Abstract
Temporal trends of serotypes from invasive pneumococcal disease (IPD) in Spain from 1979 to September 2007 under antibiotic and vaccine pressure were analyzed. A significant trend in pneumococcal conjugate 7-valent vaccine (PCV7) serotypes (except serotype 4) was found, whereby the prevalence increased from the early 1980s and decreased in the 2000s for all but serotype 23F, which began decreasing in the late 1980s. Among the major non-PCV7 serotypes, a significant decrease was observed for serotypes 1, 5, and 7F in the 1980s. From the late 1990s, serotypes 1, 5, 6A, 7F, and 19A increased significantly, while serotypes 3 and 8 showed similar but nonsignificant trends over time. The incidence of IPD cases was 10.7/100,000 for the period 1996 to 2006, with reporting coverage ranging from 18% to 43%. A significant decrease in IPD incidence due to PCV7 serotypes was observed, while the incidence of non-PCV7 serotypes increased, with the consequence that there was no clear pattern in the overall incidence of IPD. Penicillin nonsusceptibility was correlated with the proportion of PCV7 serotypes. Erythromycin nonsusceptibility increased in association with long-half-life macrolide consumption and then decreased in 2004 to 2007. The increase in PCV7 serotypes and antibiotic nonsusceptibility related to antibiotic consumption in the 1980s and 1990s was reversed in the 2000s, probably as a result of PCV7 immunization. The decrease in IPD incidence due to PCV7 serotypes was mirrored by an increase in that of non-PCV7 serotypes. The impact of various preventive/therapeutic strategies on pneumococcal evolution is serotype dependent, and the dynamics remain unpredictable.
In the United States, the introduction of the pneumococcal conjugate 7-valent vaccine (PCV7) into the childhood vaccination calendar produced a dramatic reduction in the incidence of invasive pneumococcal disease (IPD) among children and adults (35, 36). The marked decrease in PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) consequently affected penicillin and erythromycin nonsusceptibility (20). However, reduction of PCV7 types was accompanied by an increase in non-PCV7-serotype IPD incidence with a rise in penicillin nonsusceptibility, mainly due to serotype 19A (2, 18, 22, 24, 29).
In Spain, from 1979 to 2007, different therapeutic approaches (antibiotics) and preventive measures (PCV7) were introduced into medical practice. These have the potential to influence the evolution of pneumococcal populations by altering the serotype distribution and/or antibiotic nonsusceptibility patterns. PCV7 was licensed for use in June 2001 for children under 2 years of age who were at clinical risk and in 2004 for those less than 5 years old. The vaccine was not subsidized by the Spanish Health Service, although the Spanish Academy of Pediatrics recommended PCV7 immunization for all children under 2 years of age. Initially, PCV7 coverage based on vaccine sales was low, but its use increased from 2002 onwards with reported vaccine coverage below 50% before 2006 (15). Penicillin nonsusceptibility increased sharply in Spain up to 1989 (12) and then remained stable with a nonsusceptibility rate of about 42% during the 1990s (11). Selection and dissemination of penicillin and/or erythromycin nonsusceptibility in Streptococcus pneumoniae were associated with antibiotic consumption, mainly of oral cephalosporins (among β-lactams) and long-half-life macrolides (14), consumption of the latter being the more important driver of nonsusceptibility in both antibiotics (13).
Previously published Spanish studies were hampered by the lack of reliable IPD incidence rates (24, 25). Serotype and antimicrobial susceptibility data rely on the passive, laboratory-based surveillance system at the Spanish Reference Laboratory for Pneumococci (SRLP), which receives clinical isolates sent voluntarily from all over the country.
The aim of this study was to analyze trends of serotype evolution among invasive pneumococcus isolates received at the SRLP from 1979 to 2007 and to assess the potential influence of antibiotic introduction and consumption and their response to PCV7 distribution. Moreover, the present study represents a reliable approach to determining IPD incidence in Spain between 1996 and 2006.
MATERIALS AND METHODS
Strains and microbiological and molecular methods.
The study included 22,831 invasive pneumococcal isolates received at the SRLP between January 1979 and September 2007 from 190 hospitals throughout the entire country. The coverage of national hospital beds by the SRLP improved from less than 18% before 1996 and from 1996 to 1997 to almost 40% in 2006 to 2007. The proportions of analyzed isolates from children (≤14 years old) have increased over time, from less than 20% before 1996 to 25% in 2006 to 2007. Serotyping was performed with the quellung reaction, dot blot assay (10), and/or real-time PCR (34). Susceptibility to penicillin and erythromycin (over the whole period) and that to levofloxacin (from 2002 onwards) were determined by the agar dilution technique (12) in accordance with the criteria of the Clinical and Laboratory Standards Institute (CLSI) (5). Breakpoints for nonsusceptibility were those defined by the CLSI (6): penicillin, ≥0.12 μg/ml; erythromycin, ≥0.5 μg/ml; and levofloxacin, ≥4 μg/ml.
Antibiotic consumption and vaccine distribution.
