Abstract
We performed a comparative analysis of the internalization mechanisms used by three viruses causing important vesicular diseases in animals. Swine vesicular disease virus (SVDV) internalization was inhibited by treatments that affected clathrin-mediated endocytosis and required traffic through an endosomal compartment. SVDV particles were found in clathrin-coated pits by electron microscopy and colocalized with markers of early endosomes by confocal microscopy. SVDV infectivity was significantly inhibited by drugs that raised endosomal pH. When compared to foot-and-mouth disease virus (FMDV), which uses clathrin-mediated endocytosis, the early step of SVDV was dependent on the integrity of microtubules. SVDV-productive endocytosis was more sensitive to plasma membrane cholesterol extraction than that of FMDV, and differential cell signaling requirements for virus infection were also found. Vesicular stomatitis virus, a model virus internalized by clathrin-mediated endocytosis, was included as a control of drug treatments. These results suggest that different clathrin-mediated routes are responsible for the internalization of these viruses.
The ways in which viruses are internalized into host cells to initiate a productive infection are varied. Viruses gaining entry via endocytosis hijack components of different pathways used by the cell to internalize ligands such as nutrients and signaling molecules. The best-characterized route is classical clathrin-mediated endocytosis, in which ligands are internalized in clathrin-coated pits (CCPs) and subsequently sorted into early endosomes that can mature into late endosomes and lysosomes (71). Distinct CCP subpopulations, defined by using different, specific adaptor proteins, have recently been reported (7). Other non-clathrin-mediated endocytosis routes, dependent on plasma membrane cholesterol-enriched microdomains called lipid-rafts, have been also characterized (50). These lipid-rafts are enriched in glycosylphosphatidylinositol- anchored proteins and, when containing caveolin-1, this pathway is commonly referred as caveola-mediated endocytosis. In addition, viruses using alternative entry pathways that do not correspond to clathrin or lipid-raft/caveola-mediated endocytosis have been reported (11, 50, 77).
Virus infection is a reproducible and easily measurable event, so viruses can act as useful endocytic tracers (65). In fact, much of the information about caveolae-mediated endocytosis has been obtained from the study of virus entry and infection (63). Recent reports show that closely related viruses can enter into the cell via different pathways, whereas viruses of different families can exploit similar mechanisms, highlighting the importance of understanding the cell biology of virus entry (19). Comparative studies of the internalization of different viruses have revealed differential dependence on dynamin, an endocytosis-associated protein (24), as well as the differential involvement of Rab5 and Rab7 GTPases in intracellular virus trafficking and infection (76). Other studies comparing ligands for caveola-mediated endocytosis, such as that seen with viruses and cholera toxin, have uncovered diversity in caveola-mediated endocytosis routes (64, 66). In the present study, we have analyzed the internalization pathway of swine vesicular disease virus (SVDV), and we have performed a comparative analysis with foot-and-mouth disease virus (FMDV) and vesicular stomatitis virus (VSV). SVDV is a porcine pathogen and a member of the Picornaviridae family; it is included into the Enterovirus genus and is closely related to the human pathogen coxsackievirus B5 (CVB5) (91). FMDV is a typical species of the Aphthovirus genus within the Picornaviridae family (80), and VSV is a member of the genus Vesiculovirus of the family Rhabdoviridae (70). Despite the marked differences exhibited by the three viruses at the taxonomical level and in their requirements to organize replication complexes, these viruses share similar growth kinetics on IBRS-2 cells, and the vesicular lesions and clinical signs of the disease they produce in natural hosts are so similar as to require a differential diagnosis (2, 51).
SVDV and FMDV virions are composed of an icosahedral capsid of about 26 nm in diameter, built up with 60 copies of each of the four structural proteins (VP1 to VP4). The capsid contains a single-stranded RNA molecule of positive polarity that constitutes the viral genome (27, 82, 87). VSV is a negative-stranded RNA virus whose virions are much more complex and enveloped by lipid bilayers (70).
SVDV can infect cultured cells using heparan sulfate (HS) proteoglycans as surface receptors, via the coxsackievirus-and-adenovirus receptor (CAR), and some isolates retain the ability to bind human decay-accelerating factor (DAF) (26, 34). After attachment to the cell, SVDV particles undergo receptor-induced conformational alterations that expose the N-terminal portion of VP1 that is internal within the capsid (33); this interaction with CAR is responsible for the conformational capsid rearrangements in related coxsackieviruses (55). Internalization through different endocytic pathways has been reported among the SVDV-related coxsackieviruses. Thus, a clathrin-dependent mechanism is exploited by a CVB3 strain that only uses CAR as a cell receptor (18), and a caveola-mediated, dynamin-independent route that shares common features with macropinocytosis has been reported for another CVB3 strain that uses both DAF and CAR as cell receptors (15, 16). Also, a CVB3 variant entering through HS has been shown to be internalized in a pH-dependent way (90), whereas a CVB4 strain interacting with DAF and CAR is endocytosed via a lipid-raft-dependent mechanism (85).
In contrast, FMDV binds different αv integrins as surface receptors, specifically αvβ8 in IBRS-2 cells, the cell line used in the present study (12), and it is internalized following the clathrin endocytic route (8, 52, 61). Early work suggested phosphatidylserine as the VSV cell receptor (72), but recent observations do not support this hypothesis (13). However, it is well established that VSV is internalized through clathrin-mediated endocytosis and has become a model virus internalized through this route (36, 52, 54, 84).
Our results indicate that SVDV is internalized into the cell by a mechanism which can be inhibited by treatments that affect clathrin-mediated endocytosis and also requires traffic through an endosomal compartment for penetration. Supporting this hypothesis, SVDV particles were found in CCPs in electron microscopy studies, colocalized with markers of early endosomes by confocal microscopy, and SVDV infectivity could be significantly inhibited by drugs that raise endosomal pH. The early step of SVDV and VSV infection—but not that of FMDV—was dependent on the integrity of microtubules, and SVDV-productive endocytosis was more sensitive to plasma membrane cholesterol extraction than those of FMDV and VSV. Taken together, these results suggest that different endosomal routes are responsible for the internalization of these viruses into different populations of early endosomes.
MATERIALS AND METHODS
Cells and viruses.
IBRS-2 cells (22) and BHK-21 cells (ATCC) were maintained in Dulbecco modified Eagle medium (DMEM) (Gibco-BRL) supplemented with 5% fetal calf serum (Sigma), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). SVDV SPA/1/′93 isolate was grown on IBRS-2 cells. Type C FMDV C-S8c1 (81) and VSV Indiana serotype viral stocks were prepared by amplification in BHK-21 cells. Type C FMDV C-S8c1 isolate entered cells using αv integrins, but not HS (6, 53, 59). Nucleotide sequencing confirmed the absence of mutations responsible for HS binding acquisition (6) in the viral stock used in the present study, which was unable to infect CHO cells that do not express integrin receptors for FMDV but are susceptible to infection by FMDV mutants that bind HS (6; data not shown).
Antibodies and reagents.
The following antibodies against viral proteins were used: monoclonal antibody (MAb) I1 anti-VSV G protein (47), MAb 2H12 against VP1 SVDV (9), rabbit polyclonal antiserum against VP1 SVDV (33), and MAb 5C4 against FMDV antigenic site D (45). MAb against EEA1, rabbit polyclonal antiserum against caveolin-1, and a phospho-specific MAb against Tyr14-phosphorylated caveolin-1 were from BD Transduction Labs. MAbs against tubulin (DM1A) and β-actin (AC-15) were from Sigma. A rabbit polyclonal anti-βII tubulin antiserum (4) and MAb 25H8 to the cis-Golgi marker protein gp74 (1) were also used. Alexa Fluor 488-conjugated phalloidin; Alexa Fluor 488-conjugated transferrin (TF); Alexa Fluor 488-, 594-, or 555-labeled goat anti-mouse immunoglobulin G (IgG); and Alexa Fluor 488- or 594-labeled goat anti-rabbit IgG were purchased from Invitrogen. Anti-mouse and anti-rabbit horseradish peroxidase-coupled IgG secondary antibodies were from GE Healthcare.
