Abstract
Members of the genus Shewanella inhabit various environments; they are capable of synthesizing various types of low-melting-point fatty acids, including monounsaturated fatty acids (MUFA) and branched-chain fatty acids (BCFA) with and without eicosapentanoic acid (EPA). The genes involved in fatty acid synthesis in 15 whole-genome-sequenced Shewanella strains were identified and compared. A typical type II fatty acid synthesis pathway in Shewanella was constructed. A complete EPA synthesis gene cluster was found in all of the Shewanella genomes, although only a few of them were found to produce EPA. The roles and regulation of fatty acids synthesis in Shewanella were further elucidated in the Shewanella piezotolerans WP3 response to different temperatures and pressures. The EPA and BCFA contents of WP3 significantly increased when it was grown at low temperature and/or under high pressure. EPA, but not MUFA, was determined to be crucial for its growth at low temperature and high pressure. A gene cluster for a branched-chain amino acid ABC transporter (LIV-I) was found to be upregulated at low temperature. Combined approaches, including mutagenesis and an isotopic-tracer method, revealed that the LIV-I transporter played an important role in the regulation of BCFA synthesis in WP3. The LIV-I transporter was identified only in the cold-adapted Shewanella species and was assumed to supply an important strategy for Shewanella cold adaptation. This is the first time the molecular mechanism of BCFA regulation in bacteria has been elucidated.
In general, bacteria stringently control the production of a variety of fatty acids with different melting temperatures to achieve the correct physical state of the membrane lipids (33, 51). Modulation of fatty acid composition is a common strategy for most organisms to ensure sufficient membrane fluidity by increasing the amount of low-melting-point fatty acids, such as monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and branched-chain fatty acids (BCFA) (19, 23, 45). Low temperature and high pressure exert similar effects on the biological membrane, with an irreversible change from a fluid, disordered state to a nonfluid, ordered state (13, 23). The responses of bacteria to reduced temperature or elevated pressure frequently entail the increased incorporation into membrane phospholipids of BCFA and unsaturated fatty acids (UFA) (15, 16, 34, 37, 38, 49). How bacteria modulate membrane fatty acid unsaturation has been well documented (2, 31), and a novel polyketide synthesis pathway has been discovered for eicosapentanoic acid (EPA) and docosahexanoic acid synthesis in bacteria (35). For BCFA synthesis, the difference from straight-chain fatty acid synthesis lies only in the respective acylated primer acyl carrier proteins and in the condensing enzyme, β-ketoacyl-acyl carrier protein synthase III (FabH), which prefer modified primer acyl chains for elongation instead of the “normal” acetyl primer (11, 22). Although the cold-regulated BCFA synthesis has been observed in some bacteria, the underlying mechanism has not been well characterized (17, 30).
Most of the deep sea is characterized as permanently low temperature (2°C to 4°C) with high hydrostatic pressure (10 MPa to 110 MPa). To overcome the membrane problem caused by low temperature and/or high pressure, most deep-sea bacteria have been observed to increase membrane fluidity by increasing the proportion of UFA, including MUFA and PUFA (15, 16, 44, 52). However, the regulation of fatty acid synthesis in deep-sea bacteria is only partly elucidated in the psychrophilic, pressure-tolerant deep-sea bacterium Photobacterium profundum SS9. It was demonstrated for the first time in SS9 at the molecular level that MUFA, but not EPA, is essential for growth at low temperature and/or high pressure, although SS9 increased its EPA content significantly at low temperature and high pressure (4). The regulation of EPA synthesis is still not well understood; however, it has been suggested that the enzymes for EPA synthesis may possess higher activity at low temperature and/or high pressure (3). It is thus still unknown if other deep-sea bacteria utilize the same strategy as SS9, or a similar one, to adjust their membranes to low temperature and/or high pressure.
Shewanella species are widely distributed in various environments, and they are known for their versatile respiration capabilities (47). The typical low-melting-point fatty acids of the genus Shewanella include BCFA (i13:0, i15:0) and MUFA (16:1, 18:1), and some of them include PUFA (20:5, EPA) (47). Shewanella strains are among the most frequently isolated deep-sea bacteria. In this study, we searched for and compared the genes or gene clusters involved in fatty acid synthesis in 15 sequenced Shewanella genomes. Fatty acid biosynthesis and regulation in Shewanella were further investigated in a model deep-sea bacterium, Shewanella piezotolerans WP3, which grows optimally at 20°C and 20 MPa (48). Here, we describe the changes in the fatty acid profiles when WP3 was grown at different temperatures and pressures. The roles of the low-melting-point fatty acids, including MUFA, EPA, and BCFA, in the growth of the bacterium at low temperature and high pressure were elucidated, and the main regulatory strategy for BCFA synthesis in WP3 is also clarified.
MATERIALS AND METHODS
Strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. Shewanella strains were cultured in modified marine medium 2216E (5 g/liter tryptone, 1 g/liter yeast extract, 0.1 g/liter FePO4, 34 g/liter NaCl) with 0.3 mg/liter [12C]leucine and shaking at 220 rpm at different temperatures as indicated in the text. Escherichia coli strain WM3064 was incubated in Luria-Bertani medium (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl) at 37°C with the addition of 50 μg/ml dl-α,ɛ-diaminopimelic acid. For solid media, agar (Sangon Inc., Shanghai, China) was added at 15 g/liter. The antibiotics chloramphenicol (50 μg/ml for E. coli; 25 μg/ml for Shewanella strains) and ampicillin (100 μg/ml) were added to the medium when required. The antibiotic cerulenin (2,3-epoxy-4-oxo-7,10-dodecadienamide) was added at 10 μg/ml when used in inhibition studies. All antibiotics were obtained from Sigma Chemical Co. (St. Louis, MO). When required, media were supplemented with EPA (20:5ω3) at a final concentration of 0.025% and with 0.05% Tween 80 (polyoxyethylenesorbitan-monooleate) as a solubilizing agent for the EPA.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| WM3064 | Cloning and donor strain for conjugation; ΔdapA | 21 |
| S. piezotolerans | ||
| WP3 | Wild type | Laboratory stock |
| WP3ΔEPA | WP3 Δswp3547 (EPA-deficient mutant) | This study |
| WP3ΔLIV | WP3 Δswp3487 (LIV-I function-deficient mutant) | This study |
| Plasmids | ||
| pRE112 | Allelic-exchange vector | 18 |
| pRE112-EPA | pRE112 containing deletion structure for swp3547; used to make WP3ΔEPA | This study |
| pRE112-LIV | pRE112 containing deletion structure for swp3487; used to make WP3ΔLIV | This study |
High-pressure growth studies.
To examine the high-pressure growth of the Shewanella strains, each culture was grown to stationary phase in 2216E marine medium at 1 atm (1 atm = 0.101 MPa) and 20°C. The stationary-phase cultures were diluted 1,000-fold in the same medium and then transferred to fill 30-ml polyethylene transfer pipettes, which were sealed with no air space and placed inside pressure vessels. Pin closure pressure vessels (53) were used in this study (constructed by Wuxi Jinliu Petrochemical Instrument Equipment Inc., China). Pressure was applied using a hand-operated pump with a quick-fit connector to the pressure vessel. The cells were then incubated at a hydrostatic pressure of 0.1 or 20 MPa (1 and 200 atm, respectively) at 20°C.
