Abstract
Staphylococcus lugdunensis, a rare cause of severe infections such as native valve endocarditis, often causes superficial skin infections similar to Staphylococcus aureus infections. We initiated a study to optimize the identification methods in the routine laboratory, followed by a population-based epidemiologic analysis of patients infected with S. lugdunensis in Viborg County, Denmark. Recognition of a characteristic Eikenella corrodens-like odor on Columbia sheep blood agar combined with colony pleomorphism and prominent β-hemolysis after 2 days of incubation, confirmed by API-ID-32 Staph, led to an 11-fold increase in the detection of S. lugdunensis. By these methods we found 491 S. lugdunensis infections in 4 years, corresponding to an incidence of 53 per 100,000 per year, an increase from 5 infections per 100,000 inhabitants in the preceding years. Seventy-five percent of the cases were found in general practice; these were dominated by skin abscesses (36%), wound infections (25%), and paronychias (13%). Fifty-six percent of the infections occurred below the waist, and toes were the most frequently infected site (21%). Only 3% of the patients suffered from severe invasive infections. The median age was 52 years, and the male/female ratio was 0.69. Our study shows that S. lugdunensis is a common cause of skin and soft-tissue infections (SSTI) and is probably underrated by many laboratories. S. lugdunensis should be accepted as a significant pathogen in SSTI and should be looked for in all routine bacteriological examinations, and clinicians should be acquainted with the name and the pathology of the bacterium.
Staphylococcus lugdunensis, one of the coagulase-negative staphylococci (CoNS), was first described by Freney et al. 20 years ago (8). It causes severe infections similar to Staphylococcus aureus infections, especially acute endocarditis in prosthetic and native valves (14, 24, 29, 30, 36). Other infections such as osteomyelitis (17, 23), peritonitis (28), intravascular catheter infections (5, 32), prosthetic joint infections (26), and urinary tract infections have also been reported (10). S. lugdunensis has been reported as an important cause also of skin and soft tissue infections (SSTI), primarily in the groin (1, 39) and mammae (13, 22, 25, 37, 41).
S. lugdunensis is part of the normal human skin flora and commonly colonizes the perineal region (1, 37, 39); however, carriage rates of different CoNS are not comprehensively accounted for, and only a few have looked for S. lugdunensis carriage (13, 16, 39).
Our study was undertaken after two serious cases of S. lugdunensis infection, a fatal case of endocarditis with protracted bacterial identification but correct antibiotic treatment and a case of late-diagnosed osteomyelitis, which prompted an optimization of our routine microbiological methods for identification of S. lugdunensis. This subsequently increased the number of S. lugdunensis isolates significantly, especially in samples from patients in general practice, and encouraged us to conduct a prospective epidemiologic study.
We here describe the improved method for identification of S. lugdunensis, which led to an 11-fold-increased incidence, and report the epidemiology of the 491 cases identified in Viborg County, Denmark, from July 2002 through June 2006.
MATERIALS AND METHODS
Bacteriology and optimization of microbiological identification.
Prior to optimization of laboratory methods to identify S. lugdunensis, CoNS were speciated only in cases of invasive infections or if found in pure culture. In order to find a reliable method, we tested different methods from previous studies (3, 8, 11, 19, 20, 28).
In the process we noticed a prominent β-hemolysis on Columbia sheep blood agar (Becton Dickinson [BD] Glostrup, Denmark) after 2 days, whereas no β-hemolysis was seen on 5% horse blood agar (Statens Serum Institute Diagnostica [SSI], Hillerød, Denmark). We further observed that S. lugdunensis develops a characteristic odor, similar to that of Eikenella corrodens, when cultured on Columbia sheep blood agar. This odor has been described as “hypochlorite bleach-like” (40).
Differences in β-hemolysis and odor were investigated for 35 different isolates from seven staphylococcal species (5 S. lugdunensis isolates, 5 S. warneri isolates, 5 S. epidermidis isolates, 5 S. haemolyticus isolates, 5 S. capitis isolates, 5 S. simulans isolates, and 5 S. aureus isolates). Analyses were performed on Columbia agar with 5% sheep blood (BD), Columbia agar with 5% calf blood (SSI), 5% horse blood agar (SSI), 5% calf blood agar (SSI), and 5% human blood agar (SSI). Hemolysis was recorded as clear β-hemolysis, incomplete β-hemolysis, and no β-hemolysis.
