Abstract
We report on Mycobacterium tuberculosis Rv0241c and Rv3389c, representing two physiologically functional 3-hydroxyacyl-thioester dehydratases (Htd). These enzymes are potentially entrained in type 2 fatty acid synthase (FASII). Mycobacterial FASII is involved in the synthesis of mycolic acids, which are the major constituents of the protective layer around the pathogen, shielding it from noxious chemicals and the host's immune system. Mycolic acids are additionally associated with the virulence and resilience of M. tuberculosis. Here, Rv0241c and Rv3389c, which are distinct from the previously identified heterodimers Rv0635-Rv0636 (HadAB) and Rv0636-Rv0637 (HadBC) but also the homodimer Rv0130 (HtdZ), were identified by expressing the corresponding candidate open reading frames in Saccharomyces cerevisiae htd2Δ cells lacking mitochondrial 3-hydroxyacyl-acyl carrier protein dehydratase activity, followed by scoring for phenotype rescue. The htd2Δ mutant fails to produce sufficient levels of lipoic acid and does not respire or grow on nonfermentable carbon sources. Soluble protein extracts made from mutant htd2Δ cells expressing mitochondrially targeted Rv0241c or Rv3389c contained 3-hydroxyacyl-thioester hydratase activity. Moreover, mutant yeast cells expressing Rv0241c or Rv3389c were able to recover their respiratory growth on glycerol medium and efficiently reduce 2,3,5-triphenyltetrazolium chloride. Additionally, expression of mitochondrial Rv0241c or Rv3389c in htd2Δ cells also restored de novo lipoic acid synthesis to 92 and 40% of the level in the wild-type strain, respectively. We propose naming Rv0241c and Rv3389c as HtdX and HtdY, respectively, and discuss the implications of our finding with reference to Rv0098, a candidate mycobacterial FabZ homologue with intrinsic thioesterase and hydratase activities that lacks the eukaryotic-like hydratase-2 motif.
Mycobacterium tuberculosis causes immense human morbidity and mortality worldwide, and it is thought that about 30 million people have died from tuberculosis in the past decade alone (see references 1 and 41 and citations therein). The World Health Organization estimates that one-third of the human population is infected with M. tuberculosis, which kills more adults than any other single infectious agent (43). Although effective drugs against tuberculosis exist, treatment is extended and arduous, and in certain countries 36% of tuberculosis patients are now infected with isoniazid- or rifampin-resistant strains (36). Hence, there is a renewed interest and urgency in developing new therapeutics against M. tuberculosis.
An attractive target for therapeutics is represented by the essential process of bacterial fatty acid biosynthesis (10, 24). M. tuberculosis contains a type 2 fatty acid synthase system (FASII) that consists of discrete enzymes, but it also has an additional associative FASI system (5) which is comprised of several enzymatic activities within a single multifunctional synthase. The two FAS systems cooperate in the production of mycolic acids, which are very-long-chain α-branched ß-hydroxylated fatty acids (C54-63) linked to C22-24 α side chains. These lipids participate in forming the protective layer around the pathogen, thereby adding to its persistence and virulence (41).
The penultimate step in the process of fatty acid biosynthesis is represented by the action of a 3-hydroxyacyl-acyl carrier protein (ACP) dehydratase (HAD) on its cognate thioester substrate. In M. tuberculosis FASII, this step is undertaken by Rv0635-Rv0636 (HadAB) and Rv0636-Rv0637 (HadBC) (9, 37) and possibly also Rv0130 (HtdZ) (21, 29). However, several other candidate proteins with putative hydratase-like structures have been suggested as potential HADs, including Rv0098, Rv0216, Rv0241c, Rv0504c, Rv2499c, Rv2524c, Rv3389c, Rv3538, and Rv3542c (11, 41). Within the spectrum of FASII enzymes, dehydratases (along with enoyl reductases) present the highest degree of structural and sequence divergence (11, 29, 37, 38). The classical paradigm is embodied by the Escherichia coli FabA and FabZ proteins, both of which adopt a single “hotdog fold” typically found in dehydratases and thioesterases (13). Although FabA differs from FabZ in that the former harbors an additional isomerase activity and does not form a hexameric superstructure like FabZ (31), the two proteins exhibit a common FabA/Z type of active-site motif, and therefore novel proteins cannot be distinguished between being FabA- or FabZ-like enzymes based on sequence analysis alone (38). The question of whether any dehydratases with an apparent FabA/Z-type active site actually occur in M. tuberculosis is contentious (38, 41). Several mycobacterial proteins with a degree of similarity to enzymes involved in polyhydroxyalkanoate biosynthesis have been shown previously to contain 3-hydroxyacyl-coenzyme A (CoA) dehydratase activity (21, 29, 37, 38); however, these proteins belong to a subfamily of enzymes defined by the presence of an amino acid sequence termed the hydratase-2 motif of eukaryotic multifunctional enzyme type 2 (MFE2) that is distinct from that of FabA/Z. An interesting variation of the hotdog fold theme can be found in examples where two consecutive hotdog folds either have been shown or are predicted to occur in a single polypeptide, thereby mimicking a dimeric structure. Examples of this variation include yeast MFE2 (Fox2p) and also the yeast mitochondrial FASII dehydratase Htd2p (30), as well as Rv3389c in M. tuberculosis (38).
The yeast Saccharomyces cerevisiae is a convenient model system for investigating the physiological function of mycobacterial proteins (15), since this organism undertakes mitochondrial fatty acid biosynthesis in a FASII-dependent manner (27). The equivalent HAD in yeast is represented by the aforementioned Htd2p (30). A yeast mutant devoid of Htd2p contains abnormally small mitochondria, fails to assemble respiratory complexes or produce sufficient levels of lipoic acid, and is unable to respire. This phenotype can be rescued by supplying the mutant with a plasmid-borne gene encoding Htd2p but also mitochondrially targeted E. coli FabA and FabZ (30) or M. tuberculosis HtdZ (21), the last three proteins representing structurally divergent dehydratases. Mitochondrial FASII is involved in de novo synthesis of the octanoyl-thioester precursor of lipoic acid (19), whereas mycobacterial FASII is claimed to be incapable of de novo fatty acid biosynthesis (5) and instead is proposed to extend FASI-produced C20 acyl thioesters to C60-90 mycolic acids (41).
Here, we have exploited S. cerevisiae as a surrogate host for the identification of M. tuberculosis proteins with HAD activity, by expressing published candidates in the yeast htd2Δ mutant. Mutant yeast cells complemented with mycobacterial genes were examined for 3-hydroxyacyl-thioester hydratase activity and compared to an otherwise isogenic strain expressing mycobacterial HtdZ for growth on glycerol, lipoic acid production, and respiration. The implications of our findings in yeast for the issue of the types of HADs that might be involved in fatty acid biosynthesis in M. tuberculosis are discussed.
MATERIALS AND METHODS
Yeast strains, plasmids, and oligonucleotides.
The yeast strains, plasmids, and oligonucleotides used are listed in Tables 1 and 2. E. coli strain T0P10F′ was used for all plasmid amplifications and isolations. The wild-type S. cerevisiae strain BY4741 and its htd2Δ or oar1Δ derivatives were obtained from Euroscarf (www.uni-frankfurt.de). Introduction of expression plasmids pPLM264 through pPLM276 into yeast cells (resulting in strains yPLM277 through yPLM291) was performed using a published method (12), and transformants were selected on solid glucose medium lacking uracil or lacking uracil and leucine.
TABLE 1.
