Abstract
The high sensitivity and sharp frequency discrimination of hearing depend on mechanical amplification in the cochlea. To explore the basis of this active process, we examined the pharmacological sensitivity of spontaneous otoacoustic emissions (SOAEs) in a lizard, the Tokay gecko. In a quiet environment, each ear produced a complex but stable pattern of emissions. These SOAEs were reversibly modulated by drugs that affect mammalian otoacoustic emissions, the salicylates and the aminoglycoside antibiotics. The effect of a single i.p. injection of sodium salicylate depended on the initial power of the emissions: ears with strong control SOAEs displayed suppression at all frequencies, whereas those with weak control emissions showed enhancement. Repeated oral administration of acetylsalicylic acid reduced all emissions. Single i.p. doses of gentamicin or kanamycin suppressed SOAEs below 2.6 kHz, while modulating those above 2.6 kHz in either of two ways. For ears whose emission power at 2.6–5.2 kHz encompassed more than half of the total, individual emissions displayed facilitation as great as 35-fold. For the remaining ears, emissions dropped to as little as one-sixth of their initial values. The similarity of the responses of reptilian and mammalian cochleas to pharmacological intervention provides further evidence for a common mechanism of cochlear amplification.
The cochlea achieves its sensitivity and sharp tuning through an active process that boosts mechanical signals near threshold (reviewed in ref. 1). Amplification enables the cochlea to compensate for energy losses that result from viscous damping by its fluid contents (2). The enhancement of low-amplitude signals allows the mechanosensory receptors of the inner ear, hair cells, to detect subnanometer displacements of their hair bundles (3, 4) and to be detection-limited by thermal noise (5, 6).
The cochlear amplifier is thought to supply positive feedback that can produce instability and mechanical oscillations during periods of excessive gain. These oscillations manifest themselves as spontaneous otoacoustic emissions (SOAEs), sounds that are emitted from ears in the absence of stimulation (reviewed in ref. 7). Just as the active process contributes most to acoustical sensitivity for small stimuli (reviewed in ref. 8), SOAEs are generally detectable only in quiet environments. Moreover, the prevalence of SOAEs diminishes in the elderly (9) as it does in other populations whose hearing acuity is lessened (10). The correspondence between neural tuning curves and the isosuppression tuning curves of SOAEs suggests a common mechanism for amplification and SOAE generation (11–13).
SOAEs have been detected from vertebrates of disparate inner-ear structure, including amphibians, reptiles, birds, and mammals (reviewed in refs. 7, 14, and 15). The active process of mammals resides in outer hair cells, which may effect amplification by changing length in response to electrical stimulation (reviewed in refs. 16 and 17). In these animals, spontaneous oscillations of outer hair cells are thought to produce cochlear pressure changes that propagate through the middle ear to the tympanum and are then observed externally as SOAEs (18–20). For nonmammalian animals, which lack outer hair cells, voltage-dependent somatic contractions cannot mediate cochlear amplification (14, 21, 22) and another mechanism must be invoked to explain SOAEs. Nevertheless, the emissions in these species strongly resemble those of mammals. The robust emissions from both the bobtail lizard (23) and the Tokay gecko (24) maintain unique, ear-specific patterns over time. Isosuppression tuning curves for SOAEs from both species (13, 24) correspond well to the respective neural tuning curves (25, 26). Like the emissions from mammals (27), the SOAEs from lizards are reversibly suppressed by brief periods of anoxia and display temperature dependence of the emission frequency (22–24).
The intensities of SOAEs in mammals can be affected by several pharmacological agents. Large doses of salicylates, such as acetylsalicylic acid (aspirin), reversibly suppress emissions in rhesus monkeys (18) and humans (28–32). Salicylates also reversibly diminish the ear's sensitivity by elevating the sensory thresholds of individual cochlear nerve fibers and eliminating their sharp tuning (33, 34). The site at which salicylates affect SOAEs is unknown. In birds as well as mammals, bath-applied salicylate reversibly alters the appearance of hair cell membranes, producing dilatation and vesiculation of subsurface cisternae (35, 36) as well as crenulation of the plasmalemma (37). In outer hair cells of the guinea pig, these changes are accompanied by cessation of voltage-driven length changes and by a reduction in membrane capacitance (20, 35, 38). In view of the lability of the active process (3), it is not surprising that such dramatic changes are accompanied by an abatement of SOAEs.
