Abstract
We demonstrate that Mycobacterium tuberculosis GroEL1 is phosphorylated by PknF at two positions, Thr25 and Thr54. Unexpectedly, Mycobacterium smegmatis GroEL1 is not a substrate of its cognate PknF. This study shows that the phosphorylation profiles of conserved proteins are species dependent and provide insights that may explain the numerous biological functions of these important proteins.
Heat shock protein 60 (Hsp60), also known as Cpn60 or GroEL, is a prototypical molecular chaperone, which in Escherichia coli has been shown to be required for growth and necessary for the folding of several essential proteins (11, 18). Although most bacteria possess a single copy of the groEL gene (22), actinomycetes are unusual in having genes that encode two forms of the Hsp60 chaperone GroEL. The major human pathogen Mycobacterium tuberculosis GroEL1 and GroEL2 proteins (20) share 61% amino acid identity. In M. tuberculosis, both GroEL proteins are upregulated during heat shock (32), oxidative stress response (9), and macrophage infection (27). Moreover, several studies reported their involvement in the immune response to M. tuberculosis infection (21, 29). However, M. tuberculosis GroEL proteins are unusual chaperones in the sense that they exhibit very weak ATPase activity and they are poorly active in refolding substrates. Another intriguing feature of the M. tuberculosis GroEL proteins is the absence of the canonical oligomeric state, suggesting that the mycobacterial chaperones have a completely different folding mechanism than that of the prototypic GroEL (31). This raises questions about the nature of the biological functions of these chaperones and whether they are required for intracellular protein folding or associated with virulence in the infected host (30). Whereas groEL2 was found to be essential for cell survival, a groEL1 mutant was indistinguishable from the wild-type strain either in broth culture or within macrophages. However, infection studies in mice indicated the failure of the groEL1 mutant to produce a granulomatous inflammation either in mice or guinea pigs, which correlated with a reduced cytokine expression (15). Thus, GroEL1 appears to be a key factor in controlling the cytokine-dependent granulomatous response during M. tuberculosis infection. However, whether this immunomodulatory function represents the primary role of GroEL1 in mycobacteria remains uncertain, as the protein is also present in atypical and nonpathogenic species, such as Mycobacterium smegmatis. In this species, groEL1 contains the attB site for phage Bxb1 integration (19) and was found to be dispensable for normal planktonic growth (28). The unexpected role of GroEL1 in biofilm formation by physically interacting with the β-ketoacyl-AcpM synthase KasA, a key component of the type II fatty acid synthase involved in mycolic acid biosynthesis, has recently been reported (2, 28). In general, different GroEL1 proteins exhibit a bewildering variety of biological functions, and albeit the M. tuberculosis and M. smegmatis GroEL1 orthologues display very highly conserved sequences, they do not behave similarly. The extensive literature on GroEL1 proteins seems to suggest that these molecules are not unitary and that different GroEL1 proteins from different sources could express different patterns of biological activity. It appears very likely that slight changes in the sequence and/or structure of mycobacterial GroEL proteins can exert profound effects on their biological activity. Although the biochemical, functional, and structural properties of mycobacterial GroEL1 proteins have been studied (30), it is not known whether these proteins undergo posttranslational modifications. Therefore, as a first step to decipher how subtle primary sequence changes may confer different activities to the M. tuberculosis and M. smegmatis GroEL1 proteins, we investigated whether these proteins exhibit differential posttranslational modifications.
STPK-mediated phosphorylation of M. tuberculosis GroEL1.
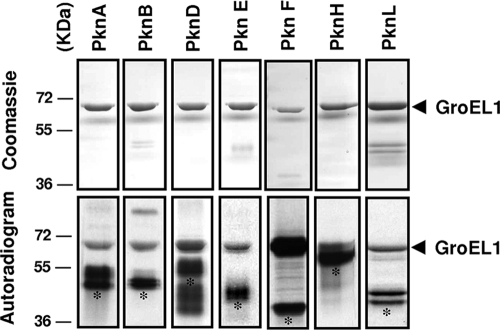