S. cerevisiae strains and plasmids used
| Strain or plasmid (parental genotype) | Description | Source or reference |
|---|---|---|
| S. cerevisiae strains | ||
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| BY4741 htd2Δ (BY4741) | yhr067c::KanMX | Euroscarf |
| yPLM35 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0098 (mit-Rv0098) | 21 |
| yPLM36 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-HtdZ (mit-Rv0130) | 21 |
| yPLM277 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0216 | This study |
| yPLM278 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0241c | This study |
| yPLM279 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0504c | This study |
| yPLM280 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0635 | This study |
| yPLM281 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0636 | This study |
| yPLM282 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv0637 | This study |
| yPLM283 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv2499c | This study |
| yPLM284 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv3389c | This study |
| yPLM285 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv3538 | This study |
| yPLM286 (BY4741 htd2Δ) | Expressing mitochondrial Coq3p-Rv3542c | This study |
| yPLM287 (yPLM280) | Expressing Coq3p-Rv0635 and multicopy Coq3p-Rv0636 | This study |
| yPLM288 (yPLM280) | Expressing Coq3p-Rv0636 from two multicopy plasmids | This study |
| yPLM289 (yPLM282) | Expressing Coq3p-Rv0637 and multicopy Coq3p-Rv0636 | This study |
| yPLM43 (BY4741) | BY4741 oar1Δ expressing mitochondrial Oar1p | This study |
| Plasmids | ||
| pBluescript KS II | pKS cloning vector | Stratagene |
| pPLM56 (pBluescript KS II) | pKS:Rv0098 mitochondrial Rv0098 in pBluescript | 21 |
| pPLM63 (pBluescript KS II) | pKS:Rv0130 mitochondrial Rv0130 in pBluescript | 21 |
| pPLM254 (pBluescript KS II) | pKS:Rv0216 mitochondrial Rv0216 in pBluescript | This study |
| pPLM255 (pBluescript KS II) | pKS:Rv0241c mitochondrial Rv0241c in pBluescript | This study |
| pPLM256 (pBluescript KS II) | pKS:Rv0504c mitochondrial Rv0504c in pBluescript | This study |
| pPLM257 (pBluescript KS II) | pKS:Rv0635 mitochondrial Rv0635 in pBluescript | This study |
| pPLM258 (pBluescript KS II) | pKS:Rv0636 mitochondrial Rv0636 in pBluescript | This study |
| pPLM259 (pBluescript KS II) | pKS:Rv0637 mitochondrial Rv0637 in pBluescript | This study |
| pPLM260 (pBluescript KS II) | pKS:Rv2499c mitochondrial Rv2499c in pBluescript | This study |
| pPLM261 (pBluescript KS II) | pKS:Rv3389c mitochondrial Rv3389c in pBluescript | This study |
| pPLM262 (pBluescript KS II) | pKS:Rv3538 mitochondrial Rv3538 in pBluescript | This study |
| pPLM263 (pBluescript KS II) | pKS:Rv3542c mitochondrial Rv3542c in pBluescript | This study |
| YEp352 | URA3-marked multicopy episomal plasmid | 26 |
| YEplac181 | LEU2-marked multicopy episomal plasmid | 16 |
| pPLM62 (YEp352) | COQ3-EcQOR fusion behind the CTA1 promoter | This study |
| pPLM65 (pPLM62) | COQ3-Rv0098 fusion behind the CTA1 promoter | 21 |
| pPLM49 (pPLM62) | COQ3-Rv0130 fusion behind the CTA1 promoter | 21 |
| pPLM264 (pPLM62) | CTA1-COQ3-EcQOR missing NcoI site in URA3 | This study |
| pPLM265 (pPLM264) | COQ3-Rv0216 fusion behind the CTA1 promoter | This study |
| pPLM266 (pPLM264) | COQ3-Rv0241c fusion behind the CTA1 promoter | This study |
| pPLM267 (pPLM264) | COQ3-Rv0504c fusion behind the CTA1 promoter | This study |
| pPLM268 (pPLM264) | COQ3-Rv0635 fusion behind the CTA1 promoter | This study |
| pPLM269 (pPLM264) | COQ3-Rv0636 fusion behind the CTA1 promoter | This study |
| pPLM270 (pPLM264) | COQ3-Rv0637 fusion behind the CTA1 promoter | This study |
| pPLM271 (pPLM264) | COQ3-Rv2499c fusion behind the CTA1 promoter | This study |
| pPLM272 (pPLM264) | COQ3-Rv3389c fusion behind the CTA1 promoter | This study |
| pPLM273 (pPLM264) | COQ3-Rv3534 fusion behind the CTA1 promoter | This study |
| pPLM274 (pPLM264) | COQ3-Rv3542c fusion behind the CTA1 promoter | This study |
| pPLM275 (YEplac181) | COQ3-Rv0636 fusion behind the CTA1 promoter | This study |
TABLE 2.
Oligonucleotides used
| Oligonucleotide | Sequence | Reference |
|---|---|---|
| Rv0098 MLS F | 5′-TTATCCATGGGCCACACCGACTTGACGCCC-3′ | 21 |
| Rv0098 R | 5′-TATTAAGCTTACGGAATGTTGAGGGCCGC-3′ | 21 |
| Rv0130 MLS F | 5′-TTATCCATGGGCACCTTCGAGTCGGTCGCCG-3′ | 21 |
| Rv0130 R | 5′-TATTAAGCTTCAGGCGACGTAGCGCACGATGC-3′ | 21 |
| Rv0216 5′ NcoI | 5′-TTATCCATGGCTAGCGGGTATGGGGGC-3′ | This study |
| Rv0216 3′ HindIII | 5′-TATTAAGCTTCTAGAATTGCAAGGCGCTAAAAC-3′ | This study |
| Rv0241c 5′ NcoI | 5′-TTATCCATGGCTCAACCCAGCGGCCTGAAG-3′ | This study |
| Rv0241c 3′ HindIII | 5′-TATTAAGCTTCTATAGACCCCGCACGGTAGC-3′ | This study |
| Rv0504c 5′ NcoI | 5′-TTATCCATGGCAGTTCCCGAAGAAGCCCAGAC-3′ | This study |
| Rv0504c 3′ HindIII | 5′-TATTAAGCTTCTAGATCGATGCAATCGCCGC-3′ | This study |
| Rv0635 5′ NcoI | 5′-TTATCCATGGCGTTGAGCGCAGACATC-3′ | This study |
| Rv0635 3′ HindIII | 5′-TATTAAGCTTTCACGCAGCGCCATCAGAAAATC-3′ | This study |
| Rv0636 5′ NcoI | 5′-TTATCCATGGCGCTGCGTGAGTTCAGC-3′ | This study |
| Rv0636 3′ HindIII | 5′-TATTAAGCTTCTACGCTAACTTCGCCGAGGC-3′ | This study |
| Rv0637 5′ NcoI | 5′-TTATCCATGGCGCTCAAGACCGATATC-3′ | This study |
| Rv0637 3′ HindIII | 5′-TATTAAGCTTTTACGCGGTCCTGATGACCTG-3′ | This study |
| Rv2499c 5′ NcoI | 5′-TTATCCATGGCAAAGCACGCCGGCGACCGTG-3′ | This study |
| Rv2499c 3′ HindIII | 5′-TATTAAGCTTTCATTGCGCCTCCTTAATGGAC-3′ | This study |
| Rv3389c 5′ NcoI | 5′-TTATCCATGGCGATTGATCCGAACTCC-3′ | This study |
| Rv3389c 3′ HindIII | 5′-TATTAAGCTTCTAACCCGCCACGTACTCCAC-3′ | This study |
| Rv3538 5′ NcoI | 5′-TTATCCATGGCCATCGACTTGGACGTCGCGC-3′ | This study |
| Rv3538 3′ HindIII | 5′-TATTAAGCTTCTATGCCGGCACCAGCTCCAC-3′ | This study |
| Rv3542c 5′ NcoI | 5′-TTATCCATGGCCGGGGTGAGCGACATTCAGG-3′ | This study |
| Rv3542c 3′ HindIII | 5′-TATTAAGCTTTCATTCGTCAGGCTCCCATGC-3′ | This study |
| XXIV-1 | 5′-GGATATCTTGACTGATTTTTCGATGGAGGGCACAGTTAAGC-3′ | This study |
| XXIV-2 | 5′-GCTTAACTGTGCCCTCCATCGAAAAATCAGTCAAGATATCC-3′ | This study |
Plasmid constructions.