Data concerning antibiotic consumption were obtained from IMS Health (Intercontinental Marketing Services, Madrid, Spain) as the total number of antibiotic wholesale sales per presentation per year. Consumption was calculated as daily defined doses (DDD) per 1,000 inhabitants per day (DDD/1,000 inhabitants/day), in accordance with the 2002 World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology recommendations (37). Details of the number of PCV7 doses delivered per year were provided by the manufacturer (Wyeth Farma SA, Madrid, Spain), and vaccine distribution was calculated by dividing the number of doses by the number of children under 5 years of age and expressed in units/1,000 inhabitants <5 years of age/year. Annual population data were obtained from the Spanish Instituto Nacional de Estadística (www.ine.es).
Statistical analysis.
A multiple stepwise linear regression analysis was performed in which antibiotic consumption (macrolides, β-lactams, and fluoroquinolones), antibiotic nonsusceptibility (penicillin and erythromycin), PCV7 distribution, and prevalence of serotypes included in PCV7 among invasive isolates were considered as independent variables. Data for antibiotic consumption were analyzed by dividing macrolides into those administered three times a day (t.i.d.) (erythromycin and spiramycin), twice a day (b.i.d.) (clarithromycin, roxithromycin, mydecamycin, and josamycin), and once a day (o.d.) (azithromycin and dirithromycin) and dividing β-lactams into cephalosporins (oral and parenteral), aminopenicillins (oral and parenteral), and penicillin G plus narrow-spectrum penicillins. The SPSS v.14 statistical program (SPSS Inc., Chicago, IL) was used to derive best-fit models (those with the highest r2 for the fewest variables).
Temporal trends of nonsusceptibility prevalence, fluoroquinolone consumption, PCV7 annual distribution, and adjusted number of IPD cases were measured by the chi-square test for trends, and nonparametric correlations (Spearman's rho) of the prevalence of nonsusceptibility with the annual distribution of PCV7 from 2001 onwards and with fluoroquinolone consumption were calculated.
The temporal trends of each PCV7 serotype and of the seven most prevalent serotypes not included in the vaccine were calculated by nonlinear regression.
Prevalences of penicillin, erythromycin, and levofloxacin nonsusceptibility during the different periods were compared using the chi-square test or, when appropriate, Fisher's exact test.
Incidence rates of IPD cases were calculated for the period 1996 to 2006 after adjustment for underascertainment. A correction factor for hospital underreporting was computed for every year by dividing the number of beds in the hospital sending isolates in a particular year by the number of beds in all hospitals in the country in the same year (31). The correction factor was applied to the number of IPD isolates received annually in order to calculate adjusted numbers of cases. Annual incidence rates were calculated by dividing the adjusted number of cases by the midyear population. Incidence rates could not be computed for other periods due to the lack of reliable data with which to estimate ascertainment before 1996 and for 2007.
RESULTS
Proportions of pneumococcal serotypes and antibiotic susceptibility over time.
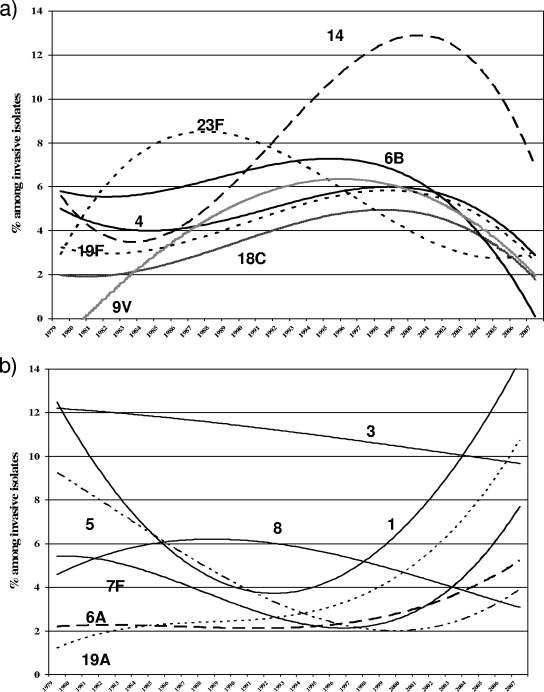
All PCV7 serotype trends (Fig. 1a) increased in the 1980s and during the 1990s and then decreased in the 2000s, except for 23F, in which the decrease began in the late 1980s and continued through the 1990s. The most frequent non-PCV7 serotypes (3 and 8; Fig. 1b) showed a slight decrease. Other serotypes showed significant trends that followed a cubic function: serotypes 5 and 7F decreased in the 1980s and increased in the 2000s; serotype 1 decreased in the 1980s and increased from the 1990s onwards, the most marked rise occurring in the 2000s; and serotype 6A showed a significant (P = 0.008) increasing trend from the late 1990s onwards, as did serotype 19A, whose incidence rose most steeply in the 2000s.
FIG. 1.