We first investigated whether the M. tuberculosis GroEL1 chaperone could be modified by phosphorylation, which would provide valuable insights into some of their functional properties. Protein phosphorylation allows extracellular signals to be translated in cellular responses and has recently emerged as a major physiological mechanism due to its link to virulence in different pathogens (14, 35). The M. tuberculosis genome encodes 11 Ser/Thr protein kinases (STPKs) (1, 6), and most are being investigated for their physiological roles and potential application for future drug development to combat tuberculosis (36). The presence of several STPKs suggests that phosphorylation influences a wide range of biological functions, such as adaptation to various environmental conditions, stress, cell wall synthesis, cell division, and pathogenicity (36). Here, we tested the hypothesis whether M. tuberculosis STPKs could phosphorylate GroEL1. PknA, PknB, PknD, PknE, PknF, PknH, and PknL were expressed as His-tagged fusions and purified from E. coli as described previously (23). Recombinant GroEL1 was expressed and purified from E. coli BL21(DE3)Star harboring the pETPhos_GroEL1 plasmid (Table 1). When the STPKs were incubated in the presence of GroEL1 and [γ-33P]ATP, phosphorylation of GroEL1 was observed with the different kinases, although the levels of the GroEL1 signal varied (Fig. 1). In fact, while all selected kinases, present at comparable concentrations, were able to phosphorylate GroEL1, PknF generated the strongest signal, thus leading to the hypothesis that GroEL1 could be preferentially recruited by PknF (Fig. 1). Together, these data indicate different levels of substrate specificity of the various STPKs, as reported previously for other mycobacterial substrates (23). A corollary arising from these experiments is that GroEL1 interacting with several STPKs may be regulated in vivo by multiple environmental signals, although this remains to be established.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and use or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG; used for general cloning | Invitrogen |
| E. coli BL21(DE3)Star | F−ompT hsdSB(rB− mB−) gal dcm (DE3); used to express recombinant proteins in E. coli | Stratagene |
| Plasmids | ||
| pETPhos | pET15b (Novagen) derivative including the replacement of the thrombin site coding sequence with a tobacco etch virus protease site and Ser-to-Gly mutagenesis in the N-terminal His tag | 4 |
| pETPhos_groEL1 | pETPhos derivative used to express His-tagged fusion of GroEL1 | This work |
| pETPhos_groEL1_T25A | pETPhos derivative used to express His-tagged fusion of GroEL1(T25A) | This work |
| pETPhos_groEL1_T54A | pETPhos derivative used to express His-tagged fusion of GroEL1(T54A) | This work |
| pETPhos_groEL1_T25A/T54A | pETPhos derivative used to express His-tagged fusion of GroEL1(T25A/T54A) | This work |
| pETPhos_pknF | pETPhos derivative used to express His-tagged fusion of the PknF cytosolic domain | This work |
| pETPhos_pknF_K41M | pETPhos derivative used to express His-tagged fusion of the PknF(K41M) cytosolic domain | This work |
| pETPhos_pknF_T173A | pETPhos derivative used to express His-tagged fusion of the PknF(T173A) cytosolic domain | This work |
| pETPhos_pknF_T175A | pETPhos derivative used to express His-tagged fusion of the PknF(T175A) cytosolic domain | This work |
| pETPhos_pknF_T173A/T175A | pETPhos derivative used to express His-tagged fusion of the PknF(T173A/T175A) cytosolic domain | This work |
| pETPhos_pknF_Msm | pETPhos derivative used to express His-tagged fusion of the PknF cytosolic domain from M. smegmatis | This work |
| pETPhos_groEL1_Msm_WT | pETPhos derivative used to express His-tagged fusion of GroEL1(WT) from M. smegmatis | This work |
| pETPhos_groEL1_Msm_A25T | pETPhos derivative used to express His-tagged fusion of GroEL1(A25T) from M. smegmatis | This work |
FIG. 1.
In vitro phosphorylation of GroEL1 by STPKs. Seven recombinant STPKs (PknA, PknB, PknD, PknE, PknF, PknH, and PknL) encoded by the M. tuberculosis genome were expressed and purified as His-tagged fusions in E. coli and incubated with the purified His-tagged M. tuberculosis GroEL1. The different proteins were incubated together with [γ-33P]ATP for 15 min, subjected to gel electrophoresis, and stained with Coomassie blue (top panel). Radioactive bands were revealed by autoradiography (bottom panel). Standard proteins of known molecular masses (in kilodaltons) were run in parallel, and their positions are shown to the left of the gels. Asterisks indicate the autophosphorylated kinases.
Contribution of the activation loop Thr173 and Thr175 residues in the autophosphorylation activity of PknF.