Aminoglycoside antibiotics also quench particular types of mammalian otoacoustic emissions. Amikacin reversibly suppresses transiently evoked otoacoustic emissions in humans (39), whereas gentamicin reversibly attenuates the cubic (2f2 − f1) and higher-order distortion products in the guinea pig (40). Because aminoglycosides block the hair cell's transduction channel (41, 42), the diminution of distortion-product otoacoustic emissions by gentamicin is consistent with the hypothesis that these emissions stem from the channels' mechanical properties (reviewed in ref. 1). Aminoglycosides have many other effects (reviewed in ref. 43), however, so the site at which they influence emissions is uncertain.
In the interest of characterizing the pharmacological sensitivity of the cochlear active process in a species without outer hair cells, we have examined the effects of salicylates and aminoglycosides on SOAEs from a lizard, the Tokay gecko.
Materials and Methods
Experimental Preparation.
SOAEs were measured from Tokay geckos (Gekko gecko) with masses of 19–69 g (Carolina Biological Supply; Tom Crutchfield's Reptile Enterprises, Bushnell, FL). Prior to recording, each animal was lightly anesthetized with an i.p. injection of 17 mg⋅kg−1 pentobarbital sodium (Nembutal Sodium; Abbott). An additional 3.5 mg⋅kg−1 of anesthetic was given to any animal that became active before a recording session was complete.
Because the intensities and frequencies of SOAEs from lizards are strongly temperature-dependent (22, 24, 44), each animal was placed on an electrical heating pad and its oral temperature was monitored by a thermocouple connected to an electrical thermometer (BAT-8; Bailey Instruments, Saddle Brook, NJ). Recordings were made at temperatures between 25°C and 28°C. Measurements made before and after drug administration were temperature-matched within 1°C.
Each experimental animal in a first group was administered a single i.p. dose of 62 mg⋅kg−1 sodium salicylate. In a second set of experiments, each gecko received 35 mg⋅kg−1 acetylsalicylic acid orally twice daily for periods of 2–7 days. After each dose, an animal was kept in isolation to ensure that the drug was not regurgitated. In a third group, each animal was administered either gentamicin sulfate or kanamycin sulfate as a single i.p. dose of 500 mg⋅kg−1. No animal served in more than one set of reported experiments. In most instances, control recordings were made immediately before drug administration. For six ears from the third group, however, control recordings were taken in the 20 min following aminoglycoside administration, a period before any changes were observed in SOAEs.
Detection of SOAEs.
Recordings of SOAEs were made with a low-noise, 25-mm diameter condenser microphone (model no. 4179; Brüel and Kjær, Nærum, Denmark), which was sealed with vacuum grease into a tight-fitting cylindrical adapter that tapered to a hollow plastic cone with a lesser inner diameter of 4.8 mm. After the cone's orifice had been gently placed around the opening of the animal's external auditory meatus, a light coat of petroleum jelly was used to seal it to the animal's head. Failure to form a tight seal was signaled by a greatly increased noise level in a broad band of frequencies around 1 kHz.
Experiments were conducted in a sound-attenuation chamber that comprised two nested boxes, each with dual walls of 5-mm and 13-mm particle board sandwiching a 1-mm sheet of lead. The chamber's 0.25 m3 interior was lined with sound-absorbent plastic foam. For 10 control experiments in which the acoustical adapter was sealed to an animal's skin adjacent to the external auditory meatus, the noise power over the frequency range of 0.5–5.2 kHz was 36.2 ± 0.1 μPa2 (mean ± SEM).
Data Collection.
Signals from the microphone were amplified by a preamplifier and a measuring amplifier (model nos. 2260 and 2609; Brüel and Kjær) employing an A-weighting filter (Standards Secretariat, Acoustical Society of America, 1983) to reject low-frequency noise. An eight-pole Butterworth bandpass filter (model no. 852; Wavetek, San Diego) with half-power frequencies of 0.25 kHz and 7 kHz further conditioned the signals. Data were sampled at 14 kHz with a 12-bit analog-to-digital converter. The interface and direct-memory-access module (NB-A2000 and NB-DMA2800; National Instruments, Austin, TX) were controlled by a computer (Quadra 800; Apple Computer, Cupertino, CA). Programs were written in labview (version 3.1; National Instruments).
