Abstract
We report a case of Epstein-Barr virus (EBV) primo infection with the development of successive infectious mononucleosis, hemophagocytic lymphohistiocytosis, and B-cell lymphoproliferative disorder in a patient treated with azathioprine for Crohn's disease. This case report suggests that specific EBV-related clinical and virological management should be considered when treating a patient with inflammatory bowel disease with azathioprine.
CASE REPORT
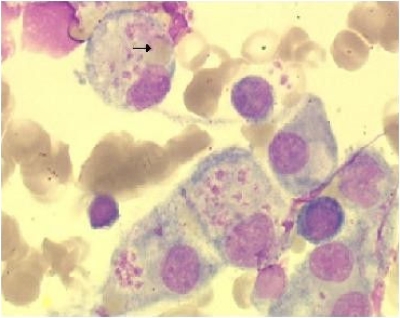
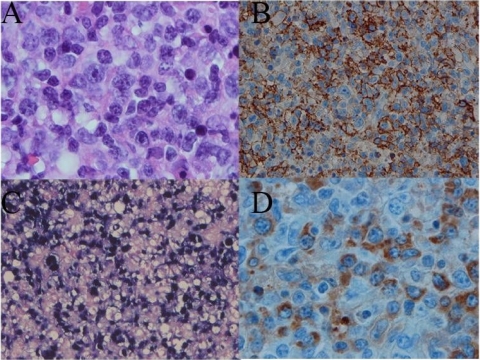
A 25-year-old man was admitted to the infectious disease department of the university medical center of Reims (France) hospital in April 2007 (day 1 of hospitalization) for a chronic fatigue syndrome going on for 5 weeks. This patient was known to suffer from uncomplicated Crohn's disease, diagnosed 6 years before and treated for 3 years with azathioprine. Five weeks before hospitalization, the patient had suffered from a clinical syndrome consisting of asthenia, weight loss, nocturnal sweats, and sore throat. His general practitioner established a diagnosis of infectious mononucleosis syndrome on the basis of the following serological Epstein-Barr virus (EBV) data: EBV viral capsid antigen immunoglobulin M (IgM) and IgG levels of 31 and 35 IU/liter, respectively (N, <15 IU/liter), and 37 and 21 UI/liter, respectively, 1 week later. Anti-Epstein Barr nuclear antigen IgG was only detected at 15 UI/liter (N, <9 IU/liter) in the second serum sample. On clinical examination, a 10% weight loss and a continuous fever of up to 40°C were reported. Eyelid edema, voluminous pharyngitis, mouth mucous membrane ulcerations, generalized lymphadenopathy, and splenomegaly were observed. The azathioprine treatment was immediately stopped. The classical laboratory findings evidenced the presence of pancytopenia (hemoglobin, 81 g/liter; thrombocyte count, 40,000/mm3; leukocyte count, 800 /mm3) without atypical leukocytes or blasts. Liver function tests showed the following: aspartate aminotransferase, 294 IU/liter (N, <40 IU/liter); alanine aminotransferase, 119 IU/liter (N, <40 IU/liter); lactate dehydrogenase, 1,111 IU/liter (N, 200 to 450 IU/liter). The C-reactive protein level was 216 mg/liter (N, <10 mg/liter). Because of the presence of pancytopenia, bone marrow aspiration was performed on day 1 and classical cytological analysis showed normal cellular counts associated with macrophage hyperplasia and signs of hemophagocytosis (specifically, phagocytosis of platelets and erythrocytes) with the absence of abnormal cells (Fig. 1). Taken together, these data allowed us to establish a diagnosis of EBV-related hemophagocytic lymphohistiocytosis (HLH). Treatment with intravenous methylprednisolone (2 mg/kg/day) in association with total human immunoglobulins (1 g/kg/day for 2 days) was started immediately (day 1). Because of an apparently nonclinical response to this therapeutic management, total human immunoglobulins associated with acyclovir and methylprednisolone intravenous bolus injection (1 g/day every 2 days for 5 days and 2 mg/kg/day every other day) were administered on day 9. Moreover, EBV genome quantification by real-time PCR (EBV-PCR; Argene Bosoft, Varhiles, France) showed high viral loads in the peripheral blood (130,000 copies/ml of serum). On day 11, a cervical lymph node biopsy was performed. Histological analysis of the surgical cervical lymph node biopsy material revealed polymorphic B-cell lymphoid proliferation with extensive ischemic necrosis. The normal lymph node architecture was masked by infiltrates of lymphoid cells of variable size demonstrating immunoblastic and plasmacytoid features associated with some sternbergoid cells (Fig. 2A). Erythrophagocytosis was prominent in macrophages. The B-cell origin was demonstrated by positive immunohistochemical staining for CD20 and CD79a (Fig. 2B). The majority of the cells were positive for EBV LMP1 antigen immunostaining and for EBV latency-associated RNA by in situ hybridization (Fig. 2C). Moreover, we determined the presence of monoclonal IgGκ production in tumor cells associated with a high EBV load in lymph node tissues (230,000 copies/μg of total extracted DNA) (Fig. 2D). The diagnosis of EBV-related B-cell lymphoproliferative disorder was confirmed on day 14 by the demonstration of a monoclonal B-cell population by PCR amplification assay of heavy-chain gene variable regions CDRII and CDRIII with a home-made PCR with known primers (1, 17). Because of respiratory distress resulting from upper airway obstruction by giant tonsils and edema of the base of the tongue, our patient was admitted to the intensive care unit, where he underwent an emergency tracheotomy and invasive mechanical ventilation assistance. A chest X-ray showed multiple bilateral alveolar opacities. Broad-spectrum