DNA manipulations and plasmid constructions were performed according to standard techniques (2). A previous mention of plasmids pPLM56 and pPLM63, representing URA3-marked YEp352 multicopy plasmids (26) carrying the nucleotide sequence for COQ3-Rv0098 or COQ3-Rv0130 fusions corresponding to mitochondrially targeted Rv0098 (mit-Rv0098) or HtdZ (mit-Rv0130) driven by the oleic acid-inducible CTA1 promoter (14), can be found elsewhere (21). PCR was applied to M. tuberculosis H37Rv genomic DNA using Phusion high-fidelity DNA polymerase (Finnzymes Oy, Espoo, Finland) and oligonucleotides Rv0098 MLS F and Rv0098 R (Table 2 lists all oligonucleotides used), which introduced a 5′ NcoI site and a 3′ HindIII site, respectively, at an annealing temperature of 55°C. Electrophoretic resolution of the PCR products on a 0.7% (wt/vol) agarose gel in a buffer comprised of 40 mM Tris-acetate and 1 mM EDTA (pH 8.0) revealed a single amplification product of the correct size (approximately 550 bp), which was excised and purified using Qiagen spin columns according to the manufacturer's instructions (Qiagen, Hilden, Germany). Ligation of this insert to a plasmid vector, pBluescript KSII (Stratagene, La Jolla, CA), that was linearized using EcoRV restriction enzyme resulted in plasmid pPLM56 (Table 1). Following digestion of pPLM56 with NcoI and HindIII restriction enzymes, the amplified Rv0098 DNA was ligated behind the CTA1 promoter to a similarly digested pYE352:mitQOR plasmid (20) from which the QOR open reading frame encoding E. coli quinone reductase was removed, leaving behind the nucleotides for the Coq3p (28) mitochondrial leader sequence (Coq3pMLS). This resulted in plasmid pPLM65. Nucleotide sequencing of the Rv0098 insert in plasmids pPLM56 and pPLM65 verified that no mutations were introduced during the amplification process and that the COQ3-Rv0098 junction remained intact. A similar strategy was used to obtain an NcoI-Rv0130-HindIII amplification product of approximately 456 bp, which was inserted into an EcoRV-digested pBluescript plasmid vector (pPLM63). The insert was sequenced and ligated behind the CTA1 promoter as a COQ3-Rv0130 fusion (pPLM49).
The strategy used here for cloning the remaining candidate dehydratases from H37Rv genomic DNA was similar but included a minor modification. Phusion-based thermocycling was undertaken at an annealing temperature of 55°C using the listed oligonucleotides (Table 2) that introduced 5′ NcoI and 3′ HindIII sites into the PCR products. Electrophoretic resolution of these amplicons revealed a single product of approximately the correct size (in kb) for each of the genes studied: Rv0216, 1.0; Rv0241c, 0.85; Rv0635, 0.48; Rv0636, 0.43; Rv0637, 0.5; Rv2499c, 0.56; Rv3389c, 0.88; Rv3538, 0.86; and Rv3542, 0.94. Excision and purification of the relevant bands were followed by ligation of the inserts to EcoRV-linearized pBluescript KSII to result in plasmids pPLM254 through pPLM263 (Table 1). Following digestion of these plasmids with NcoI and HindIII restriction enzymes to release the former amplicons, the insert DNA was ligated to the nucleotide sequence for Coq3pMLS behind the CTA1 promoter in a similarly digested new pYE352:mitQOR plasmid (pPLM264) that was missing the NcoI site in the URA3 gene. This site was removed by site-directed mutagenesis with the oligonucleotide pair XXIV-1 and -2. Insert ligations resulted in the expression plasmids listed as pPLM265 through pPLM274. To prepare a LEU2-marked expression plasmid for Rv0636 in order to enable coexpression with the URA3-marked Rv0635 or Rv0637 (i.e., HadAB or HadBC), an EcoRI fragment generated from pPLM269 that encompassed the entire fusion construct nested within the CTA1 promoter and terminator was ligated to an EcoRI-digested plasmid YEplac181 (16) to generate pPLM275. Cloning of the yeast OAR1 gene behind the CTA1 promoter will be described elsewhere.
Media and growth conditions.
Standard yeast (35) and E. coli (39) media were made up as described previously. S. cerevisiae strains were propagated on solid rich-glucose YPD medium consisting of 1% (wt/vol) yeast extract-2% (wt/vol) peptone (YP), 2% (wt/vol) d-glucose, and 2% (wt/vol) agar. Episomal and centromeric plasmids were maintained in transformed strains using solid synthetic defined (SD) media consisting of 0.67% (wt/vol) yeast nitrogen base without amino acids supplemented with yeast synthetic dropout medium (Sigma-Aldrich Inc., St. Louis, MO) without uracil (SD−Ura) or without uracil and leucine (SD−Ura−Leu), to which were added 2% (wt/vol) d-glucose and 2% (wt/vol) agar. Synthetic complete (SC) media were prepared essentially as described above but with the addition of uracil and 2% (wt/vol) d-glucose (SCglucose) or 3% (wt/vol) glycerol (SCglycerol). For enzyme assays, cells were cultivated overnight in liquid oleic acid medium consisting of YP, 0.2% (wt/vol) oleic acid (pH 7.0), and 0.02% (wt/vol) Tween 80 (22).
Miscellaneous.
For hydratase activity assays, 50-ml cultures of oleic acid-grown cells were collected by centrifugation and washed twice in cold distilled water, and the freshly pelleted cells were collected in 1.5-ml plastic tubes for further processing. Cells were broken with glass beads in 100 μl breakage buffer that consisted of 50 mM KPi (pH 7.0), 0.2 M KCl, and 0.1% (wt/vol) Triton X-100. Cells were mixed vigorously for 5 min using a vortex mixer, with several refractory periods on ice. The crude extracts were spun in a chilled microcentrifuge for 10 min, and soluble protein extracts were removed for enzyme assays. Hydratase activity was assayed spectrophotometrically at 23°C as described previously (32). The assay mixture consisted of crotonase buffer (0.166 M potassium phosphate [pH 8.0] and 0.3 mg/ml bovine serum albumin), 1.0 μl purified human D-specific 3-hydroxacyl-CoA dehydrogenase (unpublished), 1 mM NAD+, and 60 μM 2-trans-decenoyl-CoA or 2-trans-hexenoyl-CoA, which were synthesized via the mixed anhydride system (17), as the substrate. Hydratase activity was expressed as moles of substrate metabolized/mg protein per min. Respiration competence was assayed by overlaying cells grown on solid SD−Ura medium with 0.1% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC) in 0.067 M phosphate-buffered saline and 1.5% (wt/vol) low-melting-temperature agarose (6). The lipoic acid content of yeast strains was monitored by a biological assay described previously (8, 23) using the lipoic acid-deficient E. coli strain JRG33 (lipA9), with minor modifications to the protocol. Yeast strains were grown in 50 ml SCglucose, SD−Ura, or SD−Ura−Leu instead of YPD, and acid hydrolysis was carried out in 0.5 ml 9 N H2SO4. Bacterial JRG33 cultures were inoculated to an initial optical density at 600 nm of 0.015 in 2 ml of 1× basal growth medium (25) containing 50 mM sodium succinate and grown for 36 to 48 h. The growth response of the strain was linear between 0.05 and 0.5 ng·ml−1 lipoic acid in the cultures.
