribution and susceptibility to antibiotics. Eur. Heart J. 5(Suppl. C)39-44. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino, T., T. Fujiwara, and M. Kilian. 2005. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J. Clin. Microbiol. 436073-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu, R. B., and F. Y. Lin. 2006. Effect of penicillin resistance on presentation and outcome of nonenterococcal streptococcal infective endocarditis. Cardiology 105234-239. [DOI] [PubMed] [Google Scholar]
- 25.Ip, M., F. Chi, S. S. Chau, M. Hui, J. Tang, and P. K. Chan. 2006. Use of the housekeeping genes, gdh (zwf) and gki, in multilocus sequence typing to differentiate Streptococcus pneumoniae from Streptococcus mitis and Streptococcus oralis. Diagn. Microbiol. Infect. Dis. 56321-324. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs, J. A., H. G. Pietersen, E. E. Stobberingh, and P. B. Soeters. 1995. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serologic characteristics. Am. J. Clin. Pathol. 104547-553. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, J. A., C. S. Schot, and L. M. Schouls. 2000. The Streptococcus anginosus species comprises five 16S rRNA ribogroups with different phenotypic characteristics and clinical relevance. Int. J. Syst. Evol. Microbiol. 50(Pt 3)1073-1079. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura, Y., X. G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45406-408. [DOI] [PubMed] [Google Scholar]
- 29.Kilian, M., K. Poulsen, T. Blomqvist, L. S. Havarstein, M. Bek-Thomsen, H. Tettelin, and U. B. Sorensen. 2008. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3e2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiratisin, P., L. Li, P. R. Murray, and S. H. Fischer. 2005. Use of housekeeping gene sequencing for species identification of viridans streptococci. Diagn. Microbiol. Infect. Dis. 51297-301. [DOI] [PubMed] [Google Scholar]
- 31.Klein, R. S., M. T. Catalano, S. C. Edberg, J. I. Casey, and N. H. Steigbigel. 1979. Streptococcus bovis septicemia and carcinoma of the colon. Ann. Intern. Med. 91560-562. [DOI] [PubMed] [Google Scholar]
- 32.Klein, R. S., R. A. Recco, M. T. Catalano, S. C. Edberg, J. I. Casey, and N. H. Steigbigel. 1977. Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med. 297800-802. [DOI] [PubMed] [Google Scholar]
- 33.Kok, H., R. Jureen, C. Y. Soon, and B. H. Tey. 2007. Colon cancer presenting as Streptococcus gallolyticus infective endocarditis. Singapore Med. J. 48e43-e45. [PubMed] [Google Scholar]
- 34.Lu, C. H., W. N. Chang, Y. C. Lin, N. W. Tsai, P. C. Liliang, T. M. Su, C. S. Rau, Y. D. Tsai, C. L. Liang, C. J. Chang, P. Y. Lee, H. W. Chang, and J. J. Wu. 2002. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. Q. J. Med. 95501-509. [DOI] [PubMed] [Google Scholar]
- 35.Moller, K., E. H. Frederiksen, J. H. Wandall, and P. Skinhoj. 1999. Meningitis caused by streptococci other than Streptococcus pneumoniae: a retrospective clinical study. Scand. J. Infect. Dis. 31375-381. [DOI] [PubMed] [Google Scholar]
- 36.Olsen, R. J., P. A. Cernoch, C. M. Austin, E. A. Graviss, D. H. Farkas, and G. A. Land. 2007. Validation of the MycoAlign system for Mycobacterium spp. identification. Diagn. Microbiol. Infect. Dis. 59105-108. [DOI] [PubMed] [Google Scholar]
- 37.Picard, F. J., D. Ke, D. K. Boudreau, M. Boissinot, A. Huletsky, D. Richard, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2004. Use of tuf sequences for genus-specific PCR detection and phylogenetic analysis of 28 streptococcal species. J. Clin. Microbiol. 423686-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyart, C., G. Quesne, and P. Trieu-Cuot. 2002. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: reclassification of ‘Streptococcus infantarius subsp. coli’ as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype 11.2 as Streptococcus pasteurianus sp. nov. Int. J. Syst. Evol. Microbiol. 521247-1255. [DOI] [PubMed] [Google Scholar]
- 39.Schlegel, L., F. Grimont, E. Ageron, P. A. Grimont, and A. Bouvet. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Microbiol. 53631-645. [DOI] [PubMed] [Google Scholar]
- 40.Simmon, K. E., L. Hall, C. W. Woods, F. Marco, J. M. Miro, C. Cabell, B. Hoen, M. Marin, R. Utili, E. Giannitsioti, T. Doco-Lecompte, S. Bradley, S. Mirrett, A. Tambic, S. Ryan, D. Gordon, P. Jones, T. Korman, D. Wray, L. B. Reller, M. F. Tripodi, P. Plesiat, A. J. Morris, S. Lang, D. R. Murdoch, and C. A. Petti. 2008. Phylogenetic analysis of viridans group streptococci causing endocarditis. J. Clin. Microbiol. 463087-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spelleberg, B., and C. Brandt. 2007. Streptococcus, p. 412-429. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 42.Teng, L. J., P. R. Hsueh, J. C. Tsai, P. W. Chen, J. C. Hsu, H. C. Lai, C. N. Lee, and S. W. Ho. 2002. groESL sequence determination, phylogenetic analysis, and species differentiation for viridans group streptococci. J. Clin. Microbiol. 403172-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai, J. C., L. J. Teng, and P. R. Hsueh. 2008. Direct detection of bacterial pathogens in brain abscesses by polymerase chain reaction amplification and sequencing of partial 16S ribosomal deoxyribonucleic acid fragments. Neurosurgery 62(Suppl. 2)547-555. [DOI] [PubMed] [Google Scholar]
- 44.Tunkel, A. R., and K. A. Sepkowitz. 2002. Infections caused by viridans streptococci in patients with neutropenia. Clin. Infect. Dis. 341524-1529. [DOI] [PubMed] [Google Scholar]
- 45.Westling, K., I. Julander, P. Ljungman, M. Vondracek, B. Wretlind, and S. Jalal. 2008. Identification of species of viridans group streptococci in clinical blood culture isolates by sequence analysis of the RNase P RNA gene, rnpB. J. Infect. 56204-210. [DOI] [PubMed] [Google Scholar]