To produce power spectra of SOAEs, groups of 100 emission samples, each 200 ms in length, were collected and subjected to the fast Fourier transform with a uniform sampling window. The spectra from several groups of samples were then averaged. After fitting the factory-supplied calibration curve with a polynomial expression by use of mathematica (version 2.2.2; Wolfram Research, Champaign, IL), we corrected the data for minor nonlinearities in the microphone's frequency response. Further data analysis, including corrections for A-weighting and aberrations of the microphone, was performed with excel (version 5.0; Microsoft, Redmond, WA). Statistical analysis was conducted in excel using heteroscedastic, two-tailed, paired t tests on data from animals administered either sodium salicylate or acetylsalicylic acid. Similar but unpaired t tests were applied to data from animals administered aminoglycosides. Statistical results are presented as means ± SEM for the indicated numbers of samples.
Results
In agreement with a previous study (24), each ear of a Tokay gecko was found to display a unique but stable pattern of SOAEs under control conditions (Fig. 1). To compare the results from different ears, we partitioned SOAE power spectra into three frequency bands: 0.5–1.0 kHz, 1.0–2.6 kHz, and 2.6–5.2 kHz. These ranges were chosen because lizard cochleas rarely display SOAE peaks below 1.0 kHz (45) and because 2.6 kHz roughly bisects the frequency range to which the Tokay gecko's ear is sensitive (25).
Figure 1.
Averaged power spectra of SOAEs from four lightly anesthetized Tokay geckos under control conditions. Although peaks rarely occurred at frequencies below 1.0 kHz or above 5.2 kHz, emissions could be dispersed across the spectrum or grouped within specific frequency bands. Three panels demonstrate clustered emissions at frequencies in excess of 2.6 kHz. The interrupted vertical lines demarcate the three frequency bands used in subsequent analyses: 0.5–1.0 kHz, 1.0–2.6 kHz, and 2.6–5.2 kHz. Note that the abscissae and ordinates are logarithmic in this and subsequent power spectra.
Modulation of SOAEs by Salicylates.
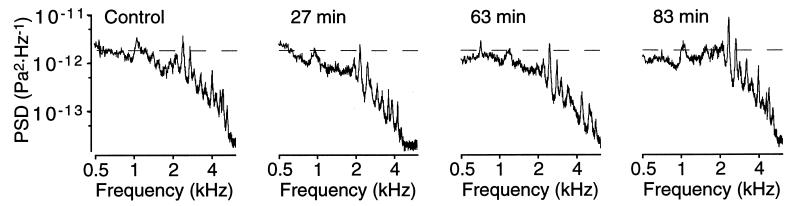
The effect on SOAEs of a single i.p. dose of sodium salicylate depended on an ear's emission power under control conditions. Sodium salicylate transiently suppressed emissions in ears with control powers greater than 3000 μPa2 (Fig. 2) (Table 1). In the hour following drug injection, emissions decreased in each of the three frequency bands; the average total power diminished to 73% of its control value. The greatest attenuation occurred in the low- and middle-frequency bands, with the power at 0.5–1.0 kHz falling to 70% of the initial value and that at 1.0–2.6 kHz declining to 73% of the control. In the second hour, the emission power at 0.5–1.0 kHz remained at 70% of the control value, whereas the power in the middle- and upper-frequency bands became statistically indistinguishable from controls. By the third hour, the power in each of the three frequency ranges returned to its control value.
Figure 2.
Time course of the effect on SOAEs of a single i.p. injection of sodium salicylate. This ear, whose initial total emission power was 700 μPa2, exhibited both suppression and facilitation of SOAEs. Within 27 min of administration of a dose of 62 mg⋅kg−1, emissions at frequencies above 1.0 kHz shifted downward in frequency and were transiently suppressed. Within 83 min, the emission originally at 1.0 kHz had largely recovered, whereas that at 2.3 kHz manifested a 123% increase over its initial value. Each trace is the average power spectrum obtained from 100 200-ms samples. In this illustration and in Figs. 4 and 5, “PSD” in the label of the ordinate axis refers to power-spectral density. In the same three figures, the interrupted horizontal lines provide fiducials against which to judge changes in emission power.
Table 1.
