Abstract
Dendritic cells (DCs) play a critical role in cell-to-cell-mediated transmission of human immunodeficiency virus type 1 (HIV-1). Interactions between intercellular adhesion molecules (ICAMs) and their ligands facilitate DC-T-cell contact. The interaction between ICAM-1 on DCs and leukocyte function-associated molecule 1 (LFA-1) on CD4+ T cells has been proposed to be important for DC-mediated HIV-1 transmission. Given that DCs and T cells express multiple ICAMs and binding ligands, the relative importance of ICAMs in DC-mediated HIV-1 transmission remains to be defined. Here, we examine the role of ICAM-1, -2, and -3 in DC-mediated HIV-1 transmission to various types of target cells including primary CD4+ T cells. The expression levels of ICAMs and their ligands on immature and mature DCs and various types of HIV-1 target cells were measured by flow cytometry. Blocking ICAM-1 in DCs with specific monoclonal antibodies and small interfering RNA impaired DC-mediated HIV-1 transmission. DC-mediated viral transmission was significantly inhibited when both ICAM-1 on DCs and LFA-1 on CD4+ T cells were blocked. However, blockade of ICAM-1 on target cells did not significantly inhibit DC-mediated HIV-1 transmission. Ectopic expression and antibody blocking suggest that DC-mediated HIV-1 transmission to primary CD4+ T cells is independent of ICAM-2 and ICAM-3. Taken together, our data clarified the role of ICAMs in DC-mediated HIV-1 transmission to CD4+ T cells.
Dendritic cells (DCs) are among the first target cells that encounter human immunodeficiency virus type 1 (HIV-1) at the mucosa and contribute to the initial stages of HIV-1 infection and dissemination (30, 46). Immature DCs (iDCs) capture HIV-1 in submucosal tissues and migrate to lymphoid tissues, where iDCs become mature DCs (mDCs) to efficiently present antigens to T cells (45, 46). The efficiency of HIV-1 transmission is increased by DC maturation (3, 7, 12, 13, 19, 23, 28, 32, 39, 42, 44, 50), suggesting that mDCs promote HIV-1 spread to CD4+ T cells in lymphoid tissues. DCs efficiently transfer HIV-1 to CD4+ T cells through virological synapses, which facilitate viral transmission by concentrating HIV-1 and viral receptors at the contact zone between DCs and CD4+ T cells (28). Compared with iDCs, mDCs more efficiently facilitate the formation of virological synapses, which contribute to mDC-enhanced HIV-1 transmission to CD4+ T cells (14, 15, 23, 39, 42, 43, 50).
Interactions between intercellular adhesion molecules (ICAMs) and their ligands likely facilitate DC-T-cell contact and HIV-1 transmission. DC maturation upregulates ICAM-1 expression, which is correlated with mDC-enhanced HIV-1 transmission to CD4+ T cells (32). Blocking ICAM-1 on DCs impairs HIV-1 transmission (32). Given that the interaction between ICAM-1 and leukocyte function-associated molecule 1 (LFA-1) is important for DC-T-cell adhesion (9), it has been proposed that the interaction between ICAM-1 on DCs and LFA-1 on CD4+ T cells is important for HIV-1 trans infection (32). Notably, LFA-1 can also interact with ICAM-2 and -3 (5, 35). ICAM-1 binds to Mac-1 (CD11b) and CD11c in addition to LFA-1 (6, 9). DCs and T cells express multiple ICAMs and ligands, which can mediate multifactorial interactions between DCs and T cells (9). Thus, the relative importance of ICAMs in DC-mediated HIV-1 transmission remains to be examined.
ICAM-3 present on T cells binds with high affinity to DC-SIGN (DC-specific ICAM-3-grabbing nonintegrin), a C-type lectin that mediates efficient HIV-1 trans infection (16, 17). Our recent results indicated that iDC-mediated HIV-1 trans infection is partially dependent on DC-SIGN, while mDCs enhance HIV-1 transmission to different types of target cells independently of DC-SIGN and C-type lectins (42). It has been proposed that DC-SIGN-ICAM-3 interactions stabilize DC-T-cell adhesion and enhance HIV-1 transmission (16, 32). However, ICAM-3 expression on target cell lines is not essential for DC-SIGN-mediated HIV-1 transmission (1, 48). It remains unclear whether ICAM-3 expressed on primary CD4+ T cells aids in DC-mediated HIV-1 transmission.
In the present study, we examined the role of ICAM-1, -2, and -3 in DC-mediated HIV-1 transmission to various types of target cells including primary CD4+ T cells. Blocking ICAM-1 on DCs and LFA-1 on CD4+ T cells significantly impaired DC-mediated HIV-1 transmission to primary CD4+ T cells, while DC-mediated HIV-1 transmission appeared to be independent of ICAM-2 and ICAM-3. Our data clarified the role of ICAMs in DC-mediated HIV-1 transmission to primary CD4+ T cells.
MATERIALS AND METHODS
Cell culture.
Human peripheral blood mononuclear cells were isolated from the buffy coat of healthy donors (provided by the Blood Center of Wisconsin, Milwaukee, WI) as previously described (41). CD14+ monocytes and CD4+ T cells were isolated separately from peripheral blood mononuclear cells using anti-CD14- and anti-CD4-coated magnetic beads (BD Bioscience) and cultured as previously described (41). Purified CD14+ monocytes were treated with granulocyte-macrophage colony-stimulating factor and interleukin-4 to generate iDCs, and mDCs were differentiated by the addition of 10 ng/ml of lipopolysaccharide (LPS; Sigma-Aldrich) to iDCs and cultured for an additional 2 days (42). Primary CD4+ T cells were cultured in the presence of 20 IU/ml of recombinant interleukin-2 (the NIH AIDS Research and Reference Reagent Program) and activated by phytohemagglutinin (PHA; 5 μg/ml) for 2 days, as previously described (41). DCs and CD4+ T cells were more than 98% pure by flow cytometry analysis of surface markers as previously described (7, 42, 48). HEK293T, Hut/CCR5, GHOST/R5, and GHOST/R5/ICAM-3 cell lines have been previously described (41).
