Abstract
The human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein trimer consists of gp120 and gp41 subunits and undergoes a series of conformational changes upon binding to the receptors, CD4 and CCR5/CXCR4, that promote virus entry. Surprisingly, we found that the envelope glycoproteins of some HIV-1 strains are functionally inactivated by prolonged incubation on ice. Serial exposure of HIV-1 to extremes of temperature, followed by expansion of replication-competent viruses, allowed selection of a temperature-resistant virus. The envelope glycoproteins of this virus resisted cold inactivation due to a single passage-associated change, H66N, in the gp120 exterior envelope glycoprotein. Histidine 66 is located within the gp41-interactive inner domain of gp120 and, in other studies, has been shown to decrease the sampling of the CD4-bound conformation by unliganded gp120. Substituting asparagine or other amino acid residues for histidine 66 in cold-sensitive HIV-1 envelope glycoproteins resulted in cold-stable phenotypes. Cold inactivation of the HIV-1 envelope glycoproteins occurred even at high pH, indicating that protonation of histidine 66 is not necessary for this process. Increased exposure of epitopes in the ectodomain of the gp41 transmembrane envelope glycoprotein accompanied cold inactivation, but shedding of gp120 did not. An amino acid change in gp120 (S375W) that promotes the CD4-bound state or treatment with soluble CD4 or a small-molecule CD4 mimic resulted in increased cold sensitivity. These results indicate that the CD4-bound intermediate of the HIV-1 envelope glycoproteins is cold labile; avoiding the CD4-bound state increases temperature stability.
Human immunodeficiency virus type 1 (HIV-1) is an enveloped virus that, like all retroviruses, contains two copies of genomic RNA and several enzymes required for viral replication (5). One of these enzymes, reverse transcriptase (RT), converts the viral RNA into DNA in the cytoplasm of the newly infected cell (4, 66). HIV-1 entry into target cells is mediated by the viral envelope glycoproteins, which are assembled into a trimeric complex on the virion surface (39, 48). Previous studies identified RT and the envelope glycoproteins as the HIV-1 components most vulnerable to inactivation by high temperature (38).
In infected, virus-producing cells, the HIV-1 envelope glycoproteins are synthesized as an approximately 860-amino-acid precursor. This precursor is extensively glycosylated to produce the gp160 envelope glycoprotein, which assembles into trimeric complexes. Proteolytic cleavage of gp160 creates the gp120 exterior envelope glycoprotein and the gp41 transmembrane envelope glycoprotein. The three gp120 envelope glycoproteins in the trimeric complex associate noncovalently with the gp41 envelope glycoproteins, which are anchored in the membrane. Following the conversion of a subset of the glycan residues to complex carbohydrates, the trimeric envelope glycoprotein complex is transported to the cell surface, from which it may be incorporated into the membranes of budding virions (3, 8, 9, 20, 43, 70).
HIV-1 entry involves the viral envelope glycoproteins and receptors on the target cell surface. The receptor CD4 and chemokine receptor CCR5 or CXCR4 are recognized by gp120 (11-13, 15, 19, 33). Receptor binding triggers conformational changes in the envelope glycoprotein complex that eventually promote the fusion of the viral and target cell membranes, a process that is essential for virus entry and that is mediated by the gp41 transmembrane glycoproteins. In this manner, the high potential energy of the unliganded HIV-1 envelope glycoprotein complex is channeled via multiple metastable intermediate states into the force required to fuse the membranes of virus and target cell (16, 44, 71, 73).
Under some circumstances, the HIV-1 envelope glycoprotein complex undergoes inactivating conformational changes. For example, soluble forms of the CD4 receptor (sCD4), in addition to competing for target cell CD4, can also trigger conformational changes in the HIV-1 envelope glycoproteins that lead to functional inactivation (H. Haim, submitted for publication). In the extreme, sCD4 binding causes the shedding of the gp120 glycoprotein from the envelope glycoprotein complex (23, 28, 45-47). The efficiency of gp120 shedding is much greater at 37°C than at room temperature (47, 52). The inactivation of the HIV-1 envelope glycoproteins in the absence of sCD4 is much slower than after sCD4 incubation (H. Haim, submitted).