Temporal trends of serotypes that were included (a) and the most prevalent serotypes not included (b) in PCV7 among invasive isolates, 1979 to 2007. Best fits were always with a cubic function. The statistical information is as follows: serotype 4, r2 = 0.207, P = 0.115; serotype 6B, r2 = 0.457, P = 0.001; serotype 9V, r2 = 0.686, P < 0.001; serotype 14, r2 = 0.758, P < 0.001; serotype 18C, r2 = 0.483, P = 0.001; serotype 19F, r2 = 0.533, P < 0.001; serotype 23F, r2 = 0.672, P < 0.001; serotype 1, r2 = 0.841, P < 0.001; serotype 3, r2 = 0.145, P = 0.262; serotype 5, r2 = 0.530, P = 0.023; serotype 6A, r2 = 0.371, P = 0.008; serotype 7F, r2 = 0.676, P < 0.001; serotype 8, r2 = 0.213, P = 0.106; serotype 19A, r2 = 0.855, P < 0.001.
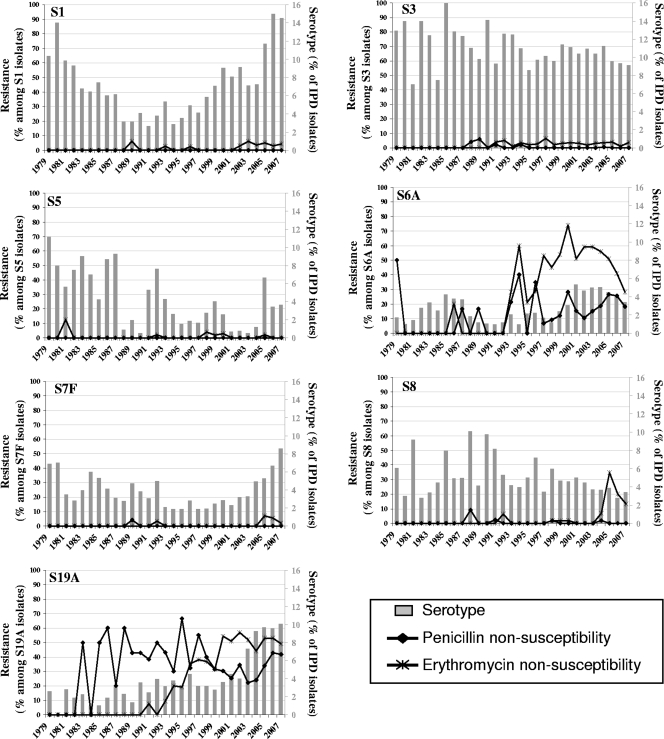
Values for penicillin-nonsusceptible pneumococci (PNSP) and erythromycin-nonsusceptible pneumococci (ENSP) were analyzed for PCV7 versus all nonvaccine serotypes for each of seven periods (PNSP and ENSP, Tables 1 and 2, respectively). The decrease in overall PNSP in the last period coincided with a significant increase in PNSP among nonvaccine serotypes (13.1% in 2001 to 2003 versus 32.3% in 2004 to 2007), mainly due to an increase in the incidence of serotype 19A strains (separated by year in Fig. 3). Similarly, a decrease in overall ENSP was observed in the last period, coinciding with a significant increase in ENSP among nonvaccine serotypes that was also mainly due to an increase in the incidence of serotype 19A strains (separated by year in Fig. 3).
TABLE 1.
Serotypes of PNSP (MIC, ≥0.12 μg/ml)
| Period (no. of invasive isolates) | No. of PNSP (% of invasive isolates) | Distribution of PNSP
|
||
|---|---|---|---|---|
| No. of PCV7 serotype isolates (% of total PNSP) | Non-PCV7 serotypes
|
|||
| No. (% of total PNSP) | Serotypes (% of total non-PCV7 PNSP) | |||
| 1979-1985 (1,031) | 175 (17.0)a | 134 (76.6)c | 41 (23.4)c | 11A (31.7), 15 (24.4), 23A (14.6), 21 (12.2), others (17.1) |
| 1986-1989 (1,154) | 420 (36.4) | 352 (83.8) | 68 (16.2) | 15 (33.8), 19A (14.7), NTd (14.7), others (36.8) |
| 1990-1992 (1,519) | 496 (32.7) | 433 (87.3) | 63 (12.7) | 19A (36.5), 15 (23.8), NT (19.1), others (20.6) |
| 1993-1997 (3,490) | 1,355 (38.8) | 1,182 (87.2) | 173 (12.8) | 19A (32.4), 15 (35.3), NT (12.1), others (20.2) |
| 1998-2000 (3,577) | 1,264 (35.3) | 1,112 (88.0) | 152 (12.0) | 15 (41.5), 19A (25.0), 6A (10.5), others (23.0) |
| 2001-2003 (4,271) | 1,381 (32.3) | 1,200 (86.9) | 181 (13.1) | 19A (33.1), 15A (11.0), 35 (11.0), 6A (16.0), others (28.9) |
| 2004-2007 (7,417) | 1,830 (24.7)b | 1,238 (67.7)b | 592 (32.3)b | 19A (44.3), 24 (15.2), 6A (12.0), 15A (10.6), others (17.9) |
P < 0.001 versus 1986-1989 data.