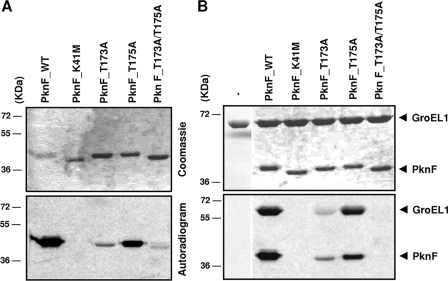
M. tuberculosis PknF participates in multiple biological processes, such as cell growth, septum formation, and glucose transport (8). It has been shown to phosphorylate the two Forkhead-associated domains of the ABC transporter protein Rv1747 (7, 26) and the Forkhead-associated domain of Rv0020 (13). The autokinase activity of PknF was demonstrated by incubating the protein with [γ-33P]ATP. Wild-type PknF [PknF(WT)] incorporated radioactive phosphate from [γ-33P]ATP, giving rise to a strong radioactive signal at the expected size of the protein, indicating that PknF undergoes autophosphorylation (Fig. 2A). To exclude the possibility of exogenous contamination that could explain labeling of PknF, Lys41 present in subdomain II of PknF was mutated to Met (Tables 1 and 2). Purified PknF(K41M) was incubated with [γ-33P]ATP, and as expected, no signal could be detected, confirming the essential role of Lys41 in catalyzing the phosphorylation reaction, in agreement with previous reports on other mycobacterial kinases (24). The transition between active and inactive forms occurring via control of access to the catalytic site and/or substrate-binding site reflects one possible regulatory mechanism in eukaryote-type kinases. This involves phosphorylation/dephosphorylation steps through an autocatalytic mechanism or by intervention of other kinases and phosphatases. The active/inactive conformational switch is driven by the activation loop in numerous kinases (16), and the conformation of the activation loop depends on the phosphorylation state of this major control element (17). Boitel et al. (3) demonstrated that Thr171 and Thr173 are essential phosphoacceptors in M. tuberculosis PknB kinase activity. The PknL activation loop residues Thr173 and Thr175, corresponding to Thr171 and Thr173 in PknB, were also identified as necessary for full PknL activity, and phosphorylation of Thr173 was required for optimal PknL-mediated phosphorylation of Rv2175c (5). Conservation of the autophosphorylation pattern in the activation loop of PknF has been reported, with Thr173 and Thr175 being the only two phosphorylated residues in the activation loop of this kinase (10). Here we addressed whether phosphorylation of the activation loop affects PknF activity by individually substituting Thr173 and Thr175 by Ala to generate the PknF(T173A) and PknF(T175A) mutants, respectively (Tables 1 and 2). In addition, a double mutant, PknF(T173A/T175A) was also created. Substitution of Thr173 by Ala severely impaired the autokinase activity of PknF (Fig. 2A). In contrast, the Thr175A mutant did not exhibit a strongly reduced signal, although it was weaker than the signal of the wild-type kinase. However, when both residues were mutated, the signal almost completely disappeared. Interestingly, the electrophoretic migration profile showed that PknF(K41M) and PknF(T173A/T175A) migrated faster than PknF(WT), PknF(T173A), or PknF(T175A), although they possess the same molecular mass (Fig. 2A). These different electrophoretic mobility properties are very likely due to their different intrinsic phosphorylation states, as already reported for other kinases (5). Together, these data indicate that double phosphorylation of the Thr173 and Thr175 activation loop residues is necessary for optimal kinase activity, although Thr173 appears to play a more prominent role than Thr175 does. These results support the notion that both phosphothreonines participate in the regulatory control of PknF activity.
FIG. 2.
In vitro phosphorylation of M. tuberculosis GroEL1 by PknF and the different PknF mutants. (A) Autokinase activity of the different derivatives of PknF. All proteins [PknF(WT), PknF(K41M), PknF(T173A), PknF(T175A), and PknF(T173A/T175A)] were overproduced in E. coli, purified as His-tagged fusions, and incubated with [γ-33P]ATP. Proteins were separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). (B) In vitro phosphorylation of GroEL1 by PknF and the different PknF mutants in the presence of [γ-33P]ATP. Proteins were separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). The positions of molecular mass markers (in kilodaltons) are shown to the left of the gels.
TABLE 2.
Primers used in this study
| Primer | Gene | Sequencea (5′ to 3′) |
|---|---|---|
| NtermGroEL1 | groEL1 | ATA TAG CTC ATA TGA GCA AGC TGA TC (NdeI) |
| CtermGroEL1 | groEL1 | ATA GGA TCC TCA GTG CGC GTG CCC GTG (BamHI) |
| NtermGroEL1_T25A | groEL1 | ATG GAC AAG CTG GCC GAC GCC GTG CGG GTG ACG CTG GGG |
| CtermGroEL1_T25A | groEL1 | CCC CAG CGT CAC CCG CAC GGC GTC GGC CAG CTT GTC CAT |
| NtermGroEL1_T54A | groEL1 | GTT ACC AAC GAC GGC GTC GCG GTG GCA CGT GAG ATC GAG |
| CtermGroEL1_T54A | groEL1 | CTC GAT CTC ACG TGC CAC CGC GAC GCC GTC GTT GGT AAC |
| NtermPknF_CD | pknF | TAATAGCT CAT ATG CCG CTC GCG GAA GGT TCG (NdeI) |
| CtermPknF_CD | pknF | TAT AAG CTT TTA CGG TTG CGA CAC CCG CGT (HindIII) |
| NtermPknF_CD_T173A | pknF | CCA AGC GGA TTG GCC GCC ACA AAC ATG |
| NtermPknF_CD_T175A | pknF | CCA AGC GGA TTG ACC GCC GCA AAC ATG ACT GTG GGC ACC |
| CtermPknF_CD_T175A | pknF | GGT GCC CAC AGT CAT GTT TGC GGC GGT CAA TCC GCT TGG |
| NtermPknF_CD_T173A/T175A | pknF | CCA AGC GGA TTG GCC GCC GCA AAC ATG ACT GTG G |
| NtermPknF_CD_smeg | pknFMsm | TA ATA GCT CAT ATG CCA CTG GCC GCT GGG GAG ACA (NdeI) |
| CtermPknF_CD_smeg | pknFMsm | TAT GGA TCC TCA GGC CGC GTG CCT GGC GAC CGG (BamHI) |
| NtermGroEL1_smeg | groEL1Msm | TA ATA GCT CAT ATG AGC AAG CAG ATT GAA TTC AAC (NdeI) |
| CtermGroEL1_smeg | groEL1Msm | TAATAGCT GCT AGC TCA GTG AGC GTG GCC GTG GTG (NheI) |
| NtermGroEL1_A25T_smeg | groEL1Msm | GTC GAC AAG CTC GCC GAC ACC GTC AAG GTC ACG CTC GGC |
| CtermGroEL1_A25T_smeg | groEL1Msm | GCC GAG CGT GAC CTT GAC GGT GTC GGC GAG CTT GTC GAC |
Restriction sites are underlined and specified in the parentheses after the sequence. Mutagenized codons are shown in bold type.