Here, we subjected a molecularly cloned HIV-1 to repeated rounds of selection at elevated temperatures. As expected, the selected virus was more stable than the parental virus at high temperatures. Surprisingly, the parental virus was inactivated on ice more rapidly than at room temperature; the heat-selected virus was resistant to this cold inactivation. Unlike heat inactivation, which involves multiple components of the virus (38), sensitivity to the cold was determined solely by the viral envelope glycoproteins. The envelope glycoproteins of the selected virus exhibited increased functional longevity at 0°C compared with the envelope glycoproteins of the parental virus. The basis for this cold stability was found to be a change of histidine 66 in the gp120 inner domain to an asparagine residue. We investigated the requirements for cold stability of the HIV-1 envelope glycoproteins and established a relationship between the CD4-bound conformation and temperature-dependent lability.
MATERIALS AND METHODS
Selection of heat-resistant virus.
The pNL4.3-KB9 plasmid, which encodes the KB9 envelope glycoproteins (32) in the background of the NL4.3 provirus (1), was used to generate infectious HIV-1. 293T cells were transfected with 25 μg of the pNL4.3-KB9 plasmid by using a calcium phosphate transfection kit (Invitrogen, Carlsbad, CA). After 10 h, medium was replaced, and virus-containing supernatants were harvested 3 days later. After clarification by low-speed (2,000 × g for 10 min) centrifugation, the amount of virus in the supernatant was quantitated by a RT assay (59). Virus-containing supernatants were aliquoted and stored at −80°C. For a selection of heat-resistant viruses, 1G5 cells, which were derived from the Jurkat T-cell line and contain a stably integrated luciferase gene under the control of the HIV-1 long terminal repeat (LTR) (2), were incubated overnight with 200,000 RT units of virus in 1 ml. Cells were washed once, resuspended in 10 ml growth medium, and kept in culture at 37°C to allow virus replication. After 3 to 4 days or when the titer of virus reached 500,000 RT units/ml, the supernatant was harvested, quantitated, and stored at −80°C. Aliquots of virus were thawed, adjusted for equivalent RT units, and kept in a water bath set at a temperature between 37°C and 56°C, while a control aliquot was directly added to cells. At various time points aliquots were removed, cooled to room temperature, and added to 1G5 Jurkat T cells. Cells were split every 3 to 4 days, and the titer of virus in the culture medium was measured. Those cultures with a detectable viral titer and derived from aliquots exposed the longest to a given temperature were expanded further, whereas the rest were discarded. When the virus titer in the selected cultures reached approximately 500,000 RT units/ml, culture media were collected, aliquoted, and kept at −80°C. These samples served as the starting stocks for the next round of selection. The incubation of virus at given temperature, the selection of a resistant virus population, and expansion on 1G5 Jurkat T cells were repeated for several cycles, with increased temperature of selection or duration of exposure at each cycle.
Site-directed mutagenesis.
Mutations in the HIV-1 env gene were introduced by site-directed mutagenesis using the QuikChange protocol (Stratagene). The H66N change was introduced into pSVIIIenv plasmids (29) expressing the KB9, ADAfl, YU2, JR-FL, and ADA-N197S envelope glycoproteins. The ADAfl, YU2, JR-FL, and ADA-N197S envelope glycoproteins are R5-tropic. The KB9 envelope glycoproteins are dualtropic. The ADAfl envelope glycoproteins are full-length ADA envelope glycoproteins and differ from the previously reported ADA-HXBc2 chimeric envelope glycoproteins (65). The ADA-N197S envelope glycoproteins are CD4-independent, R5 envelope glycoproteins derived from an ADA-HXBc2 chimeric envelope glycoprotein (34, 35). Complementary pairs of primers were used to introduce the H66N mutation into the envelope expressor plasmids; the sequence of one primer is as follows: 5′-ATATGATACAGAGGTAAATAATGTTTGGGC-3′.
Plasmids encoding the KB9 and YU2 HIV-1 envelope glycoproteins containing substitutions for histidine 66 other than asparagine were made as described above using the following primers: H66A, 5′-ATATGATACAGAGGTAGCTAATGTTTGGGC-3′; H66F, 5′-ATATGATACAGAGGTATTTAATGTTTGGGC-3′; H66D, 5′-ATATGATACAGAGGTAGATAATGTTTGGGC-3′; H66Q, 5′-ATATGATACAGAGGTACAGAATGTTTGGGC-3′; H66W, 5′-ATATGATACAGAGGTATGGAATGTTTGGGC-3′; H66K, 5′-ATATGATACAGAGGTAAAGAATGTTTGGGC-3′.
