Abstract
Progressive multifocal leukoencephalopathy (PML) is a frequently fatal disease caused by uncontrolled polyomavirus JC (JCV) in severely immunodeficient patients. We investigated the JCV-specific cellular and humoral immunity in the Swiss HIV Cohort Study. We identified PML cases (n = 29), as well as three matched controls per case (n = 87), with prospectively cryopreserved peripheral blood mononuclear cells and plasma at diagnosis. Nested controls were matched according to age, gender, CD4+ T-cell count, and decline. Survivors (n = 18) were defined as being alive for >1 year after diagnosis. Using gamma interferon enzyme-linked immunospot assays, we found that JCV-specific T-cell responses were lower in nonsurvivors than in their matched controls (P = 0.08), which was highly significant for laboratory- and histologically confirmed PML cases (P = 0.004). No difference was found between PML survivors and controls or for cytomegalovirus-specific T-cell responses. PML survivors showed significant increases in JCV-specific T cells (P = 0.04) and immunoglobulin G (IgG) responses (P = 0.005). IgG responses in survivors were positively correlated with CD4+ T-cell counts (P = 0.049) and negatively with human immunodeficiency virus RNA loads (P = 0.03). We conclude that PML nonsurvivors had selectively impaired JCV-specific T-cell responses compared to CD4+ T-cell-matched controls and failed to mount JCV-specific antibody responses. JCV-specific T-cell and IgG responses may serve as prognostic markers for patients at risk.
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system caused by lytic replication of the human polyomavirus JC (JCV) in oligodendrocytes (29). PML is observed in the setting of profound cellular immunodeficiency as encountered in hematological malignancy, after hematopoietic stem cell transplantation, and, more recently, after exposure to potent antilymphocyte drugs, such as natalizumab (16, 22, 25, 32). In the last 25 years, however, PML has been most frequently diagnosed in connection with the human immunodeficiency virus type 1 (HIV-1)/AIDS epidemic (4). Although different steps in the pathogenesis of PML have been elucidated (21, 28, 34), its somewhat erratic manifestation even in the setting of HIV/AIDS suggests that additional factors might be in play. Several lines of evidence suggest that JCV-specific cellular immunodeficiency is presumably a key factor (6, 12, 17). As no antivirals with clinically documented efficacy are available, treatment aims at improving JCV-specific immunity. This task is difficult to achieve in the settings of cancer, chemotherapy, and transplantation (9). Moreover, most patients are diagnosed when neurological signs are present, typically reflecting extensive central nervous system disease with little time for such interventions. In HIV/AIDS, however, the availability of combination antiretroviral therapy (cART) has significantly improved the prognosis and largely reduced the incidence rates of PML (3, 10). Nevertheless, mortality still approximates 50% within the first year (2, 31). Recent data show that JCV-specific cytotoxic T lymphocytes in the peripheral blood at diagnosis may be associated with a better outcome, but independent studies of prospectively collected patients are missing (12, 24).
(Presented in part at the IVth International Conference on Polyomaviruses and Human Diseases, Barcelona, Spain, 30 September to 3 October 2007.)
MATERIALS AND METHODS
Study participants and setting.
The Swiss HIV Cohort Study (SHCS) is a prospective, observational study of HIV-1-infected adults initiated in 1988 and approved by the local institutional review boards (26). The diagnosis “PML” has been encoded in the SHCS data sheet as either “definitive” if proven by histology or “presumptive” according to clinical, radiological, or laboratory evidence, i.e., JCV detected by PCR in cerebrospinal fluid. Between January 1995 and August 2006, 67 patients with the diagnosis PML were identified in the SHCS database and were confirmed by independent chart review. Accordingly, the diagnosis PML was classified as follows: (i) “possible” by typical clinical and neuroradiologic findings, (ii) “laboratory confirmed” by detection of JCV DNA in cerebrospinal fluid, or (iii) “definitive” by histology of brain biopsy or autopsy (8). The neuroradiological findings on magnetic resonance imaging were evaluated according to combined clinical and radiological criteria as elaborated in a recent consensus statement for the critical radiological distinction between multiple sclerosis and PML (21). Patients with PML were enrolled in the study if viable cryopreserved peripheral blood mononuclear cell (PBMC) samples were available at the time of PML diagnosis, which led to the exclusion of 38 PML cases. Of the included 29 PML patients (definitive, 4; laboratory confirmed, 10; possible, 15), cryopreserved PBMC samples were available within ±6 months of diagnosis for 23 (79%) patients. Since PBMC samples were cryopreserved yearly, we aimed at obtaining one PBMC sample at diagnosis, one up to 2.5 years before, and one up to 1.5 years after PML diagnosis. Plasma samples were cryopreserved every 6 months, and all available samples within an interval from 2.5 years before to 1.5 years after diagnosis were included. Cases and controls were selected based on the SHCS database of December 2006, and the follow-up data were based on the SHCS database of July 2008.