RESULTS
M. tuberculosis HadAB and HadBC rescue the growth phenotype of yeast htd2Δ cells.
Rv0635-Rv0636 (HadAB) and Rv0636-Rv0637 (HadBC) have previously been demonstrated to represent FASII HADs of mycolic acid biosynthesis (9, 37). To assess whether these two mycobacterial proteins could compensate in vivo for the missing HAD activity in the mitochondria of a yeast htd2Δ mutant strain, they were expressed as fusion constructs (mit-Rv0635, mit-Rv0636, and mit-Rv0637) that were preceded by the cleavable Coq3p mitochondrial leader sequence (Coq3pMLS), which has been demonstrated before to be sufficient to target proteins to yeast mitochondria (28). As controls, isogenic htd2Δ cells were transformed with plasmids expressing either mycobacterial HtdZ (21) or a mycobacterial protein that is considered nonrescuing, Rv0098. The corresponding genes were tethered behind the yeast oleic acid-inducible CTA1 promoter on URA3-marked YEp352 multicopy plasmids (26). In addition, the hadB construct was also ligated to a LEU2-marked multicopy plasmid, YEplac181 (16), thereby allowing for cotransformation with the aforementioned plasmids. Single and double transformants were selected for and maintained on glucose medium lacking uracil or lacking uracil and leucine. Following adjustment of the cell concentration to an optical density at 600 nm of 1.0 and serial 10-fold dilution, cells were spotted onto glycerol or glucose medium.
The results demonstrated that htd2Δ mutant cells expressing HtdZ grew well on glycerol as the sole carbon source, whereas those mutants expressing Rv0098 gave rise to only an insignificant background growth (Fig. 1), verifying the restrictiveness of the medium used for assessing the ability to rescue the mutant's respiratory growth phenotype. The results additionally showed that the htd2Δ strain expressing HadAB was also capable of substantial growth on glycerol as the sole carbon source, whereas mutant cells expressing HadBC were a great deal less efficient at respiratory growth (Fig. 1). On the other hand, those cells expressing only Rv0636, from two different plasmids (HadBB), were essentially incapable of growth on glycerol (Fig. 1). Therefore, we were able to show here for the first time that within the context of a heterologous yeast framework, HadAB, and to a lesser extent also HadBC, could function as physiological FASII HADs. This is also the first demonstration to our knowledge of expression of two separate heterologous polypeptides in yeast mitochondria that heterodimerize into an active FASII enzyme.
FIG. 1.
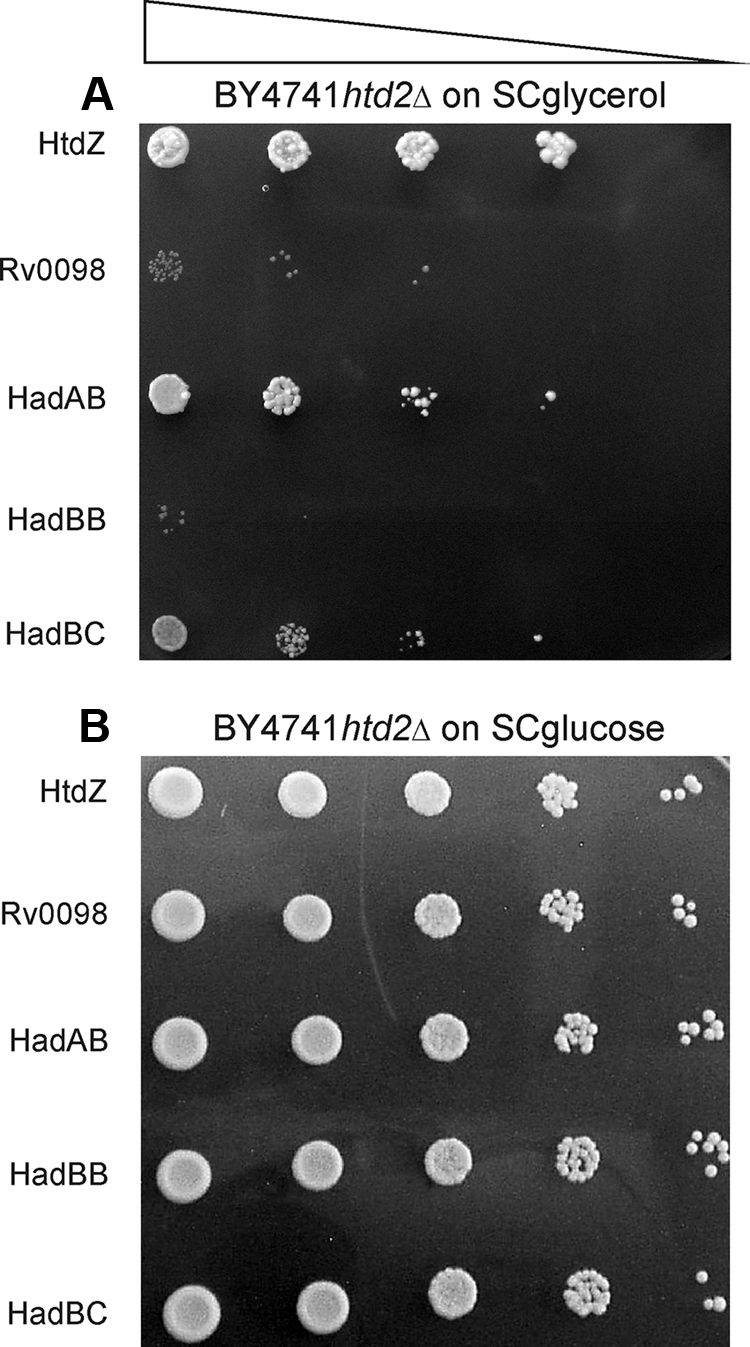
Respiratory growth of S. cerevisiae htd2Δ mutants expressing known M. tuberculosis dehydratases. Yeast htd2Δ cells synthesizing mitochondrially targeted Rv0130 (HtdZ) (representing a positive control); Rv0098 (a negative control); or Rv0635, Rv0636, or Rv0637 all dually expressed with Rv0636 on an additional multicopy plasmid to give rise to mitochondrial HadAB, HadBB (negative control), or HadBC, respectively, were grown in liquid glucose medium deficient in uracil or in uracil and leucine, as required, which selected for plasmid presence, and following serial 10-fold dilution (triangle), culture aliquots were applied to solid synthetic complete medium with 3% (wt/vol) glycerol (A) or 2% (wt/vol) glucose (B). The plates were incubated at 30°C until single colonies appeared and were recorded photographically. The strains used were yPLM35, yPLM36, yPLM287, yPLM288, and yPLM289.
S. cerevisiae htd2Δ cells expressing mycobacterial Rv0241c or Rv3389c grow on glycerol.
The success at complementing the htd2Δ mutant phenotype for growth on glycerol using the known mycolic acid biosynthesis enzymes HadAB and HadBC underscored the suitability of this method for searching for additional HADs among the potential candidates listed in the introduction. Three separate transformants expressing each of the candidates were streaked on glycerol medium, and of these, two genes were capable of rescuing the growth phenotype of the htd2Δ mutant, Rv0241c and Rv3389c (see below). Moreover, to determine whether additional HadB partners existed, an htd2Δ mutant expressing HadB from a LEU2-marked multicopy plasmid was additionally transformed with each of the seven noncomplementing candidate dehydratases, Rv0098, Rv0216, Rv0504c, Rv2499c, Rv2524c, Rv3538, and Rv3542c; however, none of these resulted in functional complementation.
