Abstract
The E2 protein of human papillomavirus (HPV) binds to specific sites in the viral genome to regulate its transcription, replication, and maintenance in infected cells. Like most regulatory proteins, E2 is rapidly turned over. A high-throughput assay was developed to quantify the expression and stability of E2 in vivo, based on its fusion to Renilla luciferase (RLuc). The steady-state levels of Rluc-E2 were quantified by measuring the amounts of associated luciferase activity, and its degradation was measured by monitoring the decrease in enzymatic activity occurring after a block of translation with cycloheximide. Using this assay, the E2 proteins from a low-risk (HPV11) and a high-risk (HPV31) human papillomavirus (HPV) type were found to have short half-lives of 60 min in C33A cervical carcinoma cells and to be ubiquitinated and degraded by the proteasome. Analysis of mutant proteins showed that the instability of E2 is independent of its DNA-binding and transcriptional activities but is encoded within its transactivation domain, the region that binds to the cellular chromatin factor bromodomain-containing protein 4 (Brd4) to regulate viral gene transcription. Overexpression of Brd4, or of its C-terminal E2-interaction domain, was found to increase the steady-state levels and stability of wild-type E2 but not of E2 mutants defective for binding Brd4. These results indicate that the stability of E2 is increased upon complex formation with Brd4 and highlight the value of the luciferase assay for the study of E2 degradation.
Papillomaviruses are a family of small double-stranded DNA viruses that induce benign and malignant hyperproliferative lesions of the differentiating epithelium (24, 25, 49). Approximately 25 types of human papillomavirus (HPV) infect the anogenital region. The oncogenic or “high-risk” types, such as HPV16, -18, and -31, are found in the majority of cervical and anal cancers and their precursor lesions (7, 25, 49). They are also present in a subset of head-and-neck cancers (22, 42). The low-risk types, including HPV6 and -11, cause benign genital warts (condylomas) (29) and laryngeal papillomatosis (6), a rare but debilitating infection acquired by infants during birth from an infected mother.
The life cycle of HPV is coupled to the cellular differentiation program that keratinocytes undergo in the epithelium (17, 25, 32). These viruses infect the basal cell layer, where they maintain their double-stranded DNA genome as a circular episome in the nucleus of infected cells. Maintenance of the HPV episome in primary keratinocyte cultures requires four viral proteins: the two oncogenes, E6 and E7, the E1 replicative helicase, and the multifunctional E2 protein (25, 32). E2 binds to specific sites in the regulatory region of the viral genome to promote its replication, regulate its transcription, and ensure its proper segregation to daughter cells at mitosis (11). E2 is comprised of two functional domains, an N-terminal transactivation domain (TAD) and a C-terminal DNA-binding/dimerization domain separated by a hinge region. The TAD is a protein interaction module that binds through one surface to the viral E1 helicase to promote replication of the genome and, through the opposite surface, to cellular transcription factors, including bromodomain-containing protein 4 (Brd4), to regulate viral gene transcription (1, 2, 43). In the context of papillomaviruses, Brd4 was first identified as the cellular chromatin component that is bound by E2 to tether the viral episome to host chromosomes in order to facilitate its segregation to daughter cells during mitosis (47, 48). In addition to mediating the function of E2 as a segregation factor, Brd4 is also needed for E2 to activate or repress transcription (8, 33, 40, 41, 44). Brd4 is a member of the BET family of proteins whose members contain two tandem bromodomains (BD1 and BD2) and an extraterminal domain (20). Brd4 binds to the E2 TAD through its C-terminal domain (2, 47) and to acetylated chromatin via its two bromodomains (16, 30). Brd4 was also identified as a positive regulator of P-TEFb, a kinase that stimulates transcriptional elongation by RNA polymerase II (26, 46). Recent evidence also indicates that Brd4 plays a role in cell cycle progression by promoting transcription of growth-associated genes in G1 and allowing progression into S phase (35, 45). Accordingly, cells in which Brd4 expression is downregulated by shRNA arrest in G1 and undergo apoptosis (35, 45).
Mutagenesis of the E2 TAD has led to the identification of amino acid substitutions that genetically separate the transcription from the replication function of the protein. Substitutions of arginine 37 for alanine (R37A) and of isoleucine 73 for leucine or alanine (I73L or I73A) have been shown to abrogate the ability of E2 to activate or repress transcription while having little effect on its ability to support viral DNA replication (3, 12, 13, 15, 19, 23, 37, 39). Later studies showed that substitutions of R37 and I73 affect the binding of E2 to Brd4 (8, 40, 41). Satisfyingly, a crystal structure of the E2 TAD in complex with a Brd4 peptide showed that R37 and I73 form part of the Brd4-binding surface (2).
The E2 proteins from both bovine papillomavirus (BPV) and HPV18 E2 have been shown to be polyubiquitinated in vivo and in vitro, respectively, and degraded by the proteasome (9, 38). Using pulse-chase analysis, the half-life of BPV E2 was measured to be 50 min (38). Comparable results were obtained for the E2 proteins from different HPV types fused to green fluorescent protein (GFP). Specifically, the half-lives of GFP-E2 were determined to be 50 min for HPV18 and HPV16, 125 min for HPV6, and 75 min for HPV11 in HeLa cells (9, 10). However, little is known about the cellular factors involved in the rapid turnover of E2.
The study of E2 degradation would be greatly facilitated by assays that could rapidly determine the stability of the protein in a quantitative manner. We report here the development of a luciferase assay to monitor the expression and degradation of the human papillomavirus E2 protein in cells. Using this quantitative assay, we provide evidence that degradation of E2 is modulated by complex formation with Brd4.
MATERIALS AND METHODS
Plasmid constructions and mutagenesis.
Plasmids to express the E2 protein of HPV11 and HPV31, as fusions with Renilla luciferase (RLuc), were constructed by inserting XhoI-BamHI digested PCR fragments encoding E2 between the XhoI and BamHI sites of plasmid pRluc-C2 (Perkin-Elmer). Primers used for amplification were designed such as to encode XhoI and BamHI restriction sites. The following pairs of primers were used: HPV11 E2, 5′-AAAACTCGAGATGGAAGCAATAGCCAAGCGTTTAG-3′ and 5′-TTTTGGATCCTTACAATAAATGTAATGACATAAACCCCACCTT-3′; and HPV31 E2, 5′-AAAACTCGAGACTCTTTCTCAACGTTTAAATGT-3′ and 5′-TTTTGGATCCCTAAATAGTCATATATCCTGTTGACAC-3′. A linker encoding a 3xFlag epitope was then inserted at the XhoI site by annealing of the following five oligonucleotides and ligation (oligonucleotides O1 and O5 were 5′ phosphorylated): O1, 5′-TCGACGGCGACTACAAGGACCACGATGGCGA-3′; O2, 5′-CTACAAGGACCATGACATCGATTATAAGGACGATGACG-3′; O3, 5′-ATAAGAGCGGCGGCGGAGGGAGCC-3′; O4, 5′-GATGTCATGGTCCTTGTAGTCGCCATCGTGGTCCTTGTAGTCGCCG-3′; and O5, 5′-TCGAGGCTCCCTCCGCCGCCGCTCTTATCGTCATCGTCCTTATAATC-3′). The 3xFlag epitope was also incorporated in the control RLuc expression plasmid. In this case, the 3xFlag linker was created by annealing five oligonucleotides—O1, O2, and O4 (described above), as well as O6 (5′-ATAAGAGCGGCGGCGGAGGGAGCG-3′) and phosphorylated O7 (5′-GATCCGCTCCCTCCGCCGCCGCTCTTATCGTCAT CGTCCTTATAATC-3′)—and inserted between the XhoI and BamHI sites of pRluc-C2. The firefly luciferase (FLuc) coding plasmid was constructed by inserting the FLuc coding region present in the pGL3-Basic plasmid (Promega) between the XhoI and XbaI restriction sites of the pCI plasmid (Promega). Truncations of HPV31 E2 fused to RLuc were created as