Molecular biology techniques.
Recombinant DNA techniques were carried out by published methods (43). Plasmid DNA was prepared with an E.Z.N.A. plasmid minikit I (Omega). PCR products and DNA fragments were purified with the E.Z.N.A. Cycle-Pure kit I (Omega) and were extracted from gels with an E.Z.N.A. gel extraction kit (Omega). All cloned inserts and DNA fragments were confirmed by DNA sequencing. Transformation of E. coli cells was carried out by using the CaCl2 method (43).
Plasmid construction and mutant generation.
The EPA-deficient mutant WP3ΔEPA and the branched-chain amino acid (BCAA) ABC transporter (LIV-I) function-deficient mutant WP3ΔLIV were created via deletion mutagenesis with the mobilizable suicide plasmid pRE112 (encoding chloramphenicol resistance) (18). orfswp3547, one of the five genes necessary for EPA biosynthesis, and orfswp3487, which encodes the only extracellular-substrate-binding protein in the LIV-I transporter, were chosen to be deleted to construct WP3ΔEPA and WP3ΔLIV, respectively. The first step was the generation of a deleted copy of the target genes using two-step crossover PCR amplification from strain WP3 genomic DNA with the following primers: 5′-GGGGGTCTAGAATCCGCCGACCTTCCATG-3′ (swp3487 Left For), 5′-TTTGTTGGCCATCTGGCTGGAGCGCCGTGTTGATTGGTTAGGT-3′ (swp3487 Left Rev), 5′-CCAGATGGCCAACAAAAATAGTTTCCTC-3′ (swp3487 Right For), and 5′-AAAAAGAGCTCGTATTGGCGGCGCTTTATATGCTCACTA-3′ (swp3487 Right Rev), as well as 5′-AATCTAGACTGGAGAAATATCTACCTAACCG-3′ (swp3547 Left For), 5′-CGTCACCGAGTATCAATTTGCGGTCAGTATGGATGGACAAC-3′ (swp3547 Left Rev), 5′-TTGATACTCGGTGACGAAGGGGTTAG-3′ (swp3547 Right For), and 5′-AAGAGCTCATTTGCACTGTTTTTCACTGAGTAGTTG-3′ (swp3547 Right Rev). Then, the PCR products were cloned into pRE112 as XbaI-SacI fragments, yielding pRE112-EPA and pRE112-LIV, respectively. Bacterial conjugations were used to transfer these two plasmids from the donor strain, E. coli WM3064, into the recipient strain, S. piezotolerans WP3, as described by Chi and Bartlett (10). Finally, the deleted mutants were selected using the method described by Edwards et al. (18).
Fatty acid extraction and analysis.
Cells were grown under different conditions in 2216E medium to early stationary phase, washed twice with 3% NaCl at 4°C, centrifuged at 8,000 × g, and then freeze-dried. Ten milligrams of dried cells was put into a Teflon-lined screw-cap tube with 2 ml anhydrous methanolic/HCl (11:1 [vol/vol]). The tubes were tightly closed and sonicated for 10 min and then heated at 100°C for 40 min. After the tubes were cooled, 1 ml of n-hexane was added to extract the fatty acid methyl esters. The extraction procedures were repeated three times. The analysis of fatty acid methyl ethers was performed by gas-liquid chromatography and mass spectrometry.
RNA extraction and reverse transcription (RT).
Total RNA was isolated from the WP3 cultures grown under different conditions with a TRI reagent-RNA/DNA/protein isolation kit (Molecular Research Center, Inc.) according to the manufacturer's instructions. The RNA samples were treated with DNase I at 37°C for 1 h and then purified with an RNeasy Mini Kit (Qiagen, Germany). The purified RNA samples were used to synthesize cDNA with the RevertAid First Strand cDNA Synthesis Kit (MBI, Fermentas) following the manufacturer's instructions.
Real-time RT-PCR.
The primer pairs for the selected genes for real-time PCR were designed using Primer Express software (Applied Biosystems, San Francisco, CA). PCR cycling was conducted using 7500 System SDS software in reaction mixtures with total volumes of 50 μl containing 1× SYBR green I Universal PCR Master Mix (ABI), 0.5 μM each primer, and 1 μl cDNA template. The amount of target was normalized to that of the reference gene swp0453, whose expression remains stable under various conditions relative to the calibrator (the transcription levels of the genes at 0.1 MPa, or 20°C, were set as 1) (29). Real-time RT-PCR assays were performed in triplicate for each sample, and a mean value and standard deviation were calculated for the relative RNA expression levels.
Isotopic-tracer method.
13C-labeled and 12C-labeled l-leucine were added to 2216E medium at a final concentration of 0.3 mg/liter, and the pH was adjusted to 7.0. The overnight-cultured Shewanella strains were diluted 200-fold in this medium and then cultured at different temperatures with shaking at 220 rpm until early stationary phase. The cells were harvested, and the fatty acid was extracted and analyzed as described previously.
Genomic-sequence analysis.
The complete genomes of the Shewanella strains analyzed in this study were downloaded from GenBank. A set of subsystem-based genomic annotations and metabolic-reconstruction tools implemented in the SEED platform were used to capture the existing knowledge of type II fatty acid synthesis pathways. The reconstruction is initiated by defining a list of functional roles (enzymes, transporters, and regulators) immediately associated with a biological process under study (40). This information is obtained by review of the literature related to fatty acid metabolism and pathway-reaction-compound information available in public resources, such as KEGG and MetaCyc. Most initial information about functional roles and the respective genes was derived from studies of E. coli (31, 42) and a group of BCFA-producing bacteria, including Bacillus subtilis and Listeria monocytogenes (26, 30). To compare the EPA gene cluster in Shewanella, the MicrobesOnline website (http://www.microbesonline.org) for comparative genomics was used (5). Domain arrangement analyses of the predicted amino acid sequences of the pfaA genes were conducted using the ProDom database of protein domain families (12), the Conserved Domain Database with Reverse Position Specific BLAST (6), and the ISREC ProfileScan server (http://hits.isb-sib.ch/cgi-bin/PFSCAN). Dinucleotide bias analysis was performed using the method proposed by Karlin (27). The dinucleotide relative abundance value, δ*, was calculated with the δρ-WEB program (http://deltarho.amc.nl/) (46).
RESULTS
Fatty acid synthesis genes and pathways in Shewanella.