Bisindolmaleymide I (Bis), cytochalasin D (Cyt D), concanamycin A, filipin III, genistein, methyl-β-cyclodextrin (MβCD), α-cyclodextrin (αCD), lovastatin, nocodazole, wortmannin, chlorpromazine (Cpmz), and sodium orthovanadate (OV) were from Sigma. PP2 in solution was purchased from Calbiochem. Sucrose and ammonium chloride (NH4Cl) were from Merck. Bis, Cyt D, concanamycin A, filipin, Geneticin, lovastatin, nocodazole, wortmannin, and dynasore were dissolved in dimethyl sulfoxide. OV and NH4Cl were dissolved in water. Cpmz, sucrose, MβCD, and αCD were directly prepared in DMEM.
Drug treatments.
Preinfection analyses were performed in the presence of drugs before infection (pretreatment) and during the whole infection period (except for sucrose, concanamycin A, MβCD, and αCD), while for postinfection analyses the drug was added 3 h postinfection. Monolayers of IBRS-2 cells grown on tissue culture plates were extensively washed with DMEM and pretreated for 30 min with Bis (5 μM), Cyt D (1 μM), filipin (1 μg/ml), concanamycin A (1,000 nM), dynasore (80 μM), genistein (100 μM), MβCD (10 mM), αCD (10 mM), nocodazole (40 μM), wortmannin (200 nM), Cpmz (30 μM), OV (1 mM), or sucrose (0.45 M) or for 1 h in the case of NH4Cl (25 or 50 mM in 25 mM HEPES [pH 7.4]), PP2 (10 μM), lovastatin (5 μM), or nystatin (25 μg/ml). For sucrose and concanamycin A treatment, the drug was maintained only during pretreatment and the first infection hour or TF internalization period. MβCD and αCD were only present during 30 min preinfection. The rest of the drugs were maintained throughout the infection to avoid cellular recovery. To evaluate the effect of the treatments on virus replication (postinfection), all of the drugs were added 3 h postinfection and maintained during the rest of infection, except MβCD, sucrose and concanamycin A, which were removed 0.5 and 1.5 h after their addition, respectively. Control cells were incubated in parallel with the same volume of drug solvent.
Infections and virus titrations.
Triplicate wells of IBRS-2 cells pretreated or not with the drugs were infected with VSV, SVDV, or FMDV using a multiplicity of infection (MOI) of 0.5 PFU/cell in experiments measuring the productive virus infection or an MOI of 100 PFU/cell in experiments tracking virus entry by immunofluorescence or to determine caveolin-1 phosphorylation. After the first infection hour, the viral inoculum was removed, and fresh medium containing 5% fetal calf serum was added (this time point was considered 1 h postinfection). For dynasore treatment, infections were performed in medium without serum since this drug binds to serum proteins, losing activity (49). Seven hours later, cells were subjected to three freeze-thaw cycles, and the total (intracellular and medium-released) virus yield was determined by plaque assay (in BHK-21 cells for VSV and FMDV or in IBRS-2 cells for SVDV), as described previously (81). Infected monolayers were also fixed and processed for immunofluorescence to determine the number of infected cells (51). Experiments in which the first hour of infection was carried out on ice to allow virus adsorption, but not internalization, are indicated in the figure legends.
TEM.
Transmission electron microscopy (TEM) was performed as described previously (51). IBRS-2 cells grown on 100-mm tissue culture plates were incubated with VSV (MOI of 300) or SVDV (MOI of 63) for 1 h on ice and subsequently transferred to 37°C for 5 min. Cells were washed with phosphate-buffered saline (PBS) and fixed for 30 min at 37°C in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) supplemented with 5 mM CaCl2, postfixed in 1% osmium tetroxide plus 1% potassium ferrocianure for 1 h at 4°C, and treated with 0.15% tanic acid in phosphate buffer (pH 7.4) for 1 min. Samples were stained with 2% uranyl acetate for 1 h at room temperature, dehydrated in ethanol according to standard protocols, and embedded in TAAB 812 resin (TAAB Laboratories). Samples were examined with a JEOL JEM-1010 electron microscope operating at 80 kV, and images were acquired by using a digital camera Bioscan792 (Gatan).
Immunofluorescence and confocal microscopy.
Cells grown on glass coverslips were washed with PBS, fixed in 4% paraformaldehyde for 15 min at room temperature (or methanol fixed for Golgi staining), blocked, and permeabilized with PBTG buffer (0.1% Triton X-100, 1% bovine serum albumin [BSA], and 1 M glycine in PBS) for 15 min at room temperature. Samples were incubated with primary antibodies diluted in 1% BSA in PBS for 1 h at room temperature, washed with PBS, and incubated with secondary antibodies 30 min at room temperature. After washing with PBS, nuclei were stained with 1 μg of DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen)/ml or by using To-Pro-3 (Invitrogen) in the case of confocal microscopy, and the samples were mounted in Fluoromount G (Southern Biotech). To determine the number of infected cells, three coverslips per treatment were examined, counting at least 300 cells per coverslip.
Samples were observed by using an Olympus BX61 epifluorescence microscope coupled to a digital camera DP70 and acquired by using Olympus DP controller software. Confocal laser scanning microscopy was performed with an Axiovert S100 TV microscope (Zeiss) coupled to a confocal Radiance 2000 system (Bio-Rad) using Lasersharp2000 5.2 software (Bio-Rad) for image acquisition. Sequential laser scanning for different laser lines was performed to avoid false colocalization. Images were processed using Adobe Photoshop 7.0.1 (Adobe Systems, Inc.).
For quantification of colocalization, the percentage of EEA1-positive structures (red) containing SVDV (green) was calculated by counting the number of EEA1-positive structures containing yellow pixels, divided by the total number of EEA1 spots in at least three different cells for each time. The same process was performed for SVDV colocalization with TF.
TF internalization assays.
Cells grown on coverslips were incubated for 15 min with 10 μg of TF-Alexa Fluor 488/ml in DMEM containing 0.5% BSA. To evaluate the effect of sucrose and Cpmz in TF endocytosis, the cells were washed with 200 mM acetic acid and 200 mM NaCl and sequentially with 150 mM NaCl, 20 mM HEPES (pH 7.4), 10 mM glucose, 5 mM KCl, 1 mM MgCl2, and 1 mM CaCl2 to eliminate extracellular TF (48) prior to fixation. Aldehyde fluorescence was quenched by incubating cells 15 min with 1 M glycine in PBS. Images of TF in cells treated or not with the drugs were acquired using the same exposure time and detector sensitivity.
AO vital staining.
Acridine orange (AO) vital staining (52) was used to qualitatively evaluate the effects of NH4Cl treatment on acidic organelles. Cells treated as described above were incubated in fresh medium, containing or not NH4Cl, and 5 μg of AO/ml at 37°C for 10 min, washed three times with the same medium without AO, and observed under a fluorescence microscope. AO fluorescence is red when it protonates and accumulates into acidic organelles.
Filipin staining.