Recruitment and phosphorylation of GroEL1 is dependent on the activation loop Thr173 and Thr175.
The identification of PknF/GroEL1 as a novel kinase/substrate pair in M. tuberculosis prompted us to assess the contribution of the PknF activation loop Thr173 and Thr175 residues in the transphosphorylation reaction between the kinase and its substrate. This was achieved by incubating the M. tuberculosis GroEL1 substrate with PknF(K41M), PknF(T173A), PknF(T175A), or PknF(T173A/T175A). As expected, PknF(K41M) was unable to phosphorylate GroEL1, indicating that phosphorylation of Lys41 is a prerequisite to the transphosphorylation reaction (Fig. 2B). More importantly, whereas the T173A mutation profoundly decreased the transphosphorylation reaction, T175A did not significantly alter PknF-dependent phosphorylation of GroEL1. Furthermore, GroEL1 could not be phosphorylated by PknF(T173A/T175A), clearly demonstrating that recruitment and phosphorylation of GroEL1 are dependent on both Thr173 and Thr175. Our results indicate that GroEL1 interacts only with the phosphorylated form of PknF and that this interaction is abolished in the double T173A/T175A mutant, suggesting that the TAT phosphopeptide recognition motif is involved in protein-protein interaction between the two partners. These results are consistent with those reported for PknB in which both Thr171 and Thr173 interact with the GarA substrate, thus indicating that both phosphorylated activation loop residues are necessary for optimal PknB-GarA interaction and maximal kinase activity (34).
GroEL1 is phosphorylated on two threonine residues.
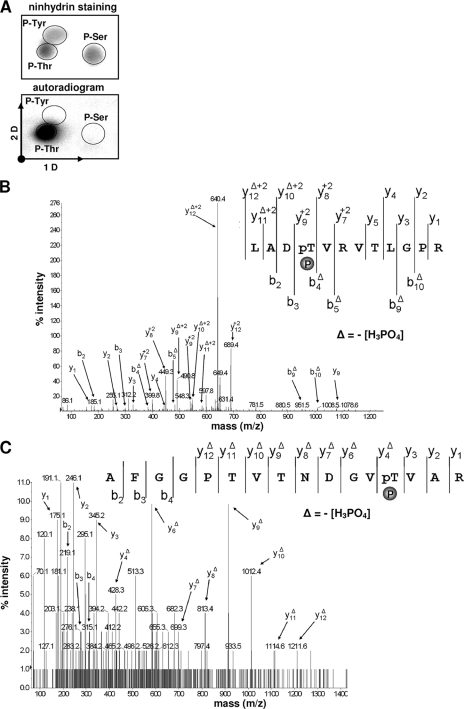
We next analyzed the phosphoamino acid content of PknF-phosphorylated GroEL1. The protein (5 μg) was labeled with [γ-33P]ATP in vitro, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), excised, and subjected to acid hydrolysis as reported previously (25). GroEL1 was found to be phosphorylated only on threonine residues (Fig. 3A). While experimentally challenging, mass spectrometry technique was then successfully developed to decipher the phosphorylation sites in a sequence-specific fashion. The M. tuberculosis GroEL1 protein contains 34 threonine residues. To identify the phosphorylated site(s), recombinant GroEL1 was incubated with cold ATP in the presence of PknF and subjected to mass spectrometry analysis after tryptic digestion. ProteinPilot database searching software (version 2.0; Applied Biosystems), using the Paragon method with phosphorylation emphasis, was used to detect and identify the phosphorylated peptides. The sequence coverage of the protein was 88%, and phosphorylation occurred only on two peptides, the peptide from positions 22 to 33 (i.e., LADTVRVTLGPR) and the peptide from positions 42 to 57 (i.e., AFGGPTVTNDGVTVAR) with an 80-Da mass increment from 1,296.73 to 1,376.91 Da, and from 1,560.78 to 1,640.74, respectively (monoisotopic mass). The tandem mass spectrometry (MS-MS) spectra unambiguously confirmed the presence of phosphate groups on Thr25 (Fig. 3B) and Thr54 (Fig. 3C), consistent with the phosphoamino analysis (Fig. 3A).
FIG. 3.