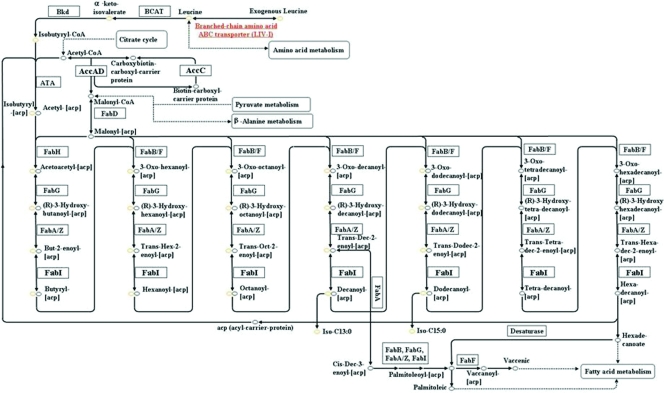
The characteristic fatty acid composition of Shewanella contains saturated fatty acids (SFA) (16:0, 18:0) and various low-melting-point fatty acids, including BCFA (i13:0, i15:0) and MUFA (16:1, 18:1), with or without EPA (20:5) (47). The genome sequences of 15 Shewanella strains, Shewanella amazonensis SB2B, Shewanella baltica OS155, Shewanella denitrificans OS217, Shewanella frigidimarina NCIMB 400, Shewanella oneidensis MR-1, Shewanella pealeana ATCC 700345, Shewanella putrefaciens 200, Shewanella sediminis HAW-EB3, Shewanella sp. strain ANA-3, Shewanella sp. strain MR-4, Shewanella sp. strain MR-7, Shewanella sp. strain W3-18-1, Shewanella loihica PV-4, Shewanella woodyi ATCC 51908, and S. piezotolerans WP3, were searched and compared for genes involved in fatty acid synthesis. All genes supposed to be involved in a typical type II fatty acid biosynthesis pathway could be easily and unambiguously identified from the genomes. The type II fatty acid biosynthesis pathway of the genus Shewanella was constructed as shown in Fig. 1. The putative pathway for BCFA synthesis is also shown in Fig. 1.
FIG. 1.
The type II fatty acid biosynthesis pathway in Shewanella strains. Red indicates that the gene was upregulated at low temperature, and underlining means the BCAA ABC transporter (LIV-I) was found only in S. piezotolerans WP3, S. loihica PV-4, S. sediminis HAW-EB3, and S. frigidimarina NCIMB 400.
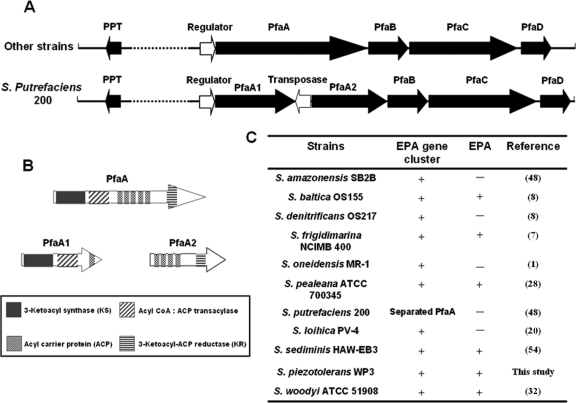
Intact EPA-synthesizing gene clusters including a phosphopantetheinyl transferase gene and pfaA, -B, -C, and -D were found to be conserved in all of the Shewanella genomes, as shown in Fig. 2A. One exception was S. putrefaciens 200, whose pfaA gene was separated by a transposase gene. The transposase gene was inserted in the middle of the S. putrefaciens pfaA gene to separate it into two parts. However, each of the small fragments had its own initiation and termination codons, implying that they might be expressed separately. Domain analyses of the two small pfaA1 and -A2 gene products also revealed that the insertion did not destroy any putative enzyme domains (Fig. 2B), which provides the possibility that they can be incorporated together and perform the full PfaA functions. Among the 11 strains whose fatty acid profiles were characterized, only S. baltica OS155, S. frigidimarina NCIMB 400, S. pealeana ATCC 700345, S. piezotolerans WP3, and S. sediminis HAW-EB3 were reported to produce EPA, while the other 6 strains could not produce EPA or produced it at extremely low levels (less than 0.1%) under the test conditions (Fig. 2C).
FIG. 2.
EPA gene cluster analysis. (A) The organizations of EPA gene clusters in all sequenced Shewanella strains are shown. Open reading frames (ORFs) are represented by arrows oriented in the direction of transcription. The putative gene product of each ORF is shown. The black arrows represent the ORFs essential for EPA synthesis, and the white arrows represent the ORFs that are not required. The dashed lines indicate different distances in different strains. (B) Comparison of enzyme domains within intact and separated pfaA gene products. CoA, coenzyme A. (C) EPA and its gene clusters in sequenced Shewanella strains.
Fatty acid profiles of WP3 at different temperatures and pressures.
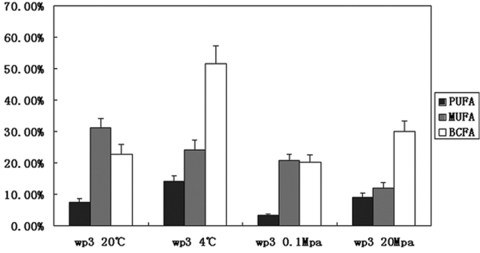
The low-melting-point fatty acid profiles of WP3 grown at different temperatures (4°C and 20°C) and pressures (0.1 MPa and 20 MPa) were characterized and are listed in Table 2. It was found that WP3 could produce three kinds of low-melting-point fatty acids: MUFA, EPA, and BCFA. The EPA and BCFA contents increased significantly as the temperature dropped or the pressure increased. When the temperature decreased from 20°C to 4°C, the proportion of BCFA increased from 22.9% to 51.6% and the BCFA/straight-chain fatty acid ratio showed significant elevation (0.30 to 1.07). The percentage of EPA increased from 6.5% at 20°C to 14.1% at 4°C. Unlike the changes in EPA and BCFA, the proportion of MUFA (16:1 and 18:1) exhibited a slight decrease with decreasing temperature. Similar to the effect of low temperature, high hydrostatic pressure also induced higher BCFA and EPA levels. The proportion of BCFA increased from 20.2% at 0.1 MPa to 30.1% at 20 MPa. Furthermore, high pressure (20 MPa) caused a threefold increase in EPA production compared to the atmospheric pressure (Table 2). The modulation of the MUFA, EPA, and BCFA contents according to the temperatures and pressures is summarized in Fig. 3.
TABLE 2.