Flow cytometry and antibodies.
Cells (1 × 105) were stained with specific monoclonal antibodies (MAbs) or isotype-matched immunoglobulin G (IgG) controls (2 μg/ml) as previously described (41). Phycoerythrin- or fluorescein isothiocyanate-conjugated mouse anti-human MAbs (BD Biosciences unless specified otherwise) against the following molecules were used in immunostaining: CD4 (clone S3.5; Caltag Laboratories), CCR5 (clone 45531; R&D Systems), CD69 (clone FN50), CD11a (clone MEM-25), CD11b (clone VIM12), CD11c (clone BU15), CD18 (clone CLB-LFA-1/1), ICAM-1 (clone HA58), ICAM-2 (clone CBR-IC2/2; BioSource), and ICAM-3 (clone TÜ41). Stained cells were analyzed using a FACSCalibur or a Guava EasyCyte flow cytometer (Guava Technologies) and the CellQuest program (Becton Dickinson) or FlowJo software (Tree Star), as previously described (42, 47).
HIV-1 stocks.
Single-cycle, luciferase reporter HIV-1 (HIV-Luc/JRFL) cells were generated by cotransfecting HEK293T cells with pLai3ΔenvLuc2 (49), an env-deleted and nef-inactivated HIV-1 proviral construct, and a construct encoding HIV-1 R5-tropic envelope protein JRFL (42). Replication-competent HIV-1NLAD8 stocks were generated by transfections of HEK293T cells with the proviral construct pNLAD8 as previously described (7, 24). The infectivity of the virus stocks was evaluated by limiting dilution on GHOST/R5 cells as previously described (42). Green fluorescent protein (GFP)-tagged infectious HIV-1 (HIV-Vpr-GFP) stocks were generated by cotransfections of HEK293T cells with pNLAD8 and a Vpr-GFP expression vector pGFP-Vpr (a gift from David McDonald, Case Western Reserve University) as previously described (27). HIV-1 p24 concentrations of viral stocks were measured by an enzyme-linked immunosorbent assay (anti-p24-coated plates were purchased from the AIDS Vaccine Program, SAIC, Frederick, MD) as previously described (7).
HIV-1 infection and transmission assays.
HIV-1 infection and transmission assays were performed as previously described (7, 41). For MAb blocking assays, DCs or other target cell types (2 × 105) were preincubated separately with purified MAbs (10 μg/ml; BD Biosciences, unless otherwise specified) of anti-ICAM-1 (clone HA58), anti-ICAM-2 (clone CBR-IC2/2; eBioscience), anti-ICAM-3 (clone TÜ41), and anti-LFA-1 (clone G43-25B) at room temperature for 0.5 h. A combination of different MAbs (10 μg/ml) was used when indicated. Culture medium was used as a negative control. To avoid potential cytotoxicity effects, sodium azide (≤0.09%) in purified antibodies was removed using Microcon centrifugal filter devices (YM-50; Millipore). Antibody-treated DCs (2 × 105) were incubated with HIV-Luc/JRFL (0.1 multiplicity of infection [MOI]), washed, and cocultured with different target cells (2 × 105) as indicated in the figures or legends. HIV-1 infection was detected at 3 days postinfection (dpi) by measuring the luciferase activity in cell lysates with a commercially available kit (Promega).
For HIV-1NLAD8 infection, iDCs and mDCs (1 × 105) were pulsed with HIV-1NLAD8 (20 ng of p24), washed thoroughly, and then cocultured separately with GHOST/R5 cells or GHOST/R5/ICAM-3 cells (1 × 105). Suspension DCs were removed after 10 h in the cocultivation by aspiration and washing, and target GHOST cells were cultured for 3 days. HIV-1 p24 levels in the supernatants of infected cells were measured by enzyme-linked immunosorbent assay at 3 dpi.
siRNA-mediated knockdown of ICAM-1 expression.
Amaxa nucleofector and cell-type-specific kits were used for the transfection of small interfering RNAs (siRNAs) as previously described (7). iDCs (2 × 106) or GHOST/R5 cells (1 × 106) were nucleofected separately with 3 μg of specific siRNA targeting ICAM-1 (iGENOME SMARTPool) and nonspecific siRNA (both were purchased from Dharmacon). Cell surface levels of ICAM-1 were measured by flow cytometry at 3 days posttransfection. To obtain ICAM-1-silenced mDCs, iDCs were nucleofected with ICAM-1-specific siRNA as described above and cultured for 48 h and then treated with LPS (100 ng/ml) for 24 h. Nonspecific siRNA was used as a negative control.
Virological synapse assay and fluorescence microscopy.
Virological synapse assays were performed as previously described (15, 43). Briefly, iDCs and mDCs (1× 105) were separately pulsed with HIV-Vpr-GFP (20 ng of p24) at 37°C for 2 h. After washes, HIV-pulsed DCs were cocultured with GHOST/R5 cells or GHOST/R5/ICAM-3 cells (1× 105) on a poly-l-lysine-coated microscope slide at 37°C for 0.5 h. Cells were then fixed with 4% paraformaldehyde for 1 h, examined using a fluorescence microscope (Nikon Eclipse TE2000U), and analyzed with MetaMorph software (version 7.0r4) as previously described (41, 43).
Statistical analyses.
Statistical analyses were performed using analysis of variance or a Wilcoxon's paired test with the Prism program.