Identification of controls.
From the SHCS database, each PML case was matched with three control patients without PML. The date of PML diagnosis was defined as “time zero.” Cases and controls were matched according to predefined criteria at time zero: (i) calendar year (±1 year), (ii) time since first positive HIV test (equal or at most 2 years longer for controls), (iii) age (±10 years), (iv) gender, and (v) CD4+ T-cell count (±25 cells/μl) at time zero and 1 or 2 years before. In addition, controls had to have PBMC samples drawn at the same time points (±6 months) as the case patients relative to time zero. For 11 (38%) PML cases, CD4+ T-cell counts prior to diagnosis were unavailable; hence, matching for these cases was performed only according to the time zero CD4+ T-cell count. To find enough controls, we relaxed the criteria for 34 (39%) of the controls as follows: calendar year (±3 years) for 14 controls and CD4+ T-cell count at time zero (±50 cells/μl) for 11 controls and for prior CD4+ T-cell counts for 20 controls despite available prior CD4+ T-cell data for the cases.
EIA and ESA.
For the enzyme immunoassay (EIA), virus-like particles (VLP) were produced by expressing the JCV capsid protein 1 in insect cells from a recombinant baculovirus, and EIAs were performed as previously described (13, 33). JCV-specific cellular immune responses were measured in 58 samples from 29 PML cases and 104 samples from 70 controls due to nonviable PBMCs. For the enzyme-linked immunospot assay (ESA), gamma interferon production by JCV-specific T cells was quantified after PBMC stimulation with overlapping 15-mer peptide pools from JCV large T antigen and VP1 capsid protein of the JCV Mad-1 strain (7). The cytomegalovirus (CMV) seroprevalence was 83% in the PML cases and 85% in the controls, which corresponded well to the overall CMV seroprevalence of 83.4% recorded in the SHCS. For JCV, the immunoglobulin G (IgG) seroprevalence was 93% in the case patients and 90% in the controls. CMV-specific cellular immune responses were measured in 51 samples from 24 CMV IgG-seropositive cases and in 98 samples from 59 IgG-seropositive controls. For CMV-specific PBMC stimulation, corresponding 15-mer peptide pools spanning CMV pp65 were used (14). Gamma interferon spots were counted by using an ESA reader (Cellular Technologies Ltd., Shaker Heights, OH). The number of spot-forming units (SFU) per well was calculated from duplicates after subtraction of the negative control.
Statistical methods.
We first visualized the courses of the four immunological markers (JCV- and CMV-specific cellular immune responses and JCV-specific IgG and IgM activities) in PML cases over time. In PML nonsurvivors, only marker values before PML diagnosis were available. In PML survivors, we tested whether marker values systematically increased or decreased at PML diagnosis by using a linear mixed model with fixed effects for the mean marker levels before and after diagnosis, random effects for the patient-specific marker value levels before and after diagnosis, and measurement error. We then concentrated on the marker values at time zero (i.e., at the date of PML diagnosis or at the respective matching date for controls), defined as the closest available measurements to that time point. We compared these marker values between cases and controls using a logistic regression with Firth bias reduction and adjustment for the matching. This method has been shown to be an alternative to exact conditional regression for matched pairs, especially for continuous markers, where the latter often leads to highly discrete or even degenerate conditional distributions (18). The analysis was separately repeated for survivors and nonsurvivors and their respective controls. As sensitivity analyses, we adjusted for (log-transformed) HIV RNA, excluded patients with possible PML diagnosis, or counted only patients who died due to PML as nonsurvivors. In a second step, we compared PML survivors and nonsurvivors with respect to their time zero immunological-marker values (with or without adjustment for CD4+ T-cell counts), again using logistic regression with Firth bias reduction. Finally, we explored correlations between immunological markers, CD4+ T-cell counts, and HIV RNA. Both correlations between patients of the measurement at time zero and correlations within patients over time were studied. For the latter, we calculated correlations for each patient contributing at least two measurements and then tested whether the proportion of patients with positive correlations was statistically different from 0.5 (the expected proportion if the true correlation is zero). Throughout, we used Spearman's rank correlation for quantifying the strength of the association.
The immunological markers were log transformed prior to all analyses, with “1” added to JCV- and CMV-specific cellular immune response values prior to log transformation to deal with values of zero. All reported confidence intervals are two-sided 95% intervals, and tests were performed at the two-sided 5% level. If not otherwise reported, P values for the comparisons of continuous or categorical covariates between groups were based on the Wilcoxon test or Fisher's exact test, respectively. We used R v2.51 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) for all analyses and the contributed R package logistf for Firth's penalized-likelihood logistic regression.
