Abstract
SigB is an alternative sigma factor that controls a large regulon in Staphylococcus aureus. Activation of SigB requires RsbU, a protein phosphatase 2C (PP2C)-type phosphatase. In a closely related organism, Bacillus subtilis, RsbU activity is stimulated upon interaction with RsbT, a kinase, which following an activating stimulus switches from a 25S high-molecular-weight complex, the stressosome, to the N-terminal domain of RsbU. Active RsbU dephosporylates RsbV and thereby triggers the release of SigB from its inhibitory complex with RsbW. While RsbU, RsbV, RsbW, and SigB are conserved in S. aureus, proteins similar to RsbT and the components of the stressosome are not, raising the question of how RsbU activity and hence SigB activity are controlled in S. aureus. We found that in contrast to the case in B. subtilis, the induced expression of RsbU was sufficient to stimulate SigB-dependent transcription in S. aureus. However, activation of SigB-dependent transcription following alkaline stress did not lead to a clear accumulation of SigB and its regulators RsbV and RsbW or to a change in the RsbV/RsbV-P ratio in S. aureus. When expressed in B. subtilis, the S. aureus RsbU displayed a high activity even in the absence of an inducing stimulus. This high activity could be transferred to the PP2C domain of the B. subtilis RsbU protein by a fusion to the N-terminal domain of the S. aureus RsbU. Collectively, the data suggest that the activity of the S. aureus RsbU and hence SigB may be subjected to different regulation in comparison to that in B. subtilis.
In eubacteria promoter recognition is mediated by a sigma factor which binds the multisubunit RNA polymerase core enzyme (α2ββ′ω) and initiates transcription. Usually, in addition to the essential housekeeping sigma factor, a variable number of alternative sigma factors with different promoter specificities are present in the bacterial cell. The availability of multiple alternative sigma factors provides the cell with an easy way to globally alter the transcriptional program in response to changing environmental conditions (25).
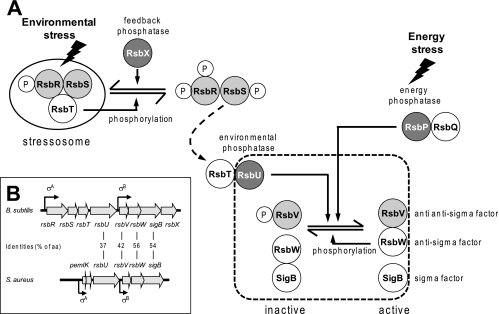
Regulation of the activity of alternative sigma factors often occurs at the posttranscriptional level by means of anti-sigma factors, which sequester sigma factors in inactive complexes (29). A very well characterized example of such a regulation is the control of the activity of the alternative sigma factor SigB in the gram-positive soil bacterium Bacillus subtilis (Fig. 1A). In this organism SigB is the master regulator of a large regulon (47, 48) which provides the cell with a multiple, unspecific, and preventive stress resistance (27, 28, 49). In the absence of stress, SigB is bound by its anti-sigma factor, RsbW, and thus cannot interact with the RNA polymerase core enzyme. SigB is normally released from the SigB/RsbW complex by the activity of RsbV, the anti-sigma factor antagonist, which competes with SigB for RsbW binding. The activity of RsbV is controlled by reversible phosphorylation at serine 56 (17, 20). Only unphosphorylated RsbV is able to compete with SigB for the binding to RsbW. The alternative binding of RsbW to RsbV ultimately leads to the release of SigB, which then can bind to core RNA polymerase and initiate transcription of the SigB-dependent stress regulon (3, 5, 17, 63). This regulatory principle composed of a target protein (SigB), an antagonist (RsbV), and a switch kinase (RsbW), with the protein interactions being controlled by reversible phosphorylation, has been named “partner switching” (2, 3).
FIG. 1.
Model of SigB activation in B. subtilis and comparison of sigB operon structures in B. subtilis and S. aureus. (A) Stress-induced activation of either of the two phosphatases RsbU or RsbP leads to dephosphorylation of the anti-anti-sigma factor RsbV. RsbV then displaces SigB from its complex with RsbW. Free SigB interacts with core RNA polymerase for transcription initiation at SigB-dependent promoters. Only RsbU and the partner-switching module comprising RsbV, RsbW, and SigB are conserved in staphylococci (dashed line). (B) Open reading frames (arrows) and experimentally demonstrated transcriptional start sites with the respective required sigma subunit are indicated. The level of amino acid (aa) conservation is shown for SigB and regulators present in both species.
In unstressed cells, most of the RsbV is phosphorylated by RsbW, which, besides being the SigB anti-sigma factor, also possesses an RsbV-specific kinase activity (14, 17). Unphosphorylated RsbV is generated by two different protein phosphatase 2C (PP2C)-type phosphatases, RsbU and RsbP, in response to environmental and energy stress, respectively (33, 60, 62, 66). The energy stress phosphatase RsbP is a two-domain protein with an N-terminal PAS domain and a C-terminal PP2C domain (60). PAS domains are frequently found in the bacterial world and have been shown to have a function in the sensing of redox potential and light or oxygen concentration but also in the control of protein-protein interactions (59). The rsbP gene forms an operon with rsbQ, a gene encoding an α/β-hydrolase. Genetic and structural studies suggest that RsbQ is essential for RsbP activity, possibly by providing a small molecule that might act as a cofactor to RsbP (32, 60).
The activity of RsbU, the environmental stress phosphatase, is controlled by a second “partner-switching” module. This second partner-switching module is composed of the switch kinase RsbT and the antagonist RsbS. Interaction of RsbT with the N-terminal domain of RsbU stimulates the C-terminally located RsbU phosphatase activity toward RsbV-P (15, 26, 34). To prevent inappropriate activation of RsbU and consequently SigB in the absence of stress, RsbT is captured in a multicomponent 25S complex, the stressosome, which is composed of the antagonist RsbS and at least one of a group of paralogous coantagonists: RsbRA (RsbR), RsbRB (YkoB), RsbRC (YojH), and RsbRD (YqhA) (1, 12, 16, 36, 40). Once again, protein interactions in this second “partner-switching” module are controlled by reversible phosphorylations at serine (RsbS) and conserved threonine (RsbRA, -RB, -RC, and -RD) residues (13, 22, 33, 35). Phosphorylation of the stressosome components by RsbT is required in order to release RsbT, allowing interaction of RsbT with RsbU and subsequent stimulation of the phosphatase activity of RsbU. A negative feedback mechanism relying on the PP2C-type phosphatase RsbX ensures that the activation of RsbU following environmental stress is only transient. RsbX is coexpressed with SigB, and the RsbX level might rise sufficiently following stress to compete with the RsbT kinase activity, thus reversing the stressosome into a form that recaptures RsbT and prevents further activation of RsbU and consequently SigB (53, 64, 66).
In B. subtilis the genes encoding regulators of the environmental stress branch are organized with the components of the SigB/RsbV/RsbW “partner-switching” module in an eight gene operon with the structure rsbRA-rsbS-rsbT-rsbU-rsbV-rsbW-sigB-rsbX (33). A truncated version of this operon, lacking genes encoding the RsbU-regulating “partner-switching” module components RsbRA, RsbS, and RsbT and the phosphatase RsbX, is present in the gram-positive pathogen Staphylococcus aureus (Fig. 1B) (43, 54, 65). Although protein interactions of the “partner-switching” module appear to be conserved, the exact regulation of SigB activity in S. aureus is less well understood. Several studies have shown that a deletion in rsbU drastically reduces SigB activity, suggesting that, similar to the case in B. subtilis, dephosphorylation of RsbV-P is a prerequisite for SigB activation in S. aureus (24, 30, 45, 46). However, the mechanism by which RsbU activity in S. aureus is regulated in the absence of genes encoding proteins similar to the stressosome components (RsbRA, RsbS, and RsbT) remains to be elucidated. The fact that on the one hand stimuli (e.g., alkaline shock) that induce SigB activity in S. aureus in an RsbU-dependent manner do not do so in B. subtilis, but on the other hand stimuli (heat shock or osmotic stress) that require the stressosome components in B. subtilis for proper signal transduction to some extent are able to activate SigB in S. aureus, suggests major differences in the regulation of RsbU activity in this organism (23, 46, 54).
In this study, alkaline shock was used to trigger the SigB response in S. aureus in an RsbU-dependent manner. The results indicate that RsbU is an RsbV-P-specific phosphatase and that the presence of dephosphorylated RsbV is required for SigB-dependent transcription. However, in contrast to the case in B. subtilis, no dramatic changes in the accumulation pattern of RsbV, RsbW, and SigB or the RsbV/RsbV-P ratio were apparent following acute stress. Furthermore, the data suggest that the S. aureus and B. subtilis RsbU phosphatases differ in their intrinsic activities.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Strains used in this study are listed in Table 1. S. aureus was cultured in Luria-Bertani (LB) medium (Gibco BRL, Wiesbaden, Germany) with vigorous agitation at 37°C. B. subtilis cells were grown at 37°C with vigorous agitation in a synthetic medium supplemented with 0.05% (wt/vol) glucose as a carbon source and l-tryptophan (0.78 mM) (58). When included, antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; chloramphenicol, 10 μg ml−1; erythromycin, 10 μg ml−1; tetracycline, 10 μg ml−1; kanamycin 20 μg ml−1; and phleomycin 0.2 μg ml−1.