P < 0.001 versus 2001-2003 data.
P = 0.038 versus 1986-1989 data.
NT, nontypeable.
TABLE 2.
Serotypes of ENSP (MIC, ≥0.5 μg/ml)
| Period (no. of invasive isolates) | No. of ENSP (% of invasive isolates) | Distribution of ENSP
|
||
|---|---|---|---|---|
| No. of PCV7 serotype isolates (% of total ENSP) | Non-PCV7 serotypes
|
|||
| No. (% of total ENSP) | Serotypes (% of total non-PCV7 ENSP) | |||
| 1979-1985 (1,031) | 25 (2.4)a | 17 (68.0) | 8 (32.0) | 15 (25.0), 11A (12.5), 2 (12.5), 9N (12.5), 5 (12.5), others (25.0) |
| 1986-1989 (1,154) | 81 (7.0) | 66 (81.5) | 15 (18.5) | 15 (40.0), NTc (20.0), 6A (13.3), others (26.7) |
| 1990-1992 (1,519) | 148 (9.7) | 113 (76.4) | 35 (23.6) | NT (25.8), 15 (20.0), 3 (17.1), others (37.1) |
| 1993-1997 (3,490) | 666 (19.1) | 513 (77.0) | 153 (23.0) | 15 (28.8), 19A (21.6), 6A (15.0), others (34.6) |
| 1998-2000 (3,577) | 871 (24.3) | 622 (71.4) | 249 (28.6) | 15 (24.1), 19A (19.3), 6A (20.9), others (35.7) |
| 2001-2003 (4,271) | 1,169 (27.4) | 759 (64.9) | 410 (35.1) | 19A (30.0), 6A (29.3), others (40.7) |
| 2004-2007 (7,417) | 1,692 (22.8)b | 683 (40.4)b | 1,009 (59.6)b | 19A (35.6), 6A (14.1), others (50.3) |
P < 0.001 versus 1986-1989 data.
P < 0.001 versus 2001-2003 data.
NT, nontypeable.
FIG. 3.
Non-PCV7 serotype and penicillin/erythromycin resistance patterns, 1979 to 2007.
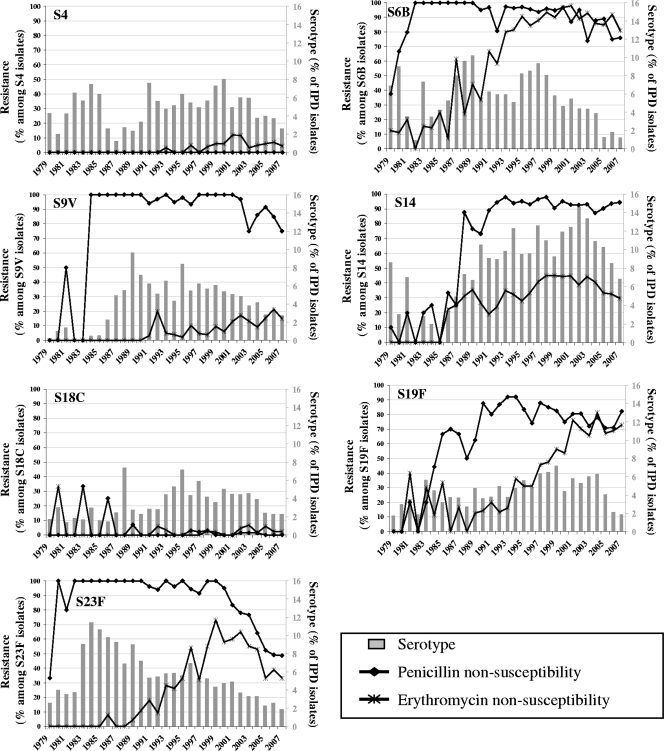
Among PCV7 serotypes (Fig. 2) and regardless of the changing patterns of serotype prevalence, PNSP and ENSP rates in serotypes 14, 6B, and 19F were high during the 1990s and 2000s. The same pattern is seen for PNSP in serotype 9V, while PNSP and ENSP rates in serotype 23F have decreased substantially since 1998. During the whole study period, serotypes 4 and 18C remained associated with antibiotic nonsusceptibility. With respect to the seven most prevalent nonvaccine serotypes (Fig. 3), only serotypes 6A and 19A presented PNSP and ENSP. PNSP rates in these two serotypes have not changed since the 1990s, while ENSP rates increased in serotype 19A.
FIG. 2.
PCV7 serotype and penicillin/erythromycin resistance patterns, 1979 to 2007.
Serotypes and nonsusceptibility to antibiotics in relation to preventive and therapeutic measures.