RESULTS
Patients' characteristics.
We identified 29 patients with cryopreserved PBMCs and plasma at the time of diagnosis. Table 1 summarizes the baseline characteristics of the enrolled PML cases. The median age was 39 years; 17% were female, and 41% had prior AIDS-defining diseases. Median CD4+ T-cell counts at diagnosis were 102 cells/μl, with plasma HIV RNA of 4.18 log10 copies/ml. Two-thirds were treated with cART before PML diagnosis. The 11 nonsurvivors (38%) died after a median of 72 days, with PML as the reason for death in 9 (82%). The 18 survivors were characterized by higher CD4+ T-cell counts at diagnosis (P = 0.02).
TABLE 1.
Baseline characteristics for 29 PML cases
| Characteristicsa | Summary statisticb
|
Pc | ||
|---|---|---|---|---|
| All patients (n = 29) | Survivors (n = 18) | Nonsurvivors (n = 11) | ||
| Age (yr) | 39 (36, 45) | 37 (34, 44) | 42 (39, 45) | 0.13 |
| Female gender | 5 (17) | 2 (11) | 3 (27) | 0.34 |
| PML diagnosis | ||||
| Definitive | 4 (14) | 2 (11) | 2 (18) | 0.09 |
| Laboratory confirmed | 10 (34) | 4 (22) | 6 (55) | |
| Possible | 15 (52) | 12 (67) | 3 (27) | |
| Yr since first positive HIV test | 8.20 (2.72, 11.34) | 7.10 (0.51, 9.14) | 10.81 (8.30,13.19) | 0.03 |
| CD4 cell count (cells/μl) | 102 (35, 152) | 119 (82, 192) | 35 (18, 107) | 0.02 |
| CD8 cell count (cells/μl) | 700 (455, 1137) | 698 (546, 1030) | 700 (425, 1168) | 0.74 |
| HIV viral load (log10 copies/ml) | 4.18 (2.62, 5.28) | 3.88 (2.62, 4.88) | 4.98 (2.79, 5.41) | 0.22 |
| Prior AIDS-defining conditions | 12 (41) | 9 (50) | 3 (27) | 0.27 |
| cART status at PML diagnosis | ||||
| cART naïve | 10 (34) | 7 (39) | 3 (27) | 0.45 |
| cART treated | 10 (34) | 7 (39) | 3 (27) | |
| cART interrupted | 9 (31) | 4 (22) | 5 (45) | |
| cART (re)initiated within 3 mo of PML diagnosis | ||||
| In cART-naïve patients | 7/10 (70) | 6/7 (86) | 1/3 (33) | 0.10 |
| In cART-pretreated patients | 4/9 (44) | 3/4 (75) | 1/5 (20) | |
Time-dependent covariates evaluated at the time of PML diagnosis.
Median (IQR) for continuous variables; n (%) for categorical variables.
Survivors versus nonsurvivors; based on Wilcoxon test for continuous variables and Fisher's exact test for categorical variables.
JCV- and CMV-specific cellular immune responses.
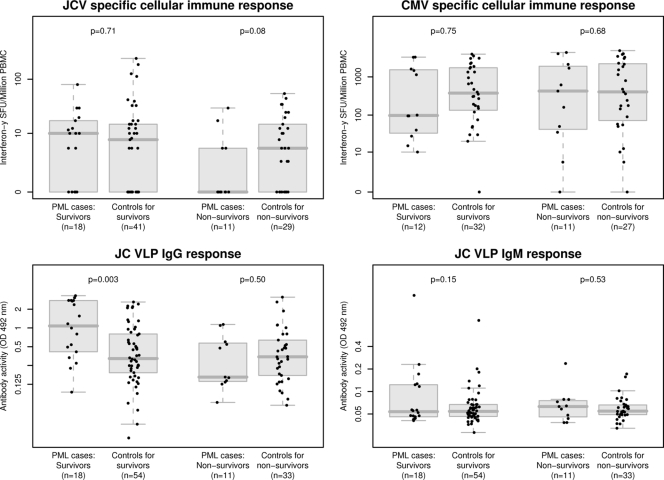
Longitudinal measurements of all parameters, including JCV- and CMV-specific T-cell responses before and after PML diagnosis, are shown in Fig. 1. In PML nonsurvivors, JCV-specific T-cell responses were low, with little variation over time. In contrast, PML survivors developed a statistically significant increase in JCV-specific T-cell responses at PML diagnosis (P = 0.04; crude median of all measurements until diagnosis, 4 [interquartile range {IQR}, 0 to 12], and after diagnosis, 10 [IQR, 5 to 30]). No significant change was observed for CMV-specific immune response in survivors (P = 0.22).