TABLE 1.
Strains used in this study
| Species and strain | Relevant genotype or features | Reference |
|---|---|---|
| Staphylococcus aureus | ||
| COL | mec, high-Mcr clinical isolate | 55 |
| COL ΔsigB | deletion of rsbVW-sigB::erm | 38 |
| RN4220 | NCTC8325-4 r− m− | 37 |
| SG001 | RN4220/p3086 | This study |
| 8325-4 | NCTC8325, cured of known prophages, 11-bp deletion in rsbU | 38, 44 |
| GP269 | 8325-4 (rsbUVW-sigB)+::tetL | 24 |
| SG002 | 8325-4 rsbVW-sigB::erm | This study |
| SG003 | 8325-4 rsbVW-sig::erm/p3086 | This study |
| SG004 | 8325-4/pXylrsbU | This study |
| Bacillus subtilis | ||
| 168 | trpC2 | 39 |
| BSM151 | trpC2 SPβ ctc::lacZ erm cat-86 | 10 |
| BSM154 | trpC2 rsbU::kan SPβ ctc::lacZ erm cat-86 | 10 |
| BSGH00 | trpC2 rsbU::kan pDG148 SPβ ctc::lacZ erm cat-86 | 26 |
| BSGH01 | trpC2 rsbU::kan pJPF01 SPβ ctc::lacZ erm cat-86 | 26 |
| BSGH08 | trpC2 rsbU::kan pBJ11 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH09 | trpC2 rsbU::kan pBJ21 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH10 | trpC2 rsbU::kan pBJ31 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH11 | trpC2 rsbU::kan pBJ41 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH12 | trpC2 rsbU::kan pBJ51 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH13 | trpC2 rsbU::kan pBJ71 SPβ ctc::lacZ erm cat-86 | This study |
| BSGH14 | trpC2 rsbU::kan pBJ81 SPβ ctc::lacZ erm cat-86 | This study |
| Escherichia coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA69 | 52 |
| BL21(DE3)/pLysS | F−lon hsdSB(rB− mB−) with DE3, a λ prophage carrying T7 RNA polymerase gene and plasmid pLysS containing T7 phage lysozyme gene and Cmr | 57 |
| C41(DE3) | Derivative of BL21(DE3) that allow synthesis of some membrane proteins and globular proteins at high levels | 42 |
S. aureus strain SG002 was generated by phage transduction of the erythromycin-linked deletion of the sigB operon from strain COL ΔsigB into strain 8325-4 (6, 38). The insertion event was confirmed by PCR using oligonucleotides MuTestpemKfor and MuTesthyporev hybridizing outside the rsbVW-sigB region (Table 2) and by the restriction pattern of the resulting PCR fragment. Transformation of plasmids into different S. aureus strains was done by a first electroporation into the restriction-negative S. aureus host strain RN4220, from which the plasmids were isolated for a second electroporation into the desired background to generate strains SG001, SG003, and SG004.
TABLE 2.
Oligonucleotides used in this study
| PCR product use and oligonucleotide name | Oligonucleotide sequence (5′ → 3′a) | Chromosomal position(s)b |
|---|---|---|
| rsbU chimeras | ||
| rsbU_SauCOL_F | ATGGTAGGTCTCAAGCTTCGGTAGGAGGTAAGAACGTGGAAGAATTTAAGCAACA | BS_520563-520579, SA_2123865-2123846 |
| Fu1_NtSau_CtBsub_F | TCTAAGGGTTTGATGCTCCTGATAAGCCATACCAAAGCCTTTAACGATTTCTTGTAAGAC | BS_520852-520814, SA_2123632-2123661 |
| Fu1_NtBsub_CtSau_R | GATTTTTTAATAGAAGTCATGATTGGCTATGGTTATAGTTATCGAGATTATCAAAGATTG | BS_520790-520818, SA_2123635-2123605 |
| rsbU_SauCOL_R | ATGGTAGGTCTCGTCGACTTAATTTACTCTTTTTATAA | SA_2122864-2122883 |
| rsbU_Bsub_F | ATGGTAGGTCTCAAGCTTCGGTAGGAGGTAAGAACGTGGATTTTAGGGAGGTTAT | BS_520563-520599 |
| Fu1_NtBsub_CtSau_F | TACCAATCTTTGATAATCTCGATAACTATATCCATAGCCAATCATGACTTCTATTAAAAA | SA_2123602-2123631, BS_520822-520793 |
| Fu1_NtSau_CtBsub_R | GATGTCTTACAAGAAATCGTTAAAGGCTTTGGAATGGCTTATCAGGAGCATCAAACCCTT | SA_2123664-2123633, BS_520822-520849 |
| rsbU_Bsub_R | ATGGTAGGTCTCGTCGACTTAAACCTTTCTCCGCAAAACAA | BS_521587-521565 |
| Fu2_NtBsub_CtSau_F | AAAGTTCCTCAGGAGGAAGCGCTGGATATCGGCGTTATTTCAGTGGCGGCACAAAAAGTA | BS_520922-520953, SA_2123500-2123473 |
| Fu2_NtSau_CtBsub_R | GCTCATCTGTTTAGCGGGAACACTGATGGCGCCAATTTGAATACTATCAAATTGTGGAAT | BS_520984-520954, SA_2123501-2123529 |
| Fu2_NtSau_CtBsub_F | GATATTCCACAATTTGATAGTATTCAAATTGGCGCCATCAGTGTTCCCGCTAAACAGATG | SA_2123532-2123501, BS_520954-520981 |
| Fu2_NtBsub_CtSau_R | ACTTACTTTTTGTGCCGCCACTGAAATAACGCCGATATCCAGCGCTTCCTCCTGAGGAAC | SA_2123470-2123500, BS_520953-520925 |
| Protein overexpression | ||
| RsbUfor01 | GGAGGATCCGTGGAAGAATTTAAGCAACA | SA_2123865-2123846 |
| RsbUrev01 | CGGAATTCTTAATTTACTCTTTTTATAA | SA_2122864-2122883 |
| RsbVfor01 | GGAGGATCCATGAATCTTAATATAGAAAC | SA_2122744-2122725 |
| RsbVrev01 | CGGAATTCTTATTCGACCTCCGTTCCTT | SA_2122418-2122437 |
| RsbW_pPR_IBA1_for | ATGGTAGGTCTCAAATGCAATCTAAAGAAGATTTTATCGAAATG | SA_2122416-2122387 |
| RsbW_pPR_IBA1_rev | ATGGTAGGTCTCAGCGCTGCTGATTTCGACTCTTTCGCCAT | SA_2121940-2121962 |
| SigB_pPR_IBA1_for | ATGGTAGGTCTCAAATGGCGAAAGAGTCGAAATCAGCTAAT | SA_2121962-2121936 |
| SigB_pPR_IBA1_rev | ATGGTAGGTCTCAGCGCTTTGATGTGCTGCTTCTTGTAATTTC | SA_2121195-2121219 |
| SArsbV.fw | GGAATTCATATGAATCTTAATATAGAAACAACC | SA_2122744-2122725 |
| SArsbV.rev | CGGGATCCTATTCGACCTCCGTTCCTTC | SA_2122418-2122437 |
| Miscellaneous | ||
| MuTestpemKfor | GATTTATCACCAGTACAGGG | SA_2124547-2124528 |
| MuTesthyporev | GAATTAATCAATTGATTGTCC | SA_ 2120736-2120756 |
| p2085_sigBSA_f | GGGTTTAAACATGGCGAAAGAGTCGAAATC | SA_2121962-2121943 |
| pRB_sigB_SA_r | GCGAATTCCTATTTATGTGCTGCTTCTTG | SA_2121192-2121212 |
Restriction sites are underlined; boldface indicates a Shine-Dalgarno sequence.
The chromosomal positions corresponding to the oligonucleotide sequences for S. aureus COL (SA) and B. subtilis 168 (BS) are according to the NCBI comprehensive microbial genome database (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) and the SubtiList database (http://genolist.pasteur.fr/SubtiList/), respectively.
Transformants of naturally competent B. subtilis were selected on LB agar plates supplemented with phleomycin (5 μg ml−1).
Construction of plasmids.
Plasmid p3086 expressing the sigB gene under the control of a tetracycline-inducible promoter was generated by ligation of a PmeI/EcoRI-digested PCR fragment amplified from COL chromosomal DNA with primers p2085_sigBSA_f and pRB_sigB_SA_r into the PmeI/EcoRI sites of p2085, a derivative of pALC2084 (4). To generate plasmids expressing wild-type rsbU and chimeric versions thereof, the S. aureus and B. subtilis rsbU genes or fragments thereof were amplified by PCR using the primers listed in Table 2 with chromosomal DNA from either S. aureus COL or B. subtilis 168 as a template. Chimeric genes were generated by mixing the appropriate DNA fragments at a 1:1 molar ratio and flanking primers in a 100-fold excess in order to perform a fusion PCR. The wild-type and fused DNA fragments were digested with BsaI, generating HindIII- and SalI-compatible ends, and ligated into HindIII- and SalI-digested pDG148 to produce plasmids pBJ11, pBJ21, pBJ31, pBJ41, pBJ51, pBJ71, and pBJ81.