Detection of cellular cholesterol by filipin staining was performed as previously reported (39). Briefly, cells treated or not with MβCD were fixed on ice and incubated with 125 μg of filipin/ml in PBS at room temperature, washed twice with PBS, and mounted in Fluoromount G. Images of cells treated or not with MβCD were acquired using the same exposure time and detector sensitivity. The fluorescence intensity of filipin staining in control and MβCD-treated cells was quantified with ImageJ software (http://rsbweb.nih.gov/ij/).
Western blot.
IBRS-2 cells grown on 35-mm tissue culture plates were scraped on ice into NP-40 lysis buffer (10 mM EGTA, 2.5 mM MgCl2, 1% NP-40, 20 mM HEPES [pH 7.4]) and sonicated. Protein concentration was determined by Bradford method, and equal amounts of protein mixed with Laemmli sample buffer were boiled, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane. The membrane was blocked, and proteins were detected by incubation with primary antibodies and with horseradish peroxidase-coupled secondary antibodies using a chemiluminescence kit (Perkin-Elmer) as previously described (28).
Data analysis.
To probe statistical significance of the data, one-way analysis of the variance was performed with statistical package SPSS 13.0 (SPSS, Inc.) for Windows. For multiple comparisons, Bonferroni's correction was applied. The data are presented as means ± the standard deviations, and statistically significant differences between control treatments and drug treatments are indicated in the figures by one or two asterisks (corresponding to P < 0.05 or P < 0.005, respectively).
RESULTS
SVDV is internalized through clathrin-mediated endocytosis.
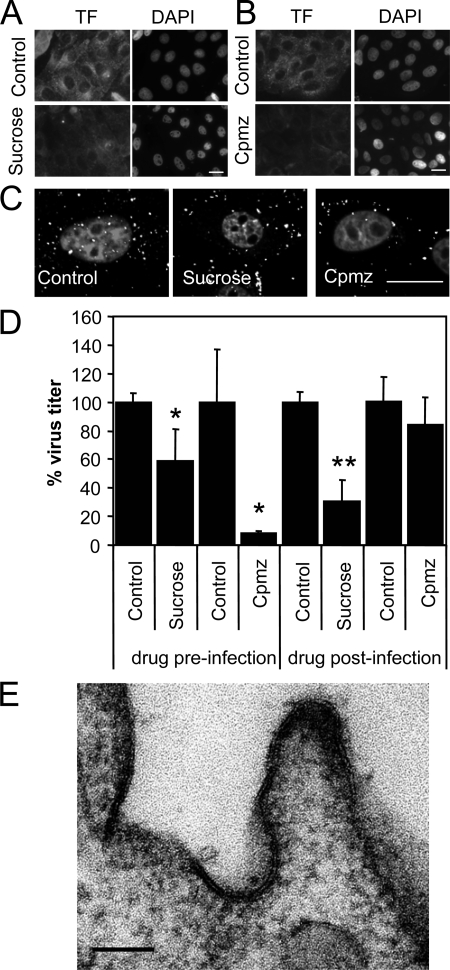
To test the role of CCPs in SVDV endocytosis, cells were treated with a hypertonic medium containing 0.45 M sucrose. This treatment has been shown to inhibit CCP assembly (30) and to reduce infection by viruses that use clathrin-mediated endocytosis (8, 38). Sucrose treatment reduced the ability of IBRS-2 cells to internalize TF (Fig. 1A), a ligand known to utilize clathrin-mediated endocytosis (29, 56). Cpmz interferes with plasma membrane CCP formation (88) and inhibits virus infection that uses clathrin mediated-endocytosis, including VSV and FMDV (36-38, 60, 61, 84). Treatment with Cpmz reduced TF internalization (Fig. 1B). As estimated by confocal microscopy, sucrose and Cpmz reduced internalization of SVDV into the cells but did not impair virus binding (Fig. 1C), since fluorescence corresponding to virions is observed at the plasma membrane. Preinfection treatment with sucrose reduced SVDV (40%) virus production (Fig. 1D). In this assay, sucrose was not present during the postinfection period; therefore, the effect observed is likely due to inhibition of entry. However, when sucrose was added 3 h postinfection, a virus titer reduction was also observed, indicating that hypertonic medium affects also virus replication. Preinfection treatment with Cpmz caused a marked inhibition of SVDV yield (Fig. 1D). When Cpmz was added postinfection, reduction in SVDV infection was not statistically significant. These results confirm that the drug was affecting an early step of SVDV infection cycle. Attempts to use more specific inhibitors such as dominant-negative Eps15, dynamin II, or Rab5 resulted in very low levels of transfection of IBRS-2 cells that impaired the analyses.
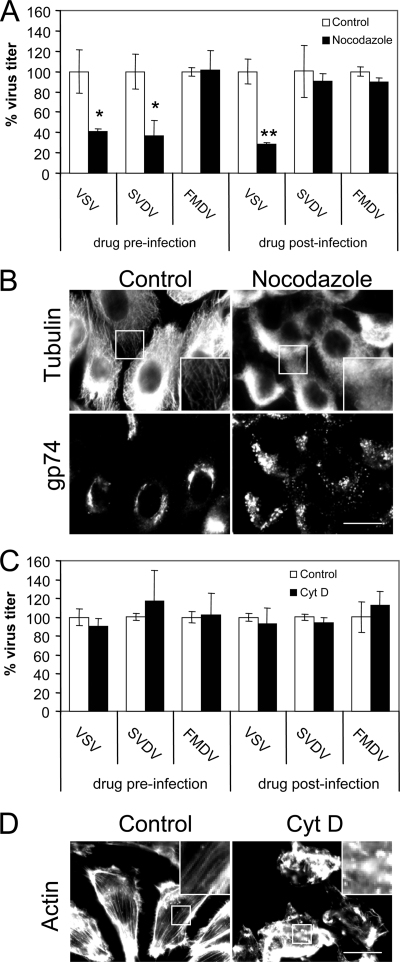
FIG. 1.
Role of clathrin for SVDV endocytosis in IBRS-2 cells. (A) Hypertonic medium inhibits TF endocytosis. Cells pretreated 30 min with sucrose were incubated 15 min with Alexa Fluor 488-labeled TF maintaining the sucrose, acid washed to eliminate extracellular TF, and fixed. (B) Cpmz inhibits TF endocytosis. Cells pretreated 30 min with chlorpromazine were incubated as for panel A with fluorescent TF. (C) Sucrose and Cpmz inhibit internalization of SVDV. Confocal sections of IBRS-2 cells were pretreated with sucrose or Cpmz and incubated with SVDV (MOI of 100) for 25 min at 37°C. SVDV was detected by immunofluorescence staining with rabbit polyclonal antiserum against VP1 and a secondary antibody labeled with Alexa Fluor 488. Nuclei were stained with To-Pro-3. (D) Effect of sucrose and Cpmz treatment on SVDV infection determined by plaque assay. (E) TEM of SVDV-infected cells. IBRS-2 cells were incubated with SVDV (MOI of 300) for 1 h on ice prior to incubation for 5 min at 37°C, fixed, and processed for electron microscopy. White bars (A to C), 20 μm; black bar (E), 100 nm. Statistically significant differences between control and drug treatments are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.005).
SVDV particle location during the internalization process was studied by means of TEM. Electrodense, spherical virus-like particles ∼26 nm in diameter, corresponding to SVDV virions, were located in the CCPs (ca. 84% of the particles undergoing endocytosis), and a representative example is shown in Fig. 1E. These particles were not found in mock-infected cells. The lack of antibodies suitable for gold-labeling impaired detection by TEM immunostaining of viral capsid proteins in these particles. Taken together, these results support a clathrin-mediated endocytosis for SVDV internalization.
Infection of SVDV requires transit through acidic endosomes.