FIG. 1.
Laboratory markers over time in PML survivors and nonsurvivors: HIV viral load (copies/ml), CD4+ T-cell count (cells/μl), JCV- and CMV-specific cellular immune responses (gamma interferon SFU/million PBMCs), and JCV-specific IgG and IgM antibody activities (OD492).
In the case-control analysis, PML nonsurvivors tended to have lower JCV-specific T-cell responses than their matched controls (P = 0.08). The odds of developing PML and surviving decreased by a factor of 0.29 if the JCV-specific T-cell response increased by a factor from 0 to 10. This trend was statistically significant when the analysis was performed with laboratory-confirmed and definitive PML cases (P = 0.004). Similarly, statistical significance was reached when adjusted for HIV RNA (P = 0.03) or restricted to the nonsurvivors who died because of PML (P = 0.049). In contrast, no difference for JCV-specific T-cell response was found when PML survivors and their controls were compared or for CMV-specific T-cell responses in any group (Table 2 and Fig. 2). JCV-specific T-cell responses correlated with 1-year survival in PML patients but reached significance only for laboratory-confirmed or definitive PML cases (P = 0.02) (Table 3). No correlation of CMV-specific immune responses with 1-year survival was observed.
TABLE 2.
Comparison of PML cases and their matched controlsa
| Parameter | All PML patients and their controls
|
Patients with laboratory-confirmed or definitive diagnosis and their controls
|
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| JCV-specific cellular immune response (estimates by 10-fold higher of parameter +1) | ||||
| All patients | 0.77 (0.36, 1.62) | 0.48 | 0.20 (0.03, 0.68) | 0.008 |
| Survivors | 1.19 (0.48, 3.05) | 0.71 | 0.50 (0.09, 2.08) | 0.34 |
| Nonsurvivors | 0.29 (0.05, 1.13) | 0.08 | 0.03 (0.00, 0.46) | 0.004 |
| CMV-specific cellular immune response (estimates by 10-fold higher of parameter +1) | ||||
| All patients | 0.85 (0.46, 1.54) | 0.60 | 0.56 (0.19, 1.47) | 0.24 |
| Survivors | 0.87 (0.35, 2.07) | 0.75 | 1.41 (0.18, 11.50) | 0.73 |
| Nonsurvivors | 0.85 (0.38, 1.88) | 0.68 | 0.44 (0.11, 1.30) | 0.14 |
| JCV VLP IgG response (estimates by 2-fold-higher parameter value) | ||||
| All patients | 1.31 (1.01,1.75) | 0.04 | 1.14 (0.83, 1.63) | 0.42 |
| Survivorsb | 1.73 (1.19, 2.70) | 0.003 | 1.46 (0.91, 2.94) | 0.13 |
| Nonsurvivors | 0.86 (0.53, 1.34) | 0.50 | 0.88 (0.52, 1.43) | 0.62 |
| JCV VLP IgM response (estimates by 2-fold-higher parameter value) | ||||
| All patients | 1.45 (0.91, 2.40) | 0.12 | 2.39 (0.99, 6.24) | 0.053 |
| Survivors | 1.44 (0.87,2.48) | 0.15 | 3.08 (1.02, 11.11) | 0.047 |
| Nonsurvivors | 1.48 (0.41, 5.32) | 0.53 | 1.51 (0.35, 6.65) | 0.56 |
Odds of developing PML based on cellular and humoral immunity at time zero in all patients and in PML survivors and nonsurvivors (and their matched controls) separately. The results are based on logistic regression using Firth's bias reduction adjusted for matching. CI, confidence interval.
If results are based on non-log-transformed JCV VLP IgG response instead, which is equally sensible based on the data, the results for survivors are significant both in all survivors (P = 0.001) and in survivors with laboratory-confirmed or definitive diagnosis and their controls (P = 0.03).
FIG. 2.
Cellular and humoral immunities in PML cases at the time of PML diagnosis and in matched controls. The P values correspond to comparisons of cases versus controls in PML survivors and nonsurvivors separately with adjustment for matching as described in Materials and Methods. The box plots of data are drawn in gray, with individual measurements shown as black dots.
TABLE 3.