Fatty acid profiles of Shewanella strains grown under different conditionsa
| Strain | Culture conditionsb | Mean % (by weight) fatty acid ± SD
|
BCFA/SC FA ratioc | UFA/SFA ratiod | ||||
|---|---|---|---|---|---|---|---|---|
| Iso-13:0 | Iso-15:0 | 16:1 | 18:1 | 20:5 | ||||
| WP3 | ||||||||
| Untreated | 20°C | 9.3 ± 2.5 | 12.3 ± 1.5 | 22.9 ± 3.8 | 8.2 ± 1.7 | 6.5 ± 1.1 | 0.3 | 0.6 |
| 4°C | 23.3 ± 4.4 | 26.2 ± 3.5 | 17.7 ± 1.9 | 6.5 ± 1.0 | 14.1 ± 1.8 | 1.07 | 0.62 | |
| 0.1 MPa | 11.2 ± 2.4 | 9.0 ± 1.6 | 7.2 ± 0.9 | 13.6 ± 1.1 | 3.2 ± 0.5 | 0.25 | 0.32 | |
| 20 MPa | 15.3 ± 1.1 | 14.8 ± 1.9 | 2.9 ± 0.4 | 9.0 ± 1.2 | 9.1 ± 1.3 | 0.43 | 0.27 | |
| Treatede | 20°C | 31.8 ± 4.8 | 9.4 ± 1.3 | 9.0 ± 1.1 | 0 | 7.1 ± 0.9 | 0.7 | 0.19 |
| 4°C | 51.9 ± 5.1 | 6.3 ± 1.0 | 7.3 ± 1.1 | 0 | 14.4 ± 1.8 | 1.39 | 0.28 | |
| 0.1 MPa | 22.3 ± 4.4 | 6.1 ± 1.3 | 4.5 ± 0.7 | 0 | 3.7 ± 0.7 | 0.55 | 0.09 | |
| 20 MPa | 27.6 ± 4.9 | 8.2 ± 2.2 | 1.9 ± 0.3 | 0 | 9.8 ± 1.6 | 0.85 | 0.13 | |
| WP3ΔEPA | 20°C | 9.4 ± 1.1 | 11.6 ± 0.9 | 28.9 ± 3.3 | 9.6 ± 0.9 | 0 | 0.31 | 0.63 |
| 4°C | 26.7 ± 3.8 | 34.7 ± 3.4 | 18.7 ± 2.5 | 6.9 ± 0.5 | 0 | 1.39 | 0.34 | |
| 0.1 MPa | 10 ± 1.2 | 8.7 ± 1.3 | 12.9 ± 2.0 | 16.9 ± 2.4 | 0 | 0.23 | 0.42 | |
| 20 MPa | 14.3 ± 2.2 | 12.7 ± 1.9 | 5.5 ± 1.1 | 11.5 ± 1.9 | 0 | 0.37 | 0.20 | |
| WP3ΔLIV | 20°C | 8 ± 1.0 | 9.3 ± 1.5 | 29.4 ± 3.3 | 10.8 ± 1.9 | 7.4 ± 0.8 | 0.21 | 0.91 |
| 4°C | 12.5 ± 1.7 | 12.3 ± 0.9 | 31.7 ± 1.6 | 10.7 ± 1.5 | 13.6 ± 1.6 | 0.33 | 1.27 | |
| 0.1 MPa | 10.2 ± 1.5 | 9.1 ± 0.8 | 8.4 ± 1.7 | 12.0 ± 1.8 | 3.9 ± 0.6 | 0.23 | 0.32 | |
| 20 MPa | 11.1 ± 1.6 | 10.3 ± 1.8 | 4.4 ± 1.0 | 11.0 ± 0.9 | 10.4 ± 1.3 | 0.27 | 0.35 | |
The data represent values derived from triplicate samples harvested in the early stationary phase of growth.
20 and 4°C cultures were grown aerobically in 2216E marine medium(with 0.3 mg/liter [12C]leucine) at 0.1 MPa; 0.1- and 20-MPa cultures were grown at 20°C in 2216E marine medium(with 0.3 mg/liter [12C]leucine).
BCFA, iso-13:0, iso-15:0, and iso-17:0; SCFA, 12:0, 13:0, 14:0, 15:0, 16:0, 16:1, 18:0, 18:1, and 20:5.
UFA, 16:1, 18:1, and 20:5; SFA, 12:0, iso-13:0, 13:0, 14:0, iso-15:0, 15:0, 16:0, iso-17:0, and 18:0.
Cerulenin was used at 10 mg/ml.
FIG. 3.
Effects of growth temperature and growth pressure on cellular fatty acid composition in S. piezotolerans WP3 (1 MPa = 10 bar ≈ 9.87 atm). The data represent mean percentages (by weight) of fatty acid species, plus standard deviations, derived from triplicate samples harvested in the early stationary phase of growth. See Materials and Methods for cultivation conditions.
EPA, but not MUFA, is required for growth of WP3 at low temperature and high pressure.
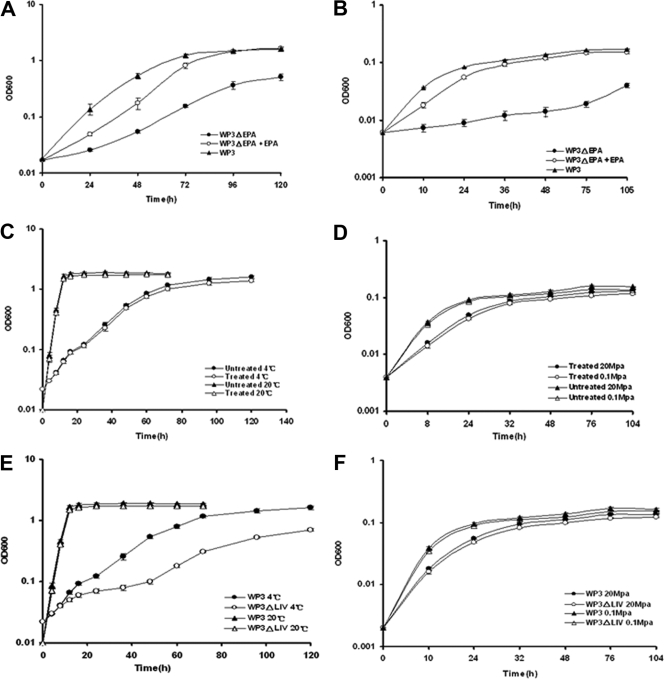
An EPA production-deficient mutant, WP3ΔEPA, was constructed, and initial analysis showed that WP3ΔEPA had defects in growth at low temperature (4°C) or at high pressure (20 MPa) (48). Upon supplementation with exogenous EPA in the form of 0.025% Tween 80, reversal of the low-temperature and high-pressure growth arrest was observed to various degrees (Fig. 4A and B). The fatty acid profiles of strain WP3ΔEPA grown under various conditions were analyzed (Table 2). In comparison with the wild-type strain, the mutant lost the ability to produce EPA, but the content of 16:1 was overexpressed at 20°C.
FIG. 4.
Growth curves of WP3 strains. (A and B) Growth characteristics of the EPA-deficient strain S. piezotolerans WP3ΔEPA at low temperature (4°C) (A) and high pressure (20 MPa) (B) in the absence or presence of exogenous EPA at a final concentration of 0.025%. (C and D) Effects of cerulenin (10 μg/ml) on the growth of S. piezotolerans WP3 at various temperatures (C) and pressures (D). (E and F) Growth of the mutant strain S. piezotolerans WP3ΔLIV at various temperatures (E) and pressures (F). OD600, optical density at 600 nm. The error bars indicate standard deviations.
To check the roles of MUFA in the growth of WP3 at low temperature and high pressure, the antibiotic cerulenin was added to the growth medium. Cerulenin irreversibly inhibits the fatty acid biosynthesis enzymes β-ketoacyl-acyl carrier protein synthases I and II in a variety of bacteria and fungi, perturbing the formation of MUFA (14, 36, 39). As shown in Table 2, the cerulenin-treated strains showed complete inhibition of 18:1 production and moderate reduction of 16:1. However, the cerulenin-treated strains had no obvious growth arrest compared with the wild type under low temperature and high pressure, except for a slight reduction in the growth yield under all conditions tested (Fig. 4C and D). These results demonstrate that under the laboratory conditions used in this study, EPA, but not MUFA, is essential for the growth of WP3 at low temperature and high pressure.