To construct Escherichia coli vectors expressing recombinant S. aureus Rsb proteins and SigB for antiserum production, appropriate DNA fragments were amplified with DNA from S. aureus COL and the primers listed in Table 2. The digested DNA fragments were ligated into pRSETA (His-RsbU and His-RsbV) or pPRIBA-1 (RsbW-Strep and SigB-Strep) to generate tagged versions of the proteins. All plasmids are listed in Table 3.
TABLE 3.
Plasmids used in this study
| Plasmid | Relevant features | Reference or source |
|---|---|---|
| pDG148 | bla kan pleo lacI Pspac | 56 |
| pJPF01 | bla kan pleo lacI Pspac::rsbUBS_1-335 | 26 |
| pBJ11 | bla kan pleo lacI Pspac::rsbUSA_1-333 | This study |
| pBJ21 | bla kan pleo lacI Pspac::rsbUBS_1-81,SA_79-333 | This study |
| pBJ31 | bla kan pleo lacI Pspac::rsbUSA_1-78,BS_82-335 | This study |
| pBJ41 | bla kan pleo lacI Pspac::rsbUSA_1-122,BS_126-335 | This study |
| pBJ51 | bla kan pleo lacI Pspac::rsbUBS_1-125,SA_123-333 | This study |
| pBJ71 | bla kan pleo lacI Pspac::rsbUSA_1-78,BS_82-125,SA_123-333 | This study |
| pBJ81 | bla kan pleo lacI Pspac::rsbUBS_1-335,SA79-122,BS_126-335 | This study |
| pALC2084 | pALC2073 with gfpuvr cloned into the EcoRI site | 4 |
| p2085 | Derivative of pALC2073 with modified restriction sites | D. Bauer and M. Fraunholz, unpublished |
| p3086 | Expressing the sigB gene under control of a tetracycline-inducible promoter | This study |
| pXylrsbU | pXyl derivative containing rsbU from COL under control of the xylose-inducible promoter Pxyl, Tcr | 54 |
| pRSETA | E. coli expression vector; His tag fusions; bla | Invitrogen |
| pRSETA-rsbU | Expressing S. aureus RsbU 1-335 as N-terminal His tag | This study |
| pRSETA-rsbV | Expressing S. aureus RsbV 1-112 as N-terminal His tag | This study |
| pPRIBA-1 | E. coli expression vector; bla | IBA |
| pPRIBA-rsbW | Expressing S. aureus RsbW 1-159 as C-terminal Strep tag | This study |
| pPRIBA-sigB | Expressing S. aureus SigB 1-256 as C-terminal Strep tag | This study |
| pET11a | E. coli expression vector; bla | Merck |
| pET11a-rsbV | Expressing S. aureus RsbV 1-112 | This study |
Production of RsbU-, RsbV-, RsbW-, and SigB-specific antisera.
For the overexpression of recombinant RsbU, RsbV, RsbW, or SigB, the respective vectors were transformed into E. coli BL21(DE3)/pLysS and a single colony was propagated in 1 liter LB medium until the culture reached an optical density at 540 nm (OD540) of 0.4. Synthesis of the proteins was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM). Cells were harvested 2 hours after induction. Purification of proteins was performed according to the recommendations of the manufacturer (His tag, Invitrogen, Germany; Strep tag, IBA, Göttingen, Germany). All proteins were purified under native conditions, with the exception of His-RsbU, which had to be solubilized by the addition of 8 M urea. Purified proteins were used for the immunization of rabbits (Pineda, Berlin, Germany). The anti-RsbV and anti-SigB sera were subjected to antigen-specific affinity purification.
Cloning, expression, purification, and phosphorylation of nontagged RsbV.
Genomic DNA from S. aureus COL was used as a template for the amplification of rsbV by PCR in preparation for ligation into the NdeI and BamHI sites of plasmid pET11a. Primers are listed in Table 2.
RsbV was overexpressed in E. coli (C41) and purified by a method similar to that for the purification of RsbV from B. subtilis (12). Cells were disrupted by sonication in 30 ml of lysis buffer [20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 0.1 mM 4-(2-aminoethyl) benzene sulfonyl fluoride] and centrifuged for 30 min at 15,000 rpm. The supernatant was then applied to a Q-Sepharose (GE Healthcare) column preequilibrated with lysis buffer, and the chromatogram was developed with an NaCl gradient from 0 to 500 mM. RsbV-containing fractions were concentrated by centrifugal filtration (Amicon) and then loaded onto a Superdex 75 gel filtration column (GE Healthcare) preequilibrated in lysis buffer supplemented with 200 mM NaCl. Fractions containing RsbV were assessed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and pure RsbV was stored at −80°C in gel filtration buffer supplemented with 10% glycerol.
RsbV-P (S. aureus) was obtained by incubating RsbV overnight at 30°C in a 20:1 molar ratio with B. subtilis RsbW (provided by R. J. Lewis) in a buffer of 20 mM Tris-HCl (pH 8.0) and 2 mM ATP. The extent of phosphorylation was monitored by nondenaturing PAGE, and following complete phosphorylation, the kinase RsbW was separated from RsbV by Superdex 75 (GE Healthcare) gel filtration chromatography. The site of the in vitro phosphorylation of RsbV was Ser-57, as determined by mass spectrometry (MS) using the procedures described below.
Two-dimensional protein electrophoresis, isoelectric focusing (IEF) slab gels, SDS-PAGE, and Western blot analysis.
S. aureus cells were harvested in ice-cold killing buffer (20 mM NaN3, 5 mM MgCl2, 20 mM Tris, pH 7.5), centrifuged (9,000 × g, 4°C, 5 min), washed in ice-cold killing buffer, resuspended in lysis buffer (50 mM NaF, 10 mM Tris, pH 8.0) and disrupted with a Ribolyser (Hybaid, Teddington, England). Briefly, 500 μl of glass beads (Sartorius Göttingen, Germany) with a diameter of 0.1 to 0.11 mm were mixed with 1 ml cell suspension in a 2-ml cell culture tube. Cells were mechanically disrupted by 30 s of shaking at a speed 6.5 ms−2. The lysis efficacy was determined to be 95%. In order to remove cell debris, the protein extracts were cleared by centrifugation at 21,000 × g for 5 min at 4°C, followed by a second centrifugation of the supernatant at 21,000 × g for 30 min at 4°C. The protein concentration was determined with the Roti-Nanoquant protein assay (Roth, Karlsruhe, Germany) according to the manufacturer's instructions. Protein extracts were stored at −20°C.
For the analysis of the B. subtilis insoluble cytoplasmic protein fraction, 1.5 ml of cell culture was harvested at various time points by centrifugation at 21,000 × g for 1 min at 4°C. The cell pellet was resuspended in TE (10 mM Tris, 1 mM EDTA, pH 7.5), where the volume of TE was calculated according to the following formula: OD500 at the sampling points × 100/2 = μl TE. Lysozyme was then added to a final concentration of 0.1 μg/μl, and the sample was incubated for 10 min at 37°C. After incubation, the lysate was mixed with an equal amount of SDS-PAGE loading buffer and incubated for 10 min at 95°C, and 20 μl was subjected to electrophoresis. The respective soluble cytoplasmic protein fractions were prepared by ultrasonic treatment of the cells followed by centrifugation (21,000 × g for 15 min at 4°C) in order to remove cell debris prior to the addition of SDS-PAGE loading buffer to the cleared supernatant. Protein extracts were stored at −20°C.
The two-dimensional electrophoresis of protein extracts (400 μg protein) was carried out using the immobilized pH gradient (IPG) technique with IPG strips (GE-Healthcare, Little Chalfont, United Kingdom) in a pH range of 3.5 to 4.5 in the first dimension and SDS-PAGE with 15% acrylamide in the second dimension as described previously (7). The gels were first stained with Diamond Pro-Q (Invitrogen, Germany) according to the manufacturer's instructions in order to detect potentially phosphorylated proteins. After scanning of the Diamond Pro-Q-stained gels, total protein was visualized by Coomassie blue staining (11).