To generate envelope glycoproteins with a deletion of the gp41 cytoplasmic tail, a stop codon was introduced just after the sequence encoding the transmembrane region by using complementary pairs of primers; the sequence of one primer is as follows: 5′-CCCTGCCTAACTCTAATTTACTATAGAAAGTACAGC-3′. The presence of the desired mutations and absence of unintended changes were confirmed by DNA sequencing of the entire Env-coding region. All plasmids expressing the wild-type and mutant envelope glycoproteins were prepared using the QIAFilter kit (Qiagen), quantified, and stored.
Production of single-round recombinant HIV-1 reporter viruses.
Recombinant HIV-1 encoding firefly luciferase and pseudotyped with the wild-type or mutant HIV-1 envelope glycoproteins was produced by cotransfecting 293T cells with a pSVIIIenv plasmid expressing the HIV-1 envelope glycoprotein variants, the pCMVΔP1ΔenvpA plasmid, and the pHIV-1Luc plasmid at a 1:2:2 weight ratio, respectively, using the Effectene reagent (Qiagen). The pCMVΔP1ΔenvpA plasmid encodes the packaging components (Gag/Pol proteins) and the Tat protein of HIV-1 (53). The pHIV-1Luc plasmid carries a packageable HIV-1 vector that is defective in all HIV-1 genes except tat and that expresses the luciferase reporter gene (77). Recombinant HIV-1 pseudotyped with vesicular stomatitis virus glycoprotein G (VSV G) was generated similarly by including pHCMV-G (79), a VSV G expression plasmid, in the transfection mixture at a weight ratio equivalent to that for the plasmid expressing the HIV-1 envelope glycoproteins. The viral stocks, which are capable of a single round of infection, were harvested 2 to 3 days later, quantitated by an RT assay, aliquoted, and stored at −80°C.
Virus infection assay.
Cf2Th cells coexpressing CD4 and coreceptor CCR5 (Cf2Th-CD4/CCR5) or CXCR4 (Cf2Th-CD4/CXCR4) or expressing only CCR5 (Cf2Th-CCR5) were seeded at a density of 8,000 cells/well in a 96-well plate and cultured overnight at 37°C. About 30,000 RT units of virus was added to the cells the next day at 37°C in 200 μl of medium, and the cells were cultured for another 2 days. Medium was removed, and cells were lysed with 50 μl of 1× luciferase lysis buffer (Promega, Madison, WI). Luciferase assays were performed using d-luciferin salt as a substrate (BD Pharmingen, San Jose, CA) with the LB 96V microplate luminometer (EG & G Berthold Technologies, Oak Ridge, TN).
To assess the effect of temperature on virus infectivity, virus preparations equalized for RT activity were incubated at different temperatures. At the end of the incubation, aliquots were removed and transferred to a −80°C freezer until infection. To measure the infectivity of the virus, aliquots were thawed at 37°C just before infection and added to target cells in triplicate. The time dependence of decay of virus infectivity at a given temperature was also assayed similarly, except that aliquots of virus were removed at different time points and transferred to −80°C. At the completion of the experiment, aliquots were thawed at 37°C and added to target cells in triplicate.
To assess the effect of pH on cold inactivation, viruses equalized for RT activity were pelleted; supernatants were replaced with medium at a specific pH (5.0 to 8.0). Viruses were resuspended in this medium and kept on ice. At regular time points, aliquots were removed and viruses were again pelleted by centrifuging them at 12,000 × g. Viruses were resuspended in medium at neutral pH just before addition to target cells to assay infection.
Analysis of envelope glycoprotein levels on virus particles.
Radiolabeled HIV-1 virions were produced by cotransfecting 293T cells with the pSVIIIenv plasmid, expressing the HIV-1 envelope glycoprotein variants, the pCMVΔP1ΔenvpA plasmid, and pSVL-tat, a Tat expression vector driven by the simian virus 40 late promoter. One day later, the medium was replaced with radiolabeling medium (10% heat-inactivated, dialyzed fetal bovine serum, 10 μg/ml of penicillin-streptomycin solution, 50 μCi/ml 35S-Express protein labeling mix [PerkinElmer, Waltham, MA], and 2 mM l-glutamine in methionine- and cysteine-free Dulbecco's modified Eagle medium). The cells were incubated at 37°C for another 2 to 3 days, and the medium containing radiolabeled virus particles was collected. The medium was clarified of cellular debris by centrifugation at low speed and then loaded onto a 20% sucrose cushion (20% sucrose in phosphate-buffered saline [PBS]). After centrifugation at 27,000 × g for 1.5 h at 4°C, the pelleted virus particles were resuspended in 0.5 ml radioimmunoprecipitation assay buffer (1% NP-40, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate in PBS). The viral proteins were precipitated by a mixture of sera from HIV-1-infected individuals.