follows. First, the adenine residue preceding the XbaI site of the Rluc-3xFlag plasmid described above was mutated to cytosine to prevent dam methylation and allow cleavage by XbaI. Different subfragments of HPV31 E2 were then inserted into this plasmid between the BamHI and XbaI sites. The following pairs of primers were used for PCR: complete HPV31 E2, 5′-TATATGGATCCGAGACTCTTTCTCAACGTTTAAATGTG-3′ and 5′-TATATTCTAGATTAAATAGTCATATATCCTGTTGACACTGATAC-3′); TAD (amino acids [aa] 1 to 208), 5′-TATATGGATCCGAGACTCTTTCTCAACGTTTAAATGTG-3′ and 5′-ATATTTCTAGATTAAGCAAAGGATATTCGTCACTGCTAAATA-3′; Hinge (aa 198 to 293), 5′-TAATTGGATCCTCTGTATTTAGCAGTGACGAAATATCCT-3′ and 5′-TTTAATCTAGATTATGTAGTTGCAGGACAACTGACAGC-3′; DBD (aa 266 to 372), 5′-ATATAGGATCCTCCGTGGACAGTGTCAACTGTGG-3′ and 5′-TATATTCTAGATTAAATAGTCATATATCCTGTTGACACTGATAC-3′; TAD+Hinge (aa 1 to 293), 5′-TATATGGATCCGAGACTCTTTCTCAACGTTTAAATGTG-3′ and 5′-TTTAATCTAGATTATGTAGTTGCAGGACAACTGACAGC-3′; and Hinge+DBD (aa 198 to 372), 5′-TAATTGGATCCTCTGTATTTAGCAGTGACGAAATATCCT-3′ and 5′-TATATTCTAGATTAAATAGTCATATATCCTGTTGACACTGATAC-3′. These different plasmids encode fusion proteins in which the 3xFlag epitope is separated from the Rluc sequence by nine residues (GDLRRALDG) and the E2 fragment is separated from the 3xFlag epitope by eight residues (SGGGGSGS). Codon-optimized HPV31 E2 was purchased from GenScript Corp. and inserted, using BamHI/HindIII cloning, into pCMV1a (Stratagene), which introduces a 3xFlag sequence at the N terminus of E2. The Brd4-C construct was created by amplifying the portion of human Brd4 encoding aa 1224 to 1362 by PCR from the pOTB7 vector (ATCC 6714018, GenBank no. BC008354) using the primers 5′-GCGGGATCCGCCACCATGGCACCGAAGAAGAAAAGGAAGGTGTTCGAGCAGTTCCGCCGCG-3′ and 5′-TTACTGCAGAAGCTTTCAGAAAAGATTTTCTTCAAATATT-3′ and inserted between the BamHI and PstI restriction sites of the pCR3.1 expression plasmid. Using this strategy, the amino acid sequence MAPKKKRKV, containing the simian virus 40 (SV40) nuclear localization signal, was inserted at the N-terminal of Brd4-C. The Flag-tagged full-length (aa 1 to 1362) and N-terminal (aa 1 to 1223) human Brd4 constructs were kind gifts from Cheng-Ming Chiang (University of Texas Southwestern Medical Center). All plasmids were verified by DNA sequencing. Further details on construction of these plasmids are available upon request.
Antibodies, Western blotting, and immunoprecipitations.
Purified mouse anti-Flag M2 antibody was obtained from Sigma (F1804, 1:2,000 dilution for Western blotting). Rabbit anti-Brd4 antibody was obtained from Abgent (AP8051a, 1:2,000 dilution). Rabbit anti-ubiquitin antibody (FL-76, sc-9133, 1:500 dilution) and rabbit anti-HA antibody (Y-11, sc-805, 1:1,250 dilution) were purchased from Santa Cruz Biotechnology. Western blotting was performed after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the transfer of proteins onto polyvinylidene difluoride membranes; results were visualized according to an enhanced chemiluminescence procedure (GE Healthcare). For immunoprecipitations, C33A cells were transfected into six-well plates, washed with ice-cold phosphate-buffered saline (PBS), and solubilized in 400 μl of cold lysis buffer (50 mM Tris-Cl [pH 7.5], 350 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 50 mM NaF, protease inhibitors, containing 20 μM MG132 and 10 mM N-ethylmaleimide). Lysates were transferred to Eppendorf tubes and rotated for 45 min at 4°C before centrifugation at 20,000 × g for 15 min. Cleared lysates were then incubated for 3 h with 20 μl of protein G-Sepharose beads (GE Healthcare) that had been previously loaded with 2.5 μg of anti-Flag M2 antibody. Beads were washed four times with 1 ml of wash buffer (50 mM Tris-Cl [pH 7.5], 350 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 50 mM NaF, and protease inhibitors and containing 20 μM MG132 and 10 mM N-ethylmaleimide) and once more with PBS, before 40 μl of 5× Laemmli sample buffer was added and the samples were boiled for 10 min to elute the proteins.
Cell culture and transfections.
C33A cells or its Brd4 knockdown derivative (cell line 1-13 from Wu et al. [44], kindly provided by Cheng-Ming Chiang, University of Texas Southwestern Medical Center) were grown in Dulbecco modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum, 0.5 IU of penicillin/ml, 50 μg of streptomycin/ml, and 2 mM l-glutamine. Transfections were performed using Lipofectamine 2000 reagent according to the manufacturer's recommendations (Invitrogen).
Luciferase assays.
Expression vectors for the RLuc-E2 fusion proteins or RLuc control were transfected together with the FLuc normalization plasmid into C33A cells. Cells were either transfected directly in white 96-well plates or transferred to 96-well plates after transfection in 100-mm dishes. FLuc and RLuc activities were measured by using the Dual-Glo luciferase system (Promega) and a GloMax 96-well luminometer (Promega) and normalized according to the instructions from the manufacturer. To measure the levels of luciferase at the steady-state, the cell culture medium in each well was replaced with 75 μl of fresh DMEM before the addition of 75 μl of the FLuc substrate. Plates were then placed on a plate shaker at room temperature for 20 min before the luminescence was read. A 75-μl portion of the substrate for RLuc was then added to each well, and the plate was agitated and read as described above. Cycloheximide chase assays were started 24 or 48 h posttransfection by replacing the cell culture medium in each well with 75 μl of DMEM containing 100 μg of cycloheximide/ml. Cells were then incubated at 37°C for the indicated time points before the addition of 75 μl of the FLuc substrate and further processing of the samples as described above.
Confocal fluorescence microscopy.
C33A cells were grown on coverslips. For immunofluorescence, cells were fixed 24 h posttransfection with 4% formaldehyde, permeabilized with 0.2% Triton X-100, and incubated with anti-Flag M2 antibody diluted 1:500 in PBS-2% milk for 1.5 h, followed by 1 h of incubation with a 1:1,000 dilution of Alexa Fluor 633-conjugated secondary antibody. Cells were washed with PBS between each step and then mounted using VectaShield mounting medium (Vector Laboratories). Images were acquired by using a LSM510 confocal laser coupled to an Axiovert 100M inverted scanning microscope (Zeiss, Toronto, Ontario, Canada) and analyzed by using LSM Image Browser version 3.2.0.70 (Zeiss).
Transactivation assays.
C33A cells (50,000 per well) were transfected in white 96-well format with either 15 ng of 3xFlag-HPV31E2 coding plasmid or 10 ng of RLuc-E2 plasmids, as indicated in the text, along with 185 ng of pK4xE2BS reporter plasmid, which contains the FLuc coding region located downstream of four E2 binding sites (kindly provided by Lou Laimins, Northwestern University). Where indicated, the control reporter plasmid pCMV-FLuc, which expresses FLuc from the cytomegalovirus (CMV) promoter, was transfected at 20 ng per well. In transfections performed with 3xFlag-HPV31E2, a plasmid expressing RLuc was used as a normalization control. To test the effect of proteasome inhibitors on E2-transactivation, cells were treated at 48 h posttransfection with increasing concentrations of MG132 or lactacystin for 6.5 h (all wells were adjusted to 0.5% dimethyl sulfoxide as a vehicle) before the FLuc and RLuc activities were measured by using a Dual-Glo luciferase system (Promega) as described above.
Downregulation of Brd4 by RNA interference.
Twenty-one-nucleotide short hairpin RNAs (shRNAs) were expressed from the pLKO.1-Puro plasmid and purchased from Open Biosystems. The targeting sequences for the Brd4 and control shRNA were 5′-CCTGGAGATGACATAGTCTTA-3′ and 5′-GCTATGAGAATAACGGTAACA-3′, respectively. C33A cells transfected with these shRNA vectors were selected for 3 days (60 to 72 h) in culture medium containing 2 μg of puromycin/ml prior to further analysis.
RESULTS
Expression of HPV11 and HPV31 E2 fused to RLuc.