IEF slab gels were prepared in a Mini-Protean cell (Bio-Rad, Munich, Germany), with the lower chamber containing 10 mM phosphoric acid as that anolyte and the upper chamber containing 20 mM sodium hydroxide as the catolyte. Fifteen-microliter samples of cell extract adjusted for equal amounts of protein were mixed with 15 μl loading buffer (8 M urea, 2.6% [vol/vol] Pharmalyte [pH range 3.5 to 5.0; GE-Healthcare, Little Chalfont, United Kingdom], 2% [vol/vol] Triton X-100, 1% [vol/vol] β-mercaptoethanol, and 0.04% [wt/vol] bromphenol blue) and loaded onto a 5% acrylamide IEF slab gel (30% acrylamide-bisacrylamide [29:1] stock solution [AppliChem, Darmstadt, Germany], 8 M urea, 4% [vol/vol] Pharmalyte [pH range 3.5 to 5.0; GE-Healthcare, Little Chalfont, United Kingdom], 0.004% [wt/vol] ammonium persulfate, and 0.02% [vol/vol] N,N,N′,N′,-tetramethylethylenediamine [TEMED]). The gel was then run at 200 V for the first 30 min and at 300 V for another 2.5 h. The analysis of the phosphorylation pattern of RsbV in B. subtilis following ethanol stress reproduced the pattern of RsbV and RsbV-P accumulation reported by Völker and colleagues (63), leading us to assume that our harvesting and IEF protocols are suitable to investigate the RsbV phosphorylation in S. aureus (data not shown).
One-dimensional SDS-PAGE was performed using 15% acrylamide gels for the analysis of RsbV and RsbW and 12.5% acrylamide gels for the analysis of RsbU and SigB according to standard procedures.
For Western blot analyses of IEF slab gels and SDS-polyacrylamide gels, the separated proteins were transferred to polyvinylidene difluoride membranes (Roth, Karlsruhe, Germany). The membranes were exposed overnight at room temperature to the primary antibody in TBS (150 mM NaCl, 50 mM Tris, pH 7.6) supplemented with 2.5% (wt/vol) nonfat dry milk and 0.05% (vol/vol) Tween 20, washed three times with TBS, and then incubated with alkaline phosphatase-conjugated secondary anti-rabbit immunoglobulin G antibody (Sigma, Germany) for 1 hour. Finally, the blots were washed and equilibrated in CDP* buffer (0.1 M diethanolamine, pH 9.5), and bound antibody was visualized using CDP* as substrate according to the instructions of the manufacturer (Perkin-Elmer, Germany), with image capture and analyses using a LumiImager and the LumiAnalyst software package (Boehringer, Mannheim, Germany).
Northern blot analysis.
For Northern blot analysis, cells were harvested as described above. Extraction, blotting, hybridization of RNA, and detection of bound probes were performed as previously described (21). Antisense RNA probes were synthesized by in vitro transcription with T7 RNA polymerase using PCR products with the T7 RNA polymerase binding site generated with primers listed in Table 3 and chromosomal DNA from S. aureus COL as the template according to the method of Gertz et al. (23).
Determination of β-galactosidase activity.
To determine the β-galactosidase activity of the ctc::lacZ transcriptional reporter gene fusion in B. subtilis, and hence infer SigB activity, 1-ml aliquots of cell cultures were harvested by centrifugation (21,000 × g, 1 min) at various time points and assayed for β-galactosidase enzyme activity as described previously (41, 61).
Identification of the phosphorylation site of RsbV.
The in-gel digestion of RsbV after separation by two-dimensional PAGE was performed according to the protocol of Eymann et al. (20). A second digestion step with endoproteinase Asp-N (Sigma, Germany) was conducted according to the instructions of the manufacturer. The phosphorylation site was identified by liquid chromatography-tandem MS analysis using a Q Trap 4000 instrument (Applied Biosystems MDS Sciex) in conjunction with a Dionex high-pressure liquid chromatography system (LC Packings).
For reversed-phase separation, the peptides were loaded onto a trap column (nano Precolumn, PepMap, C18, 300 μm [inner diameter] by 5 mm; LC Packings) and were eluted onto an analytical column (PepMap, C18, 75 μm [inner diameter] by 15 cm; LC Packings) with a binary gradient (80 or 160 min) of buffer A (0.1% [vol/vol] acetic acid) and buffer B (90% [vol/vol] acetonitrile, 0.1% [vol/vol] acetic acid) at a flow rate of 200 nl/min.
The Q Trap 4000 was used with the NanoSray II source, including the MicroIonSpray II head with a “T” inside. A mix of 80% (vol/vol) isopropanol, 10% (vol/vol) acetonitrile, and 0.1% (vol/vol) acetic acid used in the negative mode was injected via the “T” at a flow rate of 100 nl/min. Precursor ion scan for m/z 79 (PO3−) in negative mode was performed with a tandem MS experiment in positive-ion mode. The data were analyzed manually using the Analyst and Bioanalyst software for the Q-Trap 4000.
RESULTS
S. aureus RsbV is phosphorylated at Ser-57.
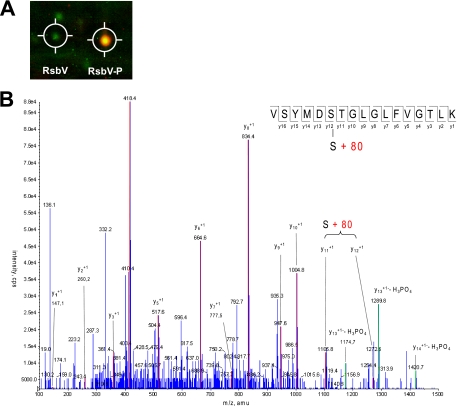
In order to determine the site of RsbV phosphorylation, the soluble cytoplasmic protein fraction from exponentially growing S. aureus COL cells was isolated and separated by two-dimensional gel electrophoresis. To identify potentially phosphorylated proteins, the gels were stained with Pro-Q, a reagent that has a preference for phosphoproteins. Protein accumulation was subsequently visualized by Coomassie blue staining. This staining procedure in combination with matrix-assisted laser desorption ionization-time-of-flight MS identified two protein spots as RsbV, of which only one was stained by Pro-Q, indicating potential phosphorylation. In agreement with this notion, the Pro-Q-stained RsbV spot migrated with a lower pI in the gel than did the RsbV spot, which showed only Coomassie blue and no Pro-Q staining (Fig. 2A). The Pro-Q-stained RsbV spot was subjected to further analysis by MS, and a phosphorylation modification was identified at serine residue 57 (Fig. 2B). Ser-57 is equivalent to Ser-56 in B. subtilis RsbV, the site of phosphorylation of this protein (20). The proteins share 42% sequence identity.
FIG. 2.
Analysis of the site of RsbV phosphorylation. (A) False-color image of a section of a two-dimensional SDS gel showing RsbV and phosphorylated RsbV. The gel image showing protein accumulation (Coomassie blue staining) is presented in green, and the gel image showing phosphorylated proteins (Diamond Pro-Q staining) is presented in red. An overlay of both colors results in a yellow staining. (B) MS identification of the RsbV phosphorylation site. The tryptic peptide containing amino acid residues Val (V) 52 to Lys (K) 68 was modified by the phosphorylation-specific mass of 80 Da. Mass peaks that match the expected bound y-ions are highlighted.
Analysis of RsbV phosphorylation following alkaline stress.
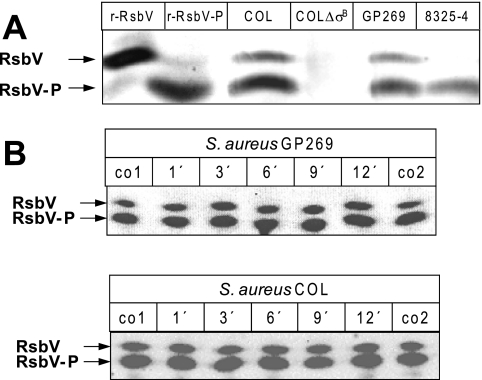
To investigate whether in S. aureus, similar to B. subtilis, stress that leads to an RsbU-dependent increase of SigB-dependent transcription is accompanied by RsbV-P accumulation and the appearance of unphosphorylated RsbV (63), we used one-dimensional IEF gels in combination with Western blotting to analyze the RsbV/RsbV-P ratio. As depicted in Fig. 3A, two RsbV-specific bands were visible when we analyzed extracts of the wild-type strain COL that were absent in the isogenic strain lacking RsbV (COL ΔsigB). A strain deficient in RsbU (8325-4) showed only the band closer to the anode. When complemented with RsbU (GP269), the band at the cathode reappeared. From these experiments we assumed that the band running closer to the anode corresponds to phosphorylated RsbV, whereas the band closer to the cathode corresponds to unphosphorylated RsbV. This conclusion is further supported by the fact that recombinant phospho-RsbV comigrated with the anode band, whereas unphosphorylated RsbV comigrated with the cathode band (Fig. 3A).
FIG. 3.
Analysis of RsbV phosphorylation following alkaline stress. (A) IEF of cytoplasmic protein extracts followed by Western blotting probed with a polyclonal anti-RsbV serum identified both forms of RsbV as indicated by arrows. Twenty micrograms of protein extracts isolated from exponentially growing S. aureus cells was separated per lane. The strains used were COL (wild type), COL ΔsigB (strain with deletion of the rsbV-rsbW-sigB operon), 8325-4 (RsbU-negative strain) and GP269 (chromosomally rsbU-complemented derivative of 8325-4). In addition, recombinant RsbV and in vitro-phosphorylated RsbV were run on the same gel (r-RsbV and r-RsbV-P, respectively). RsbV isoforms were detected with a polyclonal anti-RsbV serum. (B) For the analysis of RsbV phosphorylation following stress, S. aureus was grown to an OD540 of 0.7. At this time point, the culture was split, and one part was exposed to 30 mM potassium hydroxide whereas the other part served as an unstressed control. Samples were taken at various time points after stress. A control sample was harvested from the unstressed culture immediately before (co1) and 12 min after (co2) the beginning of the stress experiment. Twenty micrograms of protein extracts was separated per lane by IEF followed by Western blotting. RsbV isoforms were detected with a polyclonal anti-RsbV serum. All experiments were performed in triplicate.