To demonstrate more accurately that expression of mitochondrial versions of Rv0241c or Rv3389c in the htd2Δ mutant cells could compensate for the missing activity attributed to native Htd2p, four strains expressing HtdZ, Rv0098, Rv0241c, or Rv3389c were grown on uracil-deficient glucose medium, and following 10-fold serial dilution, cultures were spotted onto solid glycerol (SCglycerol) or glucose (SD−Ura) medium and the plates were incubated at 30°C until single colonies were detectable. The results in Fig. 2 demonstrate that mutant htd2Δ cells expressing Rv241c or Rv3389c resembled the HtdZ positive control in that they were capable of abundant growth on glycerol, whereas those mutant cells expressing Rv0098 were not able to grow or divide on this nonfermentable sole carbon source.
FIG. 2.
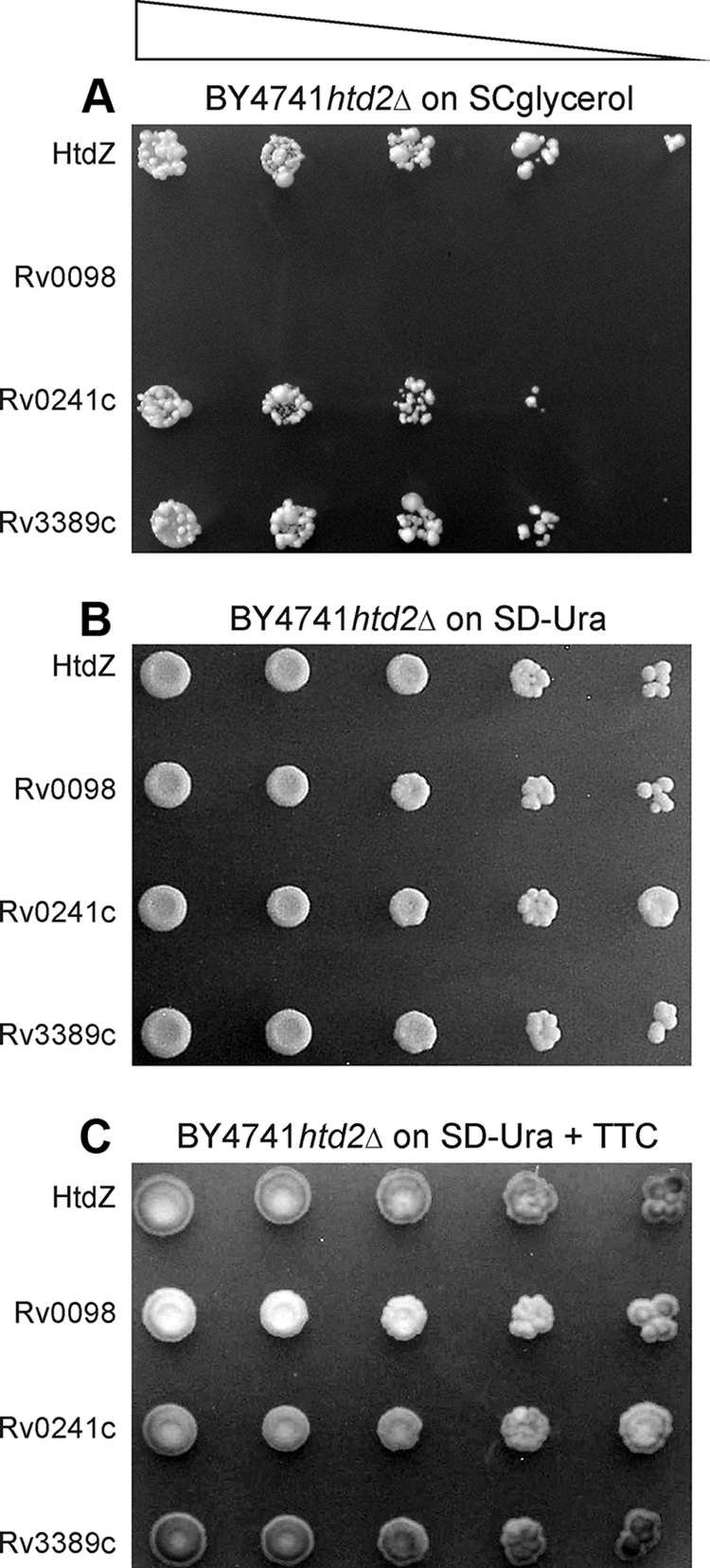
Growth on glycerol of S. cerevisiae htd2Δ mutants expressing novel M. tuberculosis dehydratases. Yeast htd2Δ cells producing mitochondrially targeted Rv0130 (HtdZ), Rv0098, Rv0241c, or Rv3389c were grown and examined essentially as detailed in the legend to Fig. 1. Tenfold serial dilutions (triangle) were spotted on synthetic complete medium containing glycerol (A) and synthetic defined glucose medium lacking uracil (B) for demonstrating plasmid presence. Application of a TTC overlay to the plate without uracil (C) underscored the efficiency of mutant cells expressing HtdZ, Rv0241c, or Rv3389c in generating the red chromophore. The strains used were yPLM35, yPLM36, yPLM278, and yPLM284.
Mitochondrial fatty acid biosynthesis deficiency is characterized in yeast mutants by a dysfunctional electron transfer chain. It follows, therefore, that the recovery of htd2Δ cells from their respiratory deficiency phenotype through complementation should be accompanied by the regeneration of this process. Hence, to demonstrate that the mitochondria of Rv0241c- or Rv3389c-expressing mutant cells contained an intact electron transfer chain, mutant cells expressing HtdZ, Rv0098, Rv0241c, or Rv3389c from URA3-marked multicopy plasmids were grown on glucose medium lacking uracil (SD−Ura) and, following 4 days of incubation at 30°C, were overlaid with TTC. The results (Fig. 2C) demonstrated that mutant htd2Δ cells expressing HtdZ were able to metabolize TTC efficiently to generate the red chromophore, whereas those cells expressing Rv0098 were not efficient. The TTC overlay additionally demonstrated that mutant cells expressing Rv0241c or Rv3389c were qualitatively as efficient as the HtdZ-expressing mutants at metabolizing TTC (Fig. 2C). These results implied that the electron transfer chain in mutant cells expressing Rv0241c or Rv3389c was restored.
Yeast htd2Δ cells expressing M. tuberculosis HadAB, HadBC, Rv0241c, or Rv3389c contain 3-hydroxacyl-thioester hydratase activity and produce lipoic acid.
To demonstrate that the two novel M. tuberculosis HAD candidates Rv0241c and Rv3389c were catalytically active, we set out to measure their enzyme activities in yeast extracts from triplicate cultures of oleic acid-induced cells. In addition, soluble protein extracts were also produced from single cultures of oleic acid-induced mutant cells expressing HadAB or HadBC. The results of the enzyme assays (Table 3) showed that a volume of 0.01 μl of soluble protein extracts containing approximately 8 mg/ml protein generated from mutant htd2Δ cells expressing the positive control HtdZ gave rise to an activity of 3.82 ± 0.42 μmol/mg protein·min−1 using trans-2-hexenoyl-CoA as the substrate. This value represents a significant improvement over that obtained following previous oleic acid inductions (21), presumably due to the freshness of the oleic acid solution added to the medium, the efficiency of the solubilization of proteins by including 0.1% Triton X-100 in the breakage buffer, and the extension of the cell breakage time by vortexing for up to 5 min. As a negative control, 1 μl of an extract consisting of approximately 4 mg/ml protein obtained from similarly treated BY4741 oar1Δ cells expressing fungal NADP(H)-dependent 3-oxoacyl-ACP reductase Oar1p, which is not capable of metabolizing the substrate in the presence of NAD(H), gave rise to an activity that was 2 orders of magnitude less (Table 3).