The E2 protein of papillomavirus is expressed at low levels, and previous studies indicated that it is rapidly ubiquitinated and degraded by the proteasome (9, 10, 38). To easily quantify the levels of HPV E2 proteins in cells without the need for overexpression, we made constructs expressing HPV11 or HPV31 E2 fused at their N terminus to RLuc. To facilitate the detection of low amounts of these fusion proteins by Western blotting, a 38-aa linker encoding a 3xFlag epitope was incorporated between the E2 and luciferase coding regions (Fig. 1A). We chose to fuse RLuc and the 3xFlag epitope at the N terminus rather than the C terminus of E2 because it was previously demonstrated that GFP, which is approximately the same size as RLuc, can be fused at the N terminus of E2 without affecting its function (10). We then tested whether the RLuc-E2 fusion proteins were appropriately expressed in C33A human cervical carcinoma cells by anti-Flag immunoblot analysis of cells transfected with increasing amounts of each plasmid (Fig. 1B and C). The two RLuc-E2 fusion proteins were readily detectable at the expected molecular mass of 80 kDa and were expressed at lower levels than RLuc alone. Transfection of ∼10-fold more RLuc-E2 expression plasmid than RLuc-plasmid was needed to obtain comparable signals in Western blots (Fig. 1B and C). E2 is known to accumulate primarily in the cell nucleus, although it can shuttle between the nucleus and cytoplasm (10). We confirmed that the RLuc-E2 fusion proteins were also localized in the nucleus of transfected C33A cells by confocal immunofluorescence microscopy using an anti-Flag antibody (Fig. 1D). In contrast, RLuc was present in both the nucleus and cytoplasm (Fig. 1D). Together, these results demonstrate that RLuc-E2 fusion proteins are produced at the expected size and are properly localized in the nucleus. Finally, to ascertain that the fusion of RLuc at the N terminus of E2 did not affect its activity and, in particular, the proper folding of the TAD, we verified that RLuc-E2 from HPV31 was still capable of transactivating an E2-responsive reporter gene and, in separate experiments, that it retained the ability to interact with E1 (see Fig. S1 in the supplemental material) and to support transient DNA replication (data not shown).
FIG. 1.
Expression of HPV11 and HPV31 E2 as fusions with RLuc. (A) Schematic representation of the fusion proteins used in the present study and showing the location of the RLuc, 3xFlag epitope, and E2 coding regions. (B and C) Western blot analysis of C33A cells transfected with the indicated amounts (in ng) of RLuc-E2 or RLuc expression plasmid. RLuc fusion proteins were detected using an anti-Flag antibody. Western blotting against tubulin was performed as a loading control. The positions and masses (in kilodaltons) are indicated to the left of each gel. (D) Intracellular localization of RLuc fusion proteins in transfected C33A cells as determined by confocal immunofluorescence microscopy using an anti-Flag antibody (top panels). The same images overlaid with phase-contrast pictures to visualize cells are also presented (bottom panels).
Expression and stability of RLuc-E2 fusion proteins measured using luciferase assays.
We then tested whether expression of RLuc-E2 fusion proteins could be detected in C33A cells by measuring the amount of associated luciferase activity at 24 h posttransfection. We took advantage of a commercially available dual luciferase assay that allows the measurement of both RLuc and FLuc activities in the same cells. In this experiment and all subsequent ones, an FLuc expression plasmid was included as an internal control to correct for potential variations such as differences in transfection efficiency. Thus, all of the luciferase activity results presented here have been normalized. Furthermore, in this assay and all subsequent ones, each data point represents the average of two to three luciferase activity measurements obtained from separate transfections. Using this protocol, we first measured the luciferase activity in cells transfected with increasing amounts of RLuc or RLuc-E2 expression plasmid and found that the correlation between enzyme activity and the amount of transfected plasmid DNA was strictly linear (Fig. 2A). Consistent with the data in Fig. 1B and C, we observed that both RLuc-E2 fusion proteins gave rise to lower amounts of luciferase activity than the control RLuc alone (Fig. 2A). Based on the enzymatic activity, RLuc-11E2 and RLuc-31E2 were 64- and 42-fold less expressed, respectively, than RLuc alone. This is consistent with E2 being rapidly targeted for degradation compared to RLuc, which has a reported half-life of ∼14 h (31). Importantly, the luciferase assay could readily detect low levels of RLuc-E2 expression, with at least a fivefold-greater sensitivity than Western blotting against the 3xFlag epitope.
FIG. 2.

Expression and stability of RLuc-E2 fusion proteins measured using luciferase activity. (A) Luciferase activity measured in C33A cells transfected with increasing amounts of RLuc-E2 vectors or RLuc control vector. For each measurement, the levels of RLuc activity were normalized to those of FLuc expressed from a cotransfected plasmid. (B) The stabilities of RLuc proteins were determined 48 h posttransfection by measuring the levels of luciferase activity remaining at different time points (in minutes) after a block of protein synthesis with 100 μg of cycloheximide/ml.
We next determined whether the half-lives of RLuc-E2 fusion proteins could be determined by measuring the levels of luciferase activity at different time points after a block of de novo protein synthesis with cycloheximide. Under these conditions, the luciferase activity of E2 fusion proteins diminished rapidly to ca. 40% after 3 h compared to the control RLuc and then remained stable (Fig. 2B). From these data, the half-lives of RLuc-E2 proteins from both HPV11 and HPV31 were measured to be ∼60 min. This value is consistent with previous studies that measured half-lives of 75 min for HPV11 and 50 min for both HPV16 and HPV18 E2 using a conventional protocol (9, 10). As an additional control, we verified that the stability of both fusion proteins was independent of the amount of expression vector transfected (see Fig. S2 in the supplemental material), indicating that the degradation machinery is not saturated under our assay conditions. Collectively, these results demonstrate the usefulness of RLuc fusion proteins to quantitatively assess the expression and stability of E2 from different HPV types.
RLuc-E2 fusion proteins are degraded by the proteasome.
Based on previous reports that BPV and HPV18 E2 are degraded by the proteasome (9, 38), we anticipated that our HPV11 and HPV31 RLuc-E2 fusion proteins would also be degraded by this pathway. To verify this assumption, we first tested whether the steady-state accumulation of RLuc-E2 could be increased by the proteasome inhibitor lactacystin. At 24 h posttransfection, C33A cells were incubated for 5 h with increasing concentrations of lactacystin and the level of each fusion protein assessed by immunoblot analysis with an anti-Flag antibody. Under these conditions, lactacystin led to a dose-dependent increase in the levels of RLuc-E2 but had little effect on the levels of RLuc alone (Fig. 3A). This increase in RLuc-E2 accumulation was accompanied by the appearance of high-molecular-weight species, which likely correspond to polyubiquitinated forms of the proteins (Fig. 3A and data below). Similar results were obtained with MG132, another commonly used proteasome inhibitor (data not shown). Importantly, lactacystin also increased the levels of luciferase activity associated with RLuc-E2 fusions, in a dose-dependent fashion (Fig. 3B), but again had little effect on the activity of RLuc alone. The luciferase activities of RLuc-E2 from HPV11 and HPV31 were increased by as much as 70 and 50%, respectively (Fig. 3B). Finally, we showed that lactacystin could prevent the degradation of both RLuc-E2 proteins in a cycloheximide chase assay (Fig. 3C). Collectively, these results indicate that RLuc-E2 fusion proteins are degraded by the proteasome, like wild-type (untagged) E2.
FIG. 3.
Effect of lactacystin on the stability of RLuc-E2. (A) Western blot analysis of C33A cells transfected with a fixed amount of expression vectors for RLuc-E2 from HPV11 and HPV31, or RLuc alone, and treated with the indicated concentrations of the proteasome inhibitor lactacystin for 5 h. Proteins were detected by using an anti-Flag antibody. (B) Luciferase activity measured from C33A cells transfected and treated with lactacystin. (C) Stability of RLuc-E2 or RLuc alone measured 24 h posttransfection in cells treated or not with 50 μM lactacystin for 1 h. The luciferase activity was determined at different time points (min) after a block of protein synthesis with cycloheximide.
RLuc-E2 fusion proteins are polyubiquitinated in vivo.