In a next step we analyzed whether alkaline stress had an impact on the RsbV/RsbV-P ratio. Upon exposure to an alkaline shock, transcription of sigB and several SigB-dependent genes increases within 10 min. It has previously been shown that induction of the solely SigB-dependent gene csb7 (SACOL2484) requires a functional RsbU protein, suggesting that dephosphorylation of RsbV-P is necessary to activate SigB-dependent transcription in response to alkaline stress (46). However, unphosphorylated RsbV was already detectable during exponential growth in the absence of stress. Furthermore, neither the relative levels nor the RsbV/RsbV-P ratio markedly changed during the course of the experiment (Fig. 3B). Quantitation of the band intensities revealed maximum changes of 1.5-fold, which was not considered significant.
Role of RsbU in SigB activation and RsbV dephosphorylation.
Several studies have shown a severe defect in SigB activity in S. aureus in the absence of RsbU (24, 30, 45). This defect in SigB-dependent transcription is likely due to the fact that in the absence of RsbV-P-specific phosphatase activity, unphosphorylated RsbV, the alternative binding partner of RsbW, is not present in the cell, and thus all SigB remains bound and inactivated by the anti-sigma factor RsbW.
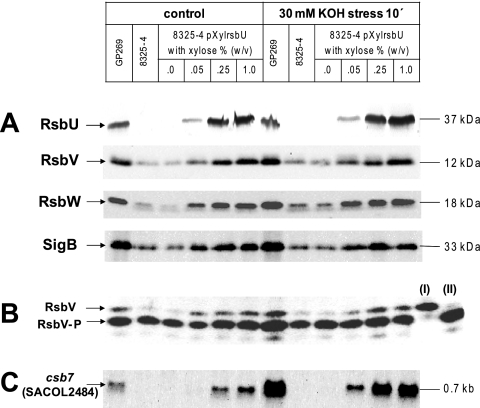
In order to test this hypothesis and further elucidate the role of RsbU in SigB activation, we analyzed the accumulation of SigB and its regulators RsbV and RsbW (Fig. 4A) and the impact of the relative level of RsbU on the RsbV phosphorylation status (Fig. 4B). In addition, as a measure of SigB activity, we assayed the transcription of the SigB-dependent gene csb7 (SACOL2484) (Fig. 4C). These experiments were done in the presence and absence of alkaline stress to distinguish between effects related to the intracellular RsbU level and stress induction. For these experiments we used derivatives of the RsbU-deficient strain 8325-4, including a strain complemented in cis with the S. aureus COL rsbU gene (GP269) and strain SG004, which carries a plasmid expressing the S. aureus COL rsbU gene under the control of a xylose-inducible promoter.
FIG. 4.
Influence of RsbU on the amounts of RsbV, RsbW, and SigB and on SigB-dependent transcription of csb7. The different S. aureus strains were grown in LB medium. If appropriate, the medium was supplemented with xylose at the indicated concentrations to induce expression of the plasmid-encoded rsbU. An unstressed control sample was taken when the culture reached an OD540 of 0.7, and the remaining culture was exposed to an alkaline shock for 10 min by the addition of potassium hydroxide to a final concentration of 30 mM. (A) For SDS-PAGE followed by Western blot analysis, 20 μg of protein extracts was separated per lane and probed with polyclonal serum specific for RsbU, RsbV, RsbW, or SigB. Molecular masses are indicated as estimated from the PageRuler prestained protein ladder (Fermentas, St. Leon-Rot, Germany) (B) IEF experiments were performed with 20 μg of protein extracts, and the blotted gels were probed with a polyclonal anti-RsbV serum. Recombinant RsbV (I) and RsbV-P (II) were run in parallel, serving as a control to monitor proper separation of the RsbV isoforms. (C) For Northern blot analysis of csb7 (SACOL2484) transcription, 10 μg of total RNA was separated per lane and the membrane was probed with a digoxigenin-labeled csb7 (SACOL2484)-specific RNA probe. All experiments were performed in triplicate.
Interestingly, we found a direct positive correlation between the level of RsbU and SigB activity even in the absence of stress. Elevating the amount of RsbU inside the cell, by increasing the concentration of xylose in the growth medium, was sufficient to (i) induce and (ii) gradually increase the level of csb7 (SACOL2484) transcription. For instance, transcription of csb7 (SACOL2484) was not detectable in strain 8325-4 (lacking RsbU) and in strain 8325-4/pXylrsbU in the absence of xylose. Furthermore, very low xylose concentrations (0.05%, wt/vol) and consequently RsbU concentrations did not support csb7 transcription. However, when grown in the presence of increasing concentrations of xylose (0.25% and 1.0%, wt/vol), strain 8325-4/pXylrsbU produced increasing amounts of RsbU followed by increased csb7 (SACOL2484) transcription (Fig. 4A and C).
A direct correlation between the amount of RsbU and the accumulation of RsbV, RsbV-P, RsbW, and SigB in the absence of stress was also evident. A low expression level of RsbV, RsbW, and SigB was, however, also detectable in the absence of RsbU. Transcription of the respective genes is not solely SigB dependent but is mediated by a SigA-dependent promoter upstream of SACOL2059 which likely contributes to a basal expression of RsbU, SigB, and its regulators RsbV and RsbW (54). However, although increasing the level of RsbU also increased the total RsbV level, the RsbV/RsbV-P ratio remained unchanged (Fig. 4B). Summarizing these results, we found that even in the absence of stress the expression of RsbU is sufficient to (i) generate unphosphorylated RsbV and (ii) allow SigB-dependent transcription. Additionally, we could show that an increase in the amount of RsbU is correlated with increased SigB activity but apparently does not affect the RsbV/RsbV-P ratio. Quantitation of the RsbV and RsbV-P signals showed an approximately constant RsbV/RsbV-P ratio of 0.22 when RsbU was present (results not shown).
Next, we asked how stress, induced by a 10-min alkaline shock, would affect SigB activity in addition to increasing RsbU concentrations. Although imposition of alkaline shock did not alter the accumulation pattern of RsbV, RsbW, and SigB, a strong increase in csb7 (SACOL2484) transcription was detectable in strains expressing RsbU. This induction significantly exceeded the increase of csb7 (SACOL2484) transcription which accompanied the increase of RsbU in unstressed cells (Fig. 4C). For instance, the small amount of RsbU in the strain grown in the presence of 0.05% (wt/vol) xylose, which did not lead to detectable csb7 (SACOL2484) transcription in the absence of stress, was sufficient to support stress-dependent induction of csb7 (SACOL2484). However, as seen in the experiments described above, stress did not markedly change the RsbV/RsbV-P ratio. Only in cells expressing high RsbU levels (0.25 and 1.0% xylose) could a slight increase in the RsbV/RsbV-P ratio, up to 0.35, be detected. However, this change in RsbV/RsbV-P ratio was not significant to affect the signaling pathway (Fig. 4B and results not shown).
Analysis of SigB-dependent transcription of csb7 (SACOL2484) in the absence of the “partner-switching” proteins RsbW and RsbV and the RsbU phosphatase.
The results presented above revealed that in contrast to the case in B. subtilis, stress that activates SigB-dependent transcription is not accompanied by a strong accumulation of RsbV, RsbW, and SigB in S. aureus. Nevertheless, we could show that RsbU is required for RsbV-P dephosphorylation and that increasing amounts of RsbU are followed by increasing SigB activity. However, even in the absence of any obvious changes in the accumulation pattern of RsbV and RsbV-P when comparing stress and nonstress conditions, SigB-dependent transcription of csb7 was strongly inducible by an alkaline shock. Taken together, these observations raise the possibility that the stress-triggered increase of SigB-dependent transcription might be mostly independent from an accumulation of free SigB triggered by increased RsbV-P dephosphorylation. Such mechanisms would require that at least a fraction of SigB remains in an unbound state in the absence of stress. In line with this notion is the observation that significant transcription from several SigB-dependent promoters already occurs during exponential growth (8, 46). The stress-dependent increase in SigB-dependent transcription could thus rather rely on mechanisms influencing transcription initiation (e.g., by additional alkali-responsive regulators) or competitiveness for core RNA polymerase.
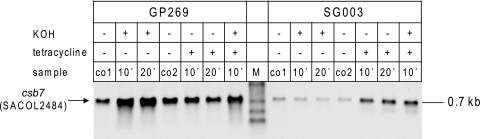
To test this hypothesis, we generated an 8325-4 derivative strain devoid of the chromosomal copies of rsbU, rsbV, rsbW, and sigB and the autoregulation at the SigB promoter in front of rsbV. This strain was transformed with a plasmid which allows expression of SigB under the control of a tetracycline-inducible promoter (S. aureus SG003) (Fig. 5). If the stress-dependent increase in SigB activity does not actually rely on the accumulation of free SigB, expression of SigB in the absence of its regulators RsbV, RsbW, and RsbU should be sufficient to facilitate stress induction of SigB-dependent transcription. To monitor SigB-dependent transcription in this system, we again analyzed the transcription of the SigB-dependent gene csb7 (SACOL2484) by Northern blotting.