After internalization, viruses release their genetic material penetrating into the host cell cytoplasm and leading to a productive infection. A common uncoating mechanism used by viruses entering trough CCP-mediated endocytosis depends on endosomal acidification (50, 58, 66). Pretreatment with NH4Cl resulted in a neutralization of intralumenal pH within acidic organelles, as estimated by the lack of detection of red spots corresponding to acidic organelles labeled by AO vital staining (Fig. 2A). As reported earlier (52, 54), preinfection treatment with NH4Cl significantly reduced VSV and to a lower extent FMDV titers, as well as the percentage of infected cells (Fig. 2B). This compound also reduced SVDV infection in a dose-dependent manner. Postinfection addition of NH4Cl had no effect on virus infection (data not shown), suggesting that a transit through an acidic compartment is required for the productive entry of these three viruses. Indeed, pretreatment with concanamycin A, a potent and specific inhibitor of the vacuolar ATPase (25), reduced SVDV infection (Fig. 2C); this was not observed when this drug was added postinfection (data not shown; for a comparison of the effects of concanamycin A in FMDV and VSV infection, see reference 52). These results led us to study SVDV infection by confocal microscopy. At very early times postinfection (5 min) most of the virus was not internalized yet and appeared attached to the cell membrane. After 15 min, virus was observed inside the cell, and the viral fluorescence colocalized (16.6% ± 2%) with that of early endosomes positive for EEA1 (Fig. 2D, circles). The percentage of early endosomes containing SVDV was reduced with the infection progress (45 min), indicating particle translocation to a different cell compartment (Fig. 2E). SVDV particles also colocalized with TF (18% ± 6%) (Fig. 2F, circles), and endosomes loaded with SVDV could be observed close to microtubules (Fig. 2F, arrowheads). Often, these microtubule-associated endosomes were negative for TF. Taken together, these results suggest that SVDV-productive infection requires transit through acidic endosomes for penetration.
FIG. 2.
Dependence on acidic endosomes for virus penetration. (A) Evaluation of treatment with increasing NH4Cl concentrations on acidic intracellular compartments by AO vital staining. AO stains in red acidic organelles and nuclei in green. (B) Effects of pretreatment with different concentrations of NH4Cl (0, 25, or 50 mM) on virus infection analyzed by plaque assay and by determination of the percentage of infected cells in immunofluorescence studies using I1, 2H12, and 5C4 antibodies to detect VSV, SVDV, and FMDV, respectively. Appropriate secondary antibodies coupled to Alexa Fluor 488 were used for detection of virus-specific antibodies. (C) Effects of pretreatment with concanamycin A on SVDV infection studied by plaque assay. (D) Colocalization assay of SVDV with the early endosomes marker EEA1. Cells were infected with SVDV (MOI of 100) for 1 h on ice and then transferred to 37°C 15 or 45 min, fixed, and processed for immunofluorescence using a mouse MAb to stain EEA1 (red) and a rabbit polyclonal antiserum to detect SVDV VP1 (green). Colocalization is shown in yellow (circles). Anti-mouse IgG secondary antibodies labeled with Alexa Fluor 594 and anti-rabbit IgG Alexa Fluor 488-coupled secondary antibodies were used to detect primary antibodies. (E) Quantification of colocalization between EEA1 and SVDV. (F) Colocalization assays of SVDV with TF and microtubules. Cells were infected as in panel D in combination with TF for 1 h on ice, transferred to 37°C 15 min, fixed, and processed for immunofluorescence using a rabbit polyclonal antiserum against SVDV VP1 and a mouse MAb against tubulin. Anti-mouse Alexa Fluor 555-labeled and anti-rabbit Alexa Fluor 647-labeled secondary antibodies were used. Alexa Fluor 488-labeled TF is shown in green, tubulin is shown in red, and SVDV is shown in blue. Colocalization between blue and green appears in aqua (circles) in the merged image. Arrowheads point to SVDV loaded endosomes in close association with microtubules. Statistically significant differences between control and drug treatments are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.005).
Differential cytoskeleton requirements for intracellular sorting of SVDV, VSV, and FMDV.
A pre-early endosome sorting that begins at the CCP, segregating cargo into two different early endosome populations has been recently described (43). One population corresponds to static and not microtubule-associated endosomes, while in the other the endosomes are dynamic and highly mobile along microtublules containing ligands that are directed preferentially to late endosomes/lysosomes. The proximity of SVDV particles to microtubules observed at early infection times (Fig. 2F) prompted us to study the requirement of intact microtubules for virus infection. When added preinfection (Fig. 3A), the microtubule-disrupting agent nocodazole reduced VSV and SVDV infection by 60%, while, as reported (8), it did not affect FMDV infection. When nocodazole was added postinfection, SVDV titers were not reduced, suggesting that microtubules play an important role at an early infection stage. Conversely, VSV infection was reduced at a level similar to that when the drug was added postinfection, indicating that VSV can be affected by microtubule disruption at later infection stages; this is likely due to the dependence on an intact secretory pathway for VSV infection progress (21, 51, 68). In fact, microtubule depolymerization induced by nocodazole (Fig. 3B) also caused Golgi fragmentation as estimated by immunostaining of the cis-Golgi marker gp74, which was dispersed in nocodazole treated cells.
FIG. 3.
Differential cytoskeleton requirements for intracellular sorting of viruses. (A) Effects of nocodazole on VSV, SVDV, and FMDV infection studied by plaque assay. (B) Evaluation of nocodazole treatment on microtubule and Golgi integrity. Cells treated or not with nocodazole were fixed and processed for immunofluorescence using rabbit polyclonal antiserum against tubulin and MAb 25H8 against cis-Golgi protein gp74. Anti-rabbit Alexa Fluor 488-coupled and anti-mouse Alexa Fluor 594-coupled secondary antibodies were used. Bar, 20 μm. (C) Effects of Cyt D on virus infection studied by plaque assay. (D) Evaluation of Cyt D treatment on actin cytoskeleton integrity. Cells treated or not with Cyt D were fixed and processed for immunofluorescence using Alexa Fluor 488-labeled phalloidin to stain the cellular actin. Bar, 20 μm. Statistically significant differences between control and drug treatments are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.005).
Disruption of actin cytoskeleton with Cyt D (which was maintained throughout the assay to avoid cellular recovery) did not induce any significant effect on VSV, SVDV, or FMDV infection (Fig. 3C). As expected, this compound resulted in actin depolymerization (Fig. 3D). These results indicate a dependence on microtubule integrity for SVDV but not for FMDV internalization. On the other hand, disruption of actin cytoskeleton does not affect the infection of any of these three viruses.
Dynamin and differential signaling events are required for SVDV, VSV, and FMDV infection.
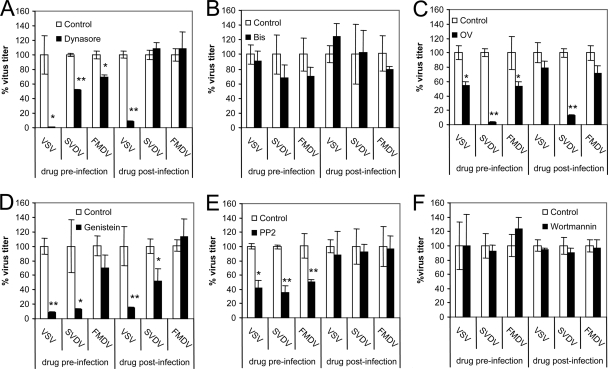
Dynamin is a GTPase involved in several types of endocytosis, including the clathrin-mediated pathway. Preinfection treatment with dynasore, a cell-permeable inhibitor of dynamin function (42, 49), inhibited the virus yield recovered upon infection of SVDV and FMDV (Fig. 4A). When added postinfection, dynasore did not inhibit SVDV and FMDV infection, indicating the requirement for dynamin is present at an early step of infection. As previously reported (36), dynasore inhibited VSV infection when added preinfection. Our results show that when added postinfection dynasore also inhibited virus growth, possibly due to effects on intracellular vesicle traffic dependent on dynamin, as vesicle formation from the Golgi (31, 40). The requirement for an intact secretory pathway in VSV infection has been reported (21, 51, 68).