The finding that RLuc-E2 proteins are degraded by the proteasome led us to investigate their modification by the ubiquitin pathway. To determine whether RLuc-E2 fusion proteins are polyubiquitinated in vivo, we examined their expression by anti-Flag immunoblot in transiently transfected C33A cells treated or not with 25 μM MG132 for 3 h to inhibit the proteasome. As can be seen in Fig. 4A, high-molecular-weight forms of RLuc-E2, likely corresponding to polyubiquitinated forms, were readily observable in cells treated with MG132. To provide more direct evidence that RLuc-E2 fusions are polyubiquitinated, they were immunoprecipitated from the different cell lysates by using an anti-Flag antibody and analyzed by Western blotting against ubiquitin or E2 (anti-Flag) as a control (Fig. 4B). As anticipated, high-molecular-weight forms of E2 were readily detected with the anti-ubiquitin antibody in the immunoprecipitates from cells treated with MG132. These results indicate that RLuc-E2 proteins are efficiently polyubiquitinated in vivo, a modification which likely underlies their rapid proteasomal degradation.
FIG. 4.

Polyubiquitination of RLuc-E2 in vivo. (A) Western blot analysis of C33A cells transfected with the indicated RLuc-E2 expression vector and then treated (+) or not (−) with 25 μM MG132 for 3 h. Cell lysates were analyzed by Western blotting with antibodies against the Flag epitope or tubulin as a loading control. The positions of RLuc-E2 fusion proteins and of molecular mass markers (in kilodaltons) are indicated on the right and left sides of the blot, respectively. (B) Western blot analysis of RLuc-E2 immunoprecipitates. RLuc-E2 proteins were immunoprecipitated from the cell lysates shown above and analyzed by Western blotting against ubiquitin and RLuc-E2 (anti-Flag). Mock-transfected cell lysates were used as a control. An unrelated control antibody (Ctl. Ab) against the large T antigen of SV40 was used to demonstrate the specificity of the immunoprecipitation step.
Mapping the domain of E2 that promotes its proteasomal degradation.
To determine which domain of E2 is responsible for its degradation, we constructed a set of deletion mutants of HPV31 E2 fused to RLuc. RLuc fusions containing the TAD, hinge, or DNA-binding domain (DBD) alone were made. Fusions containing the TAD plus hinge and the hinge plus DBD were also constructed (Fig. 5A). The stability of these different constructs was then assessed in transfected C33A cells by using a cycloheximide chase assay (Fig. 5B). These studies revealed that the two fusions containing, respectively, the TAD and the TAD+hinge regions were the least stable. In contrast, those comprised of the DBD or hinge+DBD were the most stable. These results pointed to the TAD as being a critical determinant of E2's instability. To substantiate these results, we analyzed the levels of the different RLuc fusion proteins by Western blotting in transfected C33A cells treated or not with two different concentrations of lactacystin (12.5 and 50 μM) (Fig. 5C). Fusion proteins containing the TAD (i.e., fusions comprised of the TAD, TAD+hinge, or the complete E2) accumulated as high-molecular-weight species in cells treated with lactacystin, suggesting that they were efficiently polyubiquitinated. In contrast, those lacking the TAD (i.e., fusions comprised of the hinge, DBD, or DBD+hinge) showed little evidence of polyubiquitination. Collectively, these results suggest that the TAD is the primary domain of E2 that promotes its polyubiquitination and proteasomal degradation.
FIG. 5.
Mapping the domain of HPV31 E2 responsible for its degradation. (A) Diagram of the HPV31 E2 (31E2) protein showing the location of the TAD, hinge region and DBD. The different regions of E2 that were fused to RLuc are shown below. (B) The stability of the indicated RLuc fusion proteins was determined 48 h posttransfection by measuring the levels of luciferase activity remaining at different time points (in minutes) after a block of protein synthesis with cycloheximide. (C) Western blotting analysis of C33A cells transfected with the indicated RLuc fusion expression vector and then treated or not with two concentrations of lactacystin (12.5 and 50 μM). Cell lysates were analyzed by Western blotting with antibodies against the Flag epitope and tubulin as a loading control. The positions of polyubiquitinated forms of RLuc fusion proteins and of molecular mass markers (in kilodaltons) are indicated on the right and left sides of the blot, respectively.
The steady-state levels and stability of RLuc-E2 are increased by overexpression of the Brd4 C-terminal domain.
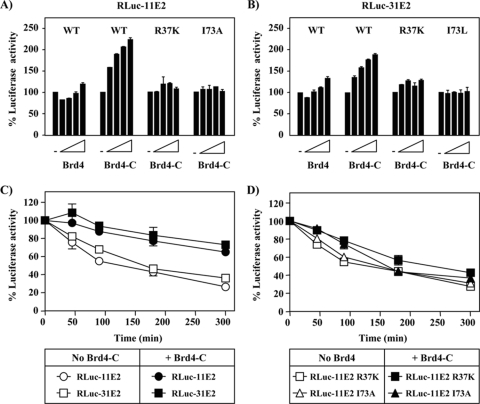
E2 interacts with the viral E1 helicase to promote replication of the HPV genome and with Brd4 to regulate viral gene transcription (11, 43). Both E1 and Brd4 bind to the TAD of E2, on opposite surfaces (1, 2). Since the TAD is the primary domain of E2 responsible for its degradation, it was of interest to test whether overexpression of these factors could affect the stability of E2. We first tested whether cotransfection of an expression vector for a codon-optimized HPV31 E1 had any effect on the levels of RLuc-31E2, but we did not observe any significant effect (data not shown). We then tested whether overexpression of Brd4 could affect the levels of RLuc-E2 using two different Brd4 expression plasmids. One plasmid encoded the full-length Brd4 (aa 1 to 1362) tagged with a Flag epitope. The other encoded only the C-terminal domain of Brd4 (Brd4-C, aa 1224 to 1362) fused to the SV40 NLS to promote its nuclear accumulation. This latter construct retains the E2-interaction domain of Brd4 and was shown previously to inhibit the transcriptional activity of E2 in a dominant-negative manner (40). Increasing amounts of both Brd4 expression vectors (8, 20, 50, and 125 ng of plasmid DNA) were transfected into 5 × 104 C33A cells, together with the plasmid encoding RLuc-11E2 (40 ng) or RLuc-31E2 (25 ng). As can be seen in Fig. 6A and B, expression of full-length Brd4 increased the levels of RLuc-E2 from both HPV11 and HPV31 by ca. 40% at the highest amount of Brd4 plasmid tested. The effect of Brd4-C was even more substantial, doubling the levels of both RLuc-E2 proteins at the highest amount of Brd4-C transfected (Fig. 6A and B). To determine whether this stimulatory effect of Brd4-C was due to its interaction with RLuc-E2, we tested its effect on mutant RLuc-E2 proteins carrying specific amino acid substitutions in the TAD, at arginine 37 (R37A) and isoleucine 73 (I73A or I73L), which abrogate binding to Brd4. The effect of Brd4-C on these mutant proteins was greatly reduced compared to its effect on wild-type RLuc-E2 (Fig. 6A and B). Based on these results, we infer that Brd4-C increases the steady-state levels of HPV11 and HPV31 RLuc-E2 by directly binding to them.
FIG. 6.
Effect of Brd4-C on the steady-state levels and stability of RLuc-E2. (A and B) The luciferase activity was measured in cells transfected with RLuc-11E2 (A) or RLuc-31E2 (B), together with increasing amounts of a second plasmid encoding either the full-length Brd4 or Brd4-C. The amount of luciferase activity measured in cells expressing RLuc-E2 in the absence of any Brd4 construct (−) was set at 100%. (C) Stability of RLuc-E2 proteins from HPV11 and HPV31 in C33A cells cotransfected or not with a Brd4-C expression vector, as indicated. RLuc-E2 stability was determined 24 h posttransfection by measuring the levels of luciferase activity remaining at different time points (in minutes) after a block of protein synthesis with cycloheximide. (D) Same as in panel C but using the indicated RLuc-11 E2 mutant proteins.
To determine whether Brd4-C was increasing the levels of HPV11 and HPV31 RLuc-E2 by reducing their degradation, we measured their stability in a cycloheximide chase assay in the presence or absence of Brd4-C. These studies revealed that Brd4-C was indeed capable of slowing down the degradation of both RLuc-E2 proteins (Fig. 6C). In fact, the increase in stability brought about by Brd4-C was comparable to that obtained with lactacystin (Fig. 3C). In contrast and as expected, Brd4-C did not stabilize the R37K and I73A RLuc-11E2 proteins as well as the wild-type protein (Fig. 6D). A weak stabilization of the R37K mutant protein was observed (Fig. 6D), a finding consistent with this substitution having a lesser effect on Brd4-binding than the I73A substitution (41). From these results, we conclude that E2 is protected from proteasomal degradation when bound to Brd4-C.