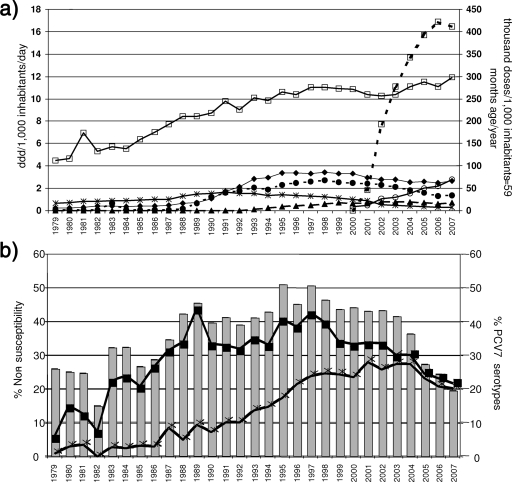
β-Lactam consumption increased continuously over time mainly due to the use of aminopenicillins and oral cephalosporins from 1989 onwards. Macrolide consumption greatly increased from 1989 (mainly due to consumption of b.i.d. and o.d. macrolides) and then dropped from 2001 onwards, while fluoroquinolone consumption increased from 2001 (when they were launched) onwards. Since the launch of PCV7 in Spain in 2001, vaccine distribution increased between 2001 and September 2007 from 46 to 412 doses per 1,000 inhabitants <5 years old/year (Fig. 4a).
FIG. 4.
Serotypes and nonsusceptibility to antibiotics in relation to antibiotic consumption and PCV7 distribution. (a) Antibiotic consumption (DDD/1,000 inhabitants/day and doses/1,000 inhabitants ≤59 months of age/year), 1979 to 2007. Symbols: solid line with open squares, aminopenicillins; solid line with filled diamonds, oral cephalosporins; solid line with asterisks, t.i.d. macrolides; dashed line with filled circles, b.i.d. macrolides; dashed line with filled triangles, o.d. macrolides; solid line with open circles, fluoroquinolones; dashed line with open squares, PCV7 distribution. (b) Percentages (bars) of serotypes included in PCV7 among invasive isolates and percentages of penicillin (filled squares) and erythromycin (asterisks) nonsusceptibility among invasive isolates, 1979 to 2007.
Penicillin nonsusceptibility ran in parallel with the prevalence of PCV7 serotypes over the whole study period (Fig. 4b). Levofloxacin nonsusceptibility was 0.1% (2002), 0.9% (2003), 0.8% (2004), 0.9% (2005), 1.3% (2006), and 1.3% (2007).
The best predictor of penicillin nonsusceptibility, based on a multiple linear regression model (R2 = 0.826; P < 0.001), was the prevalence of PCV7 serotypes among the invasive isolates received (Fig. 4b) (β = 0.909; P < 0.001). For erythromycin nonsusceptibility (Fig. 4b), the best-fit model (R2 = 0.891; P < 0.001) (Fig. 4a) identified o.d. macrolide consumption as the best predictor (β = 0.944; P < 0.001).
Before PCV7 was licensed for use, the best predictor for PCV7 serotype prevalence among invasive isolates over time (Fig. 4b), based on a multiple linear regression model (R2 = 0.720; P < 0.001), was b.i.d. macrolide consumption (β = 0.794; r2 = 0.482; P < 0.001) and, inversely, fluoroquinolone consumption (β = −0.498; r2 = 0.238; P < 0.001). Once PCV7 and fluoroquinolones became licensed for use (in 2001 and 2000, respectively), the distribution of PCV7 and consumption of fluoroquinolones were both correlated (rho, ≥0.88 or ≤−0.88; P ≤ 0.02) with (i) the decrease in penicillin nonsusceptibility from 33.6% in 2002 to 21.5% in 2007 (Fig. 4b), (ii) the decrease in the prevalence of PCV7 serotypes from 43.3% in 2002 to 19.5% in 2007 (Fig. 4b), and (iii) the significant (P < 0.001) increase in levofloxacin nonsusceptibility from 0.1% in 2002 to 1.3% in 2007. Weaker correlations were found between the decrease in erythromycin nonsusceptibility (from 26.4% in 2002 to 20.1% in 2007) and the distribution of PCV7 (rho = −0.754; P = 0.084) and fluoroquinolone consumption (r2 = −0.812; P = 0.05).
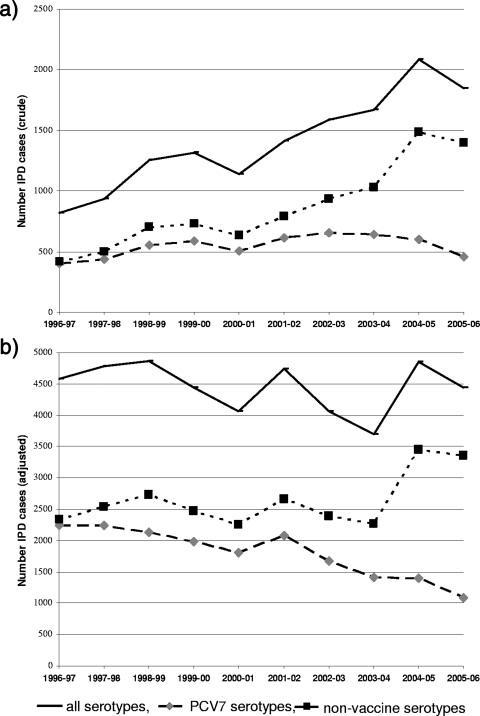
Incidence of IPD from 1996 to 2006.