Logistic survival for estimating the effects of cellular and humoral immunity at the time of PML diagnosis on 1-year survivala
| Parameter | All PML patients
|
Patients with laboratory-confirmed or definitive diagnosis
|
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| JCV-specific cellular immune response (estimates by 10-fold higher of parameter +1) | ||||
| Unadjusted | 0.36 (0.09, 1.20) | 0.10 | 0.06 (0.00, 0.62) | 0.02 |
| Adjusted for CD4 cell count | 0.45 (0.11, 1.57) | 0.21 | 0.11 (0.00, 1.56) | 0.11 |
| CMV-specific cellular immune response (estimates by 10-fold higher of parameter +1) | ||||
| Unadjusted | 1.00 (0.46, 2.19) | 1.00 | 0.67 (0.12, 2.00) | 0.49 |
| Adjusted for CD4 cell count | 1.23 (0.53, 3.04) | 0.63 | 1.15 (0.13, 19.97) | 0.88 |
| JCV VLP IgG response (estimates by 2-fold-higher parameter value) | ||||
| Unadjusted | 0.51 (0.27, 0.84) | 0.007 | 0.62 (0.30, 1.08) | 0.09 |
| Adjusted for CD4 cell count | 0.54 (0.28, 0.90) | 0.02 | 0.67 (0.28, 1.31) | 0.24 |
| JCV VLP IgM response (estimates by 2-fold-higher parameter value) | ||||
| Unadjusted | 0.84 (0.34, 1.49) | 0.58 | 0.46 (0.12, 1.39) | 0.17 |
| Adjusted for CD4 cell count | 0.88 (0.36, 1.57) | 0.68 | 0.29 (0.00, 1.30) | 0.12 |
Univariate analyses and analyses adjusted for CD4 cell counts based on logistic regression using Firth's bias reduction. CI, confidence interval.
JCV-specific T-cell responses at PML diagnosis (or at the respective time zero for controls) were detectable in 36% of nonsurviving cases compared to 67% of patients who survived PML and 61% of controls. The median JCV T-cell response measured in SFU per 106 PBMC at diagnosis was 10 (IQR, 0 to 16) in PML survivors, 0 (IQR, 0 to 5) in PML nonsurvivors, and 5 (IQR, 0 to 15) in controls. The median CMV-specific immune response at time zero was 98 (IQR, 37 to 1,494) in PML survivors, 425 (IQR, 43 to 1,898) in PML nonsurvivors, and 400 (IQR, 105 to 1,854) in controls (Fig. 2). CMV-specific T-cell responses were significantly higher than JCV-specific T-cell responses in all three groups (all P < 0.01).
JCV-specific humoral immune response.
JCV-specific antibodies were analyzed in 99 and 98 samples from 29 cases, respectively, and in 283 samples from 87 controls. IgG and IgM showed little variation over time in nonsurvivors (Fig. 1). In survivors, however, we observed a statistically significant increase in IgG (P = 0.005; crude median of all measurements until diagnosis, 0.43 [IQR, 0.32 to 1.40], and after diagnosis, 2.86 [IQR, 1.76 to 3.10]) but no significant change for IgM responses (P = 0.22).
At diagnosis, 93% of cases were JCV IgG seropositive. Seventeen of 18 (94%) PML survivors had IgG activities (optical densities at 492 nm [OD492] of ≥0.22 compared to only 5/11 (45%) PML nonsurvivors. Among the controls, 78/87 (90%) were seropositive at time zero, and 62/87 (71%) had IgG activities (OD492) of ≥0.22. The median IgG activities at time zero were 1.08 in PML survivors, 0.16 in PML nonsurvivors, and 0.33 in controls. The respective values for IgM were 0.054, 0.063, and 0.055 (Fig. 2). PML nonsurvivors had JCV-specific IgG antibody levels similar to those of matched controls. In contrast, PML survivors had significantly higher JCV-specific IgG antibody levels than matched controls (P = 0.003). The same was true if this analysis was adjusted for the HIV RNA load (P = 0.003). IgM antibody levels tended to be higher in PML patients than in matched controls without reaching statistical significance (Table 2).
Higher IgG values at diagnosis were associated with significantly better 1-year survival of PML cases in the univariate analyses, which was also true after adjustment for the CD4+ T-cell count. However, the result was not significant when cases with possible PML were excluded. No association between IgM values and survival of PML cases was observed (Table 3).
Roles of CD4+ T-cell counts and HIV RNA for JCV-specific immune responses.
Between-patient correlations of CD4+ T-cell counts, HIV RNA, and the four markers of JCV-specific and CMV-specific immune responses at time zero were generally not strong. In PML cases, the only rank correlations that exceeded 0.5 in absolute value were those between IgG and IgM (rank correlation, 0.52; P = 0.004) and between CD4+ T-cell counts and HIV RNA (rank correlation, −0.65; P < 0.001), whereas in controls, the absolute value of the rank correlations never exceeded 0.5. The examined parameters showed little systematic variation over time for PML nonsurvivors (Fig. 1). Therefore within-patient associations were calculated only for PML survivors. Significant longitudinal associations in survivors were found between IgG and CD4+ T-cell counts (positive association in 76% of the 17 analyzable patients; P = 0.049), between IgG and HIV RNA load (negative association in 80% of patients; P = 0.03), and between CD4+ T-cell counts and HIV RNA loads (negative association in 87% of patients; P = 0.007).