The steady-state levels and stability of RLuc-E2 are also increased by overexpression of full-length Brd4.
When we determined the degree of overexpression of full-length Brd4 and of Brd4-C by Western blotting, we quickly realized that full-length Brd4 was produced at much lower levels than Brd4-C (see Fig. 3 in the supplemental material). This result suggested that the weaker stabilizing effect of full-length Brd4 on RLuc-E2 levels observed in Fig. 6A and B was likely due to its lower expression. To directly address this possibility, we tested whether transfection of higher amounts of Brd4 plasmid would increase the levels of RLuc-E2. We kept the same transfection conditions as described above but this time using 200 and 400 ng of Brd4 expression plasmid. Because these are large quantities of DNA relative to the number of cells being transfected, we were concerned about possible nonspecific effect on cellular physiology and/or viability and therefore included as controls the I73 mutants RLuc-E2 and RLuc alone. As shown in Fig. 7A, high amounts of Brd4 increased the steady-state levels of wild-type E2 from both HPV11 and HPV31. This effect was robust as the levels of both RLuc-E2 were increased by 2.5-fold at the highest concentration of Brd4 expression plasmid tested. In contrast, high amounts of Brd4 had a much weaker, although not negligible, effect on the two I73 mutant proteins and on RLuc alone (Fig. 7A). Thus, full-length Brd4 can increase the levels of both RLuc-E2 proteins primarily by interacting with them. Accordingly, we found in cycloheximide chase assays that full-length Brd4 could protect HPV11 and HPV31 RLuc-E2 from degradation (Fig. 7B and 7C), albeit not as efficiently as Brd4-C (Fig. 6C). It is likely that the weaker stabilizing effect of full-length Brd4 on RLuc-E2, compared to that of Brd4-C, is due to its lower levels of expression. However, we cannot rule out that other mechanisms are also taking place. Regardless, the stabilizing effect of full-length Brd4 was dependent on its interaction with RLuc-E2, since it was abolished by the I73A/L substitutions in the TAD (Fig. 7B and C). Thus, both full-length Brd4 and Brd4-C protect E2 from degradation by directly interacting with it.
FIG. 7.

Effect of full-length Brd4 on the steady-state levels of RLuc-E2. (A) Luciferase activity was measured from C33A cells transfected with the indicated wild-type or mutant RLuc-E2 expression plasmid, together with two different amounts of a plasmid encoding full-length Brd4 as described in the text. The amount of luciferase activity measured in cells expressing RLuc-E2 in the absence of any Brd4 construct (−) was set at 100%. (B and C) Stability of wild-type and mutant RLuc-E2 proteins from HPV11 (B) and HPV31 (C) in C33A cells cotransfected or not with Brd4, as indicated. RLuc-E2 stability was determined 24 h posttransfection by measuring the levels of luciferase activity remaining at different time points (in minutes) after a block of protein synthesis with cycloheximide. WT, wild type.
Effect of a dominant-negative Brd4 on expression of RLuc-E2.
The results presented above indicate that Brd4 increases the stability of E2 primarily by directly binding to it. However, the findings that Brd4 could also increase the expression of the two I73 mutant RLuc-E2 (Fig. 7A), albeit to a lesser extent than that of the wild-type proteins, suggests that Brd4 may also act in part by a mechanism unrelated to complex formation. To further investigate this possibility, we made use of a Brd4 protein that lacks the E2-binding domain, which we refer to as Brd4-N (aa 1 to 1223). We found in reporter gene assays that Brd4-N has a dominant-negative effect on E2 transactivation. Specifically, we found that Brd4-N inhibited, in a dose-dependent manner, the transactivation by HPV31 E2 of a reporter gene under the control of four E2-binding sites (Fig. 8A, top panel). In parallel, we also tested the effect of Brd4-C (middle panel) and full-length Brd4 (lower panel) and found that they could inhibit and potentiate E2 transactivation, respectively, as reported previously (40). Thus, both Brd4-N and Brd4-C act in a dominant-negative manner. Because Brd4-N does not bind E2, we surmise that it exerts its dominant-negative effect by interfering with the function of endogenous Brd4. We speculate that Brd4-N could interfere with endogenous Brd4 by directly interacting with it, if Brd4 is a homodimer as suggested previously (43), or by competing for binding to cellular factors such as acetylated nucleosomes since Brd4-N retains both bromodomains.
FIG. 8.

Effect of Brd4-N on E2 transactivation and RLuc-E2 expression. (A) E2 transactivation assays. Levels of luciferase activity in C33A cells transfected with a 3xFlag-HPV31 E2 expression vector and an FLuc reporter gene driven by four E2-binding sites, together with increasing amounts (0, 25, 50, 100, and 200 ng) of Brd4-N (top panel), Brd4-C (middle panel), or full-length Brd4 (bottom panel). Luciferase activity values are presented as the fold induction above that measured in cells transfected with only the reporter gene (No E2), which was set at 1. All data have been normalized using an internal RLuc reporter gene. (B) RLuc-E2 expression levels. The levels of luciferase activity were measured in cells transfected with the indicated wild-type or mutant RLuc-E2 from HPV11 or HPV31 and increasing amounts of a second plasmid encoding Brd4-N. The amount of luciferase activity measured in cells expressing RLuc-E2 in the absence of any Brd4 construct (−) was set at 100%. WT, wild type.
Importantly, we tested the effect of this dominant-negative Brd4 construct on the expression levels of wild-type and mutant Rluc-E2 proteins from both HPV11 and HPV31. As shown in Fig. 8B, Brd4-N was found to increase the amount of wild-type RLuc-E2 by ca. 50% at the highest amount tested, despite being defective for binding to E2. Not surprisingly, it had a similar effect on the I73 mutant proteins. This stimulatory effect of Brd4-N appeared to be specific to E2, either wild type or mutant, since it had a negligible effect on RLuc alone (Fig. 8B). Thus, overexpression of Brd4-N increases the levels of RLuc-E2 by a mechanism that is unrelated to complex formation between both proteins and which is likely related to its dominant-negative effect on endogenous Brd4.
The stability of RLuc-E2 is not affected by downregulation of endogenous Brd4.
The results presented in Fig. 6 and 7 showed that, in the absence of Brd4 overexpression, mutant E2 proteins that are defective for Brd4-binding are as stable as wild-type E2. These results suggested that the stability of E2 is not significantly influenced by endogenous Brd4, perhaps because only a minority of E2 is in complex with Brd4 and productively engaged in transcription within an asynchronous cell population. To investigate further the role of endogenous Brd4, we set out to measure the stability of RLuc-E2 in C33A cells in which Brd4 expression would be downregulated with a shRNA. First, we tested the efficiency of several commercially available shRNA against Brd4. In Fig. 9A, we show by Western blotting that the most potent Brd4 shRNA that we identified could reduce the levels of cotransfected Flag-tagged Brd4 by ca. 90%, whereas a control shRNA had little to no effect. Importantly, we then showed in reporter gene assays that this potent Brd4 shRNA, but not the control shRNA, could inhibit E2 transactivation (Fig. 9B). This effect was specific to E2-mediated transcription since the same Brd4 shRNA had little to no effect on transcription from the CMV promoter (data not shown). From these experiments, we conclude that our Brd4 shRNA is both functional and specific. We then performed cycloheximide chase assays to measure the stability of RLuc-E2 from HPV11 and HPV31 in cells transfected with the Brd4 shRNA or the control shRNA. No significant difference in the stability of the two Rluc-E2 proteins was observed between control and Brd4-knockdown cells (Fig. 9C and D). Thus, depletion of endogenous Brd4 does not have a significant impact on the stability E2 from HPV11 and HPV31.
FIG. 9.