To verify the characteristics of the odor, a second study was made, in which 12 blindfolded technologists characterized the odor of 35 staphylococcal isolates (5 S. lugdunensis isolates, 4 S. warneri isolates, 5 S. epidermidis isolates, 5 S. haemolyticus isolates, 5 S. hominis isolates, 5 S. capitis isolates, 1 S. simulans isolate, and 5 S. aureus isolates) on Columbia sheep blood agar (BD). Two E. corrodens isolates were included for comparison. Results were recorded as the numbers of isolates that were positive for the characteristic odor on the basis of 12 independent evaluations of each plate.
New routine identification procedures.
Based on the above, new routine procedures were implemented from July 2002. All specimens were inoculated on Columbia agar plates with 5% sheep blood (BD) and read after 24 and 48 h of incubation at 35°C in 5% CO2. Catalase-positive Staphylococcus-like colonies were examined for colony pleomorphism, β-hemolysis, and the characteristic Eikenella-like odor after 48 h of incubation. ID 32 Staph (bioMérieux) was used for confirmatory identification.
Evaluation of PFGE pattern for clonal differences and characteristics.
Pulsed-field gel electrophoresis (PFGE) as described by Tenover et al. (33) was performed on 38 S. lugdunensis isolates; 19 were from Viborg, Denmark (4 from blood cultures, 13 from SSTI, and 1 each from cases of arthritis and osteitis), and 19 were from Växjö, Sweden (5 from blood cultures, 13 from SSTI, and 1 from a fistula).
Antimicrobial susceptibility testing.
Susceptibility testing was performed on Danish blood agar (SSI) using an inoculum giving semiconfluent growth, NEO-Sensitabs (Rosco Diagnostica), and overnight incubation. Antibiotics tested were oxacillin, methicillin (meticillin), penicillin, erythromycin, and fusidic acid. Interpretation of inhibition zones was performed as specified by the manufacturer (25a).
Epidemiologic setting.
Viborg County had approximately 235,500 inhabitants, about 5% of the Danish population. One department of clinical microbiology served all five hospitals and all 125 general practitioners.
All patients from whom S. lugdunensis was isolated from 1 July 2002 to 30 June 2006 were included in the epidemiological study. The type of infection, localization, and previous S. lugdunensis infection were extracted from the laboratory request form.
Additionally, for the period 1 July to 31 December 2002, all patients positive for S. lugdunensis and those with SSTI were followed up by telephone interviews by the general practitioners or by reading the hospital records of admitted patients. The following data were extracted: infection site, type of infection, clinical signs and symptoms, treatment, progress of infection, complications, and relapses. For patients with SSTI from which staphylococci other than S. lugdunensis were isolated, clinical information was extracted from the laboratory request form only.
Neighboring departments of clinical microbiology were interviewed about their methods and number of S. lugdunensis cases identified during 2002.
RESULTS
Optimization of identification methods.
Of the examined staphylococcal isolates only S. lugdunensis and S. aureus isolates showed prominent β-hemolysis around the colonies after 48 h incubation on Columbia agar with 5% sheep blood. The other staphylococcal species showed weak or no hemolysis. S. simulans and S. haemolyticus produced hemolysis on Columbia agar with 5% calf blood. S. lugdunensis did not produce β-hemolysis on human, horse, or calf blood agar without Columbia agar base. The characteristic Eikenella-like odor was present when S. lugdunensis isolates grew on sheep, calf, and human blood agar but not when they were cultured on horse blood agar.
The characteristic odor was present in 93% of the blinded odor tests of S. lugdunensis cultured on 5% Columbia sheep blood agar (Table 1). Other CoNS species were suspected to be S. lugdunensis in 3% to 17% of test situations.
TABLE 1.