FIG. 4.
Dynamin and cell signaling requirements for virus infection. The effects of dynasore (A), Bis (B), OV (C), genistein (D), PP2 (E), and wortmannin (F) on VSV, SVDV, and FMDV infection were studied by plaque assay. The following treatments induced measurable reported effects: dynasore, inhibition of TF internalization (49); wortmannin, endosomal vacuolation (46); and OV, tyrosine phosphorylation (73). Statistically significant differences between control and drug treatments are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.005).
Internalization and intracellular trafficking are governed by complex signaling events, including a galaxy of cellular kinases and phosphatases (65). We next addressed the effect of different inhibitors on the infection of the viruses studied. Bis, an inhibitor of protein kinase C (PKC) (66), did not inhibit significantly virus entry or infection (Fig. 4B). On the other hand, inhibition of SVDV infection by the tyrosine phosphatase inhibitor OV (66) was higher for SVDV than for FMDV and VSV when OV was added preinfection (Fig. 4C). However, OV also inhibited SVDV replication when added postinfection, supporting the possibility that the differential dependence on tyrosine phosphates was mainly due to postinternalization events. In the case of VSV and FMDV, OV only reduced virus infection when added preinfection, indicating that this drug affected virus entry of these two viruses. The nonspecific tyrosine kinase inhibitor genistein (15, 66) inhibited infection of VSV and SVDV, but not of FMDV, when added pre- and postinfection (Fig. 4D) This indicates that the inhibitory effect of this drug may be related to inhibition of virus replication rather than virus entry. The specific inhibitor of SRC-family tyrosine kinases PP2 (15), inhibited VSV, SVDV, and FMDV when added preinfection but not postinfection, suggesting that SRC kinases were involved at an early infection step (Fig. 5E).
FIG. 5.
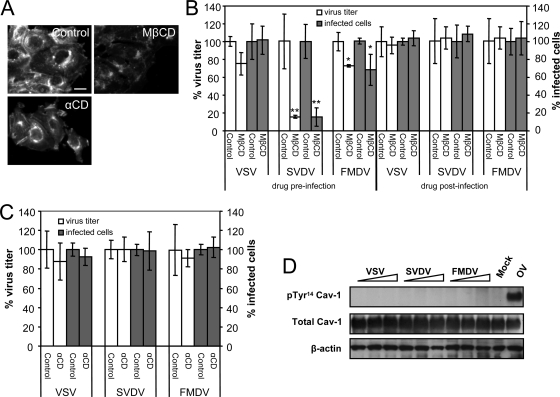
Differential requirements for cholesterol in virus infection. (A) Effects of MβCD and αCD on cellular cholesterol content of IBRS-2 cells studied by filipin staining. Bar, 20 μm. (B) Effect of cholesterol depletion by MβCD on virus infection evaluated by plaque assay and the percentage of infected cells in immunofluorescence studies using I1, 2H12, and 5C4 antibodies to detect VSV, SVDV, and FMDV, respectively, and appropriate secondary antibodies coupled to Alexa Fluor 488. (C) Effects of treatment with αCD on virus infection. (D) VSV, SVDV, and FMDV infections do not induce caveolin-1 phosphorylation on Tyr14. IBRS-2 cells were infected (MOI of 100) for 1 h on ice and then incubated for 15, 30, or 60 min at 37°C; lysed; and processed for Western blotting using MAb anti-Tyr14 phosphorylated caveolin-1, and polyclonal serum to caveolin-1 to detect total caveolin-1. An extract of IBRS-2 cells treated with 1 mM OV 60 min was included as a control of MAb anti-Tyr14 phosphorylated caveolin-1. Results obtained with β-actin are also shown as a control for protein loading. Statistically significant differences between control and drug treatments are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.005).
Inhibition of phosphatidylinositol 3-kinases with wortmannin prevents or delays ligand transfer from early to late endosomes (14). Preinfection treatment with wortmannin neither inhibited VSV and FMDV infection, as reported (8, 45), nor did it affect SVDV yields in IBRS-2 cells (Fig. 4F). This lack of effect was also observed when the drug was added postinfection. SVDV growth kinetics in the presence of wortmannin was also analyzed by plaque assay, revealing no delay in virus multiplication (data not shown). These results indicate that while dynamin is required for SVDV, VSV, and FMDV infection, differences exist in the signaling events that mediate the entry of these three viruses.
Differential cholesterol requirement for VSV, SVDV, and FMDV internalization.
Cholesterol is important for lipid-raft and caveola function and has also been implicated in clathrin-mediated endocytosis (69, 83). Plasma membrane cholesterol depletion induced by MβCD inhibits lipid-raft/caveola-dependent virus infection (66) and can also affect infection of viruses using clathrin-mediated endocytosis (52, 79, 86). In addition, cholesterol plays an important role for the endosomal penetration of different viruses (20, 32). To evaluate the role of cholesterol in the early infection steps of the viruses compared, we treated IBRS-2 cells with MβCD. As estimated by filipin staining, this drug extracted cholesterol from plasma membrane (40% ± 19% reduction in filipin staining intensity), leaving the intracellular cholesterol content intact (no reduction of filipin staining) (Fig. 5A). Treatment with αCD, a cyclodextrin that does not significantly extract cholesterol from plasma membrane (74), did not reduce filipin staining intensity.
Preinfection treatment with MβCD differentially affected virus infection (Fig. 5B). Although the VSV yield was not significantly modified, the SVDV and FMDV titers were reduced 80 and 25%, respectively, and these results correlated with the percentage of infected cells. When the drug was added postinfection, no effect was observed, confirming that MβCD affected an early step of infection (Fig. 5B). Preinfection treatment with αCD did not have any significant effect on VSV, SVDV, and FMDV infection (Fig. 5C), supporting that the inhibitory effect of MβCD was due to cholesterol depletion and not to other effects derived from the addition of a cyclodextrin.
As MβCD can inhibit virus infection through caveolae, we investigated the ability of these viruses to induce caveolin-1 phosphorylation on Tyr 14, an essential event reported for CVB3 caveola-mediated endocytosis (15) and caveola internalization (23). None of the virus tested induced caveolin-1 phosphorylation (Fig. 5D). The only positive signal was obtained in IBRS-2 cells treated with the tyrosine phosphatase inhibitor OV, whose addition results in caveolin-1 phosphorylation (73), used here as a control of the antibody used for detection of phosphorylated caveolin-1. In addition, treatment with nystatin, filipin, or lovastatin, which impair lipid-raft/caveola-mediated endocytosis (15, 85), did not inhibit SVDV or, as reported (52), VSV and FMDV infection (data not shown). These findings suggest that the role of cholesterol in SVDV and FMDV internalization is independent of caveolae.