Analysis of ligand binding to HIV-1 envelope glycoproteins on the cell surface.
Approximately 5 × 105 mock-transfected 293T cells or 293T cells expressing cytoplasmic-tail-deleted ADA-N197S, ADA-H66N-N197S, KB9, or KB9-H66N envelope glycoproteins were incubated on ice or at room temperature for 16 h. Cells were transferred to room temperature and incubated with 40 μg/ml of anti-gp120 antibodies or the C34-immunoglobulin (Ig) fusion protein (62) in fluorescence-activated cell sorter (FACS) buffer (PBS-5% fetal bovine serum) in the presence or absence of 25 μg/ml of sCD4. Cells were washed three times in FACS buffer, and bound ligands were detected by incubation for 1 h with phycoerythrin-conjugated rabbit anti-human IgG (Sigma, St. Louis, MO). The cells were washed three times with FACS buffer and analyzed with a FACScan flow cytometer with CellQuest software (Becton Dickinson, Mountain View, CA) and FLOWJO software (FlowJo, Ashland, OR).
Effects of chemokine receptor inhibitors on HIV-1 infectivity.
The neutralization sensitivity of partially cold-inactivated HIV-1 to chemokine receptor inhibitors was examined. The CCR5 inhibitor compound A (21) and CXCR4 inhibitor AMD3100 (14) were used. Compound A was kindly supplied by Martin Springer (Merck & Co., Inc.). Recombinant HIV-1, at a concentration of 150 RT units per μl, was incubated for 1 to 2 h with serial dilutions of these inhibitors in a total volume of 650 μl at room temperature. The virus-inhibitor mixture was then added to target cells in triplicate to measure infectivity.
Virus capture assay.
To analyze the structural alterations and antigenic properties of cold-inactivated envelope glycoproteins on the virus surface, we adopted a virus capture assay similar to that described by Moore et al. (48). Briefly, monoclonal antibodies (MAbs) were used to coat Immulon II enzyme-linked immunosorbent assay wells (Dynex Technologies, Chantilly, VA) overnight at 5 μg/ml in PBS. These MAbs include 7B2, 2.2B, and 2G12. The MAb 2G12 recognizes a carbohydrate-dependent epitope on the gp120 outer domain (60, 68). MAbs 7B2 and 2.2B recognize cluster I and cluster II regions in gp41, respectively (6). MAbs 7B2 and 2.2B were provided by James Robinson (Tulane University, New Orleans, LA). The wells were then washed with PBS and blocked with 3% bovine serum albumin in PBS for 30 min. Virus-containing cell supernatants were incubated at a given temperature, added to the plate in triplicate, and incubated for 3 h. Afterwards, the wells were washed three times with PBS and then overlaid with Cf2Th cells (6 × 103 cells per well) that do not express CD4 or the appropriate chemokine receptor for the HIV-1 envelope glycoproteins. Infection of target cells mediated by VSV G was allowed to proceed at 37°C overnight, and cells were split into B&W IsoPlate-96 TC plates (PerkinElmer, Waltham, MA) suitable for the luciferase assay. After another 48 h of incubation at 37°C, cells were lysed and the level of infection was quantitated.
RESULTS
Effects of temperature on the function of wild-type and H66N HIV-1 envelope glycoproteins.