The number of invasive strains recorded annually rose in parallel with the increasing coverage of national hospital beds by the SRLP, from 18% in 1996 to 1997 to 43% in 2004 to 2005 (Fig. 5a). After adjustment for underascertainment, the total number of IPD cases fluctuated over time (Fig. 5b) between 3,690 and 4,859 cases per year. The overall incidence of IPD ranged from 8.5 to 12.1 per 100,000 inhabitants, without showing any clear pattern. However, the incidence of the PCV7 serotypes dropped from 5.2 per 100,000 in the prevaccine period (1996 to 2001) to 2.4 per 100,000 in 2005 to 2006, while that of non-PCV7 serotypes increased from 6.1 to 7.5 per 100,000 from 2004 to 2005 onwards.
FIG. 5.
Numbers of IPD cases annually, per epidemiological year: all serotypes, PCV7 serotypes, and nonvaccine serotypes. (a) Crude numbers. (b) Numbers adjusted for ascertainment (31).
DISCUSSION
Three general observations can be made concerning the pneumococci received at the SRLP over the three decades considered here. First, the increase in the prevalence of antibiotic nonsusceptibility and PCV7 serotypes that was associated with antibiotic consumption in the 1980s and 1990s was reversed in the 2000s, when vaccine dose distribution increased. At the same time, non-PCV7 serotypes became more prevalent, particularly serotypes 1 and 19A. Second, some serotypes, such as 3, 4, and 8, maintained their steady secular trend over the three decades, showing no association with the introduction of therapeutic or preventive measures.
While it is a straightforward task to describe serotype and resistance patterns over time, it is much more difficult to determine the influence of the different therapeutic and preventive measures and the contribution of natural secular trends to this variation. The nonsusceptibility data for invasive S. pneumoniae isolated in hospitals from different regions of Spain between 1979 and 2007 in the present study identified the best predictor for erythromycin nonsusceptibility as being consumption of long-half-life macrolides. The best predictor for penicillin nonsusceptibility was the prevalence of PCV7 serotypes.
Between 1997 and 2001, 83% of PNSP received at the SRLP belonged to five serotypes: 6B, 9V, 14, 19F, and 23F (9) (all included in PCV7 together with serotypes 4 and 18C). The penicillin-erythromycin co-nonsusceptibility in these serotypes may be postulated as the reason for the selection and increase of PCV7 serotypes in the 1990s (after the launch of long-half-life macrolides/cephalosporins) (13) as well as for the decrease in the 2000s (after the introduction of PCV7 for children and respiratory fluoroquinolones for adults). However, other, unknown factors must be involved since the prevalence of serotypes 3, 4, and 8 was constant over the three decades despite their uniform susceptibility to β-lactams, macrolides, and quinolones and their inclusion (serotype 4) or not (serotypes 3 and 8) in the PCV7 formulation. In addition, the decrease in the incidence of serotype 23F began earlier than that of the others (in the late 1980s).
The increase in PCV7 serotypes in the 1980s and 1990s, which was associated with antibiotic consumption, was reversed after PCV7 introduction, as was noted in other countries that introduced the vaccine. However, the decrease in the prevalence of PCV7 serotypes in Spain was not as marked as that in the United States after the vaccine was introduced there (34). This was probably due to the vaccine not being included in childhood vaccination calendars throughout most of the country—it was included in the Madrid region only from November 2006—so coverage was irregular and scarce (15). Recent reports have suggested that vaccine-induced replacement by nonvaccine serotypes has arisen. This has been associated with an increasing incidence of specific serotypes such as 19A, which has become the most important cause of IPD in children in the United States as well as becoming increasingly more drug resistant (2, 3, 23, 28, 32). In Spain, the incidence of serotype 19A increased greatly after vaccine introduction, although penicillin nonsusceptibility remained as prevalent as it had been in the preceding years. Nevertheless we cannot reliably conclude that this increase was the consequence of vaccine-induced pressure, as increases in this serotype were also reported in countries where PCV7 had not been introduced, such as Israel (7) and Korea (4).
Serotype 1 is also of special interest since it has exhibited a secular trend with long-term fluctuations over the three decades of the current study, as has been reported in other parts of the world (16, 17). The incidence of serotype 1 began to increase before 2000, but this became even more marked from 2005 onwards (Fig. 1b). One possible explanation is the introduction of a new, highly pathogenic clone of serotype 1 (1). However, studies of serotype 1 disease cases in different regions did not identify any distinct “virulence” properties (8). The increase in serotype 1 has been related to the increase in pneumonia with pleural effusion observed in children older than 2 years and has been widely reported in communities in which PCV7 has and has not been introduced (8, 30, 33), a phenomenon that has also been reported in Spain (26, 27).