RESULTS
Surface expression of ICAMs and their ligands on DCs and various types of HIV-1 target cells.
To compare the expression levels of cell-surface ICAM-1, -2, and -3 and their ligands, iDCs, mDCs, and various types of HIV-1 target cells used in viral infection and transmission assays were measured by flow cytometry. These target cell types included PHA-activated primary CD4+ T cells, the human CD4+ T-cell line Hut/CCR5, and the human osteosarcoma cell line GHOST/R5, which were engineered to express high levels of HIV-1 receptors (4). The ICAM-1 expression level was increased at least fourfold on LPS-induced mDCs relative to iDCs (Fig. 1A). ICAM-2 expression was very low or nearly undetectable, while the levels of ICAM-3 were moderate and comparable on iDCs and mDCs (Fig. 1A). Compared with primary CD4+ T cells, Hut/CCR5 cells expressed high levels of ICAM-1, -2, and -3. GHOST/R5 cells expressed only ICAM-1 and not ICAM-2 and -3 (Fig. 1A).
FIG. 1.
Surface expression of ICAMs and their ligands on DCs and various types of HIV-1 target cells. iDCs, mDCs, Hut/CCR5 cells, PHA-activated primary CD4+ T cells, and GHOST/R5 cells were examined for cell surface expression of ICAM-1, -2, and -3 (A) and ligands of ICAMs (B). The asterisk in panel A indicates significantly increased expression of ICAM-1 on mDCs relative to expression on iDCs (P < 0.01, based on results from at least three independent experiments). Cells were stained with specific MAbs or isotype-matched IgG controls and analyzed by flow cytometry. Similar results with primary DCs and CD4+ T cells were obtained using cells derived from at least three different donors. One representative experiment out of three is shown.
Next, cell surface expression levels of ICAM ligands were compared (Fig. 1B). These ligands included LFA-1, Mac-1 (CD11b), and CD11c (6, 9). Two subunits of LFA-1, LFA-1α (CD11a) and β-2 integrin (CD18), were expressed on iDCs, mDCs, Hut/CCR5 cells, and primary CD4+ T cells at medium or high levels (Fig. 1B). As expected, Mac-1 (CD11b) and CD11c were highly expressed at comparable levels on iDCs and mDCs but were negative on Hut/CCR5 cells. Detection of GHOST/R5 cells was negative for all ICAM ligands examined (Fig. 1B). Primary CD4+ T cells from three different donors showed minimal nonspecific staining of CD11b and CD11c (Fig. 1B and data not shown). The purity of primary CD4+ T cells was greater than 99% according to the expression of the T-cell markers CD3 and CD4 and the absence of monocyte marker CD14 (data not shown).
Blocking ICAM-1 on DCs and LFA-1 on primary CD4+ T cells inhibits DC-mediated HIV-1 transmission.
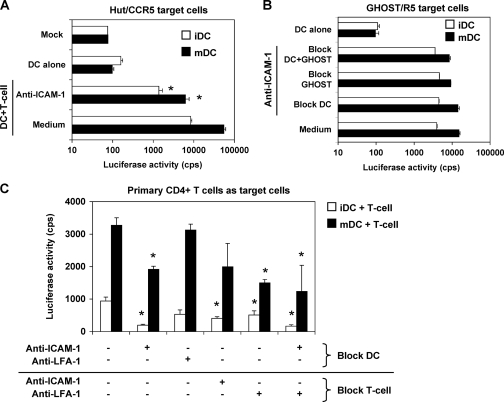
To investigate the role of ICAM-1 in DC-enhanced HIV-1 transmission efficiency, ICAM-1 was specifically blocked with neutralizing MAbs in HIV-1 transmission assays. Single-cycle, R5-tropic luciferase reporter HIV-Luc/JRFL was used, and viral infection was determined at 3 dpi by measuring the luciferase activities of cell lysates. When LFA-1-expressing Hut/CCR5 cells were used as target cells, blocking of ICAM-1 on DCs decreased iDC- and mDC-mediated HIV-1 transmission by six- and ninefold (compared with medium controls; P < 0.01), respectively (Fig. 2A). Anti-ICAM-1 treatment reduced mDC-enhanced viral transmission to iDC levels (Fig. 2A), suggesting an important role of ICAM-1 in DC-mediated HIV-1 transfer to CD4+ T cells.
FIG. 2.
Blocking ICAM-1 on DCs and LFA-1 on primary CD4+ T cells inhibits DC-mediated HIV-1 transmission. (A) ICAM-1 is important for DC-mediated HIV-1 transmission to Hut/CCR5 cells. DCs were pretreated with anti-ICAM-1 before incubation with HIV-Luc/JRFL (MOI of 0.1). HIV-1-pulsed DCs were washed and cocultured with Hut/CCR5 cells. HIV-1 infection was detected at 3 dpi by measuring the luciferase activity in cell lysates. Asterisks indicate significant differences compared with medium controls (P < 0.01). (B) DCs and GHOST/R5 cells were separately preincubated with anti-ICAM-1. DCs were incubated with HIV-Luc/JRFL, washed, and cocultured with GHOST/R5 cells for 3 days before the luciferase activity in cell lysates was measured. (C) Inhibition of DC-mediated HIV-1 transmission of HIV-Luc/JRFL to primary CD4+ T cells with MAbs against ICAM-1 and LFA-1. Medium was used as a negative control. Asterisks indicate significant differences compared with medium controls (P < 0.05). All data show the means ± standard deviations of triplicate samples. One representative experiment out of four is shown. cps, counts per second.