Odor evaluation by 12 blindfolded laboratory technologists of 35 staphylococcus and 2 Eikenella corrodens isolates
| Species tested | No. of tests resulting in detection of characteristic odora/total no. of tests |
|---|---|
| Staphylococcus lugdunensis | 56/60 |
| Staphylococcus capitis | 3/60 |
| Staphylococcus warneri | 5/48 |
| Staphylococcus haemolyticus | 10/60 |
| Staphylococcus aureus | 8/60 |
| Staphylococcus hominis | 2/60 |
| Staphylococcus epidermidis | 5/60 |
| Staphylococcus simulans | 1/12 |
| Eikenella corrodens | 17/24 |
The characteristic odor is a hypochlorite bleach-like odor, similar to that characteristic of Eikenella corrodens.
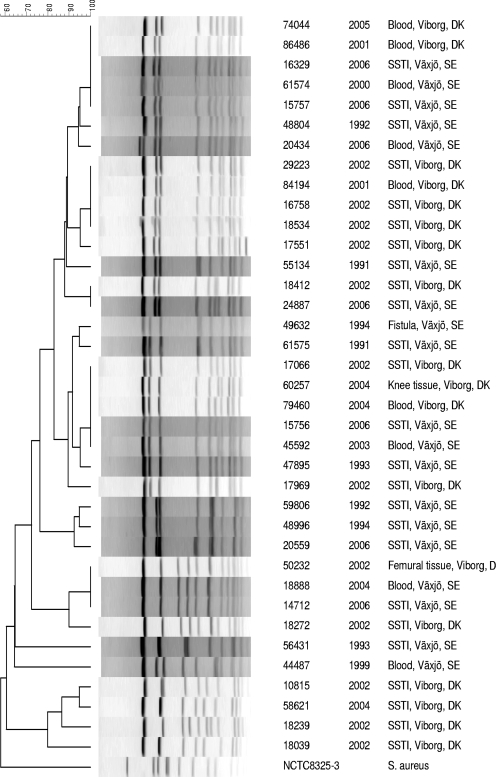
PFGE of the 19 isolates from Viborg County, Denmark, and 19 isolates from Växjö County, Sweden, showed that the same two clones dominated in both countries, and there was no difference between invasive and noninvasive strains regarding PFGE pattern (Fig. 1).
FIG. 1.
PFGE on 38 S. lugdunensis isolates from blood cultures, SSTI, and two deep infections seen in Viborg County, Denmark, and in Växjö County, Sweden, 1991 to 2006.
Epidemiology.
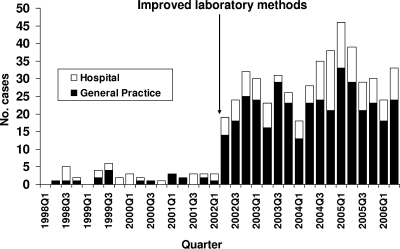
By using the improved methods from the pilot studies, it was determined that the incidence of S. lugdunensis was 53 infections per 100,000 inhabitants per year, in contrast to the value of 5 infections per 100,000 inhabitants per year previously determined (representing an increased rate of detection). Seventy-five percent of the patients were from general practice (Fig. 2). The departments of clinical microbiology in three neighboring counties, all using plating on 5% horse blood plates (SSI) for swabs from SSTI, found from zero to four S. lugdunensis infections per 100,000 inhabitants.
FIG. 2.
Numbers of Staphylococcus lugdunensis infections per quarter, Viborg County, Denmark, January 1998 through June 2006.
During a 6-month period, S. lugdunensis was found in 13% of 159 abscesses from general practice. In these specimens, S. lugdunensis was found as the only pathogen, i.e., “monoculture” in 70% (Table 2). This was similar to results for abscess specimens containing S. aureus isolates, where 86% were found in monoculture, and was significantly more frequent than the frequency for abscess specimens containing monoculture of other CoNS (20%). In swabs from infections other than abscesses, S. lugdunensis strains were found from 0.09% of 1,087 patients with impetigo, 0.16% of 616 with ear infections, 0.6% of 1,614 with unspecified wound infections, 0.7% of 587 with unspecified skin infections, 1.4% of 204 with incisional surgical wound infections, 4.5% of 22 with pustules, and 15% of 13 with infected cutaneous atheromas, respectively.
TABLE 2.