Effect of Brd4 depletion on the stability of RLuc-E2 proteins. (A) Western blot analysis showing the downregulation of Flag-tagged Brd4 (F-Brd4) by RNA interference. C33A cells were transfected with an expression vector for Flag-Brd4 and increasing amounts (1x, 2x, and 4x) of a second plasmid expressing a Brd4 shRNA or a control (Ctl.) shRNA. Flag-Brd4 was detected 24 h posttransfection using an anti-Flag antibody. Tubulin was used as a loading control. (B) Inhibition of E2-transactivation by the Brd4 shRNA. The graph shows the levels of luciferase activity in C33A cells transfected with a 3xFlag-HPV31 E2 expression vector and an FLuc reporter gene driven by four E2-binding sites, together with increasing amounts of Brd4 shRNA or control shRNA. Luciferase activity values are presented as the fold induction above that measured in cells transfected with only the reporter gene (No E2), which was set at 1. All data have been normalized using an internal RLuc reporter gene. A transcriptionally inactive E2 mutant (I73L) was used as a control. Transfected cells were selected in puromycin-containing medium for 3 days prior to analysis. (C and D) Stability of RLuc-11E2 (C) and RLuc-31E2 (D) in C33A cells transfected with the Brd4 shRNA or control shRNA. Transfected cells were selected in puromycin-containing medium for 3 days prior to analysis. The stability of RLuc-E2 was then determined by measuring the levels of luciferase activity remaining at different time points (in minutes) after a block of protein synthesis with cycloheximide.
In complementary studies, we also measured the stability of RLuc-E2 from HPV11 and HPV31 in a previously described C33A cell line that stably express a shRNA against Brd4 (cell line 1-13 described in Wu et al. [44], kindly provided by Cheng-Ming Chiang). We found that this cell line supports lower levels of E2 transactivation (see Fig. S4A in the supplemental material), a finding consistent with a reduction in Brd4 levels. RLuc-E2 from both HPV11 and HPV31 were found to be slightly more stable in C33A knockdown cells than in normal C33A cells (see Fig. S4B and C in the supplemental material). However, this was also the case for the Brd4-binding defective mutants (see Fig. S4B and C in the supplemental material). Thus, the slight stabilization of RLuc-E2 observed in Brd4 knockdown cells is unrelated to an interaction with Brd4. These results provide additional evidence that depletion of endogenous Brd4 does not have a dramatic effect on the stability E2 from HPV11 and HPV31. Satisfyingly, all of the results obtained in Brd4 knockdown experiments, either by transient transfection of shRNA or using the stable cell line, are consistent with those indicating that Brd4-binding mutant E2 have a similar stability as wild-type E2. Collectively, these results indicate that endogenous Brd4 is not a major determinant of E2's stability. As we argue in the Discussion, this conclusion does not necessarily contradict the notion that Brd4-binding protects E2 from degradation, since it may simply reflect the fact that only a small proportion of E2 proteins are bound to endogenous Brd4 in cells at any given time.
Inhibition of the proteasome reduces E2 transactivation.
The studies presented above have revealed a role for Brd4 in protecting E2 from proteasomal degradation. Since Brd4 also mediates the transcriptional activity of E2 (8, 33, 40, 41, 44), it was of interest to determine whether the proteasome was involved in E2 transactivation. To address this question, we tested the effect of two proteasome inhibitors, lactacystin and MG132, on the expression of an E2-responsive luciferase reporter gene in transfected C33A cells. Cells were transfected with an expression vector for HPV31 E2 together with the reporter gene under the control of four E2 binding sites and were then treated 48 h posttransfection, with a gradient of inhibitors for 6.5 h. As can be seen in Fig. 10A, expression of the reporter gene was increased 4.5-fold by wild-type HPV31 E2 but not by a transactivation defective mutant carrying the R37K and I73L substitutions in the TAD. In the presence of increasing concentrations of lactacystin or MG132, we observed a dose-dependent reduction in E2 transactivation, a finding consistent with a role for the proteasome in this process. To verify that the effect of the proteasome inhibitors was not due to a general reduction in RNA polymerase II transcription, we tested their effect on an unrelated luciferase reporter gene driven by the CMV promoter (Fig. 10B). In this case, neither lactacystin nor MG132 had any inhibitory effect, even at the highest concentrations tested. Rather, they both increased luciferase expression by 40 to 50% at specific concentrations. Thus, the inhibitory effect of lactacystin and MG132 on E2-transactivation is not due to a general shutdown of RNA polymerase II transcription. Collectively, these results support a role for the proteasome in E2 transactivation.
FIG. 10.

Effect of proteasome inhibitors on E2 transactivation. (A) Levels of luciferase activity in C33A cells transfected with a 3xFlag-HPV31 E2 expression vector and an FLuc reporter gene driven by four E2-binding sites. The levels of luciferase activity in cells transfected with only the reporter gene (No E2) was set at 100%. All data have been normalized using an internal RLuc reporter gene. In this assay, wild-type E2 but not the R37K/I73L double mutant activated expression of the reporter gene by 4.5-fold. Cells transfected with wild-type E2 and the reporter gene were treated with the indicated concentrations of lactacystin and MG132. (B) Levels of luciferase activity in C33A cells transfected with an FLuc reporter gene driven by the CMV promoter in cells were treated or not with the indicated concentrations of proteasome inhibitors.
DISCUSSION
Development of a quantitative luciferase assay to study E2 degradation.
For the present study, we developed a novel assay to quantify the proteasomal degradation of papillomavirus E2, based on its fusion to RLuc. This assay is both quantitative and very sensitive. In a side-by-side comparison (Fig. 1 and 2), we found that the luciferase assay is at least fivefold more sensitive than Western blotting against the 3xFlag epitope for E2 detection. This allows for the stability of E2 to be monitored under conditions of low protein expression, an important point when trying to ensure that the ubiquitin-proteasome machinery is not saturated by overexpression of the protein of interest. The luciferase assay also allows for the rapid and easy determination of the half-life of E2 with a higher throughput than traditional methods such as pulse-labeling and immunoprecipitation or Western blotting. Importantly, the assay includes an internal FLuc control for normalization purposes, an essential control when investigating the effect of overexpression or downregulation of a transcription factor such as Brd4, which affects the expression of many genes. Indeed, we have noticed that overexpression of Brd4 or Brd4-N stimulates expression from the CMV promoter, which was used in the present study to drive the expression of RLuc-E2 fusion proteins and also of the FLuc normalization control. To quantify this effect, we plotted from our existing data set the effect of Brd4, Brd4-N, and Brd4-C on the expression of the control FLuc driven from the CMV promoter. The luciferase values (see Fig. S5 in the supplemental material) clearly show that Brd4 and Brd4-N increase expression from the CMV promoter in a dose-dependent manner, whereas Brd4-C has much less of an effect. At the highest amount of Brd4 and Brd4-N tested (see Fig. S5 in the supplemental material), the levels of FLuc were almost doubled and tripled, respectively. It is unclear whether Brd4 and Brd4-N have a direct effect on transcription initiated at the CMV promoter or an indirect one on other aspects of gene expression. However, since Brd4 is required for the expression of many cellular genes, it is not surprising that it can also affect expression from the CMV promoter. Importantly, because our experiments involved normalization of the data by calculating the ratios of RLuc to FLuc activities (both expressed from the CMV promoter), we have been able to focus specifically on the effect of Brd4 on the stability of E2, independent of its effect on the CMV promoter. In summary, the use of RLuc fusions has proven extremely valuable for the study of E2 degradation not only because it is quantitative and high throughput but also because it permits the inclusion of an internal control that takes into account general effects of Brd4 on gene expression.
E2 is degraded by the proteasome via its TAD.
Using the luciferase assay, we determined that HPV11 and HPV31 E2 are degraded by the proteasome, both with a half-life of ∼60 min. This value is in agreement with previous studies that measured a half-life of 75 min for HPV11 and of 50 min for both HPV16 and 18 E2 (9, 10). Although HPV11 and HPV31 E2 have comparable half-lives, we repeatedly observed that HPV11 E2 was slightly less stable than HPV31 E2 (Fig. 2B). Accordingly, inhibition of the proteasome with lactacystin increased the steady-state levels of HPV11 E2 slightly more than those of HPV31 E2 by ca. 20% (Fig. 3B). Using a series of truncation mutants of HPV31 E2, we identified the TAD as the primary domain of E2 responsible for its proteasomal degradation. The TAD had previously been implicated in the degradation of HPV18 E2 (9). Interestingly, a growing body of evidence indicates that TADs often contain specific sequences, known as degrons, which signal their proteolysis (36). The results presented here support the notion that the E2 TAD also contains a degron. The finding that deletion of the C-terminal DBD of E2 did not significantly stabilize the protein (Fig. 5) indicates that the instability of E2 is not dependent on its ability to dimerize and bind DNA. Hence, we conclude that the instability of E2 is encoded within its TAD and that E2 does not need to be bound to DNA or productively engaged in transcription in order to be degraded by the proteasome.