Roles of BCFA and its regulation.
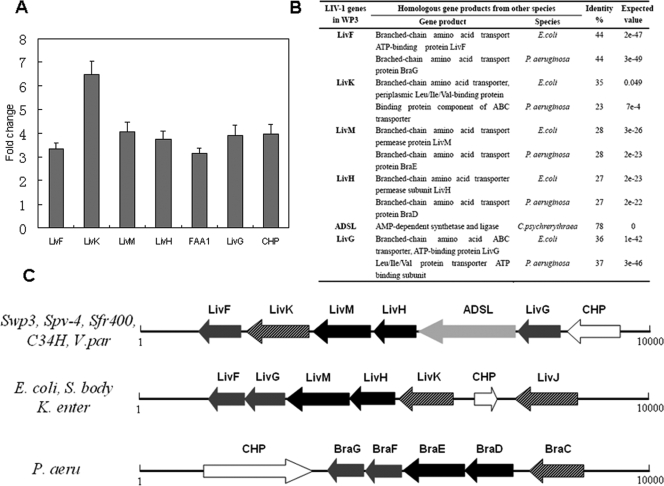
Transcriptome (microarray) analysis of WP3 grown at different temperatures (4°C and 20°C) showed that a gene cluster for the BCAA ABC transporter LIV-I (swp3487 to swp3492) was upregulated by low temperature (our unpublished data). Quantitative RT-PCR clearly verified the induction of these transporter genes by low temperature (Fig. 5A). The similarities of LIV-I genes in WP3, E. coli, and P. aeruginosa are shown in Fig. 5B. The gene organization of the LIV-I transporter in WP3 is shown in Fig. 5C. The LIV-I system belongs to the hydrophobic amino acid uptake transporter (HAAT) subfamily of the ABC superfamily (24).
FIG. 5.
(A) LIV-I gene expression levels in WP3. Upregulation of the BCAA ABC transporter gene cluster (LIV-I) at low temperature is shown. Gene expression level changes of the LIV-I system were monitored by real-time RT-PCR as discussed in Materials and Methods. The gene expression level at 20°C was set as 1, and the change represents the gene expression level at 4°C compared to the level at 20°C. The error bars indicate standard deviations. (B) The genes of the LIV-I system in WP3 are compared with the corresponding homologous genes in the two well-characterized bacteria E. coli and P. aeruginosa. (C) Organizations of BCAA ABC transporters (LIV-I) in different bacteria. The open reading frames (ORFs) are represented by arrows oriented in the direction of transcription. The putative gene product of each ORF is shown. The extracellular solute binding proteins (LivJK and BraC) are represented by hatched arrows, the integral membrane proteins (LivHM and BraDE) are represented by black arrows, and the ATPase components (LivFG and BraFG) are represented by gray arrows. Additionally, the arrow labeled ADSL represents the AMP-dependent synthetase and ligase, and the white arrows represent the conserved hypothetical protein (CHP). S. body, Shigella bodyii Sb227; K. enter, Klebsiella enterica; Swp3, S. piezotolerans WP3; Spv-4, Shewanella sp. strain PV-4; Sfr400, S. frigidimarina NCIMB 400; C34H, Colwellia psychrerythraea 34H; P. aeru, P. aeruginosa; V. par, Vibrio parahaemolyticus RIMD 2210633.
To determine if this gene cluster is responsible for the BCFA increment at low temperature, mutations were constructed in orfswp3487, which encodes the only putative substrate-binding protein of this ABC transporter, as described in Materials and Methods. The LIV-I-deficient mutant was named WP3ΔLIV. The fatty acid profiles of WP3ΔLIV grown under different conditions were characterized. The mutant strain WP3ΔLIV almost lost the low-temperature- and high-pressure-dependent BCFA regulation ability (Table 2). With a temperature decrease from 20°C to 4°C, the percentage of BCFA in the wild-type strain dramatically increased from 22.9% to 51.6%, while the percentage of BCFA in WP3ΔLIV increased only slightly, from 17.3% to 24.8%. As the pressure rose from 0.1 MPa to 20 MPa, it induced an increase of BCFA in WP3 from 20.2% to 30.1%; however, this inducement was lost in WP3ΔLIV (Table 2). The strain WP3ΔLIV showed no growth difference from the wild-type strain when grown at 20°C, at 0.1 MPa, or at 20 MPa. However, it showed significant growth arrest at low temperature (4°C) (Fig. 4E and F). These data suggest that increasing BCFA is important for the growth of WP3 at low temperature but is not essential for growth at high pressure.
WP3 and WP3ΔLIV were cultured in medium supplemented with 13C-labeled l-leucine at different temperatures. In order to determine that the supplemental l-leucine was predominant in the amount of dissociative l-leucine, the supplemental l-leucine was added at concentrations of 0.3 mg/liter and 0.6 mg/liter, respectively. The fatty acid profiles and the percentage of 13C-labeled BCFA showed almost no change no matter which concentration of supplemental l-leucine was added (data not shown). This result definitely demonstrated that when supplemental l-leucine was added at a concentration of 0.3 mg/liter, it would be predominant in the amount of dissociative l-leucine. Therefore, this concentration was used for our subsequent experiments. Then, the fatty acid profiles of these strains were characterized by gas-liquid chromatography and mass spectrometry, and the results are shown in Table 3. If the BCFA was synthesized from exogenous BCAA, it would be labeled by 13C. This clearly showed that only about 20% of the BCFA was synthesized from exogenous BCAA when WP3 was grown at 20°C; however, this percentage dramatically increased to more than 50% when the growth temperature decreased to 4°C. Moreover, it can be easily found that the portion of BCFA synthesized from exogenous BCAA was almost equal to the increasing portion of BCFA at 4°C (Table 3). Meanwhile, WP3ΔLIV showed low levels of BCFA synthesis from exogenous BCAA (10% to 20%) and nearly no increment of BCFA at 4°C.
TABLE 3.
Percentages of BCFA synthesized from exogenous [13C]leucine in Shewanella strains grown at different temperatures
| Strain | Fatty acid type | 20°C
|
4°C
|
||||
|---|---|---|---|---|---|---|---|
| RTFAa | 13C RFAb | 13C RTFAc | RTFA | 13C RFA | 13C RTFA | ||
| WP3 | i-13:0 | 9.3 | 20 | 1.9 | 23.3 | 55 | 12.8 |
| i-15:0 | 12.3 | 20 | 2.5 | 26.2 | 50 | 13.1 | |
| WP3ΔLIV | i-13:0 | 8 | 10 | 0.8 | 12.5 | 20 | 2.5 |
| i-15:0 | 9.3 | 10 | 0.9 | 12.3 | 20 | 2.5 | |
RTFA, ratio in total fatty acid.
13C RFA, ratio of the fatty acid species labeled by 13C.
13C RTFA, ratio of the 13C-labeled fatty acid in total fatty acid.
LIV-I transporter in Shewanella.
