Abstract
Carbonic anhydrase (CA) (EC 4.2.1.1) is a widespread enzyme catalyzing the reversible hydration of CO2 to bicarbonate, a reaction that participates in many biochemical and physiological processes. Mesorhizobium loti, the microsymbiont of the model legume Lotus japonicus, possesses on the symbiosis island a gene (msi040) encoding an α-type CA homologue, annotated as CAA1. In the present work, the CAA1 open reading frame from M. loti strain R7A was cloned, expressed, and biochemically characterized, and it was proven to be an active α-CA. The biochemical and physiological roles of the CAA1 gene in free-living and symbiotic rhizobia were examined by using an M. loti R7A disruption mutant strain. Our analysis revealed that CAA1 is expressed in both nitrogen-fixing bacteroids and free-living bacteria during growth in batch cultures, where gene expression was induced by increased medium pH. L. japonicus plants inoculated with the CAA1 mutant strain showed no differences in top-plant traits and nutritional status but consistently formed a higher number of nodules exhibiting higher fresh weight, N content, nitrogenase activity, and δ13C abundance. Based on these results, we propose that although CAA1 is not essential for nodule development and symbiotic nitrogen fixation, it may participate in an auxiliary mechanism that buffers the bacteroid periplasm, creating an environment favorable for NH3 protonation, thus facilitating its diffusion and transport to the plant. In addition, changes in the nodule δ13C abundance suggest the recycling of at least part of the HCO3− produced by CAA1.
The interaction of the soil bacteria belonging to the genera Mesorhizobium, Rhizobium, Bradyrhizobium, Sinorhizobium, and Azorhizobium with the root systems of leguminous plants results in the formation of a novel specialized plant organ, the root nodule (27). Symbiotic nitrogen fixation (SNF), which takes place inside nodules, represents a major mechanism for the enrichment of agricultural and natural ecosystems in assimilated nitrogen (42). During this mutually beneficial plant-microbe interaction, the plant provides the microsymbiont with photosynthate, mainly in the form of organic acids, together with other nutrients, in return for fixed nitrogen in the form of ammonium and amino acids (28, 41). Thus, in mature nitrogen-fixing nodules, both symbionts must provide the proper chemical environment for both the reduction of N2 to ammonium by bacteroids and the efficient exchange of metabolites through the peribacteroid membrane and the peribacteroid space (44). The establishment of this environment is underpinned by the coordinated expression of specialized genes in both organisms. Therefore, the elucidation of the exact physiological and biochemical roles of these genes represents a major research goal for years to come. In the case of Mesorhizobium loti, the microsymbiont of several Lotus species, including the model legume Lotus japonicus, many of the genes required for nodulation, nitrogen fixation, and nutrient exchange reside on a symbiosis island, a chromosomally integrated element that can be transferred between mesorhizobia in the environment, converting them into strains able to nodulate Lotus species (18, 36).
Carbonic anhydrase (CA) (EC 4.2.1.1) is a zinc-containing metalloenzyme that catalyzes the reverse hydration of CO2 to bicarbonate. CA is a ubiquitous enzyme, present in both prokaryotes and eukaryotes, and currently at least four distinct CA classes have been characterized, namely, α, β, γ, and δ (38). Interestingly, although the CA classes show no significant sequence or structural homology, they share similar catalytic mechanisms employing a zinc atom that is essential for catalysis (21, 22). Representatives of all CA classes are widespread in prokaryotes, indicating the ancient origins of these enzymes (33). In prokaryotes, α-CAs have been characterized from several species, including the cyanobacteria Synechococcus and Anabaena (34) and the eubacteria Neisseria gonorrhoeae (5), Helicobacter pylori (6), and Rhodopseudomonas palustris (29).
CA catalyzes the reversible hydration of CO2 to bicarbonate, a relatively simple but vital reaction, as it is involved in many diverse biochemical and physiological processes, including photosynthesis, respiration, CO2 and ion transport, and acid-base balance. Similarly, in prokaryotes, various CA isoforms are essential for a wide range of cellular functions. Smith and Ferry (32) summarized a variety of prokaryotic enzymes requiring or producing CO2/HCO3−. In Escherichia coli, the expression of the can gene, coding for a β-class CA, is required for growth in air, although it is dispensable when the partial pressure of CO2 is high or during anaerobic growth (25). cynT, the only can paralog in E. coli, is transcribed as part of the cyanase (cyn) operon and serves to provide sufficient levels of cellular HCO3− for growth with cyanate as the nitrogen source (15). In R. palustris, a periplasmic α-type CA has been proposed to be essential for bicarbonate uptake (29). Finally, a periplasmic α-type CA from H. pylori has been shown to be upregulated by low environmental pH, being essential for buffering the periplasmic acidic pH. Recent studies suggest that this CA is an integral component of the acid acclimation response that allows these neutralophile bacteria to colonize the stomach (24, 43).
In this work, we present the biochemical and physiological characterization of an α-class CA (CAA1) located in the symbiosis island of the nitrogen-fixing bacterium M. loti and showing high similarity to the periplasmic α-CAs characterized in H. pylori and R. palustris (7, 29). Our goal was to test the functionality of the encoded polypeptide and to assess the physiological role of the product of this gene during nodule development and function by using an M. loti disruption mutant. Furthermore, we aimed at studying the effect of pH on the expression of this gene in free-living M. loti. Based on preliminary findings, our hypothesis was that CAA1-catalyzed CO2 hydration might participate in a biochemical mechanism for buffering of the bacteroid periplasm at a pH favorable for NH3 protonation.
MATERIALS AND METHODS
Strains, culture conditions, and plant growth.
M. loti strains R7A and R7A CAA1::pFUS2 were kindly provided by Clive Ronson (University of Otago, Otago, New Zealand). M. loti strain R7A CAA1::pFUS2 was constructed by insertion-duplication mutagenesis. An internal fragment of the CAA1 gene was amplified from M. loti strain R7A genomic DNA and cloned into the HindIII and BamHI sites of the pFUS2 suicide vector for CAA1 inactivation by homologous recombination and generation of transcriptional fusion to lacZ (2). Gene disruption was performed by conjugation using M. loti strain R7A to generate M. loti strain R7A CAA1::pFUS2. The gene interruption was confirmed by PCR analysis and sequencing of the insertion site.