Our recent data indicated that LFA-1-negative GHOST/R5 target cells also support mDC-enhanced HIV-1 transmission (42), suggesting that an interaction between ICAM-1 and LFA-1 might not be the only factor contributing to mDC-enhanced HIV-1 transmission. Given that GHOST/R5 cells expressed only ICAM-1 and not any ICAM ligands (Fig. 1A and B), this cell line could be a useful tool in analyzing the role of ICAM-1 in DC-mediated HIV-1 transmission. When ICAM ligand-negative GHOST/R5 cells were used as targets cells in HIV-1 transmission assays, blockade of ICAM-1 on DCs did not inhibit DC-mediated HIV-1 transmission (Fig. 2B). Compared with medium controls, anti-ICAM-1 treatment of GHOST/R5 cells alone or both DCs and GHOST/R5 cells decreased DC-mediated HIV-1 transmission by 10 to 40%, but the reduction was not statistically significant (Fig. 2B). These results suggest that ICAM-1 expressed on target cells may play a less important role in DC-mediated HIV-1 transmission.
To verify the above results of HIV-1 target cell lines in a more physiologic system, activated, autologous primary CD4+ T cells were used as target cells in DC-mediated HIV-1 transmission. Specific MAbs were used to block ICAM-1 and LFA-1 on DCs and primary CD4+ T cells in HIV-1 transmission assays. Blockade of iDCs and mDCs with anti-ICAM-1 reduced HIV-1 transmission by 79% and 42% (compared with medium controls; P < 0.05), respectively (Fig. 2C). Anti-LFA-1 blocking decreased iDC-mediated HIV-1 transmission by 43% compared with medium controls, while mDC-mediated HIV-1 transmission was not affected (Fig. 2C). Compared with medium controls, DC-mediated HIV-1 transmission was decreased by 39 to 57% when primary CD4+ T cells were separately treated with anti-ICAM-1 and anti-LFA-1, but the reduction was not statistically significant in mDCs treated with anti-ICAM-1 (Fig. 2C). When both ICAM-1 on DCs and LFA-1 on primary CD4+ T cells were blocked, iDC- and mDC-mediated HIV-1 transmission was efficiently inhibited by 82% and 62% (compared with medium controls; P < 0.05), respectively (Fig. 2C). Together, these data confirm that the interaction of ICAM-1 and LFA-1 plays an important role in DC-mediated HIV-1 transmission to primary CD4+ T cells. However, increased expression of ICAM-1 on mDCs might not fully account for the enhanced HIV-1 transmission efficiency.
ICAM-1 knockdown in DCs impairs DC-mediated HIV-1 transmission to primary CD4+ T cells.
To further examine the role of ICAM-1 in DC-mediated HIV-1 transmission, siRNA-mediated ICAM-1 knockdown was performed using DCs derived from different donors. DCs were nucleofected separately with ICAM-1-specific siRNA and nonspecific siRNA controls, and the expression of cell surface ICAM-1 was measured by flow cytometry at 3 days postnucleofection. Treatment with nonspecific siRNA had no effect on ICAM-1 expression on DCs (data not shown). Compared with nonspecific siRNA controls, ICAM-1 silencing reduced ICAM-1 surface levels on iDCs and mDCs by 64% and 40%, respectively (Fig. 3A and B).
FIG. 3.
ICAM-1 knockdown in DCs impairs DC-mediated HIV-1 transmission to primary CD4+ T cells. iDCs from donor 1 (A) and iDC and mDCs from donor 2 (B) were separately nucleofected with ICAM-1-specific siRNA or nonspecific (NS) siRNA. ICAM-1 expression on cell surfaces was measured by flow cytometry at 3 days postnucleofection. Isotype-matched IgGs were used as negative controls for immunostaining. (C and D) ICAM-1 silencing in DCs significantly decreases DC-mediated HIV-1 transmission with Hut/CCR5 cells (C) or primary CD4+ T cells (D). DC-alone samples were used as negative controls. Asterisks indicate significant differences compared to nonspecific siRNA controls (P < 0.05). The data represent the means ± standard deviations. One representative experiment out of three is shown. cps, counts per second.
ICAM-1 silencing in iDCs significantly reduced HIV-1 transmission to Hut/CCR5 cells by sixfold (P < 0.01) relative to nonspecific siRNA controls (Fig. 3C). DCs alone without T-cell coculture were used as negative controls, which did not show detectable HIV-1 infection (Fig. 3C). To confirm these results, autologous primary CD4+ T cells were used as target cells in DC-mediated transmission assays. Compared with the nonspecific siRNA control, ICAM-1 silencing in iDCs and mDCs reduced HIV-1 transmission to primary CD4+ T cells by 46% and 53% (P < 0.05), respectively (Fig. 3D). These data confirm that ICAM-1 expressed on DCs plays an important role in DC-mediated HIV-1 transmission to CD4+ T cells.
ICAM-1 knockdown in target GHOST/R5 cells does not reduce DC-mediated HIV-1 transmission.
GHOST/R5 target cells express only ICAM-1 and lack any other ICAMs and ligands (Fig. 1A and B), which makes them useful as a simplified model to examine the role of ICAM-1 on target cells in DC-mediated HIV-1 transmission. siRNA-mediated ICAM-1 knockdown was performed with GHOST/R5 cells. At 3 days posttransfection with ICAM-1-specific siRNA, surface levels of ICAM-1 on GHOST/R5 cells decreased by approximately 66% relative to the nonspecific siRNA control (Fig. 4A). ICAM-1 knockdown in GHOST/R5 cells did not alter their susceptibilities to direct HIV-1 infection (Fig. 4B) and DC-mediated HIV-1 transmission at 3 dpi (Fig. 4C). These data suggest that ICAM-1 on target cells may not be essential for DC-mediated HIV-1 transmission.
FIG. 4.