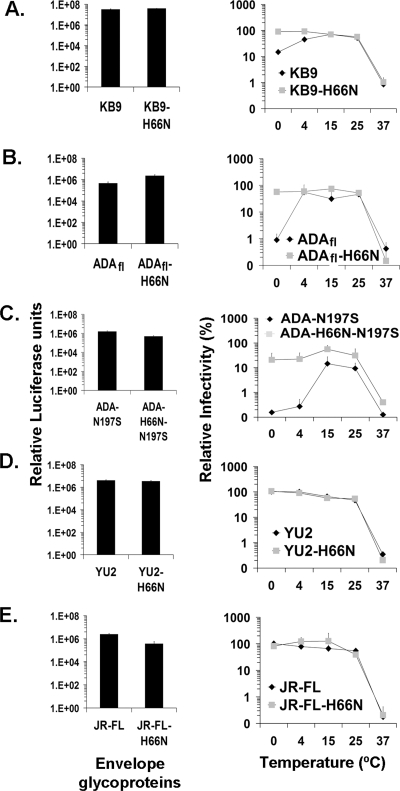
To investigate the effects of temperature extremes on HIV-1, we passaged a molecularly cloned HIV-1, NL4.3-KB9, while intermittently incubating the viruses at elevated temperatures. The NL4.3-KB9 virus is derived from the NL4.3 virus but has its envelope glycoproteins replaced by those of the pathogenic simian-human immunodeficiency virus KB9 (6). Infectious virus was generated by transfection of 1G5 indicator cells with NL4.3-KB9 proviral DNA. The 1G5 cells are Jurkat T cells that contain an integrated luciferase gene under the control of the HIV-1 LTR (2). Infection of these cells by HIV-1 results in LTR activation by the HIV-1 Tat protein and a consequent increase in luciferase expression. The NL4.3-KB9 starting virus stock was incubated at a given elevated temperature for different periods of time, cooled to room temperature, and added to 1G5 cells. Viruses that survived the most extreme temperatures were propagated and reexposed to higher temperature, and the process was repeated. A control stock of NL4.3-KB9 virus was passaged in parallel on 1G5 cells without heat selection. Once viruses that exhibited decreased heat sensitivity emerged, proviral sequences of these viruses and the control viruses were PCR amplified in fragments from infected cells. Each amplified fragment was cloned and sequenced. Comparison of the proviral sequences from heat-selected and control viruses revealed the presence of specific mutations in the former. Presumably, some combination of these mutations, which affect multiple HIV-1 genes and sequences, results in heat stability.
During the course of these studies, we observed that the parental NL4.3-KB9 virus was inactivated on ice more rapidly than at room temperature; by contrast, the heat-selected virus was resistant to this cold inactivation. We hypothesized that the major determinant of this resistance to cold inactivation was the env gene of the heat-selected virus. Several of the observed nonsynonymous changes in the env gene of the heat-resistant virus were introduced, alone or in combination, into the KB9 env in the context of a single-round recombinant virus. The temperature sensitivity of single-round recombinant HIV-1 bearing these envelope glycoproteins revealed that a single amino acid change, histidine 66 to asparagine (H66N), was responsible for the altered cold sensitivity of the envelope glycoproteins of the passaged virus. Recombinant HIV-1 with wild-type KB9 or KB9-H66N envelope glycoproteins was incubated at various temperatures for 44 h and then used to infect Cf2Th-CD4/CXCR4 cells (Fig. 1A). Both recombinant viruses exhibited similar levels of infectivity when assayed directly after thawing from −80°C storage. Both prolonged incubation on ice and at temperatures above 25°C resulted in decreased infectivity of the viruses with wild-type KB9 envelope glycoproteins. The viruses with wild-type KB9 and KB9-H66N envelope glycoproteins exhibited similar low infectivities following incubation at high temperatures. This result demonstrates that the H66N change is not sufficient to render HIV-1 heat resistant, consistent with the reported sensitivity of multiple HIV-1 proteins to heat (38). By contrast, the viruses with the KB9-H66N envelope glycoproteins were relatively resistant to the negative effects of incubation at low temperature, compared with the viruses with the wild-type KB9 envelope glycoproteins (Fig. 1A). Thus, the H66N change fully explains the resistance of the heat-selected virus to cold inactivation.
FIG. 1.
Effect of temperature on infectivity of HIV-1. Recombinant, luciferase-expressing HIV-1 bearing wild-type or H66N variants of the KB9 (A), ADAfl (B), ADA-N197S (C), YU2 (D), and JR-FL (E) envelope glycoproteins were incubated at the indicated temperatures for 44 h, and infectivity was measured. Relative infectivity is the percentage of infectivity of the same virus that was kept at −80°C as a reference control. The bar graphs show the infectivities of the control viruses. The means and standard deviations from three independent experiments performed in triplicate are shown.