Identification of the GroEL1 phosphorylation sites. (A) Phosphoamino acid content of the PknF-phosphorylated GroEL1. GroEL1 was phosphorylated in vitro in the presence of His-PknF and [γ-33P]ATP, analyzed by SDS-PAGE, electroblotted onto an Immobilon polyvinylidene difluoride membrane, excised, and hydrolyzed in acid. The phosphoamino acids thus liberated were separated by electrophoresis in the first dimension (1 D) and ascending chromatography in the second dimension (2 D). After migration, radioactive molecules were detected by autoradiography. Authentic phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) were run in parallel as internal standard controls and visualized by ninhydrin staining. (B) MS-MS spectra of the doubly charged ion [M + 2H]2+ at m/z 689.36 of the peptide from positions 22 to 33 (monoisotopic mass, 1,376.71 Da) of GroEL1. The unambiguous location of the phosphate group (P) on Thr25 was shown by observation of the “y” C-terminal daughter ion series. Starting from the C-terminal residue, all “y” ions lose phosphoric acid (−98 Da) after the phosphorylated residues. pT, phosphotyrosine. (C) MS-MS spectra of the doubly charged ion [M + 2H]2+ at m/z 821.38 of the peptide from positions 42 to 57 (monoisotopic mass, 1,640.74 Da) of GroEL1. The unambiguous location of the phosphate group on Thr54 was showed by observation of the “y” C-terminal daughter ion series. Starting from the C-terminal residue, all “y” ions lose phosphoric acid (−98 Da) after the phosphorylated residues.
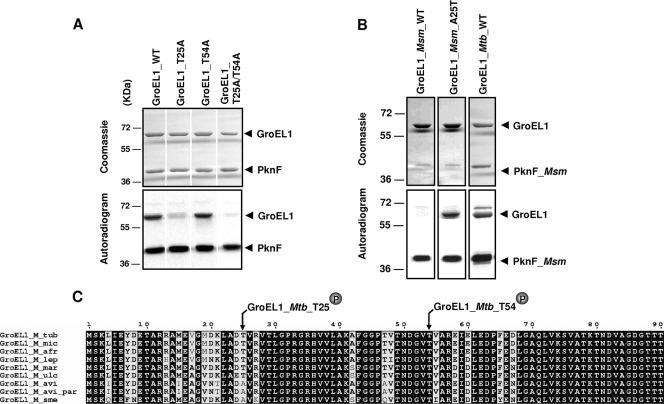
Definitive identification and localization of Thr25 and Thr54 as the two unique phosphorylation sites were achieved by site-directed mutagenesis to introduce single or double mutations that prevent specific phosphorylation (Thr-to-Ala replacements). All mutants (T25A, T54A, and T25A/T54A) were expressed, purified as a His-tagged protein in E. coli BL21(DE3)Star harboring plasmid pETPhos_groEL1_T25A, pETPhos_groEL1_T54A, or pETPhos_groEL1_T25A/T54A, and used in an in vitro kinase assay. The recombinant proteins were individually incubated along with [γ-33P]ATP and PknF. Equal amounts of proteins were separated by SDS-PAGE and analyzed by autoradiography (Fig. 4A, top panel). Unexpectedly, whereas a significant decrease of the phosphorylation signal was observed with the T25A mutant, phosphorylation of the T54A mutant was not affected (Fig. 4A, bottom panel). However, phosphorylation of the double mutant was completely abrogated compared to phosphorylation of GroEL1(WT), indicating that GroEL1 is phosphorylated only on Thr25 and Thr54. This was further supported by analysis of an additional round of mass spectrometry on GroEL1(T25A/T54A) pretreated with ATP and PknF. This failed to identify any additional phosphate group that could eventually have arisen as a compensatory mechanism to the loss of the Thr25 and Thr54 phosphorylation sites (data not shown). Overall, these data indicate that Thr25 and Thr54 are not equivalent with respect to phosphorylation by PknF, as Thr25 appears to be the primary phosphorylation site. In the absence of Thr54, GroEL1 still undergoes phosphorylation on the second site, whereas in the absence of Thr25, the protein has almost completely lost its ability to be phosphorylated.
FIG. 4.
Differential phosphorylation profiles of GroEL1 proteins from M. tuberculosis and M. smegmatis. (A) Requirement of both Thr25 and Thr54 for optimal phosphorylation of GroEL1 by PknF. GroEL1(WT) and recombinant GroEL1(T25A), GroEL1(T54A), and GroEL1(T25A T54A) proteins were purified from E. coli and used in phosphorylation assays in the presence of purified PknF and [γ-33P]ATP. Proteins were then separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). (B) GroEL1 from M. smegmatis is not phosphorylated by PknF. Recombinant GroEL1Msm(WT) and GroEL1Msm(A25T) were purified from E. coli and used in phosphorylation assays in the presence of purified PknFMsm and [γ-33P]ATP. Proteins were then separated by SDS-PAGE and stained with Coomassie blue (top panel), and radioactive bands were revealed by autoradiography (bottom panel). The positions of molecular mass markers (in kilodaltons) are shown to the left of the gels. (C) Sequence alignment of the N-terminal sequences of GroEL1 from various mycobacterial species. The alignment was performed using ClustalW and Espript programs. Residues conserved in all species are shown in white type on black. The phosphorylated sites of GroEL1 are indicated. Abbreviations: M_tub, Mycobacterium tuberculosis; M_mic, Mycobacterium microti; M_afr, Mycobacterium africanum; M_lep, Mycobacterium leprae; M_mar, Mycobacterium marinum; M_ulc, Mycobacterium ulcerans; M_avi, Mycobacterium avium; M. avi_par, M. paratuberculosis; M_sme, Mycobacterium smegmatis.