Effects of drug administration on the power of otoacoustic emissions
| Drug doses | Power (μPa2) in frequency band (kHz)
|
No. of ears | |||
|---|---|---|---|---|---|
| 0.5–1.0 | 1.0–2.6 | 2.6–5.2 | Total | ||
| Single acetylsalicylic acid | |||||
| Control (total power above 3000 μPa2) | 970 ± 100 | 2050 ± 150 | 760 ± 40 | 3780 ± 140 | 8 |
| 0 hr < t < 1 hr | 675 ± 109* | 1499 ± 217* | 578 ± 110 | 2752 ± 379* | |
| 1 hr < t < 2 hr | 683 ± 98* | 1971 ± 244 | 822 ± 131 | 3478 ± 414 | |
| Control (total power below 3000 μPa2) | 370 ± 120 | 760 ± 260 | 400 ± 120 | 1540 ± 320 | 5 |
| 0 hr < t < 1 hr | 389 ± 103 | 1098 ± 113* | 686 ± 115* | 2177 ± 110† | |
| 1 hr < t < 2 hr | 506 ± 106 | 1337 ± 142* | 509 ± 102 | 2357 ± 216* | |
| Repeated acetylsalicylic acid | |||||
| Control | 790 ± 100 | 1970 ± 190 | 560 ± 50 | 3320 ± 230 | 16 |
| 3–7 days | 752 ± 105 | 911 ± 302 | 233 ± 69† | 1896 ± 430 | |
| Single gentamicin | |||||
| 0 min < t < 20 min | 749 ± 119 | 1690 ± 408 | 742 ± 98 | 3180 ± 548 | 8 |
| 20 min < t < 80 min | 398 ± 112* | 995 ± 313 | 513 ± 164 | 1909 ± 533* | |
| 80 min < t < 140 min | 725 ± 110 | 1229 ± 227 | 621 ± 173 | 2581 ± 377 | |
| Single kanamycin | |||||
| 0 min < t < 20 min | 658 ± 97 | 2288 ± 1077 | 400 ± 143 | 3346 ± 1308 | 4 |
| 20 min < t < 80 min | 391 ± 111 | 1981 ± 138 | 592 ± 46* | 2932 ± 217 | |
| 80 min < t < 140 min | 365 ± 78* | 726 ± 889 | 296 ± 175 | 1388 ± 1112 | |
Values of emission power are expressed as means ± SEM for the indicated number of ears. * Indicates a difference from the control value significant at the level P < 0.05; † indicates a difference from the control value significant at the level P < 0.001.
For ears with control emission powers less than 3000 μPa2, SOAEs increased in strength following sodium salicylate injection. The total emission power, which reached 143% of its control value in the first hour, was dominated by increases in the middle- and high-frequency bands. The power at 1.0–2.6 kHz grew to 144% of its initial value, whereas that at 2.6–5.2 kHz climbed to 172% of the control. The total emission power continued to grow in the second hour, reaching 153% of the control value; the power at 1.0–2.6 kHz increased to 176% of its initial value. By the third hour, the power in each of the frequency bands was statistically indistinguishable from the relevant control.
The repeated oral administration of acetylsalicylic acid for 2–7 days reduced the total power of SOAEs to 57% of the control value. This protracted treatment decreased some individual emissions by as much as 85% (Fig. 3). Although the power diminished in each of the frequency bands, the decreases were greatest for middle- and high-frequency emissions. The power at 1.0–2.6 kHz declined to 46% of its initial value and that at 2.6–5.2 kHz fell to 42% of the control. Within 14 days of acetylsalicylic acid exposure, the SOAEs reverted to their original forms. After a gecko's recovery from an experiment, subsequent administration of acetylsalicylic acid failed to suppress SOAEs (data not shown). This result probably signaled enhanced salicylate metabolism in treated animals.
Figure 3.
Suppression of SOAEs from a single ear after repeated oral administration of acetylsalicylic acid. A dose of 35 mg⋅kg−1 twice daily for 2 days suppressed emissions at all frequencies in the range of 0.5–5.2 kHz. Although the power in some emissions fell to as little as 15% of its control value, the pattern of emission frequencies was largely preserved.
Modulation of SOAEs by Aminoglycosides.
Single doses of gentamicin or kanamycin elicited dramatic changes in SOAEs (Fig. 4) (Table 1). During the 2 hr following drug administration, either drug consistently suppressed both peak and baseline emissions at 0.5–2.6 kHz. The suppression of SOAEs at lower frequencies was often accompanied by the appearance of clustered emissions at frequencies above 2.6 kHz (Fig. 4B); similar patterns were occasionally observed from the ears of animals receiving only anesthetic (Fig. 1). For an emission at 3.6 kHz, as an example, the power-spectral density 24–251 min after drug administration increased 35-fold. At no time were emissions evident at frequencies above 5.2 kHz.