DISCUSSION
In this study we have attempted a sequential and comparative analysis of the entry events of three important animal viruses by using drugs inhibiting different components of cell machinery involved in viral entry. A summary of the effects on SVDV, FMDV, and VSV entry of the drugs tested for all three viruses is shown in Table 1. Our results indicate that the SVDV isolate used here, which binds CAR but not DAF (34) and is able to interact with HS (26), is internalized through dynamin-dependent, clathrin-mediated endocytosis and transported to early endosomes, as indicated by its colocalization with EEA1 and TF. Infection requires endosomal acidification, as revealed by the inhibitory effect of NH4Cl and concanamycin A. Interestingly, infection inhibition by concanamycin A was different from that observed for NH4Cl. Previous work using FMDV indicated that NH4Cl block of endosomal acidification caused a higher reduction of virus infection than concanamycin A treatment (52). This may be explained by the different conditions of NH4Cl and concanamycin treatments; while NH4Cl was present throughout the infection, concanamycin was removed after the first infection hour. However, the effect of concanamycin A, in contrast to that observed for NH4Cl, is not readily reversible (57, 62). Another differential factor is that NH4Cl blockage of endosomal acidification is produced by neutralization of endosomal pH, whereas concanamycin inhibits the proton flux into the endosome but does not neutralize remaining acid pH within the endosomes.
TABLE 1.
Summary of effects on SVDV, FMDV, and VSV entry of the drugs testeda
| Cellular target | Drug | Virusb
|
||
|---|---|---|---|---|
| SVDV | FMDV | VSV | ||
| Dynamin | Dynasore | + | + | +PI |
| Endosomal pH | NH4Cl | + | + | + |
| Microtubules | Nocodazole | + | − | +PI |
| Actin microfilaments | Cyt D | − | − | − |
| Cell signaling machinery | OV | +PI | + | + |
| Genistein | +PI | − | +PI | |
| PP2 | + | + | + | |
| Bis | − | − | − | |
| Wortmannin | − | − | − | |
| Cholesterol | MβCD | + | + | − |
| Filipin | − | − | − | |
| Lovastatin | − | − | − | |
| Nystatin | − | − | − | |
Only drugs for which results with the three viruses compared were obtained are included.
+, Statistically significant inhibitory effect of the drug on virus infection only when added preinfection; +PI, the drug also had a statistically significant inhibitory effect when added postinfection; −, no statistically significant effect when added pre- or postinfection.
Macropinocytosis has been implicated in coxsackievirus entry (16). Our results indicate that SVDV, FMDV, and VSV infections depend on dynamin but not on PKC function (Fig. 4B) and actin cytoskeleton integrity (Fig. 3C), two of the important features of macropinocytosis (3, 16), suggesting that this route is not exploited by any of the viruses studied.
Interestingly, comparison of the SVDV internalization events to those of VSV and FMDV suggests diversity in the endosomal pathways that can be exploited by these three animal viruses that use clathrin to enter cells. It has been recently reported that FMDV mutants using HS as a receptor can be internalized via caveolae in contrast to FMDV isolates interacting with integrins, whose internalization is dependent on clathrin (60). The C-S8c1 isolate used in this study does not bind HS (6), ruling out this possibility (see Materials and Methods).
Differential endocytosis requirements included sensitivity to plasma membrane cholesterol depletion with MβCD, which was higher for SVDV than for FMDV and not significant in the case of VSV. This SVDV and FMDV cholesterol requirement was independent of caveolin-1 phosphorylation, and more specific drugs affecting the caveolin pathway (nystatin, filipin, and lovastatin) did not affect virus infection, suggesting that the cholesterol requirement observed is not related to caveola function. These results could be explained by an altered location of virus receptors on plasma membrane microdomains in MβCD-treated cells (52). Supporting this hypothesis, CAR has been shown to be associated to lipid-rafts distinct from those containing caveolin-1 and glycosylphosphatidylinositol-anchored proteins (5), and infection by type 2 adenovirus, a virus internalized by clathrin-mediated endocytosis that uses CAR as a cellular receptor, is inhibited by MβCD (32). However, we cannot exclude that the different cholesterol requirements of the three viruses studied could be due to distinct endosomal escape mechanisms resulting in viral penetration, since cholesterol can play a key role in endosomal escape of different viruses, and this can be affected by cholesterol extraction with MβCD (20, 32).
Because CCPs were first associated with VSV endocytosis in a process named viropexis (78), the role of clathrin and associated proteins in viral infection has been widely investigated. Internalization of ligands by clathrin CCPs has been proposed to rely on different signaling events (89). Indeed, genistein, a tyrosine kinase inhibitor, can differentially affect clathrin-mediated internalization of ligands (44). The differences found in our experiments in the sensitivity of the viruses studied to OV (phosphatase inhibitor) and genistein (kinase inhibitor) support this notion of signaling diversity. The ability of these two molecules producing opposite effects on Tyr phosphorylation to inhibit viral infection has been also reported for echovirus (66). These observations may be explained because the signaling events driving ligand internalization are very complex and requires both regulated phosphorylation and dephosphorylation of cellular components.
Different populations of CCPs with specific adaptor proteins have been identified (35, 41, 67). Lakadamyali et al. (43) showed by means of live cell imaging that ligands for clathrin-mediated endocytosis can be sorted, in a process that begins at CCP, into two populations of early endosomes that show different microtubule-mediated mobility. Our results, obtained from the effect of different drugs on viral infection, support this specialized cargo sorting into distinct endosome populations. Treatment with nocodazole showed that SVDV may be internalized to dynamic endosomes in contrast to FMDV. VSV dependence on microtubules for intracellular sorting has been previously described (46), suggesting that it is sorted into the dynamic endosome population, although it may infect from early and late endosomes (46, 76). Since TF traffics through early and recycling endosomes but not to late endosomes (43), the observation of SVDV particles close to microtubules but not associated to TF (Fig. 2F) suggests that SVDV can be sorted to a different cell compartment in a microtubule-dependent way. These results could be explained by the possibility of SVDV and VSV (but not FMDV) being transported to late endosomes/lysosomes for productive infection. However, this possibility was ruled out because of the lack of inhibition by wortmannin, a drug that inhibits early to late endosome transport, and by Bis, an inhibitor of PKC function that has been implicated in early to late endosome viral transport (8, 10, 17, 75).
Taken together, these results indicate that SVDV and VSV are sorted to a distinct population of early endosomes different from FMDV. This pre-early endosome sorting process may begin at plasma membrane CCP as reported for other ligands (43), thus segregating cargo into different CCP populations as a mechanism to reduce competition between diverse endocytic cargo (67). A better understanding of this emerging complexity will aid in the design of new antiviral compounds targeting the clathrin-mediated endocytosis machinery of host cells.
Acknowledgments
We thank V. Ley and M. A. Jiménez-Clavero for providing SVDV isolate SPA/1/'93 and with serum to SVDV VP1, M. Dávila and E. Domingo for VSV Indiana isolate and MAb I1, I. Sandoval for MAb 25H8, E. Brocchi for MAb 2H12, and M. T. Rejas for advice in the TEM studies. Dynasore was synthesized by H. E. Pelish and was kindly provided by T. Kirchhausen.
This study was supported by Spanish grants from CICYT (Bio2008-04487-C03-01) and MEC (CSD2006-0007) and by the Fundación Severo Ochoa.
Footnotes
Published ahead of print on 18 February 2009.
Dedicated to the memory of Rosario Armas-Portela.