FIG. 5.
Analysis of csb7 (SACOL2484) transcription in the absence of RsbU, RsbV, and RsbW. S. aureus GP269 expresses chromosomally encoded SigB and the whole set of Rsb proteins (RsbU, RsbV, and RsbW). SG003 is an RsbU-, RsbV-, RsbW-, and SigB-deficient strain in the 8325-4 genetic background transformed with a plasmid expressing sigB under the control of a tetracycline-inducible promoter. The different S. aureus strains were grown in LB medium. At an OD540 of 0.7, the culture was split into three parts. The first part served as an unstressed control and was sampled at the beginning of the experiment (co1) and 20 min later (co2). The second part of the culture was stressed with potassium hydroxide (30 mM final concentration), and samples were taken 10 and 20 min after stress exposure. The third part was supplemented with tetracycline (50 ng ml−1) to induce expression of the plasmid-encoded SigB. Samples from this culture were harvested at 10 and 20 min. In addition, at 10 min after tetracycline exposure one part of the tetracycline-treated culture was transferred to a new Erlenmeyer flask and stressed with potassium hydroxide (30 mM final concentration) for another 10 min. For Northern blot analyses of csb7 (SACOL2484) transcription, 10 μg of total RNA was blotted per lane and the membrane was probed with a digoxigenin-labeled csb7 (SACOL2484)-specific RNA probe. All experiments were performed in triplicate.
Transcription of csb7 (SACOL2484) occurred even in the absence of the inducer tetracycline, indicating some leakage of the promoter. Addition of tetracycline, however, lead to an increased SigB accumulation (data not shown) and consequently csb7 (SACOL2484) transcription (Fig. 5). Interestingly, addition of potassium hydroxide to the cell culture in order to provoke alkaline stress did not trigger an increase in csb7 (SACOL2484) transcription in cells expressing solely SigB. The stress-dependent induction, however, was evident in GP269 expressing the whole set of SigB regulators. This induction was not affected by the tetracycline concentrations used to induce expression of the plasmid-encoded SigB (Fig. 5).
Analysis of RsbU and RsbU chimeras phosphatase activity.
The observation that expression of SigB alone is not sufficient to support alkaline stress induction of SigB-dependent csb7 transcription points to the importance of the partner-switching components and RsbU in the regulation of SigB activity.
Several attempts to express active recombinant S. aureus RsbU in E. coli have remained unsuccessful thus far (unpublished results). Therefore, in order to analyze the phosphatase activity of the staphylococcal RsbU and to compare it with its B. subtilis counterpart we used a B. subtilis expression system. To this end, we introduced a plasmid expressing the S. aureus RsbU protein in a B. subtilis strain carrying a deletion of its own rsbU gene and a lacZ transcriptional reporter gene fusion under the control of the SigB-dependent ctc promoter (50).
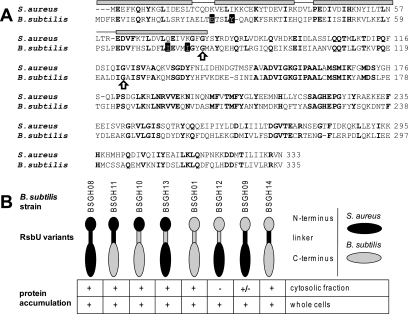
In addition to the plasmid expressing the S. aureus RsbU, we also generated vectors encoding chimeric versions of the S. aureus and B. subtilis RsbU proteins to obtain insight into the activation of the S. aureus RsbU protein. For B. subtilis it is well established that the stimulation of the C-terminal phosphatase domain requires interaction of the N-terminal regulatory domain with the RsbU-positive regulator RsbT (15, 26). Both domains are connected through a trypsin-sensitive linker of approximately 25 amino acids (19). This architecture served as a template for the design of the RsbU chimeras tested in this study. In total, six different RsbU chimeras were created, representing all possible combinations of the N termini, the C termini, and the linker region (Fig. 6). A plasmid expressing the B. subtilis RsbU protein served as a control to evaluate the functionality of the expression system.
FIG. 6.
RsbU chimeras. (A) Alignment of S. aureus and B. subtilis RsbUs. Secondary structural elements as determined for the B. subtilis RsbU N-terminal domain are shown above the sequences, with α-helices represented as gray bars. Invariant amino acids are shown in bold, and those important for RsbU-RsbT interaction in B. subtilis are shaded black. The conserved glycine residues assumed as domain boundaries for the construction of chimeric proteins are indicated by arrows. (B) Accumulation pattern of RsbU chimeras in B. subtilis as detected by Western blot analysis 120 min after induction of exponentially growing cells (OD500 = 0.3) with 1 mM IPTG. A detectable product is represented by “+” an undetectable product by “−,” and weak accumulation by “+/−.” The different RsbU proteins are schematically shown as cartoons, with the respective B. subtilis strains expressing the RsbU variants indicated.
To assess the inherent stability of the six RsbU chimeras and the two wild-type RsbU proteins from S. aureus and B. subtilis expressed in B. subtilis, we performed Western blot analyses of whole-cell extracts and the soluble cytoplasmic protein fraction. These analyses revealed that, when expressed from the plasmids, all RsbU variants accumulated to comparable amounts, with two exceptions. First, RsbUBS_1-125,SA_123-333, the chimera composed of the B. subtilis N terminus and linker region and the S. aureus phosphatase domain, although detectable in whole-cell extracts, was not present in the cytosolic protein fraction, indicating that the protein was insoluble and presumably not folded correctly (BSGH12). Second, RsbUBS_1-81,SA_79-333, the chimera with the B. subtilis N terminus and the S. aureus linker region and phosphatase domain (BSGH09), although detectable in both protein fractions, accumulated at a significantly lower level in the cytosolic protein fraction, again indicating decreased solubility of the protein (Fig. 6B). Despite the obviously reduced solubility of the two chimeras described above, all RsbU variants were analyzed for their activity in B. subtilis.
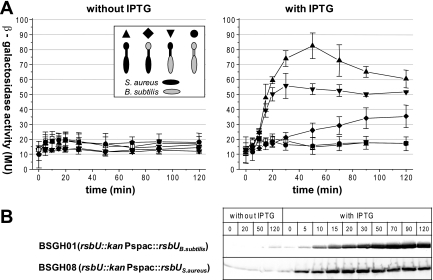
First we analyzed the stress responsiveness of B. subtilis complemented in trans with its own rsbU gene to verify the functionality of the expression system (BSGH01). When challenged with ethanol, a strong inducer of the RsbU-dependent environmental stress pathway in B. subtilis, the complemented B. subtilis strain displayed a SigB activity comparable to that of the wild-type strain (data not shown). However, when complemented with the rsbU gene from S. aureus and grown in the presence of IPTG in order to induce expression of the plasmid-encoded rsbU gene, B. subtilis displayed a severe growth defect (data not shown). Therefore, to circumvent this effect, we first cultivated the complemented strain in the absence of IPTG. After the cell culture reached an OD500 of 0.3 (exponential growth phase), IPTG was added and samples were removed to assay the SigB-dependent β-galactosidase activity.
Shortly after the addition of IPTG to the strain complemented with the rsbU gene from S. aureus, we measured a strong increase in β-galactosidase activity, which reached a maximum after 30 to 50 min (BSGH08, Fig. 7A). The kinetics of the β-galactosidase activity correlated well with the accumulation of the staphylococcal RsbU (Fig. 7B). In contrast, no induction of SigB activity could be observed during the course of the experiment when the strain was grown in the absence of IPTG (Fig. 7A). Induction of β-galactosidase activity could not be observed in the presence or absence of IPTG when B. subtilis was complemented with its own rsbU gene, confirming earlier results (15). These results indicated that when expressed in B. subtilis, the S. aureus RsbU appears to display an inherently high phosphatase activity compared to its B. subtilis counterpart. Indeed, the level of β-galactosidase activity seen in the B. subtilis strain complemented with the S. aureus rsbU gene was comparable to the level in a B. subtilis wild-type strain when challenged with 4% ethanol (data not shown).
FIG. 7.
Effect of the different RsbU chimeras on SigB activity in B. subtilis. (A) B. subtilis strains transformed with plasmids expressing variants of RsbU were grown in a synthetic medium. When the cell culture reached an OD500 of 0.3, the culture was split in two. Expression of the plasmid-encoded RsbU variants was induced by the addition of IPTG (1 mM final concentration) to one part of the culture, whereas the other, serving as a control, was grown in the absence of IPTG. Samples were taken at various time points and analyzed for β-galactosidase activity. For clarity, only the controls and chimeras displaying increased β-galactosidase activity upon IPTG addition were included in the figure. The strains shown in both panels are as follows: □, BSM154 (rsbU::kan); •, BSGH01 (rsbU::kan, Pspac::rsbU BS_1-335); ▴ BSGH08 (rsbU::kan, Pspac::rsbU SA_1-333); ⧫, BSGH09 (rsbU::kan, Pspac::rsbU BS_1-81,SA_79-333); ▾, BSGH11 (rsbU::kan, Pspac::rsbU SA_1-122,BS_126-335). Shown are means and standard deviations from three independent experiments. (B) Western blot analysis of full-length S. aureus and B. subtilis RsbU expression following addition of IPTG.