Figure 4.
Temporal evolution of SOAEs following aminoglycoside injection. (A) Intraperitoneal administration of 500 mg⋅kg−1 kanamycin sulfate suppressed emissions below 3.0 kHz and facilitated those at higher frequencies. Recovery was essentially complete less than 2 hr after drug administration. (B) Injection of the same dose of gentamicin sulfate into a different animal suppressed emissions below 3.0 kHz and facilitated of those at higher frequencies. The augmentation of high-frequency emissions progressed from a broad peak to a cluster of discrete peaks that grew in size.
Some ears manifested suppression of SOAEs across the frequency spectrum in response to aminoglycoside administration (Fig. 5) (Table 1). The power-spectral densities of individual emissions at 0.5–1.0 kHz declined to as little as 7% of their initial values. Comparable changes were seen for SOAEs at frequencies of 1.0–5.2 kHz, which dropped to as little as 3% of their control levels.
Figure 5.
Example of the time course of SOAE suppression in response to aminoglycoside administration. Intraperitoneal injection of 500 mg⋅kg−1 gentamicin sulfate initially suppressed emissions at all frequencies. Recovery commenced within 24 hr and was essentially complete by day 10. The nine peaks originally observed at frequencies above 1.3 kHz were also present at day 10 but had shifted to higher frequencies.
Not only did ears from different animals exhibit variability in their responses to aminoglycoside administration, but ears from the same animal also displayed disparate behavior. Although the power of particular emissions fluctuated severalfold, the total power emitted by each ear varied by no more than 90% from its control value (data not shown). A few ears displayed recovery of emission spectra within 4 hr of drug administration, but most did not.
Discussion
The responses of SOAEs in geckos to salicylates and aminoglycosides resembled those of mammalian emissions. The effect of i.p. administration of sodium salicylate in the gecko included both transient suppression and subsequent facilitation (Fig. 2). The time course of suppression resembled that in the rhesus monkey after injection of sodium salicylate (18), which also induces facilitation in the second hour after treatment.
As in mammals (28–32), prolonged oral administration of acetylsalicylic acid produced in geckos a marked but reversible suppression of SOAEs. In the most pronounced cases, not only were individual peaks suppressed, but baseline emissions fell as well. Suppression of baseline emissions occurs in the rhesus monkey following acute administration of sodium salicylate (18) and is inferred to reflect the nature of SOAE generators, an ensemble of oscillating units whose outputs overlap in the frequency domain and sum to an elevated baseline punctuated by discrete peaks.
In the gecko, aminoglycosides produced a reversible suppression of SOAEs that again resembled the mammalian response (39, 40). Single i.p. injections of kanamycin or gentamicin sulfate uniformly suppressed low-frequency emissions. At frequencies above 2.6 kHz, however, SOAEs displayed dramatic facilitation in some cases (Fig. 4) and suppression in others (Fig. 5). In addition, new peaks of emission appeared in the frequency range of 2.6–5.2 kHz.
The development of high-frequency SOAEs after aminoglycoside treatment may shed light on the gain control of the active process. Aminoglycosides are blockers of the hair cell's transduction channels; when present, they promote channel opening (46) but interrupt the flow of ionic current (41, 42). The drugs may thus dissociate the early steps in mechanoelectrical transduction, such as channel gating and the development of gating compliance (47), from such subsequent signals as the transduction current and receptor potential. The present results suggest that an appropriate concentration of an aminoglycoside increases the active process's gain by reducing a signal, perhaps Ca2+ entry, associated with a late step in transduction. At the same time, however, this drug concentration allows the active process to continue the mechanical exertions underlying SOAEs.
The similar effects of pharmacological treatments on the SOAEs of geckos and mammals further validate lizards as experimental models for the investigation of the cochlear active process (23, 24). Although it is possible that salicylates and aminoglycosides affect distinct cochlear amplifiers in mammals and nonmammals, the similarities of drug action are consistent with the possibility of a ubiquitous mechanism for the generation of SOAEs in vertebrates. It is plausible that this mechanism resides in an element common to all hair cells—the hair bundle (ref. 48; reviewed in ref. 1). Not only do hair bundles produce active movements spontaneously and in response to mechanical stimuli (4, 47, 49–52), but they can also amplify mechanical signals (53). An active process located in the bundle would act where energy is lost to viscous damping, thus enhancing auditory sensitivity by partially overcoming the dissipative effect of drag (2).