ICAM-1 knockdown in target GHOST/R5 cells does not reduce DC-mediated HIV-1 transmission. (A) Knockdown of ICAM-1 in GHOST/R5 cells. GHOST/R5 cells were transfected with ICAM-1-specific siRNA or nonspecific (NS) siRNA. ICAM-1 expression on cell surfaces was measured by flow cytometry at 3 days posttransfection. Isotype-matched IgGs were used as negative controls of immunostaining. (B) HIV-Luc/JRFL infection of ICAM-1-silenced GHOST/R5 cells and control cells. (C) DC-mediated transmission of HIV-Luc/JRFL to ICAM-1-silenced GHOST/R5 cells relative to control cells. Luciferase activity of cell lysates was measured at 3 dpi. The data represent the means ± standard deviations. One representative experiment out of three is shown. cps, counts per second.
Ectopic expression of ICAM-3 in GHOST/R5 target cells does not enhance their susceptibility to DC-mediated HIV-1 transmission.
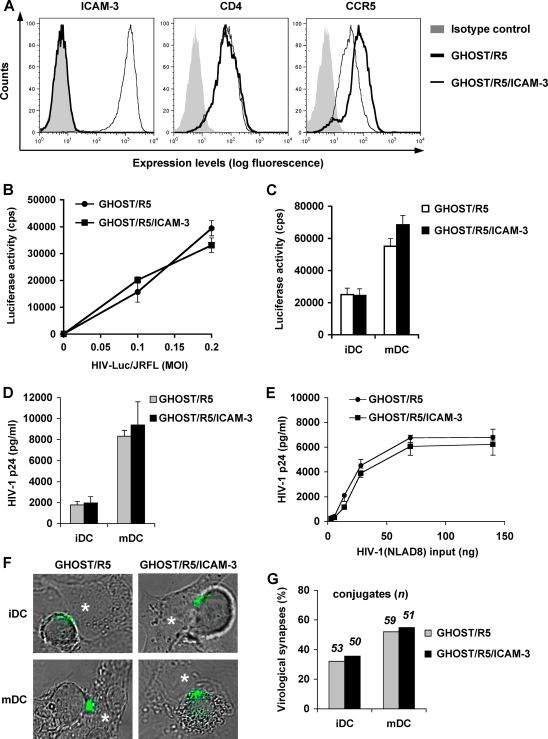
To better understand the role of ICAM-3 on target cells in DC-mediated HIV-1 transmission, GHOST/R5 cells that stably express exogenous and functional ICAM-3 (48) were used as target cells in viral transmission assays. Cell surface expression levels of ICAM-3, CD4, and CCR5 were measured by flow cytometry. Compared with ICAM-3-negative GHOST/R5 cells, GHOST/R5/ICAM-3 cells expressed high levels of ICAM-3, similar levels of CD4, and low levels of CCR5 (Fig. 5A). Despite the different levels of CCR5 expression, the susceptibilities to HIV-Luc/JRFL infection between ICAM-3-negative and -positive GHOST/R5 cells were comparable (Fig. 5B). DC-mediated HIV-1 transmission to ICAM-3-negative and -positive GHOST/R5 cells was similar (Fig. 5C), suggesting that DC-mediated HIV-1 transmission might be independent of ICAM-3 expression on target cells.
FIG. 5.
Ectopic expression of ICAM-3 in GHOST/R5 target cells does not enhance their susceptibility to DC-mediated HIV-1 transmission. (A) Surface expression levels of ICAM-3, CD4, and CCR5 on ICAM-3-negative and -positive GHOST/R5 cells were measured by flow cytometry. Isotype-matched IgGs were used as negative controls for immunostaining. (B) Comparable HIV-Luc/JRFL infection of ICAM-3-negative and -positive GHOST/R5 cells. (C) iDC- and mDC-mediated transmission of HIV-Luc/JRFL to ICAM-3-negative and -positive GHOST/R5 cells. Luciferase activity of cell lysates was measured at 3 dpi. cps, counts per second. (D) DC-mediated transmission of replication-competent HIV-1 to ICAM-3-negative and -positive GHOST/R5 cells. DCs were pulsed with HIV-1NLAD8 and then cocultured separately with ICAM-3-negative and -positive GHOST/R5 cells. Suspension DCs were removed after 10 h in the cocultivation by aspiration and washing, and adherent GHOST/R5 cells were cultured for 3 days. (E) Comparable infection of ICAM-3-negative and -positive GHOST/R5 cells with HIV-1NLAD8. In both panels D and E, HIV-1 p24 levels in supernatants were measured at 3 dpi. The data represent the means ± standard deviations. One representative experiment out of three is shown. (F) HIV-1 synapse formation between DCs and ICAM-3-negative and-positive GHOST/R5 cells. DCs were incubated with GFP-Vpr-labeled HIV-1NLAD8 (green), washed, and cultured separately with ICAM-3-negative and -positive GHOST/R5 cells for 1 h. White asterisks indicate GHOST/R5 cells and GHOST/R5/ICAM-3 cells at the virological synapses. (G) Quantitative image analysis of the virological synapses formed between DCs and ICAM-3-negative or -positive GHOST/R5 cells.
To confirm the above results of single-cycle HIV-1 infection, replication-competent, R5-tropic HIV-1NLAD8 was used in DC-mediated HIV-1 transmission assays. DCs were pulsed with a small amount of HIV-1NLAD8, washed, and then cocultured separately with ICAM-3-negative and -positive GHOST/R5 cells. The suspended DCs were removed from the cocultivation after a 10-h incubation to avoid potential HIV-1 cis infection in iDCs (7, 24). Adherent GHOST cells were washed and cultured for 3 days, and HIV-1 infection was measured by p24 quantification in the supernatants. Consistently, mDC-mediated transmission of HIV-1NLAD8 was fourfold higher than with iDCs (Fig. 5D). DC-mediated HIV-1NLAD8 transmission levels to ICAM-3-negative and -positive GHOST/R5 cells were comparable (Fig. 5D). As a control, HIV-1NLAD8 direct infection of GHOST/R5 and GHOST/R5/ICAM-3 cells showed similar virus replication levels at 3 dpi (Fig. 5E).