For the complementation of the CAA1::pFUS2 mutant strain, the CAA1 open reading frame (ORF), together with 134 bp of the terminator region, was amplified using 5′-AAATCTAGATTGCGGCTGTGCCTTGACTG-3′ and 5′-AAAGGTACCTTCCCCATGATCCGGTCA-3′ primers. The amplification product was cloned as an XbaI-KpnI fragment into the pFAJ1709 vector, yielding the plasmid pFAJCAA1. pFAJ1709 carries the spsAB symbiotic plasmid stability loci from pNGR234α of Rhizobium sp. strain NGR234, which confers plasmid stability in free-living rhizobia and bacteroids (11). pFAJCAA1 was used for transformation of the CAA1::pFUS2 strain by electroporation at 12.5 kV/cm, 25 μF, and 200 Ω. Cells competent for electroporation were prepared as previously described (17). Transformants were confirmed by retransformation of E. coli cells and restriction enzyme digestion. Plasmid stability in nodules was confirmed by plasmid rescue from antibiotic-resistant bacteroids.
All strains were grown at 28°C on yeast mannitol broth (YMB) or tryptone-yeast extract supplemented with the appropriate antibiotic (50 μg/ml gentamicin for CAA1::pFUS2; 4 μg/ml tetracycline for the pFAJCAA1 plasmid). For the construction of growth curves, 25-ml starter cultures were used to inoculate 200 ml of YMB to an initial optical density at 600 nm (OD600) of 0.05. The pH of the culture medium was adjusted to 5.5 with MES (morpholineethanesulfonic acid)/KOH (pKa, 6.1), to 7 with HEPES/NaOH (pKa, 7.5), and to 8 with Tris/H2SO4 (pKa, 8.0). All buffer concentrations were 50 mM, except for Tris (75 mM).
L. japonicus (Gifu B-129) seeds were kindly provided by Jens Stougaard (University of Aarhus, Aarhus, Denmark). The seeds were scarified for 5 min with H2SO4, sterilized for 20 min in 2% (vol/vol) NaOCl-0.02% (vol/vol) Tween 20, pregerminated at 18°C in the dark for 72 h, and spot inoculated with a suspension culture of the respective M. loti strain at an OD600 of 0.1. The plants were grown with nitrogen-free Hoagland nutrient solution in a controlled environment with an 18-h day/6-h night rotation, a 22°C day/18°C night temperature regime, and 70% air relative humidity (16).
Expression and purification of the recombinant CAA1 polypeptide.
The ORF of the CAA1 gene, excluding the stop codon, was amplified and cloned as an NcoI-XhoI fragment into the expression vector pET28a(+). Two versions of the ORF, with and without the predicted signal peptide, were amplified and cloned. The expression plasmids were transformed into E. coli strain BL21(DE3). The cells were grown at 23°C in LB broth with 50 μg/ml kanamycin to an OD600 of 0.6. CAA1 expression was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and 0.5 mM ZnSO4 for 6 h. The cells were harvested and resuspended in lysis buffer (50 mM NaH2PO4, pH 8, 300 mM NaCl, 10 mM imidazole) before cell disruption by sonication. The recombinant enzyme was purified from the resulting supernatant by nickel affinity chromatography (Qiagen, Hilden, Germany). The purified protein elutions were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie brilliant blue staining. Dialysis was performed against 25 mM MES, pH 6.3, or 20 mM HEPES, pH 7.5.
Enzyme assays.
CA activity was assayed by measuring changes in pH during the reaction by a dye indicator method (20). The assays were performed at 4°C. The buffer-indicator pairs used were (i) 50 mM MES plus 0.005% (wt/vol) bromothymol blue (pH 6.3; A615) and (ii) 50 mM HEPES plus 0.005% (wt/vol) phenol red (pH 7.5; A557). Both solutions contained 50 mM sodium sulfate to maintain a relatively constant ionic strength of the reaction medium; 10 μg of purified recombinant CAA1 protein was used in each assay. Data were acquired using a Hitachi (Tokyo, Japan) U-2800 spectrophotometer. The initial rate was measured during the first 5 seconds when the reaction was still at the linear phase. Unanalyzed rates, measured in the absence of the enzyme, were used as negative controls and were subtracted from the observed rates. An α-type bovine CA (ICN Biomedicals, Eschwege, Germany) was used as a positive control. Kinetic parameters were calculated by fitting the corrected rate data to the Michaelis-Menten equation using GraFit v3.5 (Erithacus Software, Surrey, United Kingdom).
Esterase activity was measured as p-nitrophenylacetate (4-NPA) hydrolysis at 25°C (3). The buffer used was 50 mM Tris/H2SO4, pH 7.6, and the substrate concentration was 3 mM. The reaction was monitored for 5 min at 348 nm in a Hitachi U-2800 spectrophotometer.
β-Galactosidase activity was determined by o-nitrophenyl-β-d-galactopyranoside hydrolysis at 37°C according to the method of Miller (26). The substrate concentration was 0.4 M. The reaction was monitored at 420 nm in a Hitachi U-2800 spectrophotometer.
Nitrogenase activity was estimated by acetylene reduction on whole plants incubated at 25°C in 28-ml rubber cap tubes containing 1/10 (vol/vol) acetylene. The ethylene produced at different time points was quantified with a Perkin-Elmer 8500 gas chromatograph (Perkin-Elmer Life and Analytical Science Inc., Wellesley, MA) equipped with a 2-m Porapak R column and a flame ionization detector.
The histochemical staining of β-galactosidase activity was a modification of the method described by Boivin et al. (4). The reaction buffer used was 0.1 M sodium-phosphate, pH 7.0, and nodules were flash frozen in liquid nitrogen to aid the penetration of glutaraldehyde. The nodules were then hand sectioned and observed by bright-field microscopy.
Transcript analysis using real-time reverse transcription-qPCR.
Total RNA was isolated from L. japonicus-inoculated roots and nodules and quantified by spectrophotometry and agarose gel electrophoresis. RNA samples were treated with DNase I (Promega, Madison, WI) at 37°C for 45 min to eliminate contaminating genomic DNA. First-strand cDNA was reverse transcribed using SuperScript II (Invitrogen, Paisley, United Kingdom) and random hexanucleotides (Invitrogen). CAA1 transcripts were specifically amplified using Ml0040-274F (5′-CAAATCAACATGCCGGAAGG-3′) and Ml0040-338R (5′-AACTGTGCCAGTTGGTAGACGC-3′) primers. PCRs were performed on the Stratagene MX3005P using Power SYBR green master mix (Applied Biosystems). PCR cycling started at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The primer specificity and the formation of primer dimers were monitored by dissociation curves and agarose gel electrophoresis on a 4% (wt/vol) gel. The expression levels of the M. loti polyribonucleotide nucleotidyltransferase gene (mlr5562), detected using mlr5562-499F (5′-ATCGACGAAATGCAGGAATCC-3′) and mlr5562-576R (5′-TTCCGACTCAACCATCAGCAC-3′) primers, were used as internal standards. Relative transcript levels of the gene of interest (X) were calculated as a ratio to the mlr5562 gene transcripts (S) as (1 + E)−ΔCT, where ΔCT was calculated as (CTX − CTS). The PCR efficiency (E) for each amplicon was calculated by employing the linear regression method on the log(fluorescence)-per-cycle-number data. All real-time quantitative PCRs (qPCRs) were performed on three biological repeats.