TABLE 3.
3-Hydroxyacyl-thioester dehydratase activity in yeast htd2Δ mutants expressing the listed proteins on oleic acid medium
| Proteina | Activityb on substrate:
|
|
|---|---|---|
| trans-2-Hexenoyl-CoA | 2-trans-Decenoyl-CoA | |
| HtdZ | 3.82 ± 0.42 | 0.43 ± 0.11 |
| Rv0098 | ND | 0.19 ± 0.02 |
| Rv0241c | 0.12 ± 0.02 | 0.17 ± 0.01 |
| Rv3389c | 6.08 ± 0.19 | 3.97 ± 0.20 |
| HadAB | 0.06 | 0.14 |
| HadBC | 0.09 | 0.22 |
| Oar1pc | 0.03 | 0.07 ± 0.01 |
The strains used were yPLM35, yPLM36, yPLM43, yPLM278, yPLM284, yPLM288, and 289.
Activity is expressed as μmol of substrate metabolized/mg protein·min−1; the detection limit is <0.05 nmol/mg protein·min−1. The values are means ± standard deviations (n = 3) and represent averages of enzyme activities measured in soluble protein extracts derived from yeast cells following three independent oleic acid inductions. ND, not determined.
BY4741 oar1Δ expressing native NADP(H)-dependent 3-oxoacyl-ACP reductase Oar1p from the CTA1 promoter (yPLM43).
In reference to HadAB or HadBC, soluble protein extracts produced from BY4741 htd2Δ cells expressing each one of these heterodimers yielded two- and threefold-higher activities than the negative control (Table 3), whereas those extracts obtained from cells expressing Rv0241c or Rv3389c contained activities that were 4- and 200-fold higher than that of the control using the C6 substrate; the situation with the C10 substrate was also measured and reported (Table 3). Surprisingly, soluble protein extracts generated from htd2Δ cells expressing Rv0098, which fails to complement the mutant phenotype (21), also contained 3-hydroxacyl-thioester hydratase activity that was approximately threefold higher than that of the control (Fig. 3; Table 3). Hence, in agreement with previously published data on the hydratase activity of recombinant HadABC (9, 37) and Rv3389c (38) produced in E. coli, expression of these proteins in yeast mitochondria revealed detectable levels of (FASII-like) 3-hydroxyacyl-thioester hydratase activity.
FIG. 3.
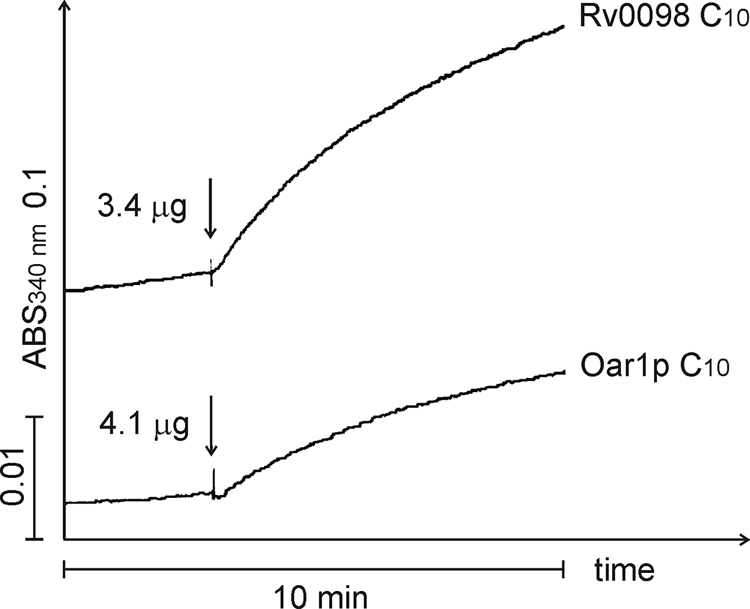
Rv0098 contains low levels of 3-hydroxyacyl-thioester hydratase activity. Soluble protein extracts from oleic acid-induced cells were added to a reaction mixture consisting of 700 μl crotonase buffer, 1 μl purified human D-specific 3-hydroxacyl-CoA dehydrogenase, and 1 mM NAD+, and the reaction was started (↓) with 60 μM 2-trans-decenoyl-CoA (C10). Reactions were monitored spectrophotometrically as the change in absorbance (ABS) on a scale of 0.1. The strains used were yPLM35 and yPLM43.
Mitochondrial FASII in yeast has been clearly linked to lipoic acid production (8, 23). To implicate directly Rv0241c or Rv3389c in fatty acid biosynthesis, production of lipoic acid in yeast mitochondria was examined, and the results are presented in Table 4.
TABLE 4.
Lipoic acid production in yeast htd2Δ mutants expressing the listed proteins on glucose medium
| Straina | Lipoic acid content (ng/g [wet wt])b |
|---|---|
| BY4741 (wild type) | 161.3 ± 31.0 |
| BY4741 htd2Δ | 35.2c |
| BY4741 htd2Δ + HtdZ | 56.1c |
| BY4741 htd2Δ + Rv0098 | 26.8 ± 1.0 |
| BY4741 htd2Δ + Rv0241c | 147.8 ± 9.3 |
| BY4741 htd2Δ + Rv3389c | 64.1 ± 5.8 |
| BY4741 htd2Δ + HadAB | 50.3 ± 7.0 |
| BY4741 htd2Δ + HadBC | 24.3 ± 6.3 |
The strains used were yPLM35, yPLM36, yPLM278, yPLM284, yPLM287, yPLM289, yPLM290, and yPLM291.
Except as indicated, the values are the means ± standard deviations (n = 3) and represent averages of three independent bacterial growth responses.
Performed in duplicate.
DISCUSSION
We showed here that the action of mitochondrially localized Rv0241c or Rv3389c could reverse the consequences to yeast of the lesion caused by the htd2Δ mutation. Previous heterologous expressions (see below) of known dehydratases in the htd2Δ mutant corrected the mutant's defective respiration and hence established its utility in screening for novel enzymes. These include bacterial FabZ and FabA (30), human HTD2 (4), trypanosome HTD2 (3), mycobacterial HtdZ (21), and, as shown here, also mycobacterial HadAB and HadBC. In light of the present complementation of the yeast HTD2 gene with Rv0241c and Rv3389c, we suggest that like the situation with the above-mentioned examples, this entitles the latter two mycobacterial genes to be referred to as htdX and htdY, respectively, as they encode bona fide M. tuberculosis FASII-like 3-hydroxyacyl-thioester dehydratases.
M. tuberculosis has been proposed to contain 13 candidate FASII dehydratases, including Rv0098, Rv0130, Rv0216, Rv0241c, Rv0504c, Rv0635, Rv0636, Rv0637, Rv2499, Rv2524c, Rv3389c, Rv3538, and Rv3542c (11, 41). Of these, Rv0130, denoted HtdZ (21), and Rv0635-Rv0636 and Rv0636-Rv0637, referred to as HadAB and HadBC (9, 37), have since been identified as physiological dehydratases. HadAB and HadBC join InhA in a growing list of M. tuberculosis enzymes implicated directly in the biosynthesis of mycolic acids that can additionally act during de novo synthesis of lipoic acid in yeast (20). The higher efficiency of yeast mutant cells expressing HadAB at growing on glycerol, compared with the situation for those expressing HadBC, is commensurate with the published data on the substrate specificities of these two heterodimers (37). HadAB is active in vitro using short-chain thioester derivatives, including C4, C4:1, C8, and to a lesser extent C8:1, whereas HadBC is not. Moreover, even using longer-chain thioesters, such as those represented by C12-20 trans-2-enoyl-CoAs, which acted as in vitro substrates for HadAB, HadBC was not particularly efficient, since as much as 10-fold concentration of substrates was required in order to demonstrate an activity for the latter enzyme (37). Hence, the previously published activity measurements (37) dovetail nicely with the ability reported here of yeast cells to differentiate between HAD species that efficiently utilize short-chain thioesters (HadAB) and those that are either less efficient (HadBC) or not at all active (HadBB).