DISCUSSION
PML is a rare disease and almost exclusively affects patients with impaired immune functions as a consequence of chemotherapy, transplantation, or therapy for autoimmune diseases. The highest rates of up to 8% were reported in HIV/AIDS in the pre-cART era and have significantly declined since the introduction of cART (1, 4, 31). Our nested case-control study of patients prospectively enrolled in the SHCS provides evidence that low T-cell immunity specifically directed at JCV is a key feature of the disease. Moreover, the study of immune responses before and after PML diagnosis supports the notion that mounting of a JCV-specific humoral and cellular immune response correlates with a better prognosis. Although limited to 29 cases, our study represents one of the largest and most comprehensive prospective, longitudinal investigations of both humoral and cellular immunities before and after PML diagnosis. Importantly, the nested controls were matched not only for age, gender, and date of manifestation, but also for CD4+ T-cell count and slope of decline. Although a declining CD4+ T-cell count is known to progressively increase the risk of opportunistic complications, including PML (2, 5), our results suggest that the frequency of JCV-specific T-cell response is indeed a critical determinant. The lack of JCV-specific immunity would explain the sporadic onset of disease in HIV-1 patients with CD4+ T-cell counts below 100/μl, as well as the exceptional cases with CD4+ T-cell counts well above 200/μl. This is further supported by the fact that T-cell responses to CMV were high and not significantly different between PML survivors, nonsurvivors, and controls.
Up to now, there has been no prospective evaluation of JCV immunity before PML diagnosis. However, the correlation of JCV-specific T-cell immunity, particularly of specific CD8+ T cells, with survival has been suggested previously (11, 12, 17). Despite methodological differences concerning the in vitro separation and expansion of T-cell subpopulations in those studies, their results seem consistent with our direct ex vivo data for PBMCs (12, 17). CD8+ T lymphocytes are critical to JCV-specific cytotoxicity, but it is now clear for CMV and adenovirus that stable antiviral control also requires specific CD4+ T cells (20).
The JCV IgG seroprevalence of 91% at PML diagnosis was comparable to the reported prevalences in other studies (23) but significantly higher than the seroprevalence of 58% found in healthy blood donors using the same assay (13). In the latter study, we observed an increasing IgG seroprevalence with age in line with significant JCV (re)exposure, which might play a role in some cases of PML (13). We found that higher JCV-specific IgG activity at diagnosis was associated with better survival and that the rise in JCV IgG levels correlated with increasing CD4+ T-cell counts and decreasing HIV RNA loads. This dynamic pattern emphasizes the role of a functional immune response, which is improved by cART by interfering with HIV replication-mediated CD4+ T-cell loss. Among the nonsurvivors, 5 of 11 patients were (re)treated with cART within 3 months of PML diagnosis, which likely was too late to modify the outcome.
PML survivors were able to mount stronger humoral immune responses than controls despite still-impaired cellular immune responses. The correlation of humoral immunity with the PML prognosis was previously investigated in 62 PML patients from the pre-cART era. Intrathecal IgG production was seen in 47 of the 62 patients without clinical and biological correlations (35). This argues against a major role of humoral immunity in the pre-cART era but should be readdressed now.
Some limitations of our study should be noted. First, given the poor prognosis of PML, the identification of surrogate markers would be desirable for predicting PML before clinical manifestation. Although prospectively collected samples from the SHCS provide a unique aspect to our case-control study, we were unable to unambiguously distinguish control patients from cases at risk for PML by immunological markers alone. Possibly, other markers or combinations of markers may be more suitable, such as the detection of JCV in plasma or in PBMCs by PCR. Second, the diagnosis of PML is generally a late-stage presentation. In the setting of HIV/AIDS, this implies missed opportunities, including HIV diagnosis, care, and cART (36). These factors also affect the availability of study samples prior to PML diagnosis. Third, a significant number of our patients, and particularly of the “survivors,” fell into the category of “possible” PML but with typical clinical and radiological magnetic resonance imaging signs. Also, we cannot exclude the possibility that patients with “possible” diagnoses were diagnosed earlier and therefore had a better outcome due to earlier introduction of cART. Fourth, immune reconstitution syndrome (IRS) has recently emerged as a fatal complication of PML in HIV/AIDS treated with cART (19) and is reported to occur in up to 23% of all PML patients (30). Therefore, some of the “nonsurvivors” with good immune responses might fall into this group. It is tempting to speculate that the higher JCV-specific humoral immune response in “survivors” could modulate a protective effect against IRS by neutralizing antigen. Interestingly, in a recent study, no difference in mortality of PML patients with or without IRS was observed (15). Finally, the overall JCV-specific T-cell responses were low. In healthy donors and HIV-1-infected patients with CD4 counts of >300/μl, the median ESA SFU was 45 per 106 PBMCs, showing a significant difference between JCV- and CMV-specific T-cell responses. Nevertheless, our results are comparable to those of earlier studies using other methods, such as in vitro-stimulation assays, to amplify responses, which are time-consuming and may introduce a bias in the activated-T-cell repertoire (27).