Determination of nodule and top-plant C isotopic composition and total C and N content.
Nodules were isolated from the plant root system and oven dried (3 days; 65°C). The same procedure was followed for the top plant (leaf and stem). All plant material was then ground with a mortar and pestle into a homogeneous fine powder, and samples of ca. 0.5 mg were transferred into tin capsules (IVA Analysentechnik, Meerbusch, Germany). Subsequently, the samples were injected into an elemental analyzer (NA 2500; CE Instruments, Milan, Italy) coupled to an isotope ratio mass spectrometer (Delta Plus; Finnigan MAT GmbH, Bremen, Germany) by a Conflo II interface (Finnigan MAT GmbH, Bremen, Germany). The carbon isotope composition (δ13C abundance) of plant tissues is an information-rich signal providing useful insights into different plant functions (1). The δ13C abundance of plant tissues is described as follows: δ13Cplant = δ13Catm − α − (b − α)Ci/Cα, where δ13C is expressed in per mille (‰), α is the discrimination during diffusion (ca. 4.4‰), b is the discrimination during carboxylation by ribulose-bisphosphate carboxylase (ca. 29‰), Ci is the CO2 concentration inside the stomatal cavities, and Catm is the atmospheric CO2 concentration (12).
Electron microscopy.
Nodules were harvested 26 days postinoculation (p.i.) and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 2 h, postfixed with 1% osmium tetroxide for 2 h, dehydrated in a graded series of alcohol followed by propylenoxide, and embedded in Spurr resin. Ultrathin sections were cut and stained with uranyl acetate, and specimens were examined and photographed with a Zeiss 9S transmission electron microscope. At least three nodules for each genotype (wild type and CAA1::pFUS2) were examined by electron microscopy.
Data analysis.
Statistical analysis was performed with SPSS 12.0 (SPSS, Inc., Chicago, IL). Comparison of mean values between the two strains (Tables 1 and 2) was conducted with independent-sample t tests at a 95% level of significance.
TABLE 1.
Comparison of parameters for L. japonicus plants inoculated with M. loti strains R7A, R7A CAA1::pFUS2, and R7A CAA1::pFUS2-pFAJ1709/CAa
| Days p.i. | M. loti strain | No. of nodules plant−1 | Wt per nodule (mg)
|
Top-plant wt (mg)
|
Nitrogenase activity (μmol ethylene h−1 mg nodule fresh wt−1) | ||
|---|---|---|---|---|---|---|---|
| Fresh | Dry | Fresh | Dry | ||||
| 14 | R7A | 1.33 ± 0.10A | 0.06 ± 0.02A | 0.05 ± 0.01A | 10.11 ± 0.61A | 1.28 ± 0.01A | 4.79 ± 0.91A |
| R7A CAA1::pFUS2 | 1.61 ± 0.10A | 0.25 ± 0.07B | 0.08 ± 0.01A | 12.76 ± 1.86A | 1.48 ± 0.32A | 7.59 ± 0.85B | |
| 28 | R7A | 2.94 ± 0.34A | 0.65 ± 0.04A | 0.10 ± 0.01A | 29.72 ± 2.50A | 3.44 ± 0.35A | 6.54 ± 0.91A |
| R7A CAA1::pFUS2 | 4.37 ± 0.31B | 1.07 ± 0.13B | 0.14 ± 0.02A | 30.17 ± 2.21A | 4.06 ± 0.40A | 11.18 ± 1.49B | |
| R7A CAA1::pFUS2-pFAJ1709/CA | 3.01 ± 0.11A | 0.61 ± 0.08A | 0.11 ± 0.02A | 27.29 ± 1.27A | 3.31 ± 0.08A | 7.48 ± 1.10A | |
Measurements were done at 14 and 28 days p.i. The values are means of four and six replications (three plants each) ± standard errors (SE) for 14 and 28 days p.i., respectively. For the numbers of nodules, the values are means of 40 plants ± SE. The statistical analysis refers to comparisons between the two strains only within the same day. Different letters indicate statistical differences at a 95% level of significance.
TABLE 2.
C and N elemental and 13C isotopic ratio analysis of L. japonicus plant parts inoculated with M. loti strain R7A or R7A CAA1::pFUS2a
| Measured parameter | Nodules
|
Top plant
|
||
|---|---|---|---|---|
| R7A | R7A CAA1::pFUS2 | R7A | R7A CAA1::pFUS2 | |
| Total C content (%) | 38.77 ± 3.06A | 44.41 ± 0.96A | 44.63 ± 0.56A | 44.57 ± 0.74A |
| Total N content (%) | 7.50 ± 0.50A | 9.44 ± 0.34B | 5.38 ± 0.10A | 5.04 ± 0.17A |
| C/N ratio | 5.16 ± 0.11B | 4.77 ± 0.13A | 8.31 ± 0.15A | 8.87 ± 0.22A |
| δ13C abundance (%) | −38.50 ± 0.18A | −37.88 ± 0.13B | −38.74 ± 0.12A | −38.61 ± 0.21A |
Measurements were done at 28 days p.i. The values are means of six replications (three plants each) ± standard errors. The statistical analysis refers to comparisons between the two strains only within the same plant part. Different letters indicate statistical differences at a 95% level of significance.
RESULTS
An α-class CA isoform is present in the symbiosis island of M. loti.
Previous work had demonstrated that genes encoding nodule-specific CA isoforms are present in various legume species, including Medicago sativa (10), Glycine max (19), and L. japonicus (14). In contrast, to our knowledge, nothing is known about the presence and the physiological roles of CA isoforms in the microsymbiont. To this end, in silico homology searches revealed the presence of a gene coding for an α-CA homologue in the symbiosis islands of both the R7A (msi040) and MAFF303099 (mll6384) strains of M. loti. In both strains, the encoded polypeptides consist of 250 amino acids, with a predicted molecular mass of 27.2 kDa. Three amino acid substitutions were detected between msi040- and mll6384-encoded polypeptides, including Gly9Ala, Thr152Met, and Lys158Asn.