E2 is protected from degradation when bound to Brd4.
The finding that the TAD contains a degron prompted us to investigate whether overexpression of the viral E1 helicase or of Brd4, which bind to opposite faces of the TAD, would affect the stability of E2. These studies revealed that overexpression of the Brd4 C-terminal domain (Brd4-C), the portion that binds to E2, protects it from degradation. Overexpression of full-length Brd4 was also found to stabilize E2, albeit not as efficiently as Brd4-C, perhaps because it is expressed at lower levels. The use of mutant E2 defective for Brd4-binding (R37 and I73) has been particularly useful for dissecting the effects of Brd4 that are due to its interaction with E2 from those that are indirect and related to a general inhibition of transcription and/or cell cycle progression. The finding that overproduction of Brd4 and Brd4-C protects wild-type E2 but not mutant E2 from degradation strongly suggests that E2 is more stable when bound to Brd4. By extension, these data also point to the Brd4-binding surface of the TAD as being involved in E2 degradation.
Interestingly, we found that the R37 and I73 mutants were as stable as wild-type E2 in the absence of Brd4 overexpression. This suggested either that the interaction of E2 with endogenous Brd4 does not affect the stability of E2 or, alternatively, that it does but that the majority of E2 in cells is not in complex with Brd4 (i.e., is in a free form) and therefore not protected from degradation. At this moment, we cannot formally rule out any one of these two possibilities, although we favor the latter one for the following reasons. The finding that overexpression of Brd4 stimulates E2 transactivation in reporter gene assays (Fig. 8A) (40) suggests that Brd4 is limiting and that its overexpression drives the assembly of a greater number of E2-Brd4 complexes capable of activating transcription. If E2 was already all bound to Brd4, it would be difficult to imagine how Brd4 overexpression could increase E2 transactivation. A similar argument can be made for why Brd4 overexpression protects E2 from degradation. Specifically, we have obtained evidence that Brd4 overproduction protects wild-type E2 but not Brd4-binding mutants, suggesting that this protective effect is indeed due to an increase in complex formation between E2 and Brd4. Again, if all of E2 was already bound to endogenous Brd4, this stabilizing effect of Brd4 would be difficult to explain. Finally, we have found that immunoprecipitation of HPV31 E2 from stable cell lines does not result in the copurification of stoichiometric amounts of Brd4, suggesting that not all E2 molecules are in complex with endogenous Brd4 (data not shown). For all of the aforementioned reasons, we favor the notions that the majority of E2 is not in complex with Brd4 in asynchronous cells and that Brd4 overproduction stimulates E2 transactivation and protects E2 from proteasomal degradation by driving the assembly of additional E2-Brd4 complexes.
We also investigated the role of endogenous Brd4 on the stability of E2 using a dominant-negative Brd4 construct (Brd4-N) and, in separate experiments, by downregulation of Brd4 with a shRNA. We found that Brd4-N inhibits E2 transactivation despite the fact that it lacks the E2-binding domain (Fig. 8A). This suggests that Brd4-N brings about its inhibitory effect by interfering with the function of endogenous Brd4. We found that overexpression of Brd4-N increased the steady-state levels of RLuc-E2 but also those of the R37 and I73 E2 mutants (Fig. 8B). From these results, we conclude that dominant-negative inhibition of endogenous Brd4 by Brd4-N does not stabilize E2 through an effect on complex formation between both proteins. To investigate more directly the role on endogenous Brd4 on the stability of E2, we depleted it by using RNA interference. The data presented in Fig. 9 clearly show that depletion of Brd4 did not affect the half-life of RLuc-E2 from HPV11 and HPV31. Comparable results were obtained when we measured the stability of RLuc-E2 from HPV11 and HPV31 in a cell line that stably expresses an shRNA (44). We surmise that this cell line still expresses some, albeit lower-than-normal, levels of Brd4 since it is essential for cell cycle progression. Accordingly, we have observed that the levels of E2 transactivation are reduced by ca. 50% in this cell line compared to normal C33A (see Fig. S4 in the supplemental material). We found that RLuc-E2 from both HPV11 and HPV31 were slightly more stable in these C33A knockdown cells than in normal C33A (see Fig. S4 in the supplemental material). However, this was also the case for the Brd4-binding defective E2 mutants (see Fig. S4 in the supplemental material). Thus, the slight stabilization of RLuc-E2 observed in Brd4 knockdown cells is likely independent of Brd4 interaction.
Collectively, our results indicate that neither depletion of endogenous Brd4 by shRNA nor its inhibition using a dominant-negative Brd4 construct has a major impact on the stability of HPV11 and HPV31 E2 that could be attributed to a reduction in E2-Brd4 complex formation. These findings are in perfect agreement with the finding that Brd4-binding mutants of E2 have a stability comparable to that of wild-type E2. We have argued above that the majority of E2 is not in complex with Brd4 in asynchronous cells and, consequently, surmise that the intrinsic instability of E2 that we have characterized in the present study is that of “free” E2 that is not productively engaged in transcription. However, we have also started to address the role of E2 degradation during transcription by testing the effect of proteasome inhibitors on E2 transactivation. These studies are discussed in the below.
E2 transactivation requires the catalytic activity of the proteasome.
There is accumulating evidence that the proteasome is involved in transcription through both its proteolytic and nonproteolytic activities (14, 27, 34). Using a reporter gene assay, we found that the proteasome inhibitors lactacystin and MG132 reduced, in a dose-dependent manner, the expression of an E2-driven reporter gene (Fig. 10A) but not that of a control reporter expressed from the CMV promoter. A similar observation was reported recently for HPV16 E2 (21). These findings indicate that the proteolytic activity of the proteasome is required for E2 transactivation, perhaps in part because E2 needs to be degraded in order for transcription to proceed. What might be the role of E2 degradation in transcription? Although we do not have a definitive answer to this question, we can suggest a few possibilities. For some transcriptional activators, it has been postulated that proteasomal degradation serves as a mechanism to limit their activity and ensure that they remain functional only when needed (14, 27, 34). By analogy, proteasomal degradation of E2 may serve as a mechanism to prevent its stable interaction and activity at the viral LCR until it can productively interact with Brd4. More recent models have emphasized the role of monoubiquitination and polyubiquitination in stimulating and inactivating, respectively, certain transcription factors (4, 5, 14, 18, 27, 28, 34). In these models, monoubiquitination has been akin to a “licensing event” allowing activators to become active while at the same time marking them for subsequent proteasomal degradation as the ubiquitin chain is extended to beyond four ubiquitin moieties. Thus, it will be important in the future to address whether E2 is activated by monoubiquitination and, if so, whether this modification has any effect on its interaction with Brd4.
Supplementary Material
Acknowledgments
We thank Cheng-Ming Chiang (Simmons Comprehensive Cancer Center, Dallas, TX) for providing the Brd4 knockdown cell line and Brd4 expression plasmids and Lou Laimins (Northwestern University, Chicago, IL) for the gift of the 4xE2 binding site reporter gene. We also thank Maria Lymberopoulos for the construction of the Brd4-C expression plasmid.
This study was supported by grants from the Cancer Research Society and the Canadian Institutes of Health Research (CIHR). H.S. and A.F.-T. hold studentships from the CIHR and Fonds de la Recherche en Santé du Québec (FRSQ), respectively. J.A. is a senior scholar from the FRSQ.