To examine whether ICAM-3 expression on GHOST/R5 target cells enhances virological synapse formation, iDCs and mDCs were pulsed with GFP-tagged infectious HIV-Vpr-GFP and cocultured separately with GHOST/R5 and GHOST/R5/ICAM-3 cells for 1 h. Both ICAM-3-negative and -positive GHOST/R5 cells could form virological synapses with iDCs and mDCs (Fig. 5F). Quantitative image analysis indicated that comparable virological synapses formed between ICAM-3-negative and -positive GHOST/R5 cells and DCs, while mDC-mediated virological synapse formation was more efficient relative to iDCs (Fig. 5G). These data suggest that ectopic ICAM-3 expression on non-T-cell target cells could not enhance the formation of virological synapses with HIV-pulsed DCs.
Blocking ICAM-2 and ICAM-3 on DCs and primary CD4+ T cells does not inhibit DC-mediated HIV-1 transmission.
To examine the role of ICAM-2 and -3 in DC-mediated HIV-1 transmission to primary CD4+ T cells, neutralizing MAbs to ICAM-2 and ICAM-3 were used. Treatment of iDCs, mDCs, and activated primary CD4+ T cells with neutralizing MAbs to ICAM-2 did not significantly inhibit DC-mediated transmission of HIV-Luc/JRFL to primary CD4+ T cells (Fig. 6A). Compared with medium controls, anti-ICAM-3 treatment of iDCs and primary CD4+ T reduced iDC-mediated HIV-1 transmission by 35 to 40%, although the reduction was not statistically significant (Fig. 6A, left panel). Moreover, anti-ICAM-3 treatment of mDCs and primary CD4+ T cells did not inhibit mDC-mediated HIV-1 transmission (Fig. 6A, right panel). To confirm the efficacy of the neutralizing MAbs, activated primary CD4+ T cells were treated separately with neutralizing MAbs against ICAM-2 (2) and ICAM-3 (34) and then stained with fluorescein isothiocyanate-conjugated anti-ICAM-2 and anti-ICAM-3, respectively. Flow cytometry analysis confirmed the efficacy of the neutralizing MAbs in blocking ICAM-2 and ICAM-3 on primary CD4+ T cells (Fig. 6B). Moreover, pretreatment of primary CD4+ T cells with MAbs to ICAM-1, ICAM-2, and ICAM-3 did not reduce HIV-1 direct infection (data not shown). Together, these data suggest that DC-mediated HIV-1 transmission to primary CD4+ T cells is independent of ICAM-2 and ICAM-3.
FIG. 6.
Blocking ICAM-2 and ICAM-3 on DCs and primary CD4+ T cells does not inhibit DC-mediated HIV-1 transmission. (A) DCs or primary CD4+ T cells were preincubated separately with neutralizing MAbs (10 μg/ml) against ICAM-2 and ICAM-3 before incubation with HIV-Luc/JRFL (MOI of 0.1). Cells were washed and cocultured, and HIV-1 transmission was detected at 3 dpi by measuring the luciferase activity in cell lysates. The data show the means ± standard deviations. One representative experiment out of three is shown. cps, counts per second. (B) Specific blocking effects of MAbs to ICAM-2 and ICAM-3. Activated primary CD4+ T cells were separately treated with medium or neutralizing MAbs (10 μg/ml) against ICAM-2 and ICAM-3 at room temperature for 30 min and then stained, respectively, with fluorescein isothiocyanate-conjugated anti-ICAM-2 or anti-ICAM-3 (2 μg/ml) at 4°C for 30 min. Isotype-matched IgGs were used as negative controls for staining.
DISCUSSION
Studying DC-mediated HIV-1 transmission is critical for understanding the mechanisms of cell-cell spread of HIV-1. The interactions between ICAMs and their ligands can facilitate DC-T-cell contact and promote the formation of immunological synapses and antigen presentation (10). However, the role of ICAMs and their ligands in DC-mediated HIV-1 transmission remains to be clarified. In the present study, we performed functional analyses to examine relative importance of ICAM-1, -2, and -3 in DC-mediated trans infection of primary CD4+ T cells.
Although multifactorial interactions between ICAMs and their ligands may facilitate DC-T-cell contact and the formation of immunological synapses, the expression of ICAM-1 on DCs and of LFA-1 on T cells appeared to be critical for DC-mediated HIV-1 trans infection. Blocking of ICAM-1 expressed on DCs significantly decreased both iDC- and mDC-mediated HIV-1 transmission to primary CD4+ T cells and a T-cell line (Fig. 2). These data imply that increased ICAM-1 expression could not fully account for enhanced HIV-1 transmission by mDCs relative to iDCs. Furthermore, ectopic expression and antibody blocking suggest that DC-mediated HIV-1 transmission to primary CD4+ T cells might be dispensable for ICAM-2 and ICAM-3. Thus, our results support a model that the interaction between ICAM-1 expressed on DCs and LFA-1 expressed on CD4+ T cells facilitates DC-mediated HIV-1 transmission to primary CD4+ T cells (Fig. 7).
FIG. 7.
A proposed model for ICAM-ligand interactions in DC-mediated HIV-1 transmission. Our results suggest that ICAM-1-LFA-1 interaction is critical for DC-mediated HIV-1 transmission to CD4+ T cells. However, multifactorial interactions between ICAMs and their ligands expressed on DCs and CD4+ T cells may play an important role in the formation of immunological synapses and the induction of immune responses. TCR, T-cell receptor; MHC-II, major histocompatibility complex class II molecules. For simplicity, additional DC-T-cell interactions are not depicted.