Comparative analysis of the M. loti R7A msi040 deduced amino acid sequence, annotated as CAA1, revealed that it shares 62%, 53% and 45% similarity with the α-type CAs from R. palustris, N. gonorrhoeae, and H. pylori, respectively (Fig. 1). Furthermore, all amino acids participating in the formation of the α-CA active site were found to be conserved in the CAA1 polypeptide, including His114, His116, and His133, which correspond to the zinc ligands. In silico topology prediction revealed that, similarly to the other bacterial α-CAs, CAA1 has an N-terminal sorting-signal peptide, which possesses the Tat (for twin-arginine translocation) motif RRNLI starting at position 3. In addition, topology analysis of the CAA1 polypeptide using the HMMTOP tool (http://www.enzim.hu/hmmtop/) (39) revealed the presence of a transmembrane helix between amino acids 9 and 26, with the N terminus localized in the cytoplasm and the C terminus, including the active site, facing the periplasm.
FIG. 1.
Comparison of the deduced amino acid sequence of the M. loti α-CA (CAA1) with those of previously characterized bacterial α-CAs. The sequences included are as follows: CAA1, M. loti (strain R7A) α-CA (CAD31445); HpαCA, H. pylori α-CA (BAE66646); NgαCA, N. gonorrhoeae α-CA (CAA72038); and RpαCA, R. palustris α-CA (BAA82053). Identical residues are black, while conservative amino acid substitutions are gray shaded. Conserved amino acids important for catalysis are marked with asterisks, and the predicted signal peptide is overlined. The sequences were aligned using ClustalX (version 2).
CAA1 codes for an active α-CA.
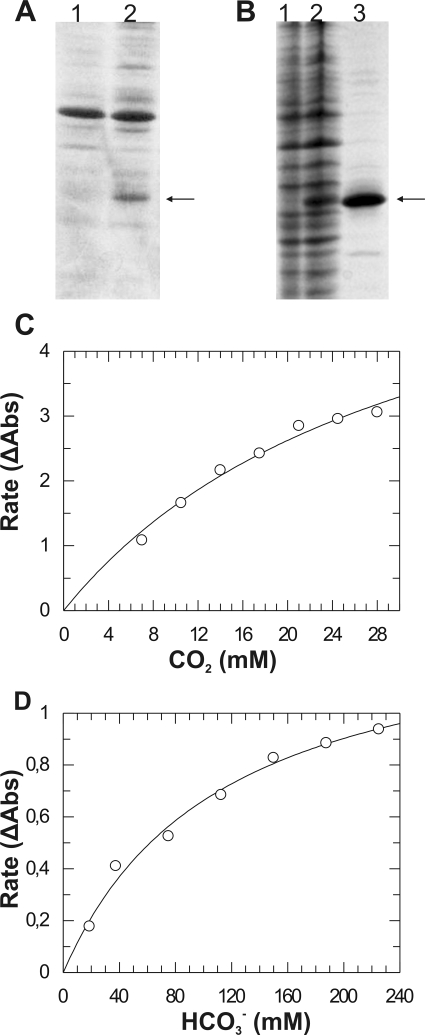
To determine the catalytic properties of CAA1, the coding region, with and without the predicted signal peptide, was subcloned into the pET-28a vector, which drives the expression of the encoded polypeptide as an N-terminal fusion to a six-His tag. When the signal peptide was included, we could not detect accumulation of the recombinant polypeptide in the soluble protein fraction of E. coli. Interestingly, low levels of the polypeptide accumulated in the periplasmic protein fraction (Fig. 2A). In contrast, the recombinant polypeptide lacking the predicted signal peptide was found to accumulate at high levels in the soluble cytoplasmic protein fraction (Fig. 2B). The expressed polypeptide lacking the signal peptide had a molecular mass of around 25 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and was purified over 90% by Ni affinity chromatography (Fig. 2B).
FIG. 2.
Heterologous expression, purification, and enzymatic assays for CA activity of the recombinant CAA1 polypeptide. (A) Accumulation of the recombinant CAA1+ polypeptide (arrow) containing the predicted N-terminal signal peptide in the E. coli periplasmic proteins (lane 2) in comparison to periplasmic proteins from E. coli cells transformed with the empty vector (lane 1). (B) Expression and purification of the recombinant CAA1− polypeptide (arrow) lacking the N-terminal signal peptide. Lane 1, total soluble proteins from E. coli cells transformed with the empty vector; lane 2, total soluble proteins from E. coli cells transformed with the CAA1− expression plasmid; lane 3, purified CAA1− recombinant polypeptide. (C) Kinetic analysis of the CO2 hydration activity was performed at pH 7.5 in HEPES/NaOH buffer. (D) Kinetic analysis of the bicarbonate dehydration activity was performed at pH 6.3 in MES/KOH buffer. Kinetic parameters were calculated by fitting the corrected rate data (change in absorbance per min [ΔAbs]) to the Michaelis-Menten equation using the program GraFit v3.5. The rates shown are means of three repeats (n = 3).
The CO2 hydration activity of the CAA1 recombinant polypeptide was measured at 4°C in a HEPES/NaOH, pH 7.5, buffer. The recombinant enzyme exhibited strong CO2 hydration activity (specific activity, 19.14·106 μmol/min/mg), while it followed Michaelis-Menten kinetics with a kcat of (8.136 ± 1.296)·106 s−1 and a Km of 31.729 ± 8.33 mM (Fig. 2C). The reverse reaction of bicarbonate dehydration was again assayed at 4°C in a MES/KOH pH 6.3 buffer (Fig. 2D). In this case, the recombinant enzyme exhibited a specific activity of 2.85·106 μmol/min/mg. Again, the recombinant CAA1 displayed Michaelis-Menten kinetics, with the following respective kinetic parameters: kcat = (1.215 ± 0.095)·106 s−1 and Km = 112.7 ± 19.5 mM. In contrast to previously characterized α-CA isoforms, the recombinant CAA1 polypeptide did not possess any measurable esterase activity with 4-NPA as a substrate.
CAA1 expression is pH regulated in free-living M. loti.