The gene cluster for the LIV-I transporter was examined in the other 14 completely sequenced Shewanella strains mentioned above. Remarkably, besides WP3, the LIV-I system was identified only in S. loihica PV-4, S. sediminis HAW-EB3, and S. frigidimarina NCIMB 400, which are cold adapted, among the sequenced Shewanella strains. Furthermore, a homology search of this gene cluster was performed using BLAST at the National Center for Biotechnology Information. Only five sequences from two orders (Alteromonadales and Vibrionales), three families (Shewanellaceae, Colwelliaceae, and Vibrionaceae), and three genera (Shewanella, Colwellia, and Vibrio) showed the highest similarity both in sequence and in continuity. Additionally, dinucleotide bias analysis was performed using the method proposed by Karlin (27). The dinucleotide relative abundance value, δ*, and the G+C variation were calculated with the δρ-WEB program (http://deltarho.amc.nl/) (46). All four LIV-I gene clusters in Shewanella showed high dinucleotide bias, and each of their δ*values was higher than those of most other genomic fragments (greater than 88%) with lengths equal to that of the LIV-I gene cluster, indicating an anomalous signature (Table 4). The G+C content of the LIV-I gene cluster also varied from that of the core genome (Table 4). All these results imply that the LIV-I system in Shewanella may have been gained through horizontal gene transfer.
TABLE 4.
Dinucleotide bias and G+C variation of the gene cluster for LIV-I transporter in four sequenced cold-adapted Shewanella strains
| Strain | Genome location | G+C content (%) (genome G+C content) | G+C plot position (%) | δ* (103)a | Genome fragments with lower δ* (%)b |
|---|---|---|---|---|---|
| S. piezotolerans WP3 | 3684100-3692345 | 45.17 (43.3) | 88.07 | 52.09 | 91.59 |
| S. loihica PV-4 | 3072142-3080399 | 56.93 (53.7) | 86.10 | 61.024 | 88.057 |
| S. sediminis HAW-EB3 | 3780533-3788928 | 50.12 (46.1) | 97.26 | 56.139 | 91.629 |
| S. frigidimarina NCIMB 400 | 2160657-2168748 | 39.76 (41.6) | 12.88 | 55.855 | 88.294 |
The value δ* denotes the dinucleotide relative-abundance difference between the LIV-I gene cluster and the complete genome and was calculated with the δρ-WEB program (http://deltarho.amc.nl/) (46). The high δ* values of these fragments indicate a likely heterologous origin.
The percentage distribution of δ* was plotted using the δρ-WEB tool with random host genomic fragments of equal length as input sequences.
DISCUSSION
The members of the genus Shewanella have been known for versatile respiration ability for a long time, which was regarded as the major reason for their wide distribution in various environments. The special fatty acid system of Shewanella may be another reason. Shewanella strains can produce more types of low-melting-point fatty acids than many other strains. Their typical low-melting-point fatty acids include BCFA (i13:0, i15:0), MUFA (16:1, 18:1), and sometimes EPA (20:5), whereas most other strains contain only one or two types. At present, more than 18 Shewanella genomes have been completely sequenced; they provide a very good basis to analyze the fatty acid synthesis system of Shewanella. In this study, we searched and analyzed the genome sequences of 15 Shewanella strains inhabiting various environments, including shallow marine water, deep-sea sediment, sea ice, freshwater lakes, and oil brine (47, 50). We were able to reconstruct the fatty acid synthesis pathway of Shewanella, including the conserved type II fatty acid biosynthesis pathway and the EPA synthesis pathway.
It is somewhat surprising to us that all of the Shewanella genomes, including S. putrefaciens 200, whose pfaA gene contains a transposase gene insertion, harbor the complete gene cluster for EPA synthesis. However, many of these Shewanella strains have not been reported to produce EPA, or they produce EPA in negligible quantities (less than 0.1%) (Fig. 2C). For example, S. oneidensis MR-1 was first reported not to be able to produce EPA, but later studies detected trace amounts of EPA (1). EPA gene induction in MR-1 was even observed at cold temperatures (3°C). It would be very interesting to further address the question of why EPA gene synthesis is active in some Shewanella strains but inactive or active only at extremely low levels in other strains. On the other hand, the regulation of EPA synthesis in EPA-producing bacteria is still largely unclear. In P. profundum SS9, it was found that the expression levels of pfa (PUFA) genes showed almost no change in response to different temperatures and even showed some decrease at high pressure, although SS9 produced more EPA at low temperature and high pressure (3). Hence, the authors predicted that the PUFA synthases might also exhibit temperature/pressure-responsive characteristics (3). We observed the same phenomenon in WP3, which implied that the regulation of EPA synthesis in WP3 may use the same strategy as in SS9.
Using S. piezotolerans WP3 as a model, the role and regulation of fatty acid synthesis in response to different temperatures and pressures were further investigated in this study. The fatty acid profiles of WP3 revealed a pronounced regulation of cellular fatty acid composition in response to changes in temperature or pressure, most notably a greater proportion of EPA and BCFA at low temperature and high pressure, while the relative amounts of MUFA decreased. In contrast to the discovery by Bartlett and coworkers, our study revealed that EPA, but not MUFA, is essential for WP3 grown at low temperature and high pressure. How can we explain these totally contrary results? First, we note that about 60% of the fatty acid production of wild-type SS9 at low temperature and high pressure was MUFA, and this percentage increased to more than 70% in the EPA-deficient strain EA10. This large amount of MUFA might have been capable of compensating for the absence of EPA. However, there is only about 20% MUFA in wild-type WP3 and no more than 30% (4°C, 25.3%; 20 MPa, 17%) in the EPA-deficient strain WP3ΔEPA. Obviously, this low level of MUFA may not provide adequate compensation for the loss of EPA, and this low level of MUFA may also be one of the reasons that no growth inhibition was observed in the cerulenin-treated cells, as the loss of MUFA may be easily compensated for by other low-melting-point fatty acids. However, no increase of EPA and BCFA were observed in cerulenin-treated strains (Table 2). Alternatively, we noticed that the average chain length of these fatty acids decreased from 15.642 to 14.424 after treatment with cerulenin at 4°C. At high pressure (20 MPa), this value decreased from 15.845 to 14.172. These decreases in the average chain length would also increase the fluidity of the cell envelope, which may be capable of compensating for the loss of MUFA in WP3.