REFERENCES
- 1.Alcalde, J., G. Egea, and I. V. Sandoval. 1994. gp74 a membrane glycoprotein of the cis-Golgi network that cycles through the endoplasmic reticulum and intermediate compartment. J. Cell Biol. 124649-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandersen, S., and N. Mowat. 2005. Foot-and-mouth disease: host range and pathogenesis. Curr. Top. Microbiol. Immunol. 2889-42. [DOI] [PubMed] [Google Scholar]
- 3.Amstutz, B., M. Gastaldelli, S. Kalin, N. Imelli, K. Boucke, E. Wandeler, J. Mercer, S. Hemmi, and U. F. Greber. 2008. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armas-Portela, R., M. A. Parrales, J. P. Albar, A. C. Martinez, and J. Avila. 1999. Distribution and characteristics of betaII tubulin-enriched microtubules in interphase cells. Exp. Cell Res. 248372-380. [DOI] [PubMed] [Google Scholar]
- 5.Ashbourne Excoffon, K. J., T. Moninger, and J. Zabner. 2003. The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain. J. Virol. 772559-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranowski, E., N. Sevilla, N. Verdaguer, C. M. Ruiz-Jarabo, E. Beck, and E. Domingo. 1998. Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J. Virol. 726362-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmerah, A., and C. Lamaze. 2007. Clathrin-coated pits: vive la difference? Traffic 8970-982. [DOI] [PubMed] [Google Scholar]
- 8.Berryman, S., S. Clark, P. Monaghan, and T. Jackson. 2005. Early events in integrin αvβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 798519-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrego, B., E. Carra, J. A. Garcia-Ranea, and E. Brocchi. 2002. Characterization of neutralization sites on the circulating variant of swine vesicular disease virus (SVDV): a new site is shared by SVDV and the related coxsackie B5 virus. J. Gen. Virol. 8335-44. [DOI] [PubMed] [Google Scholar]
- 10.Brabec, M., D. Blaas, and R. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348741-749. [DOI] [PubMed] [Google Scholar]
- 11.Brandenburg, B., L. Y. Lee, M. Lakadamyali, M. J. Rust, X. Zhuang, and J. M. Hogle. 2007. Imaging poliovirus entry in live cells. PLoS Biol. 5e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burman, A., S. Clark, N. G. Abrescia, E. E. Fry, D. I. Stuart, and T. Jackson. 2006. Specificity of the VP1 GH loop of foot-and-mouth disease virus for αv integrins. J. Virol. 809798-9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coil, D. A., and A. D. Miller. 2004. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 7810920-10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corvera, S. 2001. Phosphatidylinositol 3-kinase and the control of endosome dynamics: new players defined by structural motifs. Traffic 2859-866. [DOI] [PubMed] [Google Scholar]
- 15.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124119-131. [DOI] [PubMed] [Google Scholar]
- 16.Coyne, C. B., L. Shen, J. R. Turner, and J. M. Bergelson. 2007. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu, J. J., P. W. Leong, and M. L. Ng. 2006. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology 349463-475. [DOI] [PubMed] [Google Scholar]
- 18.Chung, S. K., J. Y. Kim, I. B. Kim, S. I. Park, K. H. Paek, and J. H. Nam. 2005. Internalization and trafficking mechanisms of coxsackievirus B3 in HeLa cells. Virology 33331-40. [DOI] [PubMed] [Google Scholar]
- 19.Damm, E. M., and L. Pelkmans. 2006. Systems biology of virus entry in mammalian cells. Cell Microbiol. 81219-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 7833-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das, S. C., D. Nayak, Y. Zhou, and A. K. Pattnaik. 2006. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J. Virol. 806368-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Castro, M. P. 1964. Behaviour of the foot-and-mouth disease virus in cell cultures: susceptibility of the IB-RS-2 cell line. Arq. Inst. Biol. Sao Paulo 3163-78. [Google Scholar]
- 23.del Pozo, M. A., N. Balasubramanian, N. B. Alderson, W. B. Kiosses, A. Grande-Garcia, R. G. Anderson, and M. A. Schwartz. 2005. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. 7901-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 174585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 2001-8. [DOI] [PubMed] [Google Scholar]
- 26.Escribano-Romero, E., M. A. Jimenez-Clavero, P. Gomes, J. A. Garcia-Ranea, and V. Ley. 2004. Heparan sulphate mediates swine vesicular disease virus attachment to the host cell. J. Gen. Virol. 85653-663. [DOI] [PubMed] [Google Scholar]
- 27.Fry, E. E., N. J. Knowles, J. W. Newman, G. Wilsden, Z. Rao, A. M. King, and D. I. Stuart. 2003. Crystal structure of Swine vesicular disease virus and implications for host adaptation. J. Virol. 775475-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Briones, M., M. F. Rosas, M. Gonzalez-Magaldi, M. A. Martin-Acebes, F. Sobrino, and R. Armas-Portela. 2006. Differential distribution of nonstructural proteins of foot-and-mouth disease virus in BHK-21 cells. Virology 349409-421. [DOI] [PubMed] [Google Scholar]
- 29.Hanover, J. A., M. C. Willingham, and I. Pastan. 1984. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell 39283-293. [DOI] [PubMed] [Google Scholar]
- 30.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinshaw, J. E. 2000. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16483-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imelli, N., O. Meier, K. Boucke, S. Hemmi, and U. F. Greber. 2004. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J. Virol. 783089-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez-Clavero, M. A., E. Escribano-Romero, A. J. Douglas, and V. Ley. 2001. The N-terminal region of the VP1 protein of swine vesicular disease virus contains a neutralization site that arises upon cell attachment and is involved in viral entry. J. Virol. 751044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimenez-Clavero, M. A., E. Escribano-Romero, V. Ley, and O. B. Spiller. 2005. More recent swine vesicular disease virus isolates retain binding to coxsackie-adenovirus receptor, but have lost the ability to bind human decay-accelerating factor (CD55). J. Gen. Virol. 861369-1377. [DOI] [PubMed] [Google Scholar]
- 35.Johannessen, L. E., N. M. Pedersen, K. W. Pedersen, I. H. Madshus, and E. Stang. 2006. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol. Cell. Biol. 26389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johannsdottir, H. K., R. Mancini, J. Kartenbeck, L. Amato, and A. Helenius. 2008. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 83440-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 751958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kee, S. H., E. J. Cho, J. W. Song, K. S. Park, L. J. Baek, and K. J. Song. 2004. Effects of endocytosis inhibitory drugs on rubella virus entry into VeroE6 cells. Microbiol. Immunol. 48823-829. [DOI] [PubMed] [Google Scholar]
- 39.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 1401357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessels, M. M., J. Dong, W. Leibig, P. Westermann, and B. Qualmann. 2006. Complexes of syndapin II with dynamin II promote vesicle formation at the trans-Golgi network. J. Cell Sci. 1191504-1516. [DOI] [PubMed] [Google Scholar]
- 41.Keyel, P. A., S. K. Mishra, R. Roth, J. E. Heuser, S. C. Watkins, and L. M. Traub. 2006. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell 174300-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchhausen, T., E. Macia, and H. E. Pelish. 2008. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 43877-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakadamyali, M., M. J. Rust, and X. Zhuang. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124997-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamaze, C., T. Baba, T. E. Redelmeier, and S. L. Schmid. 1993. Recruitment of epidermal growth factor and transferrin receptors into coated pits in vitro: differing biochemical requirements. Mol. Biol. Cell 4715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lea, S., J. Hernandez, W. Blakemore, E. Brocchi, S. Curry, E. Domingo, E. Fry, R. Abu-Ghazaleh, A. King, J. Newman, et al. 1994. The structure and antigenicity of a type C foot-and-mouth disease virus. Structure 2123-139. [DOI] [PubMed] [Google Scholar]