Swab sample results for S. lugdunensis, other CoNS, and S. aureusa
| Organism(s) | Total no. of patients (incidenceb) | No. of abscesses | % of abscesses where bacteria were found in pure culture (CIc) | No. of other SSTI |
|---|---|---|---|---|
| Staphylococcus lugdunensis | 43 (18) | 20 | 70 (46-88) | 23 |
| Other CoNS | 932 (398) | 69 | 20 (12-32) | 863 |
| Staphylococcus aureus | 2,760 (1,178) | 56 | 86 (74-94) | 2,704 |
Swabs were from soft-tissue infections and were taken in general practice in Viborg County, Denmark, during a 6-month sampling period in 2002.
Number of patients/100,000 persons/6 months.
CI, confidence interval.
SSTI caused by S. lugdunensis were clinically indistinguishable from S. aureus infections. Ninety-six percent of S. lugdunensis patients were treated with surgical incision and/or antibiotics. Cases not needing antibiotic or surgical treatment were all superficial wound infections.
During 2002 to 2006 a total of 491 cases were found. The distribution of infectious sites is shown in Table 3. In 45% of the 491 samples S. lugdunensis was found in mixed cultures; however, other significant pathogens (e.g., S. aureus and hemolytic streptococci) were found in 4% of these cultures only.
TABLE 3.
Infection type and demographic data on 491 Staphylococcus lugdunensis infections, Viborg County, July 2002 through June 2006
| Infection type | No. of patients | Median age (yr) (range) | Male/female ratio |
|---|---|---|---|
| Total | 491 | 52 (0-96) | 0.69 |
| Abscesses | 179 | 44 (3-90) | 0.50 |
| Wound infections | 122 | 64 (0-93) | 0.61 |
| Paronychia | 63 | 44 (7-90) | 1.42 |
| Incisional surgical wound | 49 | 54 (6-84) | 0.81 |
| Otitis media | 17 | 1 (0-8) | 2.4 |
| Otitis externa | 8 | 54 (22-83) | 0.15 |
| Urinary tract infections | 15 | 71 (9-89) | 0.50 |
| Deep infections | 14 | 77 (31-96) | 0.75 |
| Other | 24 | 49 (0-93) | 0.5 |
S. lugdunensis infections were primarily seen in middle-aged and elderly people (median age, 52 years) except for otitis media, where a median age of 1 year was found. The overall male/female ratio was 0.69.
Recurrent infections were seen in seven patients: three mamma infections, two genital abscesses, one wound infection, and one dialysis catheter infection.
Invasive S. lugdunensis infections were seen in two patients from general practice and 12 hospitalized patients. Six of these had necrotic toe infections, and among these three had diabetes. One of the patients with toe infection developed gangrene and died following a crus amputation. The other deep infections were two cases of knee arthritis, two deep-seated groin infections, one perineal fistula, one case of bursitis in the elbow, and two lung infections, one a case of empyema and one a case of aspergillosis with S. lugdunensis coinfection.
The antibiotic susceptibilities of the 491 isolates (% resistant) were as follows: penicillin, 20%; fusidic acid, 5%; erythromycin, 2%; methicillin-oxacillin, 0%. There was no difference in antimicrobial susceptibility between isolates from hospitalized patients and isolates from general practice.
DISCUSSION
CoNS from SSTI are seldom routinely diagnosed to species level. Although definitive identification of S. lugdunensis is well described and reliable (3, 8, 19), this study indicates that one problem is failure to suspect S. lugdunensis. Phenotypic characteristics such as colony pleomorphism and β-hemolysis are useful when detecting S. lugdunensis, in contrast to clumping and synergistic hemolysis, which characterize several CoNS species (7). In this study we found that S. lugdunensis gives a prominent β-hemolysis and a characteristic Eikenella-like odor after 2 days of incubation on Columbia agar with 5% sheep blood, which, combined with colony pleomorphism, helps in the initial recognition of S. lugdunensis. This is not described in textbooks such as the Manual of Clinical Microbiology and Topley and Wilson's Microbiology and Microbial Infections (35, 40). The hemolytic peptides with delta-toxin-like activity (SLUSH-A, SLUSH-B, and SLUSH-C) lyse certain mammalian blood cells (38), and this might be an explanation for the differences found in the evaluation of blood agars.
The characteristic odor when isolates were cultured on Columbia sheep blood agar was also present when they were cultured on calf and human blood agar, but not on horse blood agar. The decomposition of certain fatty acids in some mammal erythrocytes might be the cause of this odor (31).