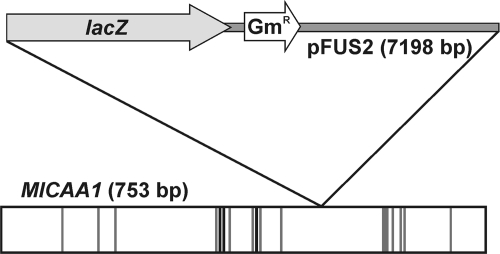
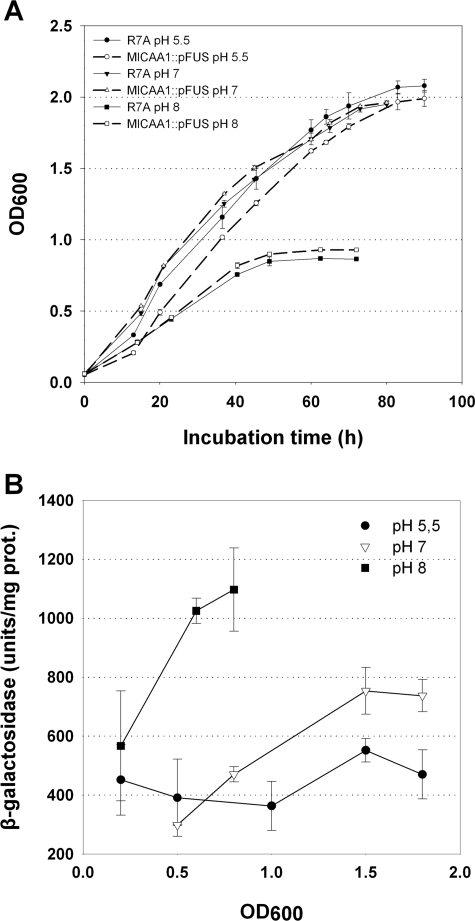
In order to gain insight into the possible physiological roles of CAA1, an M. loti strain R7A disruption mutant was obtained. The mutant was created by homologous recombination using the suicide vector pFUS2 (2), which also creates a transcriptional fusion between the interrupted gene and a promoterless lacZ gene. The disruption of CAA1 was confirmed by amplification, cloning, and sequencing of a 700-bp region including the 5′ region of CAA1 and the pFUS2 region corresponding to the N terminus of the lacZ polypeptide. The analysis revealed that the CAA1 ORF was disrupted after Val166 (Fig. 3), while pFUS2 inserted a stop codon after 19 amino acids. In a first effort, we evaluated the effect of CAA1 disruption on the free-living phenotype. Since the homologous periplasmic α-CA of H. pylori is implicated in the acid acclimation of the bacteria (24) and the gene appears to be pH regulated (43), we tested the growth of the CAA1::pFUS2 strain in batch cultures in YMB medium with various pH values (Fig. 4A). No difference in growth was observed between the CAA1::pFUS2 and the R7A control strains (Fig. 4A) at all pH values tested, while at pH 8, the growth of both strains was strongly restricted. As an indication of the CAA1 promoter activity, β-galactosidase activity was determined during the growth of the CAA1::pFUS2 strain at the different pH values (Fig. 4B). During the exponential growth phase, a twofold induction of β-galactosidase activity was detected at pH 8, while enzyme activity remained high during the stationary phase. At pH 7, a moderate induction of β-galactosidase was detected during the exponential phase, reaching a maximum during the decelerating-growth and stationary phases. The lowest levels of β-galactosidase activity were observed at pH 5.5, where a slight increase was observed during the decelerating-growth phase.
FIG. 3.
Schematic representation of the CAA1::pFUS2 disruption. The positions in the CAA1 ORF of the histidine zinc ligands are represented as black lines, while the positions of other residues participating in the formation of the active site and the hydrophobic CO2 binding pocket are represented as gray lines.
FIG. 4.
(A) Growth of the M. loti CAA1::pFUS (CAA1::lacZ transcriptional fusion) disruption mutant and R7A control strains in batch YMB cultures at pH 5.5, 7, and 8. (B) CAA1 promoter activity was estimated by β-galactosidase activity assays in samples taken at the indicated OD600 values. The values represent the means of four assays (± standard deviations). prot., protein.
Symbiotic phenotypes of the CAA1 mutant.
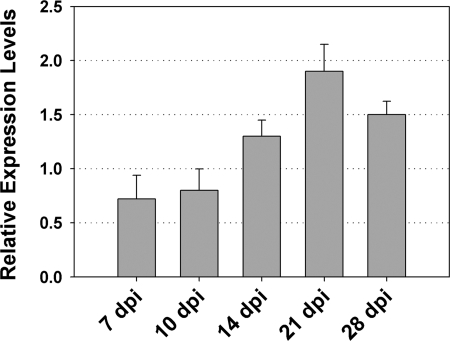
In order to study CAA1 expression during nodule development, we performed real-time reverse transcription-qPCR assays on cDNA templates reverse transcribed from total RNA isolated from nodulated root segments at 7 days p.i. and nodules at 10, 14, 21, and 28 days p.i.. This analysis revealed that CAA1 gene expression was induced twofold in mature nitrogen-fixing nodules at 21 days p.i. in comparison to the levels in nodulated roots and young developing nodules at 10 days p.i. (Fig. 5). Nitrogenase transcripts were first detectable at 14 days p.i., indicating the onset of the nitrogen fixation process (data not shown).
FIG. 5.
Accumulation of CAA1 gene transcripts during nodule development. Total RNA was isolated, reverse transcribed to cDNA, and subjected to real-time qPCR using gene-specific primers. Relative mRNA levels were calculated with respect to the level of the polynucleotide nucleotidyltransferase (mlr5562) transcripts. The bars show means plus standard deviations (n = 3). dpi, days p.i.
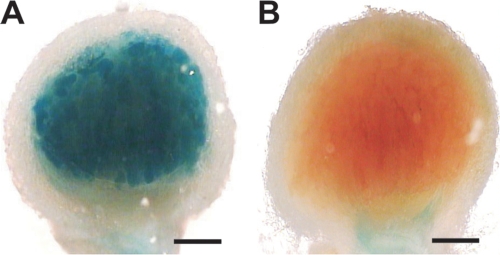
As CAA1 gene expression was found to be upregulated during SNF, we investigated the effects of CAA1 disruption on nodule development and SNF. To this end, L. japonicus seedlings were inoculated with the CAA1::pFUS2 mutant and with R7A as a control. Both strains were able to form nitrogen-fixing nodules on L. japonicus roots. To visualize the expression pattern of the CAA1::lacZ fusion, the spatial distribution of the gene fusion activity was detected with histochemical staining of nodules at 21 days p.i. Strong expression of the CAA1::lacZ fusion was detected throughout the infected cells of the nodule central tissue (Fig. 6A). In contrast, no histochemical staining was observed in nodules of the same age occupied by the M. loti R7A control strain (Fig. 6B).
FIG. 6.
Histochemical localization of β-galactosidase activity in L. japonicus nodules formed by M. loti CAA1::pFUS and M. loti R7A. Nodules at 21 days p.i. were hand sectioned and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). (A) In CAA1::pFUS-induced nodules, β-galactosidase activity was evident in the bacteroid-containing cells in the nodule central tissue. (B) No histochemical staining was detected in the nodules formed by M. loti R7A control strain. Bars, 200 μm.