Several factors and limitations of the study must be considered when assessing our results. First of all, this is an ecological study and caution is required when interpreting the associations found, since these statistical relationships do not imply causality. Although changes in serotype trends must be appraised over extended periods, long-term ecological studies increase the likelihood of capturing such events, irrespective of vaccine distribution and antibiotic consumption. Second, changes in clinical practice and surveillance interest and effort were responsible for the increasing level of ascertainment by the SRLP, a consideration that must always be taken into account in this type of comparison. Third, the study population may not be completely representative of all IPD cases in Spain with respect to age, geographical origin, and clinical conditions. These characteristics were not stable and could have led to the introduction of bias in our comparisons of the data over time. With respect to age groups, the proportion of strains typed in children significantly increased from about 2000 to 2001. This may reflect the greater awareness of IPD among pediatricians, given that PCV7 has been available since June 2001. The incidence rates are likely to be underestimated because we cannot take into account data missing due to underreporting by participating laboratories (i.e., isolates not sent to the SRLP). The number of invasive isolates received at the SRLP increased steadily over the study period. However, when adjusting for the annual coverage of the SRLP for the 1996-to-2006 period, the estimated incidence rate was relatively stable (10.7 per 100,000). This is comparable to rates in other European countries and regions, especially in studies that adjusted for underascertainment, such as those of France, in which an estimate of 11.4 cases per 100,000 people was derived for the years 1996 to 2005 (21), and of southwest England, with an estimated 10 to 13.6 cases per 100,000 between 1996 and 2005 (19).
Our study suggests that the impact of therapeutic and preventive measures on serotypes and antibiotic nonsusceptibility of invasive pneumococci from 1979 to 2007 in Spain is serotype dependent. In this sense, serotypes may act as quasispecies. Further molecular epidemiology studies are needed for us to be able to predict the trends in particular serotypes and clones.
Acknowledgments
We thank M. D. Vicioso, I. Hernandez, and O. Robledo for their technical assistance; E. Kissling; and P. Mason for her kind help in preparing the manuscript.
This work was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, Spain; grants CP05/00068 and PI 080539 from the Fondo de Investigación Sanitaria, Spain; and the Spanish Network for the Research on Infectious Diseases (REIPI RD06/0008) and by an unrestricted grant from Wyeth Farma SA, Madrid, Spain.
All authors declare no conflict of interest.
Footnotes
Published ahead of print on 18 February 2009.
REFERENCES
- 1.Brueggemann, A. B., and B. G. Spratt. 2003. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J. Clin. Microbiol. 414966-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byington, C. L., M. H. Samore, G. J. Stoddard, S. Barlow, J. Daly, K. Korgenski, S. Firth, D. Glover, J. Jensen, E. O. Mason, C. K. Shutt, and A. T. Pavia. 2005. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin. Infect. Dis. 4121-29. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2008. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998-2005. MMWR Morb. Mortal. Wkly. Rep. 57144-148. [PubMed] [Google Scholar]
- 4.Choi, E. H., S. H. Kim, B. W. Eun, S. J. Kim, N. H. Kim, J. Lee, and H. J. Lee. 2008. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg. Infect. Dis. 14275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically; 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Dagan, R., N. Givon-Lavi, E. Leibovitz, and N. Porat. 2007. Increased importance of antibiotic-resistant (AR) S. pneumoniae serotype 19A (Pn19A) in acute otitis media (AOM) occurring before introduction of 7-valent pneumococcal conjugate vaccine (PCV7) in southern Israel, abstr. G-1001, p. 277. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 8.Eastham, K. M., R. Freeman, A. M. Kearns, G. Eltringham, J. Clark, J. Leeming, and D. A. Spencer. 2004. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax 59522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenoll, A., G. Asensio, I. Jado, S. Berron, M. T. Camacho, M. Ortega, and J. Casal. 2002. Antimicrobial susceptibility and pneumococcal serotypes. J. Antimicrob. Chemother. 50(Suppl. S2)13-19. [DOI] [PubMed] [Google Scholar]
- 10.Fenoll, A., I. Jado, D. Vicioso, and J. Casal. 1997. Dot blot assay for the serotyping of pneumococci. J. Clin. Microbiol. 35764-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenoll, A., I. Jado, D. Vicioso, A. Perez, and J. Casal. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996). J. Clin. Microbiol. 363447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenoll, A., C. Martin Bourgon, R. Munoz, D. Vicioso, and J. Casal. 1991. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev. Infect. Dis. 1356-60. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Rey, C., L. Aguilar, F. Baquero, J. Casal, and R. Dal-Re. 2002. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 40159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granizo, J. J., L. Aguilar, J. Casal, R. Dal-Re, and F. Baquero. 2000. Streptococcus pyogenes resistance to erythromycin in relation to macrolide consumption in Spain (1986-1997). J. Antimicrob. Chemother. 46959-964. [DOI] [PubMed] [Google Scholar]
- 15.Grupo de Trabajo de la Ponencia de Registro y Programa de Vacunas. 2006. Enfermedad invasora por Streptococcus pneumoniae. Implicación de la vacunación con la vacuna conjugada heptavalente. Ministerio de Sanidad y Consumo, Madrid, Spain.