Acknowledgments
We thank Dr. J. Phelps and Mr. C. McKinney for computer programming, Dr. J. Zuo for constructing the recording chamber, and Ms. N. Grimes for assistance in preliminary experiments. Dr. G. A. Manley and members of our research group provided critical comments on the manuscript. This research was supported by National Institutes of Health Grant DC00241. A.J.H. is an Investigator of Howard Hughes Medical Institute.
Abbreviation
- SOAE
spontaneous otoacoustic emission
References
- 1.Hudspeth A J. Curr Opin Neurobiol. 1997;7:480–486. doi: 10.1016/s0959-4388(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 2.Gold T. Proc R Soc London Ser B. 1948;135:492–498. [Google Scholar]
- 3.Sellick P M, Patuzzi R, Johnstone B M. J Acoust Soc Am. 1982;72:131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- 4.Crawford A C, Fettiplace R. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries H L. Physica. 1948;14:48–60. [Google Scholar]
- 6.Jaramillo F, Wiesenfeld K. Nat Neurosci. 1998;1:384–388. doi: 10.1038/1597. [DOI] [PubMed] [Google Scholar]
- 7.Probst R. Adv Otorhinolaryngol. 1990;44:1–91. doi: 10.1159/000417719. [DOI] [PubMed] [Google Scholar]
- 8.Ruggero M A. Curr Opin Neurobiol. 1992;2:449–456. doi: 10.1016/0959-4388(92)90179-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebillard G, Abbou S, Lenoir M. Ann Oto-Laryng. 1987;104:363–368. [PubMed] [Google Scholar]
- 10.McFadden D, Mishra R. Hear Res. 1993;71:208–213. doi: 10.1016/0378-5955(93)90036-z. [DOI] [PubMed] [Google Scholar]
- 11.Clark W W, Kim D O, Zurek P M, Bohne B A. Hear Res. 1984;16:299–314. doi: 10.1016/0378-5955(84)90119-9. [DOI] [PubMed] [Google Scholar]
- 12.Manley G A, Yates G K, Köppl C. Hear Res. 1988;33:181–190. doi: 10.1016/0378-5955(88)90031-7. [DOI] [PubMed] [Google Scholar]
- 13.Köppl C, Manley G A. Hear Res. 1994;72:159–170. doi: 10.1016/0378-5955(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 14.Köppl C. In: Advances in Hearing Research. Manley G A, Klump G M, Köppl C, Fastl H, Oekinghaus H, editors. Singapore: World Scientific; 1995. pp. 207–216. [Google Scholar]
- 15.Köppl C, Manley G A. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- 16.Dallos P. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashmore J F, Kolston P J. Curr Opin Neurobiol. 1994;4:503–508. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 18.Martin G K, Lonsbury-Martin B L, Probst R, Coats A C. Hear Res. 1988;33:49–68. doi: 10.1016/0378-5955(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 19.Brown A M, Williams D M, Gaskill S A. J Acoust Soc Am. 1993;93:3298–3307. doi: 10.1121/1.405714. [DOI] [PubMed] [Google Scholar]
- 20.Tunstall M J, Gale J E, Ashmore J F. J Physiol. 1995;485:739–752. doi: 10.1113/jphysiol.1995.sp020765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinke R, Smolders J W T. Prog Brain Res. 1993;97:31–43. doi: 10.1016/s0079-6123(08)62260-8. [DOI] [PubMed] [Google Scholar]
- 22.Manley G A. In: Diversity in Auditory Mechanics. Lewis E R, Long G R, Lyon R F, Narins P M, Steele C R, editors. Singapore: World Scientific; 1997. pp. 32–38. [Google Scholar]
- 23.Köppl C, Manley G A. Hear Res. 1993;71:157–169. doi: 10.1016/0378-5955(93)90031-u. [DOI] [PubMed] [Google Scholar]
- 24.Manley G A, Gallo L, Köppl C. J Acoust Soc Am. 1996;99:1588–1603. doi: 10.1121/1.414680. [DOI] [PubMed] [Google Scholar]
- 25.Eatock R A, Manley G A, Pawson L. J Comp Physiol A. 1981;142:203–218. [Google Scholar]
- 26.Manley G A, Köppl C, Johnstone B M. J Comp Physiol A. 1990;167:89–99. [Google Scholar]