Interestingly, while nodule color and macroscopic structure were similar between nodules formed by the mutant and the wild-type strains, a significant increase in the number of mature nitrogen-fixing nodules formed by the CAA1::pFUS2 mutant was consistently observed at 28 days p.i. during several independent experiments (Table 1). The increase in nodule number was accompanied by a significant increase in nodule fresh weight. Although, an increase in dry weight was also observed in CAA1::pFUS2 nodules, it was statistically insignificant. In contrast to the altered nodulation kinetics, no significant differences in the top-plant growth were observed, as depicted by the determination of total hypergeous organ fresh and dry weights at both 14 and 28 days p.i. (Table 1). Nitrogenase activity in the nodules formed by both strains was estimated by measuring their acetylene reduction capacities (Table 1). In young nodules at 14 days p.i., nitrogenase activity of the CAA1 mutant was 1.5-fold higher than that of the wild-type R7A strain. In mature nodules at 28 days p.i., this difference was particularly pronounced, as in these fully developed nodules, nitrogenase activity levels were found to be almost twofold higher in the nodules occupied by the CAA1 mutant.
In an attempt to test whether the increased nodulation capacity and elevated nitrogenase activity of the CAA1 mutant had an immediate effect on the plant's nutritional status, we measured the total C and N contents of nodules and hypergeous organs from plants inoculated with the mutant and R7A wild-type strains, respectively. At 28 days p.i., the total nitrogen content of nodules formed by the CAA1 mutant was significantly higher than that of nodules of the R7A wild type (Table 2). In contrast, the total carbon content was not significantly altered between nodules formed by the two strains. This resulted in a significant decrease in the C/N ratio of the nodules formed by the α-CA mutant (Table 2). Interestingly, the excess nitrogen content found in CAA1::pFUS2 nodules did not have any effect on top-plant nutritional status, as both nitrogen and carbon total contents of the hypergeous organs were similar between plants nodulated with the CAA1 mutant and the R7A wild type, respectively (Table 2). A recent study showed that reduced levels of CA affected the carbon isotope discrimination (Δ13C) and thus the leaf organic δ13C abundance in C4 plants (8). Therefore, we aimed to test whether a similar effect on the nodulated L. japonicus plants could be detected. Interestingly, CAA1 disruption resulted in a small but significant enrichment of the nodules in 13C (higher δ13C abundance), whereas no effect was detected on the top-plant organs (Table 2).
Finally, a comparison of the ultrastructure of nodules initiated by the CAA1 mutant in comparison to the wild-type nodules failed to reveal any significant differences in bacteroid differentiation and morphology. In addition, no significant changes in bacteroid density were observed (data not shown).
In order to verify that the observed differences in nodule number, weight, and nitrogenase activity could be unambiguously attributed to the CAA1 disruption, the CAA1 coding and terminator sequences were cloned into the pFAJ1709 vector and reinserted into the CAA1::pFUS2 mutant strain. L. japonicus plants nodulated by the resulting strain did not exhibit any significant differences in nodule number, fresh and dry weight, or nitrogenase activity in comparison to the plants nodulated with the R7A control strain (Table 1).
DISCUSSION
In this report, we present biochemical and physiological studies of CAA1, an M. loti α-type CA encoded by a single gene present in the symbiosis island (18, 36). In contrast to the widespread distribution of CAs, only a few prokaryotes have been shown so far to possess active α-type CA isoforms. They include H. pylori (6), the CO2-requiring pathogen N. gonorrhoeae (5), the purple non-sulfur phototrophic bacterium R. palustris (29), and the cyanobacteria Anabaena and Synechococcus (34). Similarly, in silico homology searches revealed that α-type CA isoforms are not present in all genera of rhizobia. In addition to M. loti, α-type CA homologues are present only in the Sinorhizobium meliloti pSymA plasmid (gene SMa0045) and the Bradyrhizobium japonicum chromosome (gene bll1137 in strain USDA110).
A common feature of all bacterial α-type CAs is the presence of an N-terminal signal peptide, responsible for the periplasmic localization of the enzyme. In CAA1, the respective N-terminal signal peptide possesses the characteristic motif found in the proteins exported through the Tat pathway. Although, we do not yet have experimental confirmation for the periplasmic localization of CAA1 in M. loti, the N-terminal signal peptide resulted in the periplasmic localization of the recombinant CAA1 in E. coli (Fig. 2A).
Comparison of the CAA1 polypeptide with characterized bacterial and mammalian α-type CAs revealed that the identified zinc ligands are invariant in all α-CAs. In addition, all the hydrophobic residues forming the CO2 binding pocket near the enzyme active site, namely, Val135, Val146, Leu199, Val208, and Trp210, are identical in all bacterial α-CAs, as well as the human CAII. Our functional assays confirmed that CAA1 codes for an active α-type CA that is able to catalyze both the hydration of CO2 and the dehydration of bicarbonate. In contrast to the mammalian isoforms, we failed to detect any CAA1 esterase activity. Similarly, low 4-NPA hydrolase activity was also reported for the H. pylori α-CA (6).
The possible biochemical and physiological roles of CAA1 during symbiosis were studied by the use of a CAA1::pFUS2 disruption mutant strain. These experiments revealed that the CAA1::pFUS2 strain was able to form nitrogen-fixing nodules on L. japonicus, although inactivation of the gene caused various symbiotic effects. The mutant strain formed a higher number of nodules with increased fresh weight and nitrogenase activity (Table 1), although no differences were observed in nodule ultrastructure and bacterial distribution in infected cells. The observed effects did not improve the growth and nutritional status of plants nodulated by the mutant strain (Table 2). The only difference observed in the nodules of these plants was an increase in their nitrogen content. These findings indicate that any increase in nitrogen fixation due to increased nitrogenase activity is not beneficial for the nonsymbiotic plant organs.