When using the improved methods, we discovered 11 times more S. lugdunensis, infections than before, primarily SSTI. This in combination with the much lower rate of identification of S. lugdunensis found in three neighboring counties indicates that S. lugdunensis is vastly underrated as a cause of infection.
Clinical information supported the findings that S. lugdunensis predominantly causes abscesses and that S. lugdunensis infections are clinically indistinguishable from infections caused by S. aureus. The ability of S. lugdunensis to cause abscesses has also been described in mouse model studies (6, 18).
It has been suggested that S. lugdunensis primarily causes infections below the waist (12, 37), in particular the inguinal area (1, 39). Our results indicate a broader distribution of infected sites, which also was reported in a study by Herchline and Ayers in 1991 (13). The localization of infection seems to be age related. In small children, S. lugdunensis was predominantly found in otitis media, whereas in middle-aged patients infections were most common in armpits, groin, buttocks, mamma, and incisional sites. Among elderly people most infections were in fingers, toes, and leg ulcers. Women were more frequently infected than men, which is in contrast to S. aureus infections but in accordance with a higher carriage rate of S. lugdunensis among women (12, 39). Areas that possess apocrine sweat glands appear to the sites most frequently infected. As has been suggested for S. aureus and Staphylococcus sciuri (4), S. lugdunensis might be a commensal in areas that possesses apocrine sweat glands, i.e., axilla, anogenital region, auditory canals, eyelids, and mammary areolae.
Most of our cases with toe infections were elderly patients (median age, 60 years), and some had diabetes and fungal toe infections. Both elderly people and diabetics already have a higher prevalence of toe infections (2, 9, 27). Previous studies have described the dynamics of toe web infections, where fungal infections induce damage to the stratum corneum, which is followed by a bacterial superinfection. This might also be the case for S. lugdunensis toe infections (15, 21).
To our knowledge only one report has previously described a case of S. lugdunensis mastoiditis in relation to otitis media in a child (34). This contrasts with the 37 cases of ear infections found in the present series.
It is important that microbiologists make the general practitioners and hospital physicians acquainted with S. lugdunensis, its pathology, and risk of serious infections. In Viborg County, findings of S. lugdunensis are now followed by the comment that S. lugdunensis infections should be considered as serious as S. aureus infections.
Acknowledgments
We thank Maiken Jensen and Karen Marie Søby at the Department of Clinical Microbiology in Viborg for laboratory assistance during the study. We thank Gunnar Kahlmeter at the Department of Clinical Microbiology, Växjö, Sweden, for S. lugdunensis isolates and associated clinical information. We thank Dominique L. Monnet and Niels Frimodt-Møller for their helpful comments regarding the manuscript.
Footnotes
Published ahead of print on 25 February 2009.
REFERENCES
- 1.Bellamy, R., and T. Barkham. 2002. Staphylococcus lugdunensis infection sites: predominance of abscesses in the pelvic girdle region. Clin. Infect. Dis. 35E32-E34. [DOI] [PubMed] [Google Scholar]
- 2.Crawford, F., and J. Ferrari. 2006. Fungal toenail infections. Clin. Evid. 2212-2220. [PubMed]
- 3.De Paulis, A. N., S. C. Predari, C. D. Chazarreta, and J. E. Santoianni. 2003. Five-test simple scheme for species-level identification of clinically significant coagulase-negative staphylococci. J. Clin. Microbiol. 411219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eady, E. A., and J. H. Cove. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16103-124. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, S. P., R. Yogev, and S. T. Shulman. 2001. Staphylococcus lugdunensis: an emerging cause of ventriculoperitoneal shunt infections. Pediatr. Neurosurg. 35128-130. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson, K. P., D. W. Lambe, Jr., J. L. Keplinger, and J. H. Kalbfleisch. 1991. Comparison of the pathogenicity of three species of coagulase-negative Staphylococcus in a mouse model with and without a foreign body. Can. J. Microbiol. 37722-724. [DOI] [PubMed] [Google Scholar]