antibiotic polychemotherapy consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone associated with rituximab was administered on day 15. Quantitative EBV genome detection showed a significant increase in the peripheral blood EBV DNA load on day 17 (253,000 copies/ml). The clinical course continued to worsen, with massive upper digestive tract bleeding on day 21. Emergency gastroscopy found a pale ischemic gastric mucous membrane with numerous blood clots. Our patient died on the same day of multiple organ failure. Because X-linked lymphoproliferative disorder (XLP) was suspected, DNA was retrospectively extracted from frozen lymph node tissues. Exons and intronic flanking regions of SH2D1A were directly amplified with pairs of primers (primers and PCR conditions are available upon request). PCR products were sequenced by dideoxynucleotide termination with the Big Dye terminator kit on an ABI Prism 3130 apparatus (Applied Biosystems, Courtaboeuf, France). Sequence analysis of the PCR products revealed the wild-type genes predisposing to XLP. Moreover, human immunodeficiency virus serology testing was negative. No autopsy was performed.
FIG. 1.
High phagocytic activity among macrophages. The arrow indicates marked erythrophagocytosis.
FIG. 2.
Histopathological and immunohistological findings. (A) Infiltration of lymphoid cells of variable size with immunoblastic and plasmacytoid features. (B) Lymphoid proliferation (CD20+ staining). (C) Cells in lymph node tissue positive for EBV LMP1 antigen immunostaining and for EBV latency-associated RNA by in situ hybridization. (D) Tumor cells stained IgGκ positive (immunohistochemistry assay).
Infection with EBV (Gammaherpesviridae) is common in humans and is usually responsible for infectious mononucleosis in the general population. Moreover, EBV can induce HLH, posttransplant lymphoproliferative disorder (PTLD), and lymphoma in immunocompromised hosts (7, 10, 14). Case reports describing HLH and/or lymphoma in patients with Crohn's disease have also been published (5, 8, 13). Azathioprine and 6-mercaptopurine are the first-choice therapeutic strategy for the treatment of steroid-refractory inflammatory bowel disease. These immunomodulating drugs can be associated with the development of EBV-positive HLH or lymphoma (5, 11, 15). This is the first report of a fatal EBV primo infection case associated with the successive development of HLH and B-cell lymphoproliferative disorder in an adult patient treated with azathioprine for Crohn's disease. In the present case, the histopathological pictures were morphologically compatible with both EBV-associated fatal mononucleosis and B-cell lymphoma. Despite the tumoral morphological aspect, our case should be classified as an “early” lesion: infectious mononucleosis-like PTLD, according to the WHO 2001 classification (2).
Hügle et al. had already described a similar clinical history with lymphoma in a patient with proven XLP (6). However, in the present case, there was no familial or molecular evidence of XLP disorder. The only explanation for the fatal EBV-induced acute infection in the present report was the immunosuppressive treatment with azathioprine initiated 3 years earlier. The development of a lymphoproliferative disorder up to lymphoma (8, 13) and HLH (5) syndromes has already been associated with azathioprine treatment in patients with Crohn's disease, but this is the first reported case of simultaneous EBV-related HLH and a B-cell lymphoproliferative disorder.
The risk of lymphoma is elevated in patients treated with azathioprine for Crohn's disease (4, 9). By contrast, no association between Crohn's disease without azathioprine treatment and lymphoma has been demonstrated (12). Dayharsh et al. (3) showed the etiological role of EBV in lymphoma in patients with Crohn's disease, specifically, those treated with azathioprine. Azathioprine significantly decreases T-cell lymphocyte proliferation, thus preventing the immunological clearance of EBV-immortalized B cells. The absence of efficient control of EBV infection can lead to the successive or concomitant development of HLH and a lymphoproliferative disorder up to lymphoma (6).
In the present report, late-onset salvage cytostatic polychemotherapy did not save our patient and an acyclovir regimen was useless, in accordance with rare therapeutic success in previously published articles (7). Early treatments, specifically, etoposide for HLH patients and rituximab for PTLD patients, seems to improve survival (7, 14). We can verify that increased EBV loads despite chemotherapy are related to a poor clinical outcome (16). Our patient died because of multiple organ failure related to an upper digestive tract hemorrhage. The explanation of this hemorrhage and the fundus aspect in gastroscopy remains unclear.