As claimed above, this is the first demonstration of a physiological function for two novel dehydratases, HtdX (Rv0241c) and HtdY (Rv3389c). HtdX, whose gene lies in proximity to that encoding FabG4, a putative 3-oxoacyl-ACP reductase, was shown here to contain 3-hydroxyacyl-thioester dehydratase activity, albeit it was previously predicted to contain the signature double hot dog structure of type 2 dehydratases, and its amino acid residue sequence was shown before to adhere to the strictly conserved hydratase-2 motif (37). In addition to containing measurable levels of hydratase activity, HtdX restored to the mutant the ability to grow on glycerol and produce ample levels of lipoic acid. HtdX has been chronicled in the literature on at least two previous occasions. In one study, Rv0241c was identified together with Rv3389c, Rv0635, Rv0637, and Rv0098 during proteomic profiling of the mycobacterial membrane (18). In a second study, transcription of mycobacterial genes in response to drug treatment was investigated, and this revealed gene clusters that were specifically regulated by certain drugs. In particular, 6PP and 8PP, two high-affinity alkyl-substituted diphenyl ethers, upregulated hallmark genes associated with cell wall synthesis, including fas, the kas operon, and Rv0241c (7). This finding implicates HtdX indirectly in mycolic acid biosynthesis within the pathogen.
The second physiological dehydratase identified here, HtdY, was shown previously to contain 3-hydroxyacyl-thioester dehydratase activity (37). However, since the enzyme exhibited a reduced preference for ACP compared to CoA thioesters (40%) and the Rv3389c knockout mutant remained viable without showing significant differences in fatty acid composition (including mycolic acids), the authors claimed that it is not part of the ACP-dependent FASII system but that instead it might be involved in CoA-dependent fatty acid elongation pathways or polyhydroxyalkanoate synthesis (37). The significance of the finding presented here that HtdY could nevertheless restore the respiratory growth of the yeast htd2Δ mutant has several implications. First, HtdY not only accepted ACP thioesters as substrates in vitro (37), but since yeast mitochondrial FASII is an ACP-dependent process, this means that HtdY additionally accepted ACP thioesters also in vivo. Second, if possible, simultaneous knocking out of multiple combinations of htd genes should be undertaken in M. tuberculosis.
In the course of analyzing the M. tuberculosis genome for FabZ homologues, Rv0098, a long-chain fatty acyl-CoA thioesterase (FcoT) with only low catalytic activity (42), was revealed as a promising candidate (38, 41). Rv0098 is 36% identical and 54% similar to Streptococcus pneumoniae FabZ and contains the latter's conserved active-site residues that are also present in E. coli FabZ and FabA (41). This is important, since by possessing a FabA/Z-like active site but not the eukaryotic hydratase-2 motif, Rv0098 is placed evolutionarily closer to FabZ than any other one of the dehydratases listed, including Rv0130 and Rv3389c. Rv0098 is thought to be one of only 219 essential core genes in mycobacteria (33). As an aside, Rv0216, whose gene product was also tested here for complementation, is also a core gene (11). Furthermore, Rv0098 is required for the survival of M. tuberculosis in mouse lung macrophages (40), and has homologues in M. leprae, M. bovis, and M. smegmatis (41).
In the present study, we were able to improve significantly the oleic acid-dependent transcriptional induction of the CTA1 promoter driving the expression of Rv0098, compared with our previous attempt (21). As a result, it was possible to measure a low level of specific 3-hydroxyacyl-thioester hydratase activity for this protein in yeast extracts (Fig. 3; Table 3), although Rv0098 still failed to rescue the mutant phenotype in a convincing manner. It is attractive to postulate that, perhaps by using other creative approaches, Rv0098 might nevertheless be revealed as the elusive M. tuberculosis FabZ after all. If an Rv0098 gene knockout experiment was undertaken in an M. tuberculosis strain harboring a plasmid-borne copy of Rv0098 tethered behind an inducible promoter rather than the native promoter (34), this would help to expose whether the gene is important for mycolic acid biosynthesis. Although the observations and conclusions presented here may or may not apply to the biochemistry of mycobacteria, the combined data nevertheless call into question the previous assertion made with the discovery of the essential proteins HadAB and HadBC that M. tuberculosis does not have additional dehydratases.
Acknowledgments
We dedicate this work to Otto Scheiner, former Director of the Center for Physiology, Pathophysiology, and Immunology at the Medical University of Vienna, on the occasion of his being awarded the title of Honorary Senator.
We thank Johanna Mäkinen from the Mycobacterial Reference Laboratory at the National Public Health Institute in Turku, Finland, for providing us with M. tuberculosis genomic DNA. We thank Zhi-Jun Chen for the plasmid expressing yeast Oar1p.
This work was supported by grants from the Academy of Finland and the Sigrid Jusélius Foundation to J.K.H. and grants P19378-B03 and P19399-B03 from the Austrian Science Fund (FWF) to A.G.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Arcus, V. L., J. S. Lott, J. M. Johnston, and E. N. Baker. 2006. The potential impact of structural genomics on tuberculosis drug discovery. Drug Discov. Today 1128-34. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 3.Autio, K. J., J. L. Guler, A. J. Kastaniotis, P. T. Englund, and J. K. Hiltunen. 2008. The 3-hydroxyacyl-ACP dehydratase of mitochondrial fatty acid synthesis in Trypanosoma brucei. FEBS Lett. 582729-733. [DOI] [PubMed] [Google Scholar]
- 4.Autio, K. J., A. J. Kastaniotis, H. Pospiech, I. J. Miinalainen, M. S. Schonauer, C. L. Dieckmann, and J. K. Hiltunen. 2008. An ancient genetic link between vertebrate mitochondrial fatty acid synthesis and RNA processing. FASEB J. 22569-578. [DOI] [PubMed] [Google Scholar]
- 5.Bloch, K. 1977. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv. Enzymol. 451-84. [DOI] [PubMed] [Google Scholar]
- 6.Böker-Schmitt, E., S. Francisci, and R. J. Schweyen. 1982. Mutations releasing mitochondrial biogenesis from glucose repression in Saccharomyces cerevisiae. J. Bacteriol. 151303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshoff, H. I., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 27940174-40184. [DOI] [PubMed] [Google Scholar]
- 8.Brody, S., C. Oh, U. Hoja, and E. Schweizer. 1997. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 408217-220. [DOI] [PubMed] [Google Scholar]
- 9.Brown, A. K., A. Bhatt, A. Singh, E. Saparia, A. F. Evans, and G. S. Besra. 2007. Identification of the dehydratase component of the mycobacterial mycolic acid-synthesizing fatty acid synthase-II complex. Microbiology 1534166-4173. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, J. W., and J. E. Cronan, Jr. 2001. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu. Rev. Microbiol. 55305-332. [DOI] [PubMed] [Google Scholar]