M. tuberculosis GroEL1 has been demonstrated to be an active stimulator of human monocytes, and this effect was dependent on CD14 (21). Structure/function relationship studies of GroEL1 have shown that the signaling activity of the protein, as well as its capacity to interact with CD14 to induce monocyte cytokine secretion, resides in its equatorial domain (33). Interestingly, both phosphorylated Thr residues are present in helical regions of the equatorial domain of GroEL1, and the localization of the two sites in the domain responsible for GroEL1 activity raises the attractive hypothesis that they may play an important role in regulating GroEL1. Further work will be required to assess whether phosphorylation of this structural domain contributes to the control of GroEL1 activity in vitro and in an in vivo infection model.
GroEL1 from M. smegmatis is not phosphorylated by PknF.
Using a groEL1 mutant of M. smegmatis, Ojha et al. demonstrated that loss of M. smegmatis GroEL1 (GroEL1Msm) function does not affect planktonic growth but specifically interferes with biofilm maturation (28). The behavior of the mutant revealed that mycolic acid synthesis is regulated during biofilm maturation and that GroEL1Msm interacts physically with the condensing enzymes KasA of the fatty acid synthase type II system, a central element of the mycolic acid biosynthetic pathway. Moreover, it has been demonstrated that the condensing activity of KasA is regulated via STPK phosphorylation (23). This prompted us to evaluate and compare the phosphorylation profile of GroEL1Msm incubated with radiolabeled ATP and M. smegmatis PknF (PknFMsm). PknFMsm (MSMEG3677) has recently been identified as the closest homologue (64%) to PknF of M. tuberculosis (12), and overexpression of PknFMsm in M. smegmatis showed reduced growth associated with irregular cell structure and a defect in sliding motility and biofilm formation (12). As shown in Fig. 4B, GroEL1Msm was not phosphorylated by PknFMsm despite a strong autokinase activity for the kinase, whereas M. tuberculosis GroEL1 (GroEL1Mtb) was phosphorylated by PknFMsm. This result was rather unexpected, given the 81% identity shared by GroEL1Msm and GroEL1Mtb. Comparison of the primary sequences of GroEL1Msm and GroEL1Mtb revealed that Thr54 was conserved in both proteins, but Thr25 was substituted by Ala in GroEL1Msm (Fig. 4C). We next examined whether the absence of a threonine at position 25 could explain the lack of phosphorylation of GroEL1Msm by PknFMsm. Site-directed mutagenesis was used to replace Ala25 by Thr25, thus generating GroEL1Msm(A25T). Importantly, the A25T replacement restored the capacity of GroEL1Msm to be phosphorylated by PknFMsm (Fig. 4B), thus connecting the lack of phosphorylation of GroEL1Msm to the absence of a threonine residue at position 25.
Together, these results suggest the presence of various degrees of interaction between GroEL1 proteins and mycobacterial kinases. This is further supported by the observation that GroEL1Msm could be phosphorylated to some extent by several M. tuberculosis STPKs (including PknA, PknB, PknE, and PknF) in vitro, thus pointing out a possible cross talk between mycobacterial species (data not shown).
Our study indicates that GroEL1 is differentially phosphorylated in various mycobacterial species. Whether these different GroEL1 isoforms affect/regulate the function(s) of GroEL1 remains to be investigated, but it is anticipated that GroEL1 expresses different activities that depend directly on its phosphorylation profile, and hence on the species in which the GroEL1 is synthesized. Interestingly, the Thr25 residue was found to be conserved in all pathogenic species analyzed, including all members of the Mycobacterium tuberculosis complex, Mycobacterium leprae, Mycobacterium marinum, and Mycobacterium ulcerans (Fig. 4C). One could therefore hypothesize that this particular residue is somehow critical for GroEL1 activity in pathogenic species.
In conclusion, although mycobacterial GroEL1 proteins have been extensively studied, no data were available with respect to their potential posttranslational modifications. We report here, for the first time, phosphorylation of the M. tuberculosis GroEL1 chaperone and identified Thr25 as a critical phosphoacceptor. The results of the present study suggest that environmental signals could trigger GroEL1 phosphorylation and presumably influence/regulate the biological activity of the protein. Whether polymorphism at position 25 contributes to the different functions associated to GroEL1 in pathogenic versus nonpathogenic species is an attractive hypothesis that remains to be addressed. The generation of an isogenic strain of M. tuberculosis possessing a groEL1(T25A) allele would be necessary in order to examine the putative link between Thr25 phosphorylation and the capacity to induce an inflammatory response in infected animals. Conversely, whether the phosphorylation profile of GroEL1(A25T) alters the interaction with KasA in M. smegmatis and subsequently influences the production of mature biofilms would also be of interest. In contrast to groEL1, groEL2 is essential for mycobacterial growth (28). We have thus investigated whether GroEL2 also represents a substrate for M. tuberculosis kinases. The results of our preliminary studies suggest that GroEL2 can be phosphorylated in vitro (data not shown). Work is currently under progress to identify the phosphorylated site(s) in GroEL2 and to determine how phosphorylation may affect the GroEL2 functions.