An interesting hint about the possible physiological role of CAA1 during symbiosis comes from the physiological role of the H. pylori periplasmic α-CA homologue. H. pylori and other Helicobacter spp. are able to colonize the acidic mammalian stomach, although they are neutralophiles. This phenotype is mainly dependent on urease activity and urea influx through the UreI proton-gated channel (31). The products of urease activity in the cytoplasm are 2NH3 plus CO2, which diffuse very rapidly across the inner membrane into the periplasmic space. There, the α-CA-catalyzed hydration of CO2 provides both protons for NH4+ formation and bicarbonate, which can buffer the periplasm at a pH value close to its pKa of 6.1, within the range of bacterial growth ability (24). Interestingly, a very similar situation exists in the symbiotic nitrogen-fixing bacteroids, as large amounts of NH3 and CO2 diffuse across the inner membrane into an acidified symbiosome space, where NH3 is protonated to NH4+ for transport across the symbiosome membrane (9, 44). The acidification of the symbiosome space is facilitated by a proton pumping P-type H+-ATPase (13, 37, 40), although proteomic studies of the symbiosome membrane fraction of L. japonicus and pea also revealed the presence of V-type H+-ATPases (30, 45). The presence of an active α-CA enzyme in the periplasm of the nitrogen-fixing bacteroids could provide a complementary source of protons for the acidification of the periplasm and the protonation of NH3. In the presence of the periplasmic CA, the hydration of the CO2 produced by bacteroid respiration could be accelerated several thousandfold. The protons produced during the CA-catalyzed formation of HCO3− could subsequently be used for the protonation of NH3. The rapid conversion of NH3 to NH4+ could serve as a trap, which in turn facilitates the further diffusion of NH3 from the bacteroid across a steep concentration gradient. In addition, similarly to H. pylori, the bicarbonate produced can buffer the periplasm at a slightly acidic pH, favoring NH3 protonation. The absence of a suitable buffering system, for maintaining the acidic external pH could result in a less stable and unpredictable periplasmic pH (NH4+/NH3 pKa, 9.2). The possible role of CAA1 in maintaining an acidic periplasm during SNF is supported by the observation that the expression of the respective gene is upregulated both upon the onset of nitrogen fixation and at alkaline pH values in free-living bacteria. Similarly, the expression of the H. pylori α-CA was shown to be pH regulated by the ArsRS two-component system (43). In the CAA1::pFUS2 nodules, the formation of NH4+ outside the bacteroid inner membrane could result in a localized pH increase, which in turn would reduce the rate of NH3 protonation, diffusion from the bacteroid cytosol, and transport to the plant cytosol, resulting in the observed increase in nodule N content. The increase in nodule number observed at later stages after the onset of SNF (28 days p.i.), in size, and in nitrogenase activity could represent a response to compensate for the reduced nitrogen supplied by the microsymbiont. The increased nodulation kinetics observed after the onset of nitrogen fixation is a typical feature found in ineffective rhizobium-legume symbiosis (23, 35). In our plants, the increased nodulation kinetics was accompanied by an increase in nitrogenase activity, indicating that CAA1 disruption may limit the supply of symbiotically fixed nitrogen to the plant at the step of transport and assimilation, rather than N2 reduction. It should be pointed out that if such a CA-based mechanism for facilitated NH3 protonation and buffering of the bacteroid periplasm exists, this mechanism would probably have an auxiliary role, as is indicated by the ability of the CAA1::pFUS2 mutant to form nitrogen-fixing nodules and the absence of homologous genes from the genomes of certain rhizobium species.
An interesting clue to the possible physiological roles of CAA1 comes from the altered δ13C of nodules occupied by the CAA1::pFUS2 mutant strain. A recent study indicated that CA is involved in carbon isotope discrimination (Δ13C) in the leaves of the C4 plant Flaveria bidentis (8). In particular, it was demonstrated that reduced CA activity results in increased Δ13C and thus 13C-depleted leaves. In the present study, nodules lacking the M. loti α-type CA were 13C enriched compared to the wild-type leaves, possibly indicating that at least part of the inorganic carbon channeled through CAA1 could be used as a substrate for carboxylation reactions from either the rhizobia or the plant. Although further research is needed in this direction, it indicates that CAA1 is involved in carbon isotope discrimination during dark CO2 fixation in nodulated roots.
In this report, we have studied the biochemical properties and the possible physiological roles of the CAA1 α-class CA located in the symbiosis island of M. loti. Although plant CA isoforms are upregulated in nodules, to our knowledge, this is the first report addressing the role of the bacterial CA isoforms during SNF. Nevertheless, further work is needed in order to provide additional support for the proposed model of CA-facilitated NH3 protonation. In addition, the study of CAA1 involvement in nodule carbon economy would be greatly facilitated by L. japonicus lines with the plant CAs silenced, currently under construction in our laboratory.
Acknowledgments
The M. loti R7A CAA1::pFUS2 disruption mutant strain was kindly provided by Clive Ronson (University of Otago, Otago, New Zealand).
Footnotes
Published ahead of print on 13 February 2009.
REFERENCES
- 1.Adams, M. A., and P. F. Grierson. 2001. Stable isotopes at natural abundance in terrestrial plant ecology and ecophysiology: an update. Plant Biol. 3299-310. [Google Scholar]
- 2.Antoine, R., S. Alonso, D. Raze, L. Coutte, S. Lesjean, E. Willery, C. Locht, and F. Jacob-Dubuisson. 2000. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 1825902-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, M. J., D. V. Meyers, J. A. Verpoorte, and J. T. Edsall. 1966. Purification and properties of human erythrocyte carbonic anhydrases. J. Biol. Chem. 2415137-5149. [PubMed] [Google Scholar]
- 4.Boivin, C., S. Camut, C. A. Malpica, G. Truchet, and C. Rosenberg. 1990. Rhizobium meliloti genes encoding catabolism of trigoneline are induced under symbiotic conditions. Plant Cell 21157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirica, L. C., B. Elleby, B. H. Jonsson, and S. Lindskog. 1997. The complete sequence, expression in Escherichia coli, purification and some properties of carbonic anhydrase from Neisseria gonorrhoeae. Eur. J. Biochem. 244755-760. [DOI] [PubMed] [Google Scholar]
- 6.Chirica, L. C., B. Elleby, and S. Lindskog. 2001. Cloning, expression and some properties of alpha-carbonic anhydrase from Helicobacter pylori. Biochim. Biophys. Acta 154455-63. [DOI] [PubMed] [Google Scholar]
- 7.Chirica, L. C., C. Petersson, M. Hurtig, B. H. Jonsson, T. Boren, and S. Lindskog. 2002. Expression and localization of alpha- and beta-carbonic anhydrase in Helicobacter pylori. Biochim. Biophys. Acta 1601192-199. [DOI] [PubMed] [Google Scholar]