- 16.Hausdorff, W. P. 2007. The roles of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine 252406-2412. [DOI] [PubMed] [Google Scholar]
- 17.Hedlund, J., M. Sorberg, B. H. Normark, and G. Kronvall. 2003. Capsular types and antibiotic susceptibility of invasive Streptococcus pneumoniae among children in Sweden. Scand. J. Infect. Dis. 35452-458. [DOI] [PubMed] [Google Scholar]
- 18.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 1961346-1354. [DOI] [PubMed] [Google Scholar]
- 19.Ihekweazu, C. A., D. A. Dance, R. Pebody, R. C. George, M. D. Smith, P. Waight, H. Christensen, K. A. Cartwright, and J. M. Stuart. 2008. Trends in incidence of pneumococcal disease before introduction of conjugate vaccine: South West England, 1996-2005. Epidemiol. Infect. 1361096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, and C. G. Whitney. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 3541455-1463. [DOI] [PubMed] [Google Scholar]
- 21.Lepoutre, A., S. Georges, and E. Varon. 2007. Évolution de l'incidence des infections invasives à pneumocoques, France, 2005. Bull. Épidémiol. Hebdomadaire 537-39. [Google Scholar]
- 22.Messina, A. F., K. Katz-Gaynor, T. Barton, N. Ahmad, F. Ghaffar, D. Rasko, and G. H. McCracken, Jr. 2007. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr. Infect. Dis. J. 26461-467. [DOI] [PubMed] [Google Scholar]
- 23.Moore, M. R., R. E. Gertz, Jr., R. L. Woodbury, G. A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L. H. Harrison, J. L. Hadler, N. M. Bennett, A. R. Thomas, L. McGee, T. Pilishvili, A. B. Brueggemann, C. G. Whitney, J. H. Jorgensen, and B. Beall. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 1971016-1027. [DOI] [PubMed] [Google Scholar]
- 24.Moore, M. R., and C. G. Whitney. 2008. Emergence of nonvaccine serotypes following introduction of pneumococcal conjugate vaccine: cause and effect? Clin. Infect. Dis. 46183-185. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Almagro, C., I. Jordan, A. Gene, C. Latorre, J. J. Garcia-Garcia, and R. Pallares. 2008. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin. Infect. Dis. 46174-182. [DOI] [PubMed] [Google Scholar]
- 26.Obando, I., L. A. Arroyo, D. Sanchez-Tatay, D. Moreno, W. P. Hausdorff, and A. B. Brueggemann. 2006. Molecular typing of pneumococci causing parapneumonic empyema in Spanish children using multilocus sequence typing directly on pleural fluid samples. Pediatr. Infect. Dis. J. 25962-963. [DOI] [PubMed] [Google Scholar]
- 27.Obando, I., C. Muñoz-Almagro, L. A. Arroyo, D. Tarragó, D. Sánchez-Tatay, D. Moreno, S. Dhillon, C. Esteva, S. Hernández-Bou, J. J. Garcia-Garcia, W. P. Hausdorff, and A. B. Brueggemann. 2008. Pediatric parapneumonic empyema, Spain. Emerg. Infect. Dis. 141390-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 1921988-1995. [DOI] [PubMed] [Google Scholar]
- 29.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26468-472. [DOI] [PubMed] [Google Scholar]
- 30.Rees, J. H., D. A. Spencer, D. Parikh, and P. Weller. 1997. Increase in incidence of childhood empyema in West Midlands, UK. Lancet 349402. [DOI] [PubMed] [Google Scholar]
- 31.Reinert, R. R., S. Haupts, M. Van der Linden, C. Heeg, M. Y. Cil, A. Al-Lahham, and D. S. Fedson. 2005. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001-2003. Clin. Microbiol. Infect. 11985-991. [DOI] [PubMed] [Google Scholar]
- 32.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2971784-1792. [DOI] [PubMed] [Google Scholar]
- 33.Spencer, D. A., S. M. Iqbal, A. Hasan, and L. Hamilton. 2006. Empyema thoracis is still increasing in UK children. BMJ 3321333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarragó, D., A. Fenoll, D. Sánchez-Tatay, L. A. Arroyo, C. Muñoz-Almagro, C. Esteva, W. P. Hausdorff, J. Casal, and I. Obando. 2008. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin. Microbiol. Infect. 14828-834. [DOI] [PubMed] [Google Scholar]
- 35.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 3481737-1746. [DOI] [PubMed] [Google Scholar]
- 36.Whitney, C. G., and M. R. Moore. 2008. Direct and indirect effectiveness and safety of pneumococcal conjugate vaccine in practice, p. 353-368. In G. R. Siber, K. P. Klugman, and P. H. Mäkelä (ed.), Pneumococcal vaccines: the impact of conjugate vaccine. ASM Press, Washington, DC.
- 37.World Health Organization Collaborating Centre for Drug Statistics Methodology. 2002. ATC index with DDDs. World Health Organization Collaborating Centre for Drug Statistics Methodology, Oslo, Norway.