To investigate the generality of the effect of the H66N change on cold sensitivity of HIV-1, this change was introduced into the envelope glycoproteins derived from several primary, CCR5-using HIV-1 isolates: YU2, JR-FL, and ADAfl. We also introduced the H66N change into the envelope glycoproteins of a CD4-independent HIV-1 variant, ADA-N197S, that had been derived by passage of HIV-1 in CD4-negative, CCR5-expressing cells (34, 35). Recombinant HIV-1 encoding luciferase was pseudotyped with the wild-type and H66N variants of the above envelope glycoproteins, incubated for 44 h at various temperatures, and then used to infect Cf2Th-CD4/CCR5 cells. As was seen for viruses with the KB9 envelope glycoproteins, viruses with the wild-type ADAfl and ADA-N197S envelope glycoproteins exhibited significant reductions in infectivity after prolonged incubation on ice, as well as at temperatures above 30°C (Fig. 1B and C). By contrast, viruses with the ADAfl-H66N and ADA-H66N-N197S envelope glycoproteins, which contain the H66N change, retained their infectivity after cold incubation. Thus, the H66N change can rescue the envelope glycoproteins of several HIV-1 strains from functional inactivation due to incubation in the cold.
Viruses with the envelope glycoproteins derived from the YU2 and JR-FL HIV-1 isolates were sensitive to prolonged incubation at the higher temperatures but were not affected by incubation on ice. The H66N change did not alter these phenotypes (Fig. 1D and E).
We conclude that some, but not all, HIV-1 envelope glycoproteins are functionally inactivated by prolonged incubation at 0°C; the H66N change renders the sensitive envelope glycoproteins resistant to cold inactivation.
The H66N change reduces the rate of HIV-1 envelope glycoprotein inactivation in the cold.
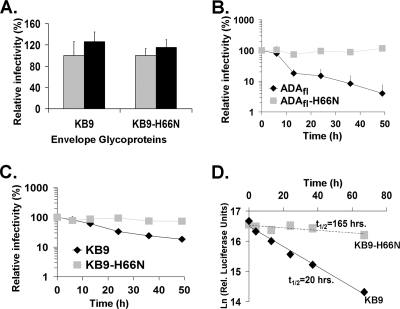
The above results suggest that the H66N change might reduce the rate of functional inactivation of some HIV-1 envelope glycoproteins in the cold. To examine this possibility directly, recombinant HIV-1 bearing the wild-type ADAfl and ADAfl-H66N (Fig. 2B) or wild-type KB9 and KB9-H66N (Fig. 2C) envelope glycoproteins was incubated on ice and, at different times, portions of the virus suspension were removed and frozen. After the virus suspension was thawed, the infectivity of the virus was assessed by incubation with Cf2Th-CD4/CCR5 cells (for the ADAfl variants) or Cf2Th-CD4/CXCR4 cells (for the KB9 variants). A pilot experiment demonstrated that a single freeze-thaw cycle did not significantly affect the infectivity of viruses with the KB9 and KB9-H66N envelope glycoproteins (Fig. 2A). The rate at which infectivity declined on ice was faster for viruses with the wild-type KB9 or ADAfl envelope glycoproteins than for viruses with the H66N change (Fig. 2B and C). The H66N change prolonged the functional half-life of the KB9 envelope glycoproteins by more than eightfold (Fig. 2D). Thus, the H66N change reduces the rate of functional inactivation of the HIV-1 envelope glycoproteins at 0°C.
FIG. 2.
Rate of cold inactivation of virus infectivity. (A) Recombinant viruses with wild-type KB9 or KB9-H66N envelope glycoproteins were kept at room temperature (gray) or frozen at −80°C (black). Frozen viruses were thawed, and their infectivities were compared with those of viruses kept at room temperature. (B and C) Recombinant viruses bearing the wild-type ADAfl or ADAfl-H66N (B) and wild-type KB9 or KB9-H66N (C) envelope glycoproteins were incubated on ice for various lengths of time. At the indicated time points, aliquots were removed and frozen at −80°C. After completion of the longest incubation, all samples were thawed and infectivity was assayed on Cf2Th-CD4/CCR5 (for ADAfl and ADAfl-H66N) and on Cf2Th-CD4/CXCR4 cells (for KB9 and KB9-H66N). Relative infectivity is the percentage of infection of the same virus not incubated on ice but kept at −80°C. Data are representative of results from three independent experiments performed in triplicate. (D) The half-lives of inactivation of viruses bearing the wild-type KB9 or KB9-H66N envelope glycoproteins after exposure to 0°C were calculated.