In conclusion, we report a fatal case of EBV primo infection with the development of successive infectious mononucleosis, HLH, and a B-cell lymphoproliferative disorder in a 25-year-old man treated with azathioprine for Crohn's disease. This case report suggests that specific EBV-related clinical and virological management should be considered when treating a patient with inflammatory bowel disease with azathioprine. Serological EBV assays have to be performed to obtain the EBV immunological status of steroid-refractory Crohn's disease patients before beginning azathioprine treatment. Moreover, in order to stop azathioprine and to quickly begin aggressive cytostatic chemotherapy and rituximab, peripheral blood viral load monitoring must be performed to diagnose EBV-induced HLH or lymphoproliferative disorders in patients treated with azathioprine for Crohn's disease.
Acknowledgments
We thank Vince Nicolas (EA-3963) for his help in sequencing the SH2D1A gene region.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Aubin, J., F. Davi, F. Nguyen-Salomon, D. Leboeuf, C. Debert, M. Taher, F. Valensi, D. Canioni, N. Brousse, and B. Varet. 1995. Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia 9471-479. [PubMed] [Google Scholar]
- 2.Chan, J. K. 2001. The new World Health Organization classification of lymphomas: the past, the present and the future. Hematol. Oncol. 19129-150. [DOI] [PubMed] [Google Scholar]
- 3.Dayharsh, G. A., E. V. Loftus, Jr., W. J. Sandborn, W. J. Tremaine, A. R. Zinsmeister, T. E. Witzig, W. R. Macon, and L. J. Burgart. 2002. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 12272-77. [DOI] [PubMed] [Google Scholar]
- 4.Farrell, R. J., Y. Ang, P. Kileen, D. S. O'Briain, D. Kelleher, P. W. Keeling, and D. G. Weir. 2000. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut 47514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido Serrano, A., F. Pérez Martín, F. J. Guerrero Igea, J. Galbarro Muñoz, and S. Palomo Gil. 2000. Fatal infectious mononucleosis during azathioprine treatment in Crohn's disease. Gastroenterol. Hepatol. 237-8. (In Spanish.) [PubMed] [Google Scholar]
- 6.Hügle, B., I. Astigarraga, J. I. Henter, A. Porwit-MacDonald, A. Meindl, and V. Schuster. 2007. Simultaneous manifestation of fulminant infectious mononucleosis with haemophagocytic syndrome and B-cell lymphoma in X-linked lymphoproliferative disease. Eur. J. Pediatr. 166589-593. [DOI] [PubMed] [Google Scholar]
- 7.Imashuku, S. 2002. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit. Rev. Oncol. Hematol. 44259-272. [DOI] [PubMed] [Google Scholar]
- 8.Juffermans, N. P., A. Jager, M. J. Kersten, van M. H. Oers, and D. W. Hommes. 2005. Epstein-Barr virus-related lymphomas in patients with inflammatory bowel disease. Ned. Tijdschr. Geneeskd. 1491859-1863. [PubMed] [Google Scholar]
- 9.Kandiel, A., A. G. Fraser, B. I. Korelitz, C. Brensinger, and J. D. Lewis. 2005. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 541121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobbervig, C., D. Norback, and B. Kahl. 2003. Infectious mononucleosis progressing to fatal malignant lymphoma: a case report and review of the literature. Leuk. Lymphoma 441215-1221. [DOI] [PubMed] [Google Scholar]
- 11.Larvol, L., J. C. Soule, and A. Le Tourneau. 1994. Reversible lymphoma in the setting of azathioprine therapy for Crohn's disease. N. Engl. J. Med. 331883-884. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, J. D., W. B. Bilker, C. Brensinger, J. J. Deren, D. J. Vaughn, and B. L. Strom. 2001. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology 1211080-1087. [DOI] [PubMed] [Google Scholar]
- 13.Losco, A., U. Gianelli, B. Cassani, L. Baldini, D. Conte, and G. Basilisco. 2004. Epstein-Barr virus-associated lymphoma in Crohn's disease. Inflamm. Bowel Dis. 10425-429. [DOI] [PubMed] [Google Scholar]
- 14.Meijer, E., and J. J. Cornelissen. 2008. Epstein-Barr virus-associated lymphoproliferative disease after allogeneic haematopoietic stem cell transplantation: molecular monitoring and early treatment of high-risk patients. Curr. Opin. Hematol. 15576-585. [DOI] [PubMed] [Google Scholar]
- 15.Posthuma, E. F., R. G. Westendorp, A. van der Sluys Veer, J. C. Kluin-Nelemans, P. M. Kluin, and C. B. H. W. Lamers. 1995. Fatal infectious mononucleosis: a severe complication in the treatment of Crohn's disease with azathioprine. Gut 36311-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Laar, J. A., C. M. Buysse, A. C. Vossen, B. Hjálmarsson, B. Van Den Berg, K. Van Lom, and J. Deinum. 2002. Epstein-Barr viral load assessment in immunocompetent patients with fulminant infectious mononucleosis. Arch. Intern. Med. 162837-839. [DOI] [PubMed] [Google Scholar]
- 17.Yamada, M., S. Hudson, O. Tournay, S. Bittenbender, S. S. Shane, B. Lange, Y. Tsujimoto, A. J. Caton, and G. Rovera. 1989. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc. Natl. Acad. Sci. USA 865123-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]