In conclusion, PML remains a significant threat in severely immunocompromised patients, including those with HIV/AIDS and other immunodeficiencies. Our data revealed JCV-specific defects in the T-cell repertoire, indicating that JCV-specific cellular immunity is a critical determinant in PML patients irrespective of the CD4+ T-cell counts. Both cellular and humoral immunities might be used as prognostic markers in future clinical studies.
Acknowledgments
We thank Igor Koralnik, Boston, MA, for critical comments; Ingrid Ziekau, Jacqueline Samarides, and the members of the Molecular Diagnostics Laboratory and Simone Binggeli, Alexis Dumoulin, and Adrian Egli of the Transplantation Virology Laboratory at the Institute for Medical Microbiology, Basel, Switzerland, for encouraging discussions; and Sohrab Boaghi for providing the VLP.
The members of the SHCS are M. Battegay, E. Bernasconi, J. Böni, H. C. Bucher, P. Bürgisser, A. Calmy, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS, Centre Hospitalier Universitaire Vaudois, CH-1011 Lausanne, Switzerland), H. Furrer (Chairman of the Clinical and Laboratory Committee), C. Fux, M. Gorgievski, H. Günthard (Chairman of the Scientific Board), H. H. Hirsch, B. Hirschel, I. Hösli, C. Kahlert, L. Kaiser, U. Karrer, C. Kind, T. Klimkait, B. Ledergerber, G. Martinetti, B. Martinez, N. Müller, D. Nadal, M. Opravil, F. Paccaud, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach (Head of the Data Center), C. Rudin (Chairman of the Mother and Child Substudy), P. Schmid, D. Schultze, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, and S. Yerly.
This work was supported by an SHCS grant (512/07) from the Swiss National Science Foundation (SNF); by unrestricted grants from the Stiftung Forschung Infektionskrankheiten, Basel, Switzerland; and by research funds from the Department of Internal Medicine, Basel, Switzerland, to N.K. and H.H.H. The funding institutions had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have declared that no competing interests exist.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Albrecht, H., C. Hoffmann, O. Degen, et al. 1998. Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV-associated progressive multifocal leukoencephalopathy. AIDS 121149-1154. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer, J., P. Miralles, J. Arri-abalaga, et al. 2003. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin. Infect. Dis. 361047-1052. [DOI] [PubMed] [Google Scholar]
- 3.Berger, J. R., and S. Houff. 2006. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res. 28299-305. [DOI] [PubMed] [Google Scholar]
- 4.Berger, J. R., B. Kaszovitz, M. J. Post, and G. Dickinson. 1987. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern. Med. 10778-87. [DOI] [PubMed] [Google Scholar]
- 5.Berger, J. R., R. M. Levy, D. Flomenhoft, and M. Dobbs. 1998. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann. Neurol. 44341-349. [DOI] [PubMed] [Google Scholar]
- 6.Berger, J. R. 2003. JCV-specific CD4 T cell response: another piece of the puzzle in explaining some aspects of AIDS associated PML. AIDS 171557-1559. [DOI] [PubMed] [Google Scholar]
- 7.Binggeli, S., A. Egli, S. Schaub, et al. 2007. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am. J. Transplant. 71131-1139. [DOI] [PubMed] [Google Scholar]
- 8.Cinque, P., I. J. Koralnik, and D. B. Clifford. 2003. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J. Neurovirol. 9(Suppl. 1)88-92. [DOI] [PubMed] [Google Scholar]
- 9.Crowder, C. D., K. A. Gyure, C. B. Drachenberg, et al. 2005. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am. J. Transplant. 51151-1158. [DOI] [PubMed] [Google Scholar]
- 10.De Luca, A., A. Ammassari, P. Pezzotti, et al. 2008. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS 221759-1767. [DOI] [PubMed] [Google Scholar]
- 11.Du Pasquier, R. A., K. W. Clark, P. S. Smith, et al. 2001. JCV-specific cellular immune response correlates with a favorable clinical outcome in HIV-infected individuals with progressive multifocal leukoencephalopathy. J. Neurovirol. 7318-322. [DOI] [PubMed] [Google Scholar]
- 12.Du Pasquier, R. A., M. J. Kuroda, Y. Zheng, J. Jean-Jacques, N. L. Letvin, and I. J. Koralnik. 2004. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 1271970-1978. [DOI] [PubMed] [Google Scholar]