Influence of subsequent exposure to 37°C on inactivation of the HIV-1 envelope glycoproteins in the cold.
The functional inactivation of HIV-1 in the cold occurs at a slower rate than heat inactivation. For example, the inactivation of the wild-type HIV-1 KB9 envelope glycoproteins proceeded with a half-life of approximately 20 h at 0°C (see above), whereas HIV-1 infectivity is eliminated within 1 hour at 56°C (64). Because virus diffusion to the target cell constitutes a slow, rate-limiting step in the virus infection process (26), it is formally possible that incubation of the virus and cells at 37°C contributes to the inactivation of HIV-1 following incubation at 0°C. To investigate this possibility, we asked whether shortening or prolonging the time during the infection procedure that the virus spends at 37°C influences the inactivation.
To reduce the time required for virus attachment to target cells, spinoculation of viruses and cells was utilized. Recombinant viruses with wild-type KB9 or KB9-H66N envelope glycoproteins were either incubated for 44 h on ice or kept at −80°C. Afterwards, half of each virus preparation was mixed with Cf2Th-CD4/CXCR4 cells and centrifuged at 1,400 rpm for 2 hours at room temperature in a Beckman Alegra centrifuge. The spinoculated virus-cell mixture was then incubated at 37°C, and the level of infection was assessed 48 h later. The other half of each virus preparation was incubated with Cf2Th-CD4/CXCR4 cells at 37°C without spinoculation. Spinoculation resulted in an enhanced efficiency of infection over the range of preincubation temperatures examined (see Fig. S1 in the supplemental material). However, the negative impact of cold incubation on the infectivity of viruses with the wild-type KB9 envelope glycoproteins, relative to that of viruses with the KB9-H66N envelope glycoproteins, was still evident following spinoculation. Thus, shortening the period of time that the virus spends at 37°C during the infection process did not significantly influence the outcome of cold-induced inactivation.
We also extended the time after incubation in the cold that the viruses spent at 37°C before contacting the target cell. Recombinant viruses containing the wild-type KB9, KB9-H66N, ADA-N197S, and ADA-H66N-N197S envelope glycoproteins were incubated on ice for 44 h and then incubated at 37°C for various lengths of time before incubation with target cells. For target cells expressing CD4, the large difference in infectivity after cold incubation between viruses with and without the H66N change was observed even in the absence of incubation at 37°C and changed only moderately with extended incubation at 37°C (see Fig. S2A and B in the supplemental material). By contrast, the low level of infectivity for CD4-negative Cf2Th-CCR5 cells retained by viruses with the ADA-H66N-N197S envelope glycoproteins following cold incubation did decay after incubation at 37°C (see Fig. S2C in the supplemental material). Thus, in most instances, events occurring at 37°C contribute only minimally to the functional inactivation of HIV-1 envelope glycoproteins following incubation at 0°C.
In other experiments, recombinant viruses with wild-type KB9 and KB9-H66N envelope glycoproteins that had been incubated on ice for 48 h were held at room temperature for 20 h before assessing their infectivity. As observed previously, the infectivity of viruses with the wild-type KB9 envelope glycoproteins was reduced approximately 10-fold as a result of the cold incubation, whereas that of viruses with the KB9-H66N envelope glycoproteins was reduced minimally by incubation on ice (see Fig. S3 in the supplemental material). The subsequent room temperature incubation resulted in a moderate (approximately twofold) decrease in infectivity for both viruses (see Fig. S3 in the supplemental material). These results, along with those described above, indicate that the effects of cold incubation are not reversed even by prolonged incubation at room temperature or 37°C.
In summary, our results indicate that the length of time that the virus is exposed to 0°C rather than the duration of the exposure to 20 to 37°C primarily determines the degree of functional inactivation. These observations are consistent with a model in which the inactivation of HIV-1 envelope glycoprotein function occurs primarily in the cold.
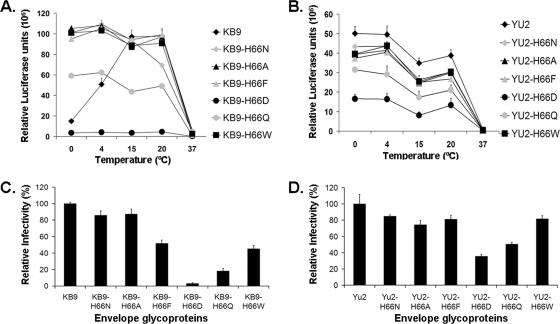
Effects on resistance to cold inactivation of amino acids other than histidine at position 66.