The analyses of the different RsbU chimeras highlighted two constructs with an elevated β-galactosidase activity. RsbUSA_1-122,BS_126-335, the RsbU chimera composed of the S. aureus N-terminal domain and linker region connected to the B. subtilis C-terminal phosphatase domain, displayed a β-galactosidase activity reaching up to 70% of that of the full-length S. aureus RsbU (BSGH11). Interestingly, RsbUBS_1-81,SA_79-333, the chimera with the B. subtilis N terminus and the S. aureus linker and phosphatase domain, despite its obvious defect in accumulation, also led to an increased β-galactosidase activity during the course of the experiment (BSGH09) (Fig. 7A). Finally, in addition to the analyses of the inherent activities of the different RsbU phosphatases and the respective chimeras, we also investigated whether they would support environmental stress induction, triggered by the addition of ethanol, in B. subtilis. These experiments revealed that the B. subtilis wild-type RsbU was the only RsbU protein tested in this study that allowed ethanol-dependent induction of SigB activity (data not shown).
DISCUSSION
The obvious differences in the repertoire of regulators of SigB activity between S. aureus and B. subtilis suggest different control mechanisms of the activity of this alternative sigma factor in these species. Most strikingly, the components of the stressosome, RsbR, RsbS, and RsbT, are absent from the S. aureus genome. The RsbU phosphatase, however, which is the regulatory target of the stressosome in B. subtilis, is conserved in both species.
In this study, we analyzed the activity of the S. aureus RsbU protein using a heterologous B. subtilis expression system. By expressing the S. aureus RsbU protein and chimeric versions thereof in an rsbU-deficient B. subtilis strain carrying a SigB-dependent reporter gene fusion, we were able to compare the activities of the S. aureus and B. subtilis RsbU proteins and to gain a first insight into the possible significance of the differences in sequence conservation between the two RsbU proteins in the two species. When expressed in B. subtilis the S. aureus RsbU displayed a high inherent activity leading to immediate SigB activation. Interestingly, a fusion of the staphylococcal N terminus and linker region to the B. subtilis RsbU PP2C domain also produced a highly active protein. Furthermore, albeit to a much lesser extent, the chimera with the B. subtilis N terminus connected to the S. aureus linker and PP2C domain also displayed some degree of activity. However, when we introduced only the S. aureus linker region into the B. subtilis RsbU, the protein remained inactive, suggesting that the linker region alone was not sufficient to transfer the high activity of the S. aureus RsbU to the B. subtilis protein. Interestingly, this construct also failed to transmit signals of environmental stress in B. subtilis (data not shown), suggesting that in B. subtilis the linker region may also play an important role in the signal transmission process. These observations suggest that the S. aureus RsbU protein is highly active even in the absence of any stimulation and that the linker region may at least in part account for this high activity. In the absence of a high-resolution structure of full-length RsbU, we cannot exclude that the chimeric RsbU proteins have adopted structures that alter the activity of the proteins in a way that leads to an artificially high or low activity. The results shown here represent only a starting point for more detailed analyses including mutations of individual amino acids in the linker region to unravel its mechanistic role in the signaling process.
The postulated high basal activity of the S. aureus RsbU is supported by the finding reported here that complementation of an RsbU-deficient S. aureus strain with its own RsbU protein is sufficient to activate SigB-dependent transcription in a dose-dependent manner and, most importantly, in the absence of a SigB inducing stimulus. Similar results have been reported by Senn and colleagues (54). A possible explanation for this observation would be that a constitutively active RsbU sets a basal level of SigB activity. Thus, even if the RsbV/RsbV-P ratio remains constant, the level of free (active) SigB may well increase in parallel with a rise in RsbU concentration.
However, we were able to show that the SigB-dependent transcription caused by simply expressing RsbU can be further increased by a stress such as alkaline shock. A prediction from the model of SigB regulation in B. subtilis is that a stress-dependent increase of RsbU phosphatase activity in S. aureus should trigger a positive feedback loop that provokes the appearance of the unphosphorylated form of RsbV and an accumulation of total RsbV, RsbW, and SigB. Surprisingly, despite a strong induction of the 1.6-kb sigB transcript following alkaline stress (46), no significant accumulation of total RsbV, RsbW, or SigB was observed following alkaline stress in S. aureus. These findings stand in contrast to what has been reported for B. subtilis, where stresses that activate SigB lead not only to a shift in the RsbV/RsbV-P ratio and a strong induction at the internal SigB promoter in front of the rsbV-rsbW-sigB-rsbX transcript (5, 9) but also to a rapid accumulation of the encoded proteins (5, 9, 18). For S. aureus, a discrepancy between the increased transcription of the sigB operon and the accumulation of SigB has also been observed in experiments monitoring the growth phase-dependent expression of this alternative sigma factor, suggesting posttranscriptional control of rsbV-rsbW-sigB expression upon entry into stationary phase or alkaline stress (54).
Taken together, these data imply that compared to that in B. subtilis, the positive feedback in S. aureus appears to be less pronounced as demonstrated by the lack of RsbV, RsbW, and SigB accumulation following alkaline stress or entry into stationary phase.
In this context a recent study by Igoshin et al., who investigated the system properties of the SigB regulation signaling network of B. subtilis using mathematical models, is of interest (31). These authors explored how alterations in the network architecture, such as, for example, the lack of positive feedback regulation, will affect the system properties. In their model, in the absence of a positive feedback, a higher prestress level of free SigB was necessary in order to get maximum stress induction comparable to that in the reference system with intact positive feedback circuits. Thus, S. aureus cells may exhibit a relatively high prestress level of free SigB able to form a transcriptionally competent complex with core RNA polymerase. Consistent with this hypothesis is the considerable amount of nonphosphorylated RsbV present in nonstressed S. aureus cells.
Although RsbU is necessary to generate nonphosphorylated RsbV, a prerequisite for significant SigB-dependent transcription in S. aureus, we did not observe any striking difference in the ratio of RsbV to RsbV-P between stressed and unstressed cells. This surprising result suggests, as was already proposed by Senn and colleagues (54), that further regulatory elements may be present in S. aureus. For example, RsbW or SigB itself could be subject to RsbU-independent posttranslational control by yet-to-be discovered mechanisms.
In addition, further, more indirect regulatory processes may account for the stress induction of transcription at SigB-dependent promoters. These processes may influence the transcription cycle or the ability of SigB to compete with SigA for limited core RNA polymerase and may not necessarily require that the phosphatase activity of RsbU increases in response to stress. For B. subtilis it has been suggested from analyses of the in vivo levels of SigA, SigB, and core RNA polymerase in combination with in vitro measurements of the affinity of both sigma factors for the core polymerase that the accumulation of free SigB following stress may not be sufficient to displace SigA and induce SigB-dependent transcription, thus requiring additional mechanisms of regulation (51). However, when an S. aureus strain expressing solely SigB and not RsbU, RsbV, or RsbW was challenged with an alkaline shock, we found no induction of SigB-dependent transcription, pointing to the important role of the partner-switching module and RsbU in the perception and transmission of signals of alkaline stress.
We cannot yet totally exclude the possibility that subtle changes in the RsbV/RsbV-P ratio and accumulation of SigB and its regulators RsbV and RsbW, which may escape detection by our IEF-based and Western blot assays, are sufficient to account for the observed increase of SigB activity following alkaline stress. Since we observed a high activity of the S. aureus RsbU protein when expressed in a B. subtilis background, control of the phosphatase activity may involve an as-yet-unidentified negative regulator in S. aureus.
In summary, our result supports a model in which RsbU and the components of the partner-switching module are crucial for the control of SigB activity in S. aureus by influencing its availability for core polymerase interactions. However, in the absence of the RsbU activity-controlling stressosome complex, the S. aureus RsbU may have evolved a high intrinsic activity that may not be subjected to a tight regulation, and as a consequence many SigB-dependent genes are expressed throughout growth. The partner-switching module may have been retained during evolution to provide a buffer that limits SigB activity, which would be deleterious to the cell if not restricted. It remains to be clarified under which conditions high SigB activity is beneficial to the cell and how such an increase in activity, e.g., during entry into stationary phase or alkaline stress, is controlled at the molecular level.
Acknowledgments
This work was supported by the SFB/TR34 (DFG), the PathoGenoMik-Plus network (BMBF), and EU (Staphdynamics).
U. Völker and W. G. Haldenwang are acknowledged for strains and the B. subtilis RsbU antibody. We thank A. Cheung, D. Bauer, and M. Fraunholz for plasmids; D. Becher and D. Albrecht for MS analysis; S. Reiss for assistance with the expression and purification of the Rsb proteins for antibody production; and T. Meier and A. Harang for excellent technical assistance.