- 27.Ohyama K, Wada H, Kobayashi T, Takasaka T. Hear Res. 1991;56:111–121. doi: 10.1016/0378-5955(91)90160-b. [DOI] [PubMed] [Google Scholar]
- 28.McFadden D, Plattsmier H S. J Acoust Soc Am. 1984;76:443–448. doi: 10.1121/1.391585. [DOI] [PubMed] [Google Scholar]
- 29.Long G R, Tubis A, Jones K. In: Peripheral Auditory Mechanisms. Allen J B, Hall J L, Hubbard A, Neely S T, Tubis A, editors. Berlin: Springer; 1985. pp. 213–220. [Google Scholar]
- 30.Long G R, Tubis A. J Acoust Soc Am. 1988;84:1343–1353. doi: 10.1121/1.396633. [DOI] [PubMed] [Google Scholar]
- 31.Wier C C, Pasanen E G, McFadden D. J Acoust Soc Am. 1988;84:230–237. doi: 10.1121/1.396970. [DOI] [PubMed] [Google Scholar]
- 32.Penner M J. Arch Otolaryngol Head Neck Surg. 1989;115:871–875. doi: 10.1001/archotol.1989.01860310109034. [DOI] [PubMed] [Google Scholar]
- 33.Evans E F, Wilson J P, Borerwe T A. In: Tinnitus. Evered D, Lawrensen G, editors. London: Pitman Books; 1981. pp. 108–129. [Google Scholar]
- 34.Stypulkowski P H. Hear Res. 1990;46:113–146. doi: 10.1016/0378-5955(90)90144-e. [DOI] [PubMed] [Google Scholar]
- 35.Dieler R, Shehata-Dieler W E, Brownell W E. J Neurocytol. 1991;20:637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- 36.Dieler R, Shehata-Dieler W E, Richter C-P, Klinke R. Hear Res. 1994;74:85–98. doi: 10.1016/0378-5955(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 37.Shehata W E, Brownell W E, Dieler R. Acta Otolaryngol. 1991;111:707–718. doi: 10.3109/00016489109138403. [DOI] [PubMed] [Google Scholar]
- 38.Kakehata S, Santos-Sacchi J. J Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotz M A, Harris F P, Probst R. Laryngoscope. 1994;104:1130–1134. doi: 10.1288/00005537-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Brown A M, McDowell B, Forge A. Hear Res. 1989;42:143–156. doi: 10.1016/0378-5955(89)90140-8. [DOI] [PubMed] [Google Scholar]
- 41.Ohmori H. J Physiol. 1985;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroese A B A, Das A, Hudspeth A J. Hear Res. 1989;37:203–218. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 43.Schacht J. Otolaryngol Clin N Am. 1993;26:845–856. [PubMed] [Google Scholar]
- 44.Manley G A, Köppl C. Hear Res. 1994;72:171–180. doi: 10.1016/0378-5955(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 45.Manley G A, Gallo L. J Acoust Soc Am. 1997;102:1049–1055. doi: 10.1121/1.419858. [DOI] [PubMed] [Google Scholar]
- 46.Denk W, Keolian R M, Webb W W. J Neurophysiol. 1992;68:927–932. doi: 10.1152/jn.1992.68.3.927. [DOI] [PubMed] [Google Scholar]
- 47.Howard J, Hudspeth A J. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 48.Yates G K, Kirk D L. J Neurosci. 1998;18:1996–2003. doi: 10.1523/JNEUROSCI.18-06-01996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howard J, Hudspeth A J. Proc Natl Acad Sci USA. 1987;84:3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaramillo F, Howard J, Hudspeth A J. In: The Mechanics and Biophysics of Hearing. Dallos P, Geisler C D, Matthews J W, Ruggero M A, Steele C R, editors. Berlin: Springer; 1990. pp. 26–33. [Google Scholar]
- 51.Benser M E, Marquis R E, Hudspeth A J. J Neurosci. 1996;16:5629–5643. doi: 10.1523/JNEUROSCI.16-18-05629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquis R E, Hudspeth A J. Proc Natl Acad Sci USA. 1997;94:11923–11928. doi: 10.1073/pnas.94.22.11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin P, Hudspeth A J. Proc Natl Acad Sci USA. 1999;96:14306–14311. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]