- 13.Egli, A., L. Infanti, A. Dumoulin, A. Buser, J. Samarides, C. Stebler, R. Gosert, and H. H. Hirsch. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199837-846. [DOI] [PubMed] [Google Scholar]
- 14.Egli, A., I. Binet, S. Binggeli, et al. 2008. Cytomegalovirus-specific T-cell responses and viral replication in kidney transplant recipients. J. Transl. Med. 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falco, V., M. Olmo, S. V. del Saz, et al. 2008. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J. Acquir. Immune Defic. Syndr. 4926-31. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Suarez, J., D. de Miguel, I. Krsnik, H. Banas, I. Arribas, and C. Burgaleta. 2005. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am. J. Hematol. 80271-281. [DOI] [PubMed] [Google Scholar]
- 17.Gasnault, J., M. Kahraman, M. G. de Goer de Herve, D. Durali, J. F. Delfraissy, and Y. Taoufik. 2003. Critical role of JC virus-specific CD4 T-cell responses in preventing progressive multifocal leukoencephalopathy. AIDS 171443-1449. [DOI] [PubMed] [Google Scholar]
- 18.Heinze, G., and M. Schemper. 2002. A solution to the problem of separation in logistic regression. Stat. Med. 212409-2419. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, H. H., G. Kaufmann, P. Sendi, and M. Battegay. 2004. Immune reconstitution in HIV-infected patients. Clin. Infect. Dis. 381159-1166. [DOI] [PubMed] [Google Scholar]
- 20.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1882199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos, L., D. Bates, H. P. Hartung, et al. 2007. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol. 6431-441. [DOI] [PubMed] [Google Scholar]
- 22.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353369-374. [DOI] [PubMed] [Google Scholar]
- 23.Knowles, W. A., P. Pipkin, N. Andrews, et al. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71115-123. [DOI] [PubMed] [Google Scholar]
- 24.Koralnik, I. J., R. A. Du Pasquier, M. J. Kuroda, et al. 2002. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J. Immunol. 168499-504. [DOI] [PubMed] [Google Scholar]
- 25.Langer-Gould, A., S. W. Atlas, A. J. Green, A. W. Bollen, and D. Pelletier. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353375-381. [DOI] [PubMed] [Google Scholar]
- 26.Ledergerber, B., J. von Overbeck, M. Egger, and R. Luthy. 1994. The Swiss HIV Cohort Study: rationale, organization and selected baseline characteristics. Soz. Praventivmed. 39387-394. [DOI] [PubMed] [Google Scholar]
- 27.Lima, M. A., A. Marzocchetti, P. Autissier, et al. 2007. Frequency and phenotype of JC virus-specific CD8+ T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J. Virol. 813361-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Major, E. O., K. Amemiya, C. S. Tornatore, S. A. Houff, and J. R. Berger. 1992. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 549-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padgett, B. L., D. L. Walker, G. M. ZuRhein, R. J. Eckroade, and B. H. Dessel. 1971. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet i1257-1260. [DOI] [PubMed] [Google Scholar]
- 30.Tan, K., R. Roda, L. Ostrow, J. McArthur, and A. Nath. 2009. PML-IRIS in patients with HIV infection. Clinical manifestations and treatment with steroids. Neurology 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tassie, J. M., J. Gasnault, M. Bentata, et al. 1999. Survival improvement of AIDS-related progressive multifocal leukoencephalopathy in the era of protease inhibitors. Clinical Epidemiology Group. French Hospital Database on HIV. AIDS 131881-1887. [DOI] [PubMed] [Google Scholar]
- 32.Van Assche, G., M. Van Ranst, R. Sciot, et al. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353362-368. [DOI] [PubMed] [Google Scholar]
- 33.Viscidi, R. P., D. E. Rollison, E. Viscidi, et al. 2003. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin. Diagn. Lab. Immunol. 10278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber, F., C. Goldmann, M. Kramer, et al. 2001. Cellular and humoral immune response in progressive multifocal leukoencephalopathy. Ann. Neurol. 49636-642. [PubMed] [Google Scholar]
- 35.Weber, T., C. Trebst, S. Frye, et al. 1997. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J. Infect. Dis. 176250-254. [DOI] [PubMed] [Google Scholar]
- 36.Wolbers, M., H. C. Bucher, H. Furrer, et al. 2008. Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med. 9397-405. [DOI] [PubMed] [Google Scholar]