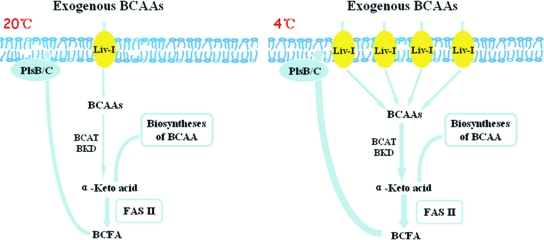
The typical type II fatty acid synthesis pathway could be easily constructed in Shewanella. BCFA synthesis has also been known to take the type II pathway. Up to now, studies of BCFA have mainly focused on gram-positive bacteria because most BCFA-producing strains are gram positive. Previous studies have revealed that BCFA is necessary for the growth of gram-positive bacteria at low temperatures (9, 41). In the regulation of BCFA synthesis, although β-ketoacyl-acyl carrier protein synthase III (FabH) was substantiated as a determining factor in BCFA synthesis based on its preference, there was no evidence that its preference in substrates was controlled by temperature or pressure. Therefore, we still have no idea as to how bacteria regulate BCFA to fulfill different requirements. There are studies showing that exogenous BCAA can largely influence the fatty acid profiles in some bacteria (25, 55). However, the molecular mechanism for this phenomenon has not been revealed. In this study, we discovered that the increase of BCFA in WP3 grown at low temperature was dramatically inhibited by blocking the function of the LIV-I system. Subsequent isotope tracer analysis indicated that the increased proportion of BCFA at low temperature was mainly synthesized from exogenous BCAA (Table 3), whereas in the LIV-I function-deficient strain WP3ΔLIV, no increment of BCFA was observed at low temperature. These results demonstrated the important role of the LIV-I transporter system in the regulation of BCFA synthesis. Taking this together with the data showing the low-temperature induction of genes of the LIV-I system, we could now propose that the increase of BCFA in WP3 at low temperature was mainly achieved through the induction of the BCAA ABC transporter LIV-I. As the model in Fig. 6 shows, when WP3 cells were grown at low temperature, the LIV-I transporter was induced to transport large numbers of exogenous BCAA into the cells for BCFA synthesis. To our knowledge, this is the first model that can clearly explain how bacteria adjust BCFA synthesis responses to different temperatures. The applicability of the model needs to be checked and tested in different bacterial strains.
FIG. 6.
Model for the regulation of BCFA synthesis in response to different temperatures in S. piezotolerans WP3. The substrates for BCFA synthesis can be generated via the degradation pathway from BCAA or from intermediates of the biosynthesis of these BCAA. The increment of BCFA synthesis at low temperature (4°C) in WP3 is achieved by induction of the BCAA ABC transporter gene cluster, which increases substantially the cell contents of BCAA for BCFA synthesis. BCAT, branched-chain amino acid transaminase; BKD, branched-chain α-keto acid dehydrogenase; PlsB, glycerol-3-phosphate acyltransferase; PlsC, 1-acyl-sn-glycerol-3-phosphate acyltransferase.
This BCAA ABC transporter belongs to the HAAT subfamily of the ABC superfamily, whose members are involved in the uptake of BCAA (24). Currently, homologues from three bacterial species (E. coli, Salmonella enterica serovar Typhimurium, and P. aeruginosa) have been characterized (24). The high-affinity BCAA transporters (LIV-I) of E. coli and S. enterica serovar Typhimurium are very similar: they consist of six gene products, with two extracellular solute binding proteins, two integral membrane proteins, and two ATP binding proteins. There are two integral membrane proteins, two ATP binding proteins, and only one extracellular solute binding protein in the LIV-I system of WP3 (Fig. 5C). It is very interesting that the BCAA LIV-I ABC transporter could be found only in cold-adapted Shewanella strains. Dinucleotide analysis showed a high δ* value and abnormal G+C content surrounding the gene cluster, which strongly imply that the ABC gene cluster has features suggestive of a foreign origin. Obtaining the LIV-I ABC transporter is thought to produce strains with greater ability to adapt to low temperatures.
Acknowledgments
This work was financially supported by the Chinese National High-Tech R&D Program (2006AA09Z428), the China Ocean Mineral Resources Research and Development Association fund (DYXM-115-02-2-03), and the National Science Foundation of China (40625016 and 30700013/C010103).
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Abboud, R., R. Popa, V. Souza-Egipsy, C. S. Giometti, S. Tollaksen, J. J. Mosher, R. H. Findlay, and K. H. Nealson. 2005. Low-temperature growth of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 71811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, E. E., and D. H. Bartlett. 2000. FabF is required for piezoregulation of cis-vaccenic acid levels and piezophilic growth of the deep-sea bacterium Photobacterium profundum strain SS9. J. Bacteriol. 1821264-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, E. E., and D. H. Bartlett. 2002. Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology 1481903-1913. [DOI] [PubMed] [Google Scholar]
- 4.Allen, E. E., D. Facciotti, and D. H. Bartlett. 1999. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at low temperature and high pressure. Appl. Environ. Microbiol. 651710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alm, E. J., K. H. Huang, M. N. Price, R. P. Koche, K. Keller, I. L. Dubchak, and A. P. Arkin. 2005. The MicrobesOnline web site for comparative genomics. Genome Res. 151015-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea, P. D. Nichols, and T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5 omega 3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 471040-1047. [DOI] [PubMed] [Google Scholar]
- 8.Brettar, I., R. Christen, and M. G. Hofle. 2002. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 522211-2217. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay, M. K., and M. V. Jagannadham. 2001. Maintenance of membrane fluidity in Antarctic bacteria. Polar Biol. 24386-388. [Google Scholar]
- 10.Chi, E., and D. H. Bartlett. 1993. Use of a reporter gene to follow high-pressure signal transduction in the deep-sea bacterium Photobacterium sp. strain SS9. J. Bacteriol. 1757533-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, K. H., R. J. Heath, and C. O. Rock. 2000. Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpet, F., J. Gouzy, and D. Kahn. 1999. Recent improvements of the ProDom database of protein domain families. Nucleic Acids Res. 27263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossins, A. R., and A. G. MacDonald. 1984. Homeoviscous theory under pressure. II. The molecular order of membranes from deep-sea fish. Biochim. Biophys. Acta 776144-150. [Google Scholar]
- 14.D'Agnolo, G., I. S. Rosenfeld, J. Awaya, S. Omura, and P. R. Vagelos. 1973. Inhibition of fatty acid biosynthesis by the antibiotic cerulenin. Specific inactivation of β-ketoacyl-acyl carrier protein synthetase. Biochim. Biophys. Acta 326155-166. [DOI] [PubMed] [Google Scholar]
- 15.DeLong, E. F., and A. A. Yayanos. 1985. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science 2281101-1103. [DOI] [PubMed] [Google Scholar]
- 16.Delong, E. F., and A. A. Yayanos. 1986. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl. Environ. Microbiol. 51730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mendoza, D., G. E. Schujman, and P. S. Aguilar. 2002. Biosynthesis and function of membrane lipids, p. 43-55. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 18.Edwards, R. A., L. H. Keller, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207149-157. [DOI] [PubMed] [Google Scholar]