- 7.Fleurette, J., M. Bes, Y. Brun, J. Freney, F. Forey, M. Coulet, M. E. Reverdy, and J. Etienne. 1989. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res. Microbiol. 140107-118. [DOI] [PubMed] [Google Scholar]
- 8.Freney, J., Y. Brun, M. Bes, M. Meugnier, et al. 1988. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Bacteriol. 38168-172. [Google Scholar]
- 9.Gupta, A. K., N. Konnikov, P. MacDonald, P. Rich, N. W. Rodger, M. W. Edmonds, R. McManus, and R. C. Summerbell. 1998. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey. Br. J. Dermatol. 139665-671. [DOI] [PubMed] [Google Scholar]
- 10.Haile, D. T., J. Hughes, E. Vetter, P. Kohner, R. Snyder, R. Patel, and F. R. Cockerill III. 2002. Frequency of isolation of Staphylococcus lugdunensis in consecutive urine cultures and relationship to urinary tract infection. J. Clin. Microbiol. 40654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert, G. A. 1990. Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi. J. Clin. Microbiol. 282425-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellbacher, C., E. Tornqvist, and B. Soderquist. 2006. Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin. Microbiol. Infect. 1243-49. [DOI] [PubMed] [Google Scholar]
- 13.Herchline, T. E., and L. W. Ayers. 1991. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation to infection. J. Clin. Microbiol. 29419-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R. M., M. A. Jackson, C. Ong, and G. K. Lofland. 2002. Endocarditis caused by Staphylococcus lugdunensis. Pediatr. Infect. Dis. J. 21265-268. [DOI] [PubMed] [Google Scholar]
- 15.Kates, S. G., K. M. Nordstrom, K. J. McGinley, and J. J. Leyden. 1990. Microbial ecology of interdigital infections of toe web spaces. J. Am. Acad. Dermatol. 22578-582. [DOI] [PubMed] [Google Scholar]
- 16.Koziol-Montewka, M., A. Szczepanik, I. Baranowicz, L. Jozwiak, A. Ksiazek, and D. Kaczor. 2006. The investigation of Staphylococcus aureus and coagulase-negative staphylococci nasal carriage among patients undergoing haemodialysis. Microbiol. Res. 161281-287. [DOI] [PubMed] [Google Scholar]
- 17.Kragsbjerg, P., J. Bomfim-Loogna, E. Tornqvist, and B. Soderquist. 2000. Development of antimicrobial resistance in Staphylococcus lugdunensis during treatment-report of a case of bacterial arthritis, vertebral osteomyelitis and infective endocarditis. Clin. Microbiol. Infect. 6496-499. [DOI] [PubMed] [Google Scholar]
- 18.Lambe, D. W., Jr., K. P. Ferguson, J. L. Keplinger, C. G. Gemmell, and J. H. Kalbfleisch. 1990. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can. J. Microbiol. 36455-463. [DOI] [PubMed] [Google Scholar]
- 19.Leung, M. J., N. Nuttall, M. Mazur, T. L. Taddei, M. McComish, and J. W. Pearman. 1999. Case of Staphylococcus schleiferi endocarditis and a simple scheme to identify clumping factor-positive staphylococci. J. Clin. Microbiol. 373353-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung, M. J., N. Nuttall, T. M. Pryce, G. W. Coombs, and J. W. Pearman. 1998. Colony variation in Staphylococcus lugdunensis. J. Clin. Microbiol. 363096-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyden, J. J. 1993. Progression of interdigital infections from simplex to complex. J. Am. Acad. Dermatol. 28S7-S11. [DOI] [PubMed] [Google Scholar]
- 22.Lina, B., F. Vandenesch, M. E. Reverdy, T. Greenland, J. Fleurette, and J. Etienne. 1994. Non-puerperal breast infections due to Staphylococcus lugdunensis. Eur. J. Clin. Microbiol. Infect. Dis. 13686-687. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch, D. R., R. J. Everts, S. T. Chambers, and I. A. Cowan. 1996. Vertebral osteomyelitis due to Staphylococcus lugdunensis. J. Clin. Microbiol. 34993-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel, R., K. E. Piper, M. S. Rouse, J. R. Uhl, F. R. Cockerill III, and J. M. Steckelberg. 2000. Frequency of isolation of Staphylococcus lugdunensis among staphylococcal isolates causing endocarditis: a 20-year experience. J. Clin. Microbiol. 384262-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ros, M. J., A. Ramirez, E. Arteaga, C. Alberto, J. Gil, and J. Reina. 1999. Infection by Staphylococcus lugdunensis: clinico-microbiologic characterization of 25 cases. Enferm. Infecc. Microbiol. Clin. 17223-226. [PubMed] [Google Scholar]
- 25a.Rosco Diagnostica. 2002. Rosco's user's guide 15th ed. Rosco Diagnostica, Taastrup, Denmark.