- 46.Le Blanc, I., P. P. Luyet, V. Pons, C. Ferguson, N. Emans, A. Petiot, N. Mayran, N. Demaurex, J. Faure, R. Sadoul, R. G. Parton, and J. Gruenberg. 2005. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 7653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefrancois, L., and D. S. Lyles. 1982. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology 121168-174. [DOI] [PubMed] [Google Scholar]
- 48.Lukacs, G. L., G. Segal, N. Kartner, S. Grinstein, and F. Zhang. 1997. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem. J. 328(Pt. 2)353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macia, E., M. Ehrlich, R. Massol, E. Boucrot, C. Brunner, and T. Kirchhausen. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10839-850. [DOI] [PubMed] [Google Scholar]
- 50.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Acebes, M. A., M. Gonzalez-Magaldi, M. F. Rosas, B. Borrego, E. Brocchi, R. Armas-Portela, and F. Sobrino. 2008. Subcellular distribution of swine vesicular disease virus proteins and alterations induced in infected cells: a comparative study with foot-and-mouth disease virus and vesicular stomatitis virus. Virology 374432-443. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Acebes, M. A., M. Gonzalez-Magaldi, K. Sandvig, F. Sobrino, and R. Armas-Portela. 2007. Productive entry of type C foot-and-mouth disease virus into susceptible cultured cells requires clathrin and is dependent on the presence of plasma membrane cholesterol. Virology 369105-118. [DOI] [PubMed] [Google Scholar]
- 53.Mateu, M. G., M. L. Valero, D. Andreu, and E. Domingo. 1996. Systematic replacement of amino acid residues within an Arg-Gly-Asp-containing loop of foot-and-mouth disease virus and effect on cell recognition. J. Biol. Chem. 27112814-12819. [DOI] [PubMed] [Google Scholar]
- 54.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1982. Pathway of vesicular stomatitis virus entry leading to infection. J. Mol. Biol. 156609-631. [DOI] [PubMed] [Google Scholar]
- 55.Milstone, A. M., J. Petrella, M. D. Sanchez, M. Mahmud, J. C. Whitbeck, and J. M. Bergelson. 2005. Interaction with coxsackievirus and adenovirus receptor, but not with decay-accelerating factor (DAF), induces A-particle formation in a DAF-binding coxsackievirus B3 isolate. J. Virol. 79655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller, K., M. Shipman, I. S. Trowbridge, and C. R. Hopkins. 1991. Transferrin receptors promote the formation of clathrin lattices. Cell 65621-632. [DOI] [PubMed] [Google Scholar]
- 57.Muroi, M., N. Shiragami, and A. Takatsuki. 1994. Destruxin B, a specific and readily reversible inhibitor of vacuolar-type H(+)-translocating ATPase. Biochem. Biophys. Res. Commun. 2051358-1365. [DOI] [PubMed] [Google Scholar]
- 58.Norkin, L. C., H. A. Anderson, S. A. Wolfrom, and A. Oppenheim. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 765156-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunez, J. I., N. Molina, E. Baranowski, E. Domingo, S. Clark, A. Burman, S. Berryman, T. Jackson, and F. Sobrino. 2007. Guinea pig-adapted foot-and-mouth disease virus with altered receptor recognition can productively infect a natural host. J. Virol. 818497-8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Donnell, V., M. Larocco, and B. Baxt. 2008. Heparan sulfate-binding foot-and-mouth disease virus enters cells via caveola-mediated endocytosis. J. Virol. 829075-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Donnell, V., M. LaRocco, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J. Virol. 798506-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 753327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelkmans, L. 2005. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 1746295-304. [DOI] [PubMed] [Google Scholar]
- 64.Pelkmans, L., T. Burli, M. Zerial, and A. Helenius. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118767-780. [DOI] [PubMed] [Google Scholar]
- 65.Pelkmans, L., E. Fava, H. Grabner, M. Hannus, B. Habermann, E. Krausz, and M. Zerial. 2005. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 43678-86. [DOI] [PubMed] [Google Scholar]
- 66.Pietiainen, V., V. Marjomaki, P. Upla, L. Pelkmans, A. Helenius, and T. Hyypia. 2004. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 154911-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puthenveedu, M. A., and M. von Zastrow. 2006. Cargo regulates clathrin-coated pit dynamics. Cell 127113-124. [DOI] [PubMed] [Google Scholar]
- 68.Rindler, M. J., I. E. Ivanov, H. Plesken, E. Rodriguez-Boulan, and D. D. Sabatini. 1984. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J. Cell Biol. 981304-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1224. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 71.Roth, M. G. 2006. Clathrin-mediated endocytosis before fluorescent proteins. Nat. Rev. Mol. Cell. Biol. 763-68. [DOI] [PubMed] [Google Scholar]
- 72.Schlegel, R., T. S. Tralka, M. C. Willingham, and I. Pastan. 1983. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell 32639-646. [DOI] [PubMed] [Google Scholar]
- 73.Shin, J., H. Jo, and H. Park. 2006. Caveolin-1 is transiently dephosphorylated by shear stress-activated protein tyrosine phosphatase mu. Biochem. Biophys. Res. Commun. 339737-741. [DOI] [PubMed] [Google Scholar]
- 74.Shvartsman, D. E., O. Gutman, A. Tietz, and Y. I. Henis. 2006. Cyclodextrins but not compactin inhibit the lateral diffusion of membrane proteins independent of cholesterol. Traffic 7917-926. [DOI] [PubMed] [Google Scholar]
- 75.Sieczkarski, S. B., H. A. Brown, and G. R. Whittaker. 2003. Role of protein kinase C βII in influenza virus entry via late endosomes. J. Virol. 77460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sieczkarski, S. B., and G. R. Whittaker. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4333-343. [DOI] [PubMed] [Google Scholar]
- 77.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 831535-1545. [DOI] [PubMed] [Google Scholar]
- 78.Simpson, R. W., R. E. Hauser, and S. Dales. 1969. Viropexis of vesicular stomatitis virus by L cells. Virology 37285-290. [DOI] [PubMed] [Google Scholar]
- 79.Snyers, L., H. Zwickl, and D. Blaas. 2003. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J. Virol. 775360-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sobrino, F., and E. Domingo (ed.). 2004. Foot-and-mouth disease: current perspectives. Horizon Bioscience, Norfolk, United Kingdom.
- 81.Sobrino, F., M. Davila, J. Ortin, and E. Domingo. 1983. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology 128310-318. [DOI] [PubMed] [Google Scholar]
- 82.Sobrino, F., M. Saiz, M. A. Jimenez-Clavero, J. I. Nunez, M. F. Rosas, E. Baranowski, and V. Ley. 2001. Foot-and-mouth disease virus: a long known virus, but a current threat. Vet. Res. 321-30. [DOI] [PubMed] [Google Scholar]
- 83.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 966775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 33853-60. [DOI] [PubMed] [Google Scholar]
- 85.Triantafilou, K., and M. Triantafilou. 2004. Lipid-raft-dependent coxsackievirus B4 internalization and rapid targeting to the Golgi. Virology 3266-19. [DOI] [PubMed] [Google Scholar]
- 86.Vela, E. M., L. Zhang, T. M. Colpitts, R. A. Davey, and J. F. Aronson. 2007. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 3691-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verdaguer, N., M. A. Jimenez-Clavero, I. Fita, and V. Ley. 2003. Structure of swine vesicular disease virus: mapping of changes occurring during adaptation of human coxsackie B5 virus to infect swine. J. Virol. 779780-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warren, R. A., F. A. Green, P. E. Stenberg, and C. A. Enns. 1998. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J. Biol. Chem. 27317056-17063. [DOI] [PubMed] [Google Scholar]
- 90.Zautner, A. E., B. Jahn, E. Hammerschmidt, P. Wutzler, and M. Schmidtke. 2006. N- and 6-O-sulfated heparan sulfates mediate internalization of coxsackievirus B3 variant PD into CHO-K1 cells. J. Virol. 806629-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang, G., D. T. Haydon, N. J. Knowles, and J. W. McCauley. 1999. Molecular evolution of swine vesicular disease virus. J. Gen. Virol. 80(Pt. 3)639-651. [DOI] [PubMed] [Google Scholar]