ICAM-1 binding to LFA-1 can enhance T-cell receptor-dependent proliferation of T cells by upregulating various signaling pathways (29). This activation may contribute to DC-enhanced HIV-1 trans infection of CD4+ T cells. LFA-1 expression on target cells has been shown to contribute to HIV-1 transmission to CD4+ T cells mediated by DCs (18, 20, 21) and T cells (22, 25, 26, 37, 38). By contrast, a recent study suggested that HIV-1 transfer between CD4+ T cells does not require LFA-1 binding to ICAM-1 and is mediated by the interaction of HIV-1 envelope with CD4 (31). It remains to be examined whether HIV-1 infection of DCs and CD4+ T cells modulates the expression and function of ICAMs and binding ligands.
The ICAM-1-LFA-1 interaction may enhance the formation of virological synapses between HIV-1-associated DCs and CD4+ T cells. A recent study using the lipid bilayers containing ICAM-1 indicated an important role of ICAM-1 in forming virological synapses between CD4+ T cells (40). We have examined the formation of virological synapses between primary CD4+ T cells and ICAM-1-silenced iDCs and mDCs by fluorescence microscopy. However, no significant difference was observed relative to nonspecific siRNA controls (data not shown). This might be due to the limited sensitivity of the virological synapse assay and low efficiency of ICAM-1 silencing in DCs. Although ICAM-1 silencing could reduce ICAM-1 surface levels on DCs by 40 to 64%, medium to high levels of ICAM-1 remained on the cell surfaces (Fig. 3A and B). Further improvement of siRNA knockdown techniques is required to examine the mechanisms by which ICAM-1 silencing inhibits DC-mediated HIV-1 transmission.
Our data suggest that ICAM-2 and ICAM-3 do not significantly contribute to DC-mediated HIV transmission. Interestingly, structural and functional studies of DC-SIGN by Snyder et al. (33) and Su et al. (36) indicate that DC-SIGN binds to HIV-1 gp120 more than 100- and 50-fold efficiently than ICAM-2 and ICAM-3, respectively. Moreover, a previous study indicated that replication of X4-tropic HIV-1 is enhanced two- to threefold in ICAM-3-negative Jurkat T cells after 10 dpi (1), suggesting that ICAM-3 may limit HIV-1 replication even though the mechanism is unknown. However, in our viral infection assays using single-cycle infection and replication-competent HIV-1, no significant difference was observed between ICAM-3-negative and -positive GHOST/R5 cells at 3 dpi (Fig. 5B and E). These different observations may result from using different cell lines, HIV-1 strains, and experimental procedures.
Our recent results suggest that intact cytoskeleton is essential for DC-mediated HIV-1 transmission to CD4+ T cells (43). Altered HIV-1 trafficking and impaired formation of virological synapses primarily accounted for the inhibition of viral transmission by cytoskeletal inhibitors (43). The actin cytoskeleton contributes to T-cell activation by forming immunological synapses between antigen-presenting cells and T cells (8). Interestingly, the immunological synapses appear to share structural similarities with the HIV-1 virological synapses and may play a role in viral pathogenesis (11).
In summary, our results clarified the role of ICAMs in DC-mediated HIV-1 transmission and provided helpful information in understanding the mechanisms of cell-cell spread of HIV-1. We showed that the interaction of ICAM-1 and LFA-1 plays an important role in DC-mediated HIV-1 transmission to primary CD4+ T cells. Moreover, DC-mediated HIV-1 transmission appears to be independent of ICAM-2 and ICAM-3. Further understanding of HIV-1 and host-cell interactions and the mechanisms of DC-mediated virus transmission will aid in the development of effective strategies to combat HIV-1 infection.
Acknowledgments
We thank the members of the Wu laboratory for helpful discussions. We thank David McDonald, Michael Emerman, and Vineet KewalRamani for the kind gift of reagents. Interleukin-2 was obtained from the NIH AIDS Research and Reference Reagent Program.