- 8.Cousins, A. B., M. R. Badger, and S. von Caemmerer. 2006. Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis. Insights from antisense RNA in Flaveria bidentis. Plant Physiol. 141232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, D. A., P. S. Poole, S. D. Tyerman, and L. Rosendahl. 2001. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 5861-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Pena, T. C., F. Frugier, H. I. McKhann, P. Bauer, S. Brown, A. Kondorosi, and M. Crespi. 1997. A carbonic anhydrase gene is induced in the nodule primordium and its cell-specific expression is controlled by the presence of Rhizobium during development. Plant J. 11407-420. [DOI] [PubMed] [Google Scholar]
- 11.Dombrecht, B., J. Vanderleyden, and J. Michiels. 2001. Stable RK2-derived cloning vectors for the analysis of gene expression and gene function in gram-negative bacteria. Mol. Plant-Microbe Interact. 14426-430. [DOI] [PubMed] [Google Scholar]
- 12.Farquhar, G. D., J. R. Ehleringer, and K. T. Hubick. 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40503-537. [Google Scholar]
- 13.Fedorova, E., R. Thomson, L. F. Whitehead, O. Maudoux, M. K. Udvardi, and D. A. Day. 1999. Localization of H+-ATPases in soybean root nodules. Planta 20925-32. [DOI] [PubMed] [Google Scholar]
- 14.Flemetakis, E., M. Dimou, D. Cotzur, G. Aivalakis, R. C. Efrose, C. Kenoutis, M. Udvardi, and P. Katinakis. 2003. A Lotus japonicus beta-type carbonic anhydrase gene expression pattern suggests distinct physiological roles during nodule development. Biochim. Biophys. Acta 1628186-194. [DOI] [PubMed] [Google Scholar]
- 15.Guilloton, M. B., A. F. Lamblin, E. I. Kozliak, M. Geraminejad, C. Tu, D. Silverman, P. M. Anderson, and J. A. Fuchs. 1993. A physiological role for cyanate-induced carbonic anhydrase in Escherichia coli. J. Bacteriol. 1751443-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handberg, K., and J. Stougaard. 1992. Lotus japonicus, an autogamous diploid legume species for classical and molecular genetics. Plant J. 2487-496. [Google Scholar]
- 17.Hayashi, M., Y. Maeda, Y. Hashimoto, and Y. Murooka. 2000. Efficient transformation of Mesorhizobium huakuii subsp. rengei and Rhizobium species. J. Biosci. Bioeng. 89550-553. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7331-338. [DOI] [PubMed] [Google Scholar]
- 19.Kavroulakis, N., E. Flemetakis, G. Aivalakis, and P. Katinakis. 2000. Carbon metabolism in developing soybean root nodules: the role of carbonic anhydrase. Mol. Plant-Microbe Interact. 1314-22. [DOI] [PubMed] [Google Scholar]
- 20.Khalifah, R. G. 1971. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 2462561-2573. [PubMed] [Google Scholar]
- 21.Kimber, M. S., and E. F. Pai. 2000. The active site architecture of Pisum sativum beta-carbonic anhydrase is a mirror image of that of alpha-carbonic anhydrases. EMBO J. 191407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisker, C., H. Schindelin, B. E. Alber, J. G. Ferry, and D. C. Rees. 1996. A left-handed beta-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila. EMBO J. 152323-2330. [PMC free article] [PubMed] [Google Scholar]
- 23.Krusell, L., K. Krause, T. Ott, G. Desbrosses, U. Kramer, S. Sato, Y. Nakamura, S. Tabata, E. K. James, N. Sandal, J. Stougaard, M. Kawaguchi, A. Miyamoto, N. Suganuma, and M. K. Udvardi. 2005. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 171625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus, E. A., A. P. Moshfegh, G. Sachs, and D. R. Scott. 2005. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 187729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlin, C., M. Masters, S. McAteer, and A. Coulson. 2003. Why is carbonic anhydrase essential to Escherichia coli? J. Bacteriol. 1856415-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 27.Oldroyd, G. E., M. J. Harrison, and M. Udvardi. 2005. Peace talks and trade deals. Keys to long-term harmony in legume-microbe symbioses. Plant Physiol. 1371205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prell, J., and P. Poole. 2006. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14161-168. [DOI] [PubMed] [Google Scholar]
- 29.Puskas, L. G., M. Inui, K. Zahn, and H. Yukawa. 2000. A periplasmic, alpha-type carbonic anhydrase from Rhodopseudomonas palustris is essential for bicarbonate uptake. Microbiology 1462957-2966. [DOI] [PubMed] [Google Scholar]
- 30.Saalbach, G., P. Erik, and S. Wienkoop. 2002. Characterisation by proteomics of peribacteroid space and peribacteroid membrane preparations from pea (Pisum sativum) symbiosomes. Proteomics 2325-337. [DOI] [PubMed] [Google Scholar]
- 31.Sachs, G., J. A. Kraut, Y. Wen, J. Feng, and D. R. Scott. 2006. Urea transport in bacteria: acid acclimation by gastric Helicobacter spp. J. Membr. Biol. 21271-82. [DOI] [PubMed] [Google Scholar]
- 32.Smith, K. S., and J. G. Ferry. 2000. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 24335-366. [DOI] [PubMed] [Google Scholar]
- 33.Smith, K. S., C. Jakubzick, T. S. Whittam, and J. G. Ferry. 1999. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc. Natl. Acad. Sci. USA 9615184-15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SoltesRak, E., M. E. Mulligan, and J. R. Coleman. 1997. Identification and characterization of a gene encoding a vertebrate-type carbonic anhydrase in cyanobacteria. J. Bacteriol. 179769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suganuma, N., Y. Nakamura, M. Yamamoto, T. Ohta, H. Koiwa, S. Akao, and M. Kawaguchi. 2003. The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol. Genet. Genomics 269312-320. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan, J. T., J. R. Trzebiatowski, R. W. Cruickshank, J. Gouzy, S. D. Brown, R. M. Elliot, D. J. Fleetwood, N. G. McCallum, U. Rossbach, G. S. Stuart, J. E. Weaver, R. J. Webby, F. J. De Bruijn, and C. W. Ronson. 2002. Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J. Bacteriol. 1843086-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szafran, M. M., and H. Haaker. 1995. Properties of the peribacteroid membrane ATPase of pea root nodules and its effect on the nitrogenase activity. Plant Physiol. 1081227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripp, B. C., K. Smith, and J. G. Ferry. 2001. Carbonic anhydrase: new insights for an ancient enzyme. J. Biol. Chem. 27648615-48618. [DOI] [PubMed] [Google Scholar]
- 39.Tusnady, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17849-850. [DOI] [PubMed] [Google Scholar]
- 40.Udvardi, M. K., and D. A. Day. 1989. Electrogenic ATPase activity on the peribacteroid membrane of soybean (Glycine max L.) root nodules. Plant Physiol. 90982-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udvardi, M. K., and D. A. Day. 1997. Metabolite transport across symbiotic membranes of legume nodules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48493-523. [DOI] [PubMed] [Google Scholar]
- 42.Vance, C. P. 2001. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 127390-397. [PMC free article] [PubMed] [Google Scholar]
- 43.Wen, Y., J. Feng, D. R. Scott, E. A. Marcus, and G. Sachs. 2007. The HP0165-HP0166 two-component system (ArsRS) regulates acid-induced expression of HP1186 alpha-carbonic anhydrase in Helicobacter pylori by activating the pH-dependent promoter. J. Bacteriol. 1892426-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White, J., J. Prell, E. K. James, and P. Poole. 2007. Nutrient sharing between symbionts. Plant Physiol. 144604-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wienkoop, S., and G. Saalbach. 2003. Proteome analysis. Novel proteins identified at the peribacteroid membrane from Lotus japonicus root nodules. Plant Physiol. 1311080-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]