- 19.Fulco, A. J. 1983. Fatty acid metabolism in bacteria. Prog. Lipid Res. 22133-160. [DOI] [PubMed] [Google Scholar]
- 20.Gao, H., A. Obraztova, N. Stewart, R. Popa, J. K. Fredrickson, J. M. Tiedje, K. H. Nealson, and J. Zhou. 2006. Shewanella loihica sp. nov., isolated from iron-rich microbial mats in the Pacific Ocean. Int. J. Syst. Evol. Microbiol. 561911-1916. [DOI] [PubMed] [Google Scholar]
- 21.Gao, H., Z. K. Yang, L. Wu, D. K. Thompson, and J. Zhou. 2006. Global transcriptome analysis of the cold shock response of Shewanella oneidensis MR-1 and mutational analysis of its classical cold shock proteins. J. Bacteriol. 1884560-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, L., S. Lobo, and K. A. Reynolds. 1998. Characterization of beta-ketoacyl-acyl carrier protein synthase III from Streptomyces glaucescens and its role in initiation of fatty acid biosynthesis. J. Bacteriol. 1804481-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazel, J. R., and E. E. Williams. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29167-227. [DOI] [PubMed] [Google Scholar]
- 24.Hosie, A. H., and P. S. Poole. 2001. Bacterial ABC transporters of amino acids. Res. Microbiol. 152259-270. [DOI] [PubMed] [Google Scholar]
- 25.Kaneda, T. 1966. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can. J. Microbiol. 12501-514. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda, T. 1977. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol. Rev. 41391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlin, S. 2001. Detecting anomalous gene clusters and pathogenicity islands in diverse bacterial genomes. Trends Microbiol. 9335-343. [DOI] [PubMed] [Google Scholar]
- 28.Kato, C., and Y. Nogi. 2001. Correlation between phylogenetic structure and function: examples from deep-sea Shewanella. FEMS Microbiol. Ecol. 35223-230. [DOI] [PubMed] [Google Scholar]
- 29.Li, S., X. Xiao, P. Sun, and F. Wang. 2008. Screening of genes regulated by cold shock in Shewanella piezotolerans WP3 and time course expression of cold-regulated genes. Arch. Microbiol. 189549-556. [DOI] [PubMed] [Google Scholar]
- 30.Lu, Y. J., Y. M. Zhang, and C. O. Rock. 2004. Product diversity and regulation of type II fatty acid synthases. Biochem. Cell Biol. 82145-155. [DOI] [PubMed] [Google Scholar]
- 31.Magnuson, K., S. Jackowski, C. O. Rock, and J. E. Cronan, Jr. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makemson, J. C., N. R. Fulayfil, W. Landry, L. M. Van Ert, C. F. Wimpee, E. A. Widder, and J. F. Case. 1997. Shewanella woodyi sp. nov., an exclusively respiratory luminous bacterium isolated from the Alboran Sea. Int. J. Syst. Bacteriol. 471034-1039. [DOI] [PubMed] [Google Scholar]
- 33.Mansilla, M. C., L. E. Cybulski, D. Albanesi, and D. de Mendoza. 2004. Control of membrane lipid fluidity by molecular thermosensors. J. Bacteriol. 1866681-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marr, A. G., and J. L. Ingraham. 1962. Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 841260-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metz, J. G., P. Roessler, D. Facciotti, C. Levering, F. Dittrich, M. Lassner, R. Valentine, K. Lardizabal, F. Domergue, A. Yamada, K. Yazawa, V. Knauf, and J. Browse. 2001. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293290-293. [DOI] [PubMed] [Google Scholar]
- 36.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 2746031-6034. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, D. S., J. L. Brown, P. D. Nichols, and T. A. McMeekin. 1997. Production of eicosapentaenoic acid and arachidonic acids by an Antarctic bacterium: response to growth temperature. FEMS Microbiol. 152349-354. [Google Scholar]
- 38.Nichols, D. S., T. A. McMeekin, and P. D. Nichols. 1994. Manipulation of polyunsaturated, branched-chain and trans-fatty acid production in Shewanella putrefaciens strain ACAM 342. Microbiology 140577-584. [Google Scholar]
- 39.Omura, S. 1981. Cerulenin. Methods Enzymol. 72520-532. [PubMed] [Google Scholar]
- 40.Overbeek, R., T. Begley, R. M. Butler, J. V. Choudhuri, H. Y. Chuang, M. Cohoon, V. de Crecy-Lagard, N. Diaz, T. Disz, R. Edwards, M. Fonstein, E. D. Frank, S. Gerdes, E. M. Glass, A. Goesmann, A. Hanson, D. Iwata-Reuyl, R. Jensen, N. Jamshidi, L. Krause, M. Kubal, N. Larsen, B. Linke, A. C. McHardy, F. Meyer, H. Neuweger, G. Olsen, R. Olson, A. Osterman, V. Portnoy, G. D. Pusch, D. A. Rodionov, C. Ruckert, J. Steiner, R. Stevens, I. Thiele, O. Vassieva, Y. Ye, O. Zagnitko, and V. Vonstein. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 335691-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puttman, M., N. Ade, and H. Hof. 1993. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 144279-283. [DOI] [PubMed] [Google Scholar]
- 42.Rock, C. O., and J. E. Cronan. 1996. Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim. Biophys. Acta 13021-16. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 44.Simonato, F., S. Campanaro, F. M. Lauro, A. Vezzi, M. D'Angelo, N. Vitulo, G. Valle, and D. H. Bartlett. 2006. Piezophilic adaptation: a genomic point of view. J. Biotechnol. 12611-25. [DOI] [PubMed] [Google Scholar]
- 45.Suutari, M., and S. Laakso. 1994. Microbial fatty acids and thermal adaptation. Crit. Rev. Microbiol. 20285-328. [DOI] [PubMed] [Google Scholar]
- 46.van Passel, M. W., A. C. Luyf, A. H. van Kampen, A. Bart, and A. van der Ende. 2005. Deltarho-web, an online tool to assess composition similarity of individual nucleic acid sequences. Bioinformatics 213053-3055. [DOI] [PubMed] [Google Scholar]
- 47.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49705-724. [DOI] [PubMed] [Google Scholar]
- 48.Wang, F., J. Wang, H. Jian, B. Zhang, S. Li, F. Wang, X. Zeng, L. Gao, D. H. Bartlett, J. Yu, S. Hu, and X. Xiao. 2008. Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PloS ONE. doi: 10.1371/journal.pone.0001937. [DOI] [PMC free article] [PubMed]
- 49.Wirsen, C. O., H. W. Jannasch, S. G. Wakeham, and E. A. Canuel. 1986. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr. Microbiol. 14319-322. [Google Scholar]
- 50.Xiao, X., P. Wang, X. Zeng, D. H. Bartlett, and F. Wang. 2007. Shewanella psychrophila sp. nov. and Shewanella piezotolerans sp. nov., isolated from west Pacific deep-sea sediment. Int. J. Syst. Evol. Microbiol. 5760-65. [DOI] [PubMed] [Google Scholar]
- 51.Yano, Y., A. Nakayama, K. Ishihara, and H. Saito. 1998. Adaptive changes in membrane lipids of piezophilic bacteria in response to changes in growth pressure. Appl. Environ. Microbiol. 64479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yayanos, A. A. 1998. Empirical and theoretical aspects of life at high pressure in the deep sea, p. 47-92. In K. Horikoshi and W. D. Grant (ed.), Extremophiles, microbial life in extreme environments. Wiley-Liss, New York, NY.
- 53.Yayanos, A. A., and A. S. Dietz. 1982. Thermal inactivation of a deep-sea barophilic bacterium, isolate CNPT-3. Appl. Environ. Microbiol. 431481-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, J. S., D. Manno, C. Beaulieu, L. Paquet, and J. Hawari. 2005. Shewanella sediminis sp. nov., a novel Na+-requiring and hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading bacterium from marine sediment. Int. J. Syst. Evol. Microbiol. 551511-1520. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, K., X. Ding, M. Julotok, and B. J. Wilkinson. 2005. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl. Environ. Microbiol. 718002-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]