This work was supported by a grant (R01-AI068493) to L.W. from the National Institutes of Health.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Biggins, J. E., T. Biesinger, M. T. Yu Kimata, R. Arora, and J. T. Kimata. 2007. ICAM-3 influences human immunodeficiency virus type 1 replication in CD4+ T cells independent of DC-SIGN-mediated transmission. Virology 364383-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butini, L., A. R. De Fougerolles, M. Vaccarezza, C. Graziosi, D. I. Cohen, M. Montroni, T. A. Springer, G. Pantaleo, and A. S. Fauci. 1994. Intercellular adhesion molecules (ICAM)-1 ICAM-2 and ICAM-3 function as counter-receptors for lymphocyte function-associated molecule 1 in human immunodeficiency virus-mediated syncytia formation. Eur. J. Immunol. 242191-2195. [DOI] [PubMed] [Google Scholar]
- 3.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 3e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 726988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Fougerolles, A. R., X. Qin, and T. A. Springer. 1994. Characterization of the function of intercellular adhesion molecule (ICAM)-3 and comparison with ICAM-1 and ICAM-2 in immune responses. J. Exp. Med. 179619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond, M. S., D. E. Staunton, A. R. de Fougerolles, S. A. Stacker, J. Garcia-Aguilar, M. L. Hibbs, and T. A. Springer. 1990. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 1113129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, C., A. M. Janas, J.-H. Wang, W. J. Olson, and L. Wu. 2007. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J. Virol. 8111352-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dustin, M. L., and J. A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 123-29. [DOI] [PubMed] [Google Scholar]
- 9.Dustin, M. L., and T. A. Springer. 1991. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu. Rev. Immunol. 927-66. [DOI] [PubMed] [Google Scholar]
- 10.Dustin, M. L., S. Y. Tseng, R. Varma, and G. Campi. 2006. T cell-dendritic cell immunological synapses. Curr. Opin. Immunol. 18512-516. [DOI] [PubMed] [Google Scholar]
- 11.Fackler, O. T., A. Alcover, and O. Schwartz. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7310-317. [DOI] [PubMed] [Google Scholar]
- 12.Fahrbach, K. M., S. M. Barry, S. Ayehunie, S. Lamore, M. Klausner, and T. J. Hope. 2007. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J. Virol. 816858-6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 7811980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia, E., D. S. Nikolic, and V. Piguet. 2008. HIV-1 replication in dendritic cells occurs via a tetraspanin-containing compartment enriched in AP-3. Traffic 9200-214. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6488-501. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100587-597. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100575-585. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert, C., R. Cantin, C. Barat, and M. J. Tremblay. 2007. Human immunodeficiency virus type 1 replication in dendritic cell-T-cell cocultures is increased upon incorporation of host LFA-1 due to higher levels of virus production in immature dendritic cells. J. Virol. 817672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 722733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groot, F., T. W. Kuijpers, B. Berkhout, and E. C. de Jong. 2006. Dendritic cell-mediated HIV-1 transmission to T cells of LAD-1 patients is impaired due to the defect in LFA-1. Retrovirology 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gummuluru, S., V. N. KewalRamani, and M. Emerman. 2002. Dendritic cell-mediated viral transfer to T cells is required for human immunodeficiency virus type 1 persistence in the face of rapid cell turnover. J. Virol. 7610692-10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hioe, C. E., P. C. Chien, Jr., C. Lu, T. A. Springer, X. H. Wang, J. Bandres, and M. Tuen. 2001. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J. Virol. 751077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izquierdo-Useros, N., J. Blanco, I. Erkizia, M. T. Fernandez-Figueras, F. E. Borras, M. Naranjo-Gomez, M. Bofill, L. Ruiz, B. Clotet, and J. Martinez-Picado. 2007. Maturation of blood derived dendritic cells enhances HIV-1 capture and transmission. J. Virol. 817559-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janas, A. M., C. Dong, J. H. Wang, and L. Wu. 2008. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology 375442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolly, C., I. Mitar, and Q. J. Sattentau. 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol. 8113916-13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 3001295-1297. [DOI] [PubMed] [Google Scholar]
- 29.Ni, H. T., M. J. Deeths, W. Li, D. L. Mueller, and M. F. Mescher. 1999. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J. Immunol. 1625183-5189. [PubMed] [Google Scholar]
- 30.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puigdomenech, I., M. Massanella, N. Izquierdo-Useros, R. Ruiz-Hernandez, M. Curriu, M. Bofill, J. Martinez-Picado, M. Juan, B. Clotet, and J. Blanco. 2008. HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 767812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder, G. A., J. Ford, P. Torabi-Parizi, J. A. Arthos, P. Schuck, M. Colonna, and P. D. Sun. 2005. Characterization of DC-SIGN/R interaction with human immunodeficiency virus type 1 gp120 and ICAM molecules favors the receptor's role as an antigen-capturing rather than an adhesion receptor. J. Virol. 794589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starling, G. C., A. D. McLellan, W. Egner, R. V. Sorg, J. Fawcett, D. L. Simmons, and D. N. Hart. 1995. Intercellular adhesion molecule-3 is the predominant co-stimulatory ligand for leukocyte function antigen-1 on human blood dendritic cells. Eur. J. Immunol. 252528-2532. [DOI] [PubMed] [Google Scholar]
- 35.Staunton, D. E., M. L. Dustin, and T. A. Springer. 1989. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature 33961-64. [DOI] [PubMed] [Google Scholar]
- 36.Su, S. V., P. Hong, S. Baik, O. A. Negrete, K. B. Gurney, and B. Lee. 2004. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J. Biol. Chem. 27919122-19132. [DOI] [PubMed] [Google Scholar]
- 37.Tardif, M. R., and M. J. Tremblay. 2005. LFA-1 is a key determinant for preferential infection of memory CD4+ T cells by human immunodeficiency virus type 1. J. Virol. 7913714-13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 7712299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 1032170-2179. [DOI] [PubMed] [Google Scholar]
- 40.Vasiliver-Shamis, G., M. Tuen, T. W. Wu, T. Starr, T. O. Cameron, R. Thomson, G. Kaur, J. Liu, M. L. Visciano, H. Li, R. Kumar, R. Ansari, D. P. Han, M. W. Cho, M. L. Dustin, and C. E. Hioe. 2008. HIV-1 envelope gp120 induces a stop signal and virological synapse formation in non-infected CD4+ T cells. J. Virol. 829445-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, J. H., A. M. Janas, W. J. Olson, V. N. KewalRamani, and L. Wu. 2007. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J. Virol. 812497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, J. H., A. M. Janas, W. J. Olson, and L. Wu. 2007. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J. Virol. 818933-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, J. H., C. Wells, and L. Wu. 2008. Macropinocytosis and cytoskeleton contribute to dendritic cell-mediated HIV-1 transmission to CD4+ T cells. Virology 381143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissman, D., Y. Li, J. M. Orenstein, and A. S. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 1554111-4117. [PubMed] [Google Scholar]
- 45.Wu, L. 2008. Biology of HIV mucosal transmission. Curr. Opin. HIV AIDS 3534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, L., and V. N. KewalRamani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 31817-23. [DOI] [PubMed] [Google Scholar]
- 48.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 765905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita, M., and M. Emerman. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 785670-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, H. J., M. A. Reuter, and D. McDonald. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans infection of T cells by mature dendritic cells. PLoS Pathog 4e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]