- 11.Castell, A., P. Johansson, T. Unge, T. A. Jones, and K. Bäckbro. 2005. Rv0216, a conserved hypothetical protein from Mycobacterium tuberculosis that is essential for bacterial survival during infection, has a double hotdog fold. Protein Sci. 141850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, D.-C., B.-C. Yang, and T.-T. Kuo. 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 2183-84. [DOI] [PubMed] [Google Scholar]
- 13.Dillon, S. C., and A. Bateman. 2004. The Hotdog fold: wrapping up a superfamily of thioesterases and dehydratases. BMC Bioinformatics 5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filppula, S. A., R. T. Sormunen, A. Hartig, W.-H. Kunau, and J. K. Hiltunen. 1995. Changing stereochemistry for a metabolic pathway in vivo. Experiments with the peroxisomal ß-oxidation in yeast. J. Biol. Chem. 27027453-27457. [DOI] [PubMed] [Google Scholar]
- 15.Gerum, A. B., J. E. Ulmer, D. P. Jacobus, N. P. Jensen, D. R. Sherman, and C. H. Sibley. 2002. Novel Saccharomyces cerevisiae screen identifies WR99210 analogues that inhibit Mycobacterium tuberculosis dihydrofolate reductase. Antimicrob. Agents Chemother. 463362-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74527-534. [DOI] [PubMed] [Google Scholar]
- 17.Goldman, P., and P. R. Vagelos. 1961. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J. Biol. Chem. 2362620-2623. [PubMed] [Google Scholar]
- 18.Gu, S., J. Chen, K. M. Dobos, E. M. Bradbury, J. T. Belisle, and X. Chen. 2003. Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol. Cell Proteomics 21284-1296. [DOI] [PubMed] [Google Scholar]
- 19.Gueguen, V., D. Macherel, M. Jaquinod, R. Douce, and J. Bourguignon. 2000. Fatty acid and lipoic acid biosynthesis in higher plant mitochondria. J. Biol. Chem. 2755016-5025. [DOI] [PubMed] [Google Scholar]
- 20.Gurvitz, A., J. K. Hiltunen, and A. J. Kastaniotis. 2008. Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 745078-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurvitz, A., J. K. Hiltunen, and A. J. Kastaniotis. 2008. Identification of a novel mycobacterial 3-hydroxyacyl-thioester dehydratase HtdZ (Rv0130) by functional complementation in yeast. J. Bacteriol. 1904088-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurvitz, A., H. Rottensteiner, S. H. Kilpeläinen, A. Hartig, J. K. Hiltunen, M. Binder, I. W. Dawes, and B. Hamilton. 1997. The Saccharomyces cerevisiae peroxisomal 2,4-dienoyl-CoA reductase is encoded by the oleate-inducible gene SPS19. J. Biol. Chem. 27222140-22147. [DOI] [PubMed] [Google Scholar]
- 23.Hayden, M. A., I. Y. Huang, G. Iliopoulos, M. Orozco, and G. W. Ashley. 1993. Biosynthesis of lipoic acid: characterization of the lipoic acid auxotrophs Escherichia coli W1485-lip2 and JRG33-lip9. Biochemistry 323778-3782. [DOI] [PubMed] [Google Scholar]
- 24.Heath, R. J., Y. T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 27330316-30320. [DOI] [PubMed] [Google Scholar]
- 25.Herbert, A. A., and J. R. Guest. 1970. Turbidimetric and polarographic assays for lipoic acid using mutants of Escherichia coli. Methods Enzymol. 18269-272. [Google Scholar]
- 26.Hill, J. E., A. M. Myers, T. J. Koerner, and A. Tzagoloff. 1986. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2163-167. [DOI] [PubMed] [Google Scholar]
- 27.Hiltunen, J. K., F. Okubo, V. A. Kursu, K. J. Autio, and A. J. Kastaniotis. 2005. Mitochondrial fatty acid synthesis and maintenance of respiratory competent mitochondria in yeast. Biochem. Soc. Trans. 331162-1165. [DOI] [PubMed] [Google Scholar]
- 28.Hsu, A. Y., W. W. Poon, J. A. Shepherd, D. C. Myles, and C. F. Clarke. 1996. Complementation of coq3 mutant yeast by mitochondrial targeting of the Escherichia coli UbiG polypeptide: evidence that UbiG catalyzes both O-methylation steps in ubiquinone biosynthesis. Biochemistry 359797-9806. [DOI] [PubMed] [Google Scholar]
- 29.Johansson, P., A. Castell, T. A. Jones, and K. Bäckbro. 2006. Structure and function of Rv0130, a conserved hypothetical protein from Mycobacterium tuberculosis. Protein Sci. 152300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastaniotis, A. J., K. J. Autio, R. T. Sormunen, and J. K. Hiltunen. 2004. Htd2p/Yhr067p is a yeast 3-hydroxyacyl-ACP dehydratase essential for mitochondrial function and morphology. Mol. Microbiol. 531407-1421. [DOI] [PubMed] [Google Scholar]
- 31.Kimber, M. S., F. Martin, Y. Lu, S. Houston, M. Vedadi, A. Dharamsi, K. M. Fiebig, M. Schmid, and C. O. Rock. 2004. The structure of (3R)-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Pseudomonas aeruginosa. J. Biol. Chem. 27952593-52602. [DOI] [PubMed] [Google Scholar]
- 32.Malila, L. H., K. M. Siivari, M. J. Mäkelä, J. E. Jalonen, P. M. Latipää, W.-H. Kunau, and J. K. Hiltunen. 1993. Enzymes converting D-3-hydroxyacyl-CoA to trans-2-enoyl-CoA. Microsomal and peroxisomal isoenzymes in rat liver. J. Biol. Chem. 26821578-21585. [PubMed] [Google Scholar]
- 33.Marmiesse, M., P. Brodin, C. Buchrieser, C. Gutierrez, N. Simoes, V. Vincent, P. Glaser, S. T. Cole, and R. Brosch. 2004. Macro-array and bioinformatic analyses reveal mycobacterial ‘core’ genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiology 150483-496. [DOI] [PubMed] [Google Scholar]
- 34.Parish, T., G. Roberts, F. Laval, M. Schaeffer, M. Daffé, and K. Duncan. 2007. Functional complementation of the essential gene fabG1 of Mycobacterium tuberculosis by Mycobacterium smegmatis fabG but not Escherichia coli fabG. J. Bacteriol. 1893721-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose, M. D., F. Winston, and P. Heiter. 1990. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Rozwarski, D. A., C. Vilcheze, M. Sugantino, R. Bittman, and J. C. Sacchettini. 1999. Crystal structure of the Mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD+ and a C16 fatty acyl substrate. J. Biol. Chem. 27415582-15589. [DOI] [PubMed] [Google Scholar]
- 37.Sacco, E., A. S. Covarrubias, H. M. O'Hare, P. Carroll, N. Eynard, T. A. Jones, T. Parish, M. Daffé, K. Bäckbro, and A. Quémard. 2007. The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 10414628-14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacco, E., V. Legendre, F. Laval, D. Zerbib, H. Montrozier, N. Eynard, C. Guilhot, M. Daffé, and A. Quémard. 2007. Rv3389C from Mycobacterium tuberculosis, a member of the (R)-specific hydratase/dehydratase family. Biochim. Biophys. Acta 1774303-311. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 40.Sassetti, C. M., and E. J. Rubin. 2003. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 10012989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama, K., C. Wang, and G. S. Besra. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 1881-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, F., R. Langley, G. Gulten, L. Wang, and J. C. Sacchettini. 2007. Identification of a type III thioesterase reveals the function of an operon crucial for Mtb virulence. Chem. Biol. 14543-551. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organisation. 1997. Anti-tuberculosis drug resistance in the world: the WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. W. H. O. Global Tuberculosis Programme, Geneva, Switzerland.