Footnotes
Published ahead of print on 11 February 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abbate, E. A., J. M. Berger, and M. R. Botchan. 2004. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 181981-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbate, E. A., C. Voitenleitner, and M. R. Botchan. 2006. Structure of the papillomavirus DNA-tethering complex E2:Brd4 and a peptide that ablates HPV chromosomal association. Mol. Cell 24877-889. [DOI] [PubMed] [Google Scholar]
- 3.Abroi, A., R. Kurg, and M. Ustav. 1996. Transcriptional and replicational activation functions in the bovine papillomavirus type 1 E2 protein are encoded by different structural determinants. J. Virol. 706169-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer, C. T., L. Burdine, B. Liu, A. Ferdous, S. A. Johnston, and T. Kodadek. 2008. Physical and functional interactions of monoubiquitylated transactivators with the proteasome. J. Biol. Chem. 28321789-21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer, C. T., A. Delahodde, F. Gonzalez, S. A. Johnston, and T. Kodadek. 2008. Activation domain-dependent monoubiquitylation of Gal4 protein is essential for promoter binding in vivo. J. Biol. Chem. 28312614-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auborn, K. J. 2002. Therapy for recurrent respiratory papillomatosis. Antivir. Ther. 71-9. [PubMed] [Google Scholar]
- 7.Baseman, J. G., and L. A. Koutsky. 2005. The epidemiology of human papillomavirus infections. J. Clin. Virol. 32(Suppl. 1)S16-S24. [DOI] [PubMed] [Google Scholar]
- 8.Baxter, M. K., M. G. McPhillips, K. Ozato, and A. A. McBride. 2005. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J. Virol. 794806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellanger, S., C. Demeret, S. Goyat, and F. Thierry. 2001. Stability of the human papillomavirus type 18 E2 protein is regulated by a proteasome degradation pathway through its amino-terminal transactivation domain. J. Virol. 757244-7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blachon, S., S. Bellanger, C. Demeret, and F. Thierry. 2005. Nucleo-cytoplasmic shuttling of high risk human papillomavirus E2 proteins induces apoptosis. J. Biol. Chem. 28036088-36098. [DOI] [PubMed] [Google Scholar]
- 11.Blachon, S., and C. Demeret. 2003. The regulatory E2 proteins of human genital papillomaviruses are proapoptotic. Biochimie 85813-819. [DOI] [PubMed] [Google Scholar]
- 12.Breiding, D. E., M. J. Grossel, and E. J. Androphy. 1996. Genetic analysis of the bovine papillomavirus E2 transcriptional activation domain. Virology 22134-43. [DOI] [PubMed] [Google Scholar]
- 13.Brokaw, J. L., M. Blanco, and A. A. McBride. 1996. Amino acids critical for the functions of the bovine papillomavirus type 1 E2 transactivator. J. Virol. 7023-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins, G. A., and W. P. Tansey. 2006. The proteasome: a utility tool for transcription? Curr. Opin. Genet. Dev. 16197-202. [DOI] [PubMed] [Google Scholar]
- 15.Cooper, C. S., S. N. Upmeyer, and P. L. Winokur. 1998. Identification of single amino acids in the human papillomavirus 11 E2 protein critical for the transactivation or replication functions. Virology 241312-322. [DOI] [PubMed] [Google Scholar]
- 16.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 1008758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doorbar, J. 2005. The papillomavirus life cycle. J. Clin. Virol. 32(Suppl. 1)S7-S15. [DOI] [PubMed] [Google Scholar]
- 18.Ferdous, A., D. Sikder, T. Gillette, K. Nalley, T. Kodadek, and S. A. Johnston. 2007. The role of the proteasomal ATPases and activator monoubiquitylation in regulating Gal4 binding to promoters. Genes Dev. 21112-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson, M. K., and M. R. Botchan. 1996. Genetic analysis of the activation domain of bovine papillomavirus protein E2: its role in transcription and replication. J. Virol. 704193-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florence, B., and D. V. Faller. 2001. You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 6D1008-D1018. [DOI] [PubMed] [Google Scholar]
- 21.Gammoh, N., D. Gardiol, P. Massimi, and L. Banks. 2009. The Mdm2 ubiquitin ligase enhances HPV E2 transcriptional activation. J. Virol. 831538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillison, M. L. 2004. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 31744-754. [DOI] [PubMed] [Google Scholar]
- 23.Grossel, M. J., F. Sverdrup, D. E. Breiding, and E. J. Androphy. 1996. Transcriptional activation function is not required for stimulation of DNA replication by bovine papillomavirus type 1 E2. J. Virol. 707264-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebner, C. M., and L. A. Laimins. 2006. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 1683-97. [DOI] [PubMed] [Google Scholar]
- 25.Howley, P. M., and D. R. Lowy. 2001. Papillomaviruses, p. 2231-2264. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 26.Jang, M. K., K. Mochizuki, M. Zhou, H. S. Jeong, J. N. Brady, and K. Ozato. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19523-534. [DOI] [PubMed] [Google Scholar]
- 27.Kodadek, T., D. Sikder, and K. Nalley. 2006. Keeping transcriptional activators under control. Cell 127261-264. [DOI] [PubMed] [Google Scholar]
- 28.Kurosu, T., and B. M. Peterlin. 2004. VP16 and ubiquitin; binding of P-TEFb via its activation domain and ubiquitin facilitates elongation of transcription of target genes. Curr. Biol. 141112-1116. [DOI] [PubMed] [Google Scholar]
- 29.Lacey, C. J. 2005. Therapy for genital human papillomavirus-related disease. J. Clin. Virol. 32(Suppl. 1)S82-S90. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., X. Wang, J. Zhang, H. Huang, B. Ding, J. Wu, and Y. Shi. 2008. Structural basis and binding properties of the second bromodomain of Brd4 with acetylated histone tails. Biochemistry 476403-6417. [DOI] [PubMed] [Google Scholar]
- 31.Loening, A. M., A. M. Wu, and S. S. Gambhir. 2007. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat. Methods 4641-643. [DOI] [PubMed] [Google Scholar]
- 32.Longworth, M. S., and L. A. Laimins. 2004. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McPhillips, M. G., J. G. Oliveira, J. E. Spindler, R. Mitra, and A. A. McBride. 2006. Brd4 is required for e2-mediated transcriptional activation but not genome partitioning of all papillomaviruses. J. Virol. 809530-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittenberg, A. G., T. N. Moiseeva, and N. A. Barlev. 2008. Role of proteasomes in transcription and their regulation by covalent modifications. Front. Biosci. 137184-7192. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki, K., A. Nishiyama, M. K. Jang, A. Dey, A. Ghosh, T. Tamura, H. Natsume, H. Yao, and K. Ozato. 2008. The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 2839040-9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muratani, M., and W. P. Tansey. 2003. How the ubiquitin-proteasome system controls transcription. Nat. Rev. Mol. Cell. Biol. 4192-201. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura, A., T. Ono, A. Ishimoto, J. J. Dowhanick, M. A. Frizzell, P. M. Howley, and H. Sakai. 2000. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol. 743752-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penrose, K. J., and A. A. McBride. 2000. Proteasome-mediated degradation of the papillomavirus E2-TA protein is regulated by phosphorylation and can modulate viral genome copy number. J. Virol. 746031-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai, H., T. Yasugi, J. D. Benson, J. J. Dowhanick, and P. M. Howley. 1996. Targeted mutagenesis of the human papillomavirus type 16 E2 transactivation domain reveals separable transcriptional activation and DNA replication functions. J. Virol. 701602-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweiger, M. R., J. You, and P. M. Howley. 2006. Bromodomain protein 4 mediates the papillomavirus E2 transcriptional activation function. J. Virol. 804276-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senechal, H., G. G. Poirier, B. Coulombe, L. A. Laimins, and J. Archambault. 2007. Amino acid substitutions that specifically impair the transcriptional activity of papillomavirus E2 affect binding to the long isoform of Brd4. Virology 35810-17. [DOI] [PubMed] [Google Scholar]
- 42.Syrjanen, S. 2005. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 32(Suppl. 1)S59-S66. [DOI] [PubMed] [Google Scholar]
- 43.Wu, S. Y., and C. M. Chiang. 2007. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 28213141-13145. [DOI] [PubMed] [Google Scholar]
- 44.Wu, S. Y., A. Y. Lee, S. Y. Hou, J. K. Kemper, H. Erdjument-Bromage, P. Tempst, and C. M. Chiang. 2006. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 202383-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, Z., N. He, and Q. Zhou. 2008. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell. Biol. 28967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, Z., J. H. Yik, R. Chen, N. He, M. K. Jang, K. Ozato, and Q. Zhou. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19535-545. [DOI] [PubMed] [Google Scholar]
- 47.You, J., J. L. Croyle, A. Nishimura, K. Ozato, and P. M. Howley. 2004. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117349-360. [DOI] [PubMed] [Google Scholar]
- 48.You, J., M. R. Schweiger, and P. M. Howley. 2005. Inhibition of E2 binding to Brd4 enhances viral genome loss and phenotypic reversion of bovine papillomavirus-transformed cells. J. Virol. 7914956-14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.zur Hausen, H., and E. M. de Villiers. 1994. Human papillomaviruses. Annu. Rev. Microbiol. 48427-447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.