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 1831329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper, S., L. Duncan, and R. Losick. 1994. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in Bacillus subtilis. Cell 22195-205. [DOI] [PubMed] [Google Scholar]
- 3.Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260165-177. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, B. T., N. P. Donegan, T. M. Jarry, M. Palma, and A. L. Cheung. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 697851-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis sigma B is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 152330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger-Bächi, B. 1983. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J. Bacteriol. 154533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt, J., K. Büttner, C. Scharf, and M. Hecker. 1999. Dual channel imaging of two-dimensional electropherograms in Bacillus subtilis. Electrophoresis 202225-2240. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bächi, and S. Projan. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 1864085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 1757931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigulla, M., T. Hoffmann, A. Krisp, A. Völker, E. Bremer, and U. Völker. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 1854305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candiano, G., M. Bruschi, L. Musante, L. Santucci, G. M. Ghiggeri, B. Carnemolla, P. Orecchia, L. Zardi, and P. G. Righetti. 2004. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 251327-1333. [DOI] [PubMed] [Google Scholar]
- 12.Chen, C. C., R. J. Lewis, R. Harris, M. D. Yudkin, and O. Delumeau. 2003. A supramolecular complex in the environmental stress signaling pathway of Bacillus subtilis. Mol. Microbiol. 491657-1669. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. C., M. D. Yudkin, and O. Delumeau. 2004. Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis. J. Bacteriol. 1866830-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delumeau, O., R. J. Lewis, and M. D. Yudkin. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 1845583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delumeau, O., S. Dutta, M. Brigulla, G. Kuhnke, S. W. Hardwick, U. Völker, M. D. Yudkin, and R. J. Lewis. 2004. Functional and structural characterization of RsbU, a stress signaling protein phosphatase 2C. J. Biol. Chem. 27940927-40937. [DOI] [PubMed] [Google Scholar]
- 16.Delumeau, O., C. C. Chen, J. W. Murray, M. D. Yudkin, and R. J. Lewis. 2006. High-molecular-weight complexes of RsbR and paralogues in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 1887885-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 1761813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufour, A., U. Völker, A. Völker, and W. G. Haldenwang. 1996. Relative levels and fraction properties of Bacillus subtilis σB and its regulators during balanced growth and stress. J. Bacteriol. 1783701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta, S., and R. J. Lewis. 2003. Crystallization and preliminary crystallographic analysis of the kinase-recruitment domain of the PP2C-type phosphatase RsbU. Acta Crystallogr. D 59191-193. [DOI] [PubMed] [Google Scholar]
- 20.Eymann, C., D. Becher, J. Bernhardt, K. Gronau, A. Klutzny, and M. Hecker. 2007. Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 73509-3526. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs, S., J. Pané-Farré, C. Kohler, M. Hecker, and S. Engelmann. 2007. Anaerobic gene expression in Staphylococcus aureus. J. Bacteriol. 1894275-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaidenko, T. A., X. Yang, Y. M. Lee, and C. W. Price. 1999. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the environmental stress signaling pathway of Bacillus subtilis. J. Mol. Biol. 28829-39. [DOI] [PubMed] [Google Scholar]
- 23.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261558-566. [DOI] [PubMed] [Google Scholar]
- 24.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 1831843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber, T. M., and C. A. Gross. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57441-466. [DOI] [PubMed] [Google Scholar]
- 26.Hardwick, S. W., J. Pané-Farré, O. Delumeau, J. Marles-Wright, J. W. Murray, M. Hecker, and R. J. Lewis. 2007. Structural and functional characterization of partner switching regulating the environmental stress response in Bacillus subtilis. J. Biol. Chem. 28211562-11572. [DOI] [PubMed] [Google Scholar]
- 27.Hecker, M., and U. Völker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 4435-91. [DOI] [PubMed] [Google Scholar]
- 28.Hecker, M., J. Pané-Farré, and U. Völker. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61215-236. [DOI] [PubMed] [Google Scholar]
- 29.Helmann, J. D. 1999. Anti-sigma factors. Curr. Opin. Microbiol. 2135-141. [DOI] [PubMed] [Google Scholar]
- 30.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igoshin, O. A., M. S. Brody, C. W. Price, and M. A. Savageau. 2007. Distinctive topologies of partner-switching signaling networks correlate with their physiological roles. J. Mol. Biol. 3691333-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko, T., N. Tanaka, and T. Kumasaka. 2005. Crystal structures of RsbQ, a stress-response regulator in Bacillus subtilis. Protein Sci. 14558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang, C. M., M. S. Brody, S. Akbar, X. Yang, and C. W. Price. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 1783846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang, C. M., K. Vijay, and C. W. Price. 1998. Serine kinase activity of a Bacillus subtilis switch protein is required to transduce environmental stress signals but not to activate its target PP2C phosphatase. Mol. Microbiol. 30189-196. [DOI] [PubMed] [Google Scholar]
- 35.Kim, T. J., T. A. Gaidenko, and C. W. Price. 2004. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 1866124-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, T. J., T. A. Gaidenko, and C. W. Price. 2004. A multicomponent protein complex mediates environmental stress signaling in Bacillus subtilis. J. Mol. Biol. 341135-150. [DOI] [PubMed] [Google Scholar]
- 37.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock exotoxin structural gene is not detectably transmitted by a prophage. Nature 305709-712. [DOI] [PubMed] [Google Scholar]
- 38.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 1804814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessières, A. Bolotin, S. Borchert, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390249-256. [DOI] [PubMed] [Google Scholar]
- 40.Marles-Wright, J., T. Grant, O. Delumeau, G. van Duinen, S. J. Firbank, P. J. Lewis, J. W. Murray, J. A. Newman, M. B. Quin, P. R. Race, A. Rohou, W. Tichelaar, M. van Heel, and R. J. Lewis. 2008. Molecular architecture of the “Stressosome,” a signal integration and transduction hub. Science 32292-96. [DOI] [PubMed] [Google Scholar]
- 41.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 42.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260289-298. [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki, E., J. M. Chen, C. Ko, and W. R. Bishai. 1999. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J. Bacteriol. 1812846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33155-166. [DOI] [PubMed] [Google Scholar]
- 45.Palma, M., and A. L. Cheung. 2001. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 697858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pané-Farré, J., B. Jonas, K. Förstner, S. Engelmann, and M. Hecker. 2006. The sigmaB regulon in Staphylococcus aureus and its regulation. Int. J. Med. Microbiol. 296237-258. [DOI] [PubMed] [Google Scholar]
- 47.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 1835617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price, C. W., P. Fawcett, H. Cérémonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41757-774. [DOI] [PubMed] [Google Scholar]
- 49.Price, C. W. 2002. General stress response, p. 161-178. In A. L. Sonenshein, R. Losick, and J. A. Hoch (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 50.Ray, C., M. Igo, W. Shafer, R. Losick, and C. P. Moran, Jr. 1988. Suppression of ctc promoter mutations in Bacillus subtilis. J. Bacteriol. 170900-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rollenhagen, C., H. Antelmann, J. Kirstein, O. Delumeau, M. Hecker, and M. D. Yudkin. 2003. Binding of σA and σB to core RNA polymerase after environmental stress in Bacillus subtilis. J. Bacteriol. 18535-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 53.Scott, J. M., T. Mitchell, and W. G. Haldenwang. 2000. Stress triggers a process that limits activation of the Bacillus subtilis stress transcription factor σB. J. Bacteriol. 1821452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senn, M. M., P. Giachino, D. Homerova, A. Steinhuber, J. Strassner, J. Kormanec, U. Fluckiger, B. Berger-Bächi, and M. Bischoff. 2005. Molecular analysis and organization of the sigmaB operon in Staphylococcus aureus. J. Bacteriol. 1878006-8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shafer, W. M., and J. J. Iandolo. 1979. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect. Immun. 25902-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52697-704. [DOI] [PubMed] [Google Scholar]
- 57.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 18560-89. [DOI] [PubMed] [Google Scholar]
- 58.Stülke, J., R. Hanschke, and M. Hecker. 1993. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J. Gen. Microbiol. 1392041-2045. [DOI] [PubMed] [Google Scholar]
- 59.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the sigmaB transcription factor of Bacillus subtilis. Mol. Microbiol. 35180-188. [DOI] [PubMed] [Google Scholar]
- 61.Völker, U., A. Dufour, and W. G. Haldenwang. 1995. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J. Bacteriol. 177114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Völker, U., A. Völker, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate sigma B of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 1773771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Völker, U., A. Völker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-sigma B antagonist, RsbV, by stress- or starvation-induced phosphatase activities. J. Bacteriol. 1785456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Völker, U., T. Luo, N. Smirnova, and W. G. Haldenwang. 1997. Stress activation of Bacillus subtilis σB can occur in the absence of the σB negative regulator RsbX. J. Bacteriol. 1791980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 1786036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 102265-2275. [DOI] [PubMed] [Google Scholar]