Acknowledgments
We thank M. Becchi, I. Zanella-Cléon, and A. Cornut (IBCP, Lyon, France) for their excellent expertise and technical assistance in mass spectrometry analysis.
This work was supported by grants from the Region Rhone-Alpes (M.J.C.), the CNRS, the University of Lyon (France), and the National Research Agency (ANR-06-MIME-027-01 to V.M. and L.K.).
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Av-Gay, Y., and M. Everett. 2000. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 8238-244. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt, A., V. Molle, G. S. Besra, W. R. Jacobs, Jr., and L. Kremer. 2007. The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol. Microbiol. 641442-1454. [DOI] [PubMed] [Google Scholar]
- 3.Boitel, B., M. Ortiz-Lombardia, R. Duran, F. Pompeo, S. T. Cole, C. Cervenansky, and P. M. Alzari. 2003. PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis. Mol. Microbiol. 491493-1508. [DOI] [PubMed] [Google Scholar]
- 4.Canova, M. J., L. Kremer, and V. Molle. 2008. pETPhos: a customized expression vector designed for further characterization of Ser/Thr/Tyr protein kinases and their substrates. Plasmid 60149-153. [DOI] [PubMed] [Google Scholar]
- 5.Canova, M. J., R. Veyron-Churlet, I. Zanella-Cleon, M. Cohen-Gonsaud, A. J. Cozzone, M. Becchi, L. Kremer, and V. Molle. 2008. The Mycobacterium tuberculosis serine/threonine kinase PknL phosphorylates Rv2175c: mass spectrometric profiling of the activation loop phosphorylation sites and their role in the recruitment of Rv2175c. Proteomics 8521-533. [DOI] [PubMed] [Google Scholar]
- 6.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. [DOI] [PubMed] [Google Scholar]
- 7.Curry, J. M., R. Whalan, D. M. Hunt, K. Gohil, M. Strom, L. Rickman, M. J. Colston, S. J. Smerdon, and R. S. Buxton. 2005. An ABC transporter containing a forkhead-associated domain interacts with a serine-threonine protein kinase and is required for growth of Mycobacterium tuberculosis in mice. Infect. Immun. 734471-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deol, P., R. Vohra, A. K. Saini, A. Singh, H. Chandra, P. Chopra, T. K. Das, A. K. Tyagi, and Y. Singh. 2005. Role of Mycobacterium tuberculosis Ser/Thr kinase PknF: implications in glucose transport and cell division. J. Bacteriol. 1873415-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dosanjh, N. S., M. Rawat, J. H. Chung, and Y. Av-Gay. 2005. Thiol specific oxidative stress response in mycobacteria. FEMS Microbiol. Lett. 24987-94. [DOI] [PubMed] [Google Scholar]
- 10.Duran, R., A. Villarino, M. Bellinzoni, A. Wehenkel, P. Fernandez, B. Boitel, S. T. Cole, P. M. Alzari, and C. Cervenansky. 2005. Conserved autophosphorylation pattern in activation loops and juxtamembrane regions of Mycobacterium tuberculosis Ser/Thr protein kinases. Biochem. Biophys. Res. Commun. 333858-867. [DOI] [PubMed] [Google Scholar]
- 11.Fayet, O., T. Ziegelhoffer, and C. Georgopoulos. 1989. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J. Bacteriol. 1711379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalaswamy, R., S. Narayanan, W. R. Jacobs, Jr., and Y. Av-Gay. 2008. Mycobacterium smegmatis biofilm formation and sliding motility are affected by the serine/threonine protein kinase PknF. FEMS Microbiol. Lett. 278121-127. [DOI] [PubMed] [Google Scholar]
- 13.Grundner, C., L. M. Gay, and T. Alber. 2005. Mycobacterium tuberculosis serine/threonine kinases PknB, PknD, PknE, and PknF phosphorylate multiple FHA domains. Protein Sci. 141918-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakansson, S., E. E. Galyov, R. Rosqvist, and H. Wolf-Watz. 1996. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20593-603. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Y., B. Henderson, P. A. Lund, P. Tormay, M. T. Ahmed, S. S. Gurcha, G. S. Besra, and A. R. Coates. 2008. A Mycobacterium tuberculosis mutant lacking the groEL homologue cpn60.1 is viable but fails to induce an inflammatory response in animal models of infection. Infect. Immun. 761535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huse, M., and J. Kuriyan. 2002. The conformational plasticity of protein kinases. Cell 109275-282. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, L. N., M. E. Noble, and D. J. Owen. 1996. Active and inactive protein kinases: structural basis for regulation. Cell 85149-158. [DOI] [PubMed] [Google Scholar]