- 26.Sampathkumar, P., D. R. Osmon, and F. R. Cockerill III. 2000. Prosthetic joint infection due to Staphylococcus lugdunensis. Mayo Clin. Proc. 75511-512. [DOI] [PubMed] [Google Scholar]
- 27.Saunte, D. M., J. B. Holgersen, M. Haedersdal, G. Strauss, M. Bitsch, O. L. Svendsen, M. C. Arendrup, and E. L. Svejgaard. 2006. Prevalence of toe nail onychomycosis in diabetic patients. Acta Derm. Venereol. 86425-428. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzler, N., R. Meilicke, G. Conrads, D. Frank, and G. Haase. 1998. Staphylococcus lugdunensis: report of a case of peritonitis and an easy-to-perform screening strategy. J. Clin. Microbiol. 36812-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonheyder, H. C., V. K. Hansen, P. Asschenfeldt, and V. T. Rosdahl. 1993. Staphylococcus lugdunensis: an important cause of endocarditis. A case report. APMIS 101802-804. [DOI] [PubMed] [Google Scholar]
- 30.Sotutu, V., J. Carapetis, J. Wilkinson, A. Davis, and N. Curtis. 2002. The “surreptitious staphylococcus”: Staphylococcus lugdunensis endocarditis in a child. Pediatr. Infect. Dis. J. 21984-986. [DOI] [PubMed] [Google Scholar]
- 31.Stoakes, L., M. A. John, R. Lannigan, B. C. Schieven, M. Ramos, D. Harley, and Z. Hussain. 1994. Gas-liquid chromatography of cellular fatty acids for identification of staphylococci. J. Clin. Microbiol. 321908-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tee, W. S., S. Y. Soh, R. Lin, and L. H. Loo. 2003. Staphylococcus lugdunensis carrying the mecA gene causes catheter-associated bloodstream infection in premature neonate. J. Clin. Microbiol. 41519-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 332233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas, S., C. Hoy, and R. Capper. 2006. Osteomyelitis of the ear canal caused by Staphylococcus lugdunensis. J. Infect. 53e227-e229. [DOI] [PubMed] [Google Scholar]
- 35.Topley, W. W. C., G. S. Wilson, and L. Collier. 1998. Topley and Wilson's microbiology and microbial infections, 9th ed., vol. 2. Hodder Arnold, London, United Kingdom.
- 36.Vandenesch, F., J. Etienne, M. E. Reverdy, and S. J. Eykyn. 1993. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin. Infect. Dis. 17871-876. [DOI] [PubMed] [Google Scholar]
- 37.Vandenesch, F., S. J. Eykyn, J. Etienne, and J. Lemozy. 1995. Skin and post-surgical wound infections due to Staphylococcus lugdunensis. Clin. Microbiol. Infect. 173-74. [DOI] [PubMed] [Google Scholar]
- 38.Vandenesch, F., M. J. Storrs, F. Poitevin-Later, J. Etienne, P. Courvalin, and J. Fleurette. 1991. Delta-like haemolysin produced by Staphylococcus lugdunensis. FEMS Microbiol. Lett. 6265-68. [DOI] [PubMed] [Google Scholar]
- 39.van der Mee-Marquet, N., A. Achard, L. Mereghetti, A. Danton, M. Minier, and R. Quentin. 2003. Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J. Clin. Microbiol. 411404-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Gravenitz, A., R. Zbinden, and R. Mutters. 2007. Actinobacillus, Capnocytophaga, Eikenella, Kingella, Pasteurella, and other fastidious or rarely encountered gram-negative rods, p. 621-635. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 41.Waghorn, D. J. 1994. Staphylococcus lugdunensis as a cause of breast abscess. Clin. Infect. Dis. 19814-815. [DOI] [PubMed] [Google Scholar]