- 18.Kerner, M. J., D. J. Naylor, Y. Ishihama, T. Maier, H. C. Chang, A. P. Stines, C. Georgopoulos, D. Frishman, M. Hayer-Hartl, M. Mann, and F. U. Hartl. 2005. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell 122209-220. [DOI] [PubMed] [Google Scholar]
- 19.Kim, A. I., P. Ghosh, M. A. Aaron, L. A. Bibb, S. Jain, and G. F. Hatfull. 2003. Mycobacteriophage Bxb1 integrates into the Mycobacterium smegmatis groEL1 gene. Mol. Microbiol. 50463-473. [DOI] [PubMed] [Google Scholar]
- 20.Kong, T. H., A. R. Coates, P. D. Butcher, C. J. Hickman, and T. M. Shinnick. 1993. Mycobacterium tuberculosis expresses two chaperonin-60 homologs. Proc. Natl. Acad. Sci. USA 902608-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewthwaite, J. C., A. R. Coates, P. Tormay, M. Singh, P. Mascagni, S. Poole, M. Roberts, L. Sharp, and B. Henderson. 2001. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect. Immun. 697349-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund, P. A. 2001. Microbial molecular chaperones. Adv. Microb. Physiol. 4493-140. [DOI] [PubMed] [Google Scholar]
- 23.Molle, V., A. K. Brown, G. S. Besra, A. J. Cozzone, and L. Kremer. 2006. The condensing activities of the Mycobacterium tuberculosis type II fatty acid synthase are differentially regulated by phosphorylation. J. Biol. Chem. 28130094-30103. [DOI] [PubMed] [Google Scholar]
- 24.Molle, V., C. Girard-Blanc, L. Kremer, P. Doublet, A. J. Cozzone, and J. F. Prost. 2003. Protein PknE, a novel transmembrane eukaryotic-like serine/threonine kinase from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 308820-825. [DOI] [PubMed] [Google Scholar]
- 25.Molle, V., L. Kremer, C. Girard-Blanc, G. S. Besra, A. J. Cozzone, and J. F. Prost. 2003. An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry 4215300-15309. [DOI] [PubMed] [Google Scholar]
- 26.Molle, V., D. Soulat, J. M. Jault, C. Grangeasse, A. J. Cozzone, and J. F. Prost. 2004. Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS Microbiol. Lett. 234215-223. [DOI] [PubMed] [Google Scholar]
- 27.Monahan, I. M., J. Betts, D. K. Banerjee, and P. D. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147459-471. [DOI] [PubMed] [Google Scholar]
- 28.Ojha, A., M. Anand, A. Bhatt, L. Kremer, W. R. Jacobs, Jr., and G. F. Hatfull. 2005. GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123861-873. [DOI] [PubMed] [Google Scholar]
- 29.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151518-525. [PubMed] [Google Scholar]
- 30.Qamra, R., S. C. Mande, A. R. Coates, and B. Henderson. 2005. The unusual chaperonins of Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 85385-394. [DOI] [PubMed] [Google Scholar]
- 31.Qamra, R., V. Srinivas, and S. C. Mande. 2004. Mycobacterium tuberculosis GroEL homologues unusually exist as lower oligomers and retain the ability to suppress aggregation of substrate proteins. J. Mol. Biol. 342605-617. [DOI] [PubMed] [Google Scholar]
- 32.Stewart, G. R., L. Wernisch, R. Stabler, J. A. Mangan, J. Hinds, K. G. Laing, D. B. Young, and P. D. Butcher. 2002. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 1483129-3138. [DOI] [PubMed] [Google Scholar]
- 33.Tormay, P., A. R. Coates, and B. Henderson. 2005. The intercellular signaling activity of the Mycobacterium tuberculosis chaperonin 60.1 protein resides in the equatorial domain. J. Biol. Chem. 28014272-14277. [DOI] [PubMed] [Google Scholar]
- 34.Villarino, A., R. Duran, A. Wehenkel, P. Fernandez, P. England, P. Brodin, S. T. Cole, U. Zimny-Arndt, P. R. Jungblut, C. Cervenansky, and P. M. Alzari. 2005. Proteomic identification of M. tuberculosis protein kinase substrates: PknB recruits GarA, a FHA domain-containing protein, through activation loop-mediated interactions. J. Mol. Biol. 350953-963. [DOI] [PubMed] [Google Scholar]
- 35.Wang, J., C. Li, H. Yang, A. Mushegian, and S. Jin. 1998. A novel serine/threonine protein kinase homologue of Pseudomonas aeruginosa is specifically inducible within the host infection site and is required for full virulence in neutropenic mice. J. Bacteriol. 1806764-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehenkel, A., M. Bellinzoni, M. Grana, R. Duran, A. Villarino, P. Fernandez, G. Andre-Leroux, P. England, H. Takiff, C. Cervenansky, S. T. Cole, and P. M. Alzari. 2008. Mycobacterial Ser/Thr protein kinases and phosphatases: physiological roles and therapeutic potential. Biochim. Biophys. Acta 1784193-202. [DOI] [PubMed] [Google Scholar]