Histidine 66 is invariant in all of the HIV-1 and simian immunodeficiency virus SIVcpz sequences deposited in the Los Alamos National HIV Sequence Database to date (http://www.hiv.lanl.gov/content/sequence/HIV/mainpage.html). The substitution of asparagine at position 66 does not create a consensus site for N-linked glycosylation. We wished to determine if the substitution of residues other than asparagine for histidine 66 would influence the phenotype of resistance to cold inactivation. Six amino acids with a range of properties were substituted for histidine 66 in the KB9 and YU2 HIV-1 envelope glycoproteins. Recombinant viruses bearing the wild-type or mutant envelope glycoproteins were incubated at different temperatures for 48 h and then incubated at 37°C with Cf2Th-CD4/CXCR4 cells (for the KB9 envelope glycoproteins) or with Cf2Th-CD4/CCR5 cells (for the YU2 envelope glycoproteins). With the exception of the H66K mutant, all of the mutant envelope glycoproteins supported HIV-1 infection to some degree (Fig. 3C and D). Viruses with the wild-type KB9 envelope glycoproteins exhibited a significantly decreased infectivity after incubation on ice; this reduction in infectivity was not observed for the viruses with the KB9-H66N, -H66A, -H66F, -H66Q, and -H66W envelope glycoproteins (Fig. 3A). Although the basal level of replication of the virus with the KB9-H66D envelope glycoproteins was low, replication of this virus was unaffected by incubation at temperatures below 20°C. The viruses with the wild-type YU2 envelope glycoproteins, as well as the viruses with the changes in histidine 66, efficiently supported HIV-1 entry after prolonged incubation on ice (Fig. 3B). We conclude that several different amino acids at position 66 can allow the HIV-1 envelope glycoproteins to resist cold inactivation.
FIG. 3.
Effects of substitution of different residues for histidine 66 on cold inactivation. (A) Recombinant HIV-1 bearing the wild-type KB9 envelope glycoproteins or the indicated mutant envelope glycoproteins was incubated at different temperatures for 48 h, and infectivity on Cf2Th-CD4/CXCR4 cells was measured. (B) Recombinant HIV-1 bearing wild-type or mutant YU2 envelope glycoproteins was incubated at different temperatures for 48 h, and infectivity on Cf2Th-CD4/CCR5 cells was measured. (C and D) The basal levels of infectivity without extended incubation at the different temperatures, relative to that of the virus with the respective wild-type envelope glycoproteins, were as follows: KB9-H66N, 86%; KB9-H66A, 87%; KB9-H66F, 52%; KB9-H66D, 3%; KB9-H66Q, 18%; KB9-H66W, 45% YU2-H66N, 85%; YU2-H66A, 74%; YU2-H66F, 81%; YU2-H66D, 35%; YU2-H66Q, 51%; YU2-H66W, 82%. The KB9-H66K and YU2-H66K envelope glycoproteins did not support detectable virus infection. Data are representative of results from three independent experiments performed in triplicate.
Effects of pH on HIV-1 sensitivity to cold inactivation.
The above results suggest that the cold-stabilizing effect of the H66N change is due to the absence of the histidine residue rather than to the presence of the substituted asparagine residue. The pKR of histidine is near 7, raising the possibility that the phenotype associated with histidine 66 might be modulated by pH changes in the physiologic range. To examine this possibility, recombinant HIV-1 with the wild-type KB9 and KB9-H66N envelope glycoproteins was prepared and shown to exhibit similar infectivities at pH 7.5 (Fig. 4A). The viruses with the KB9 and KB9-H66N envelope glycoproteins were incubated in Dulbecco's modified Eagle medium at a specific pH (range, 5.0 to 8.5) on ice for 48 h. The medium was returned to pH 7.5, and the infectivities of the viruses on Cf2Th-CD4/CXCR4 cells were assessed. The difference in infectivities of the viruses with wild-type KB9 envelope glycoproteins and viruses with the KB9-H66N envelope glycoproteins was most pronounced at high pH values (Fig. 4B). Although differences in the rates of decay of infectivity between the wild-type KB9 and KB9-H66N viruses were modest at pH 5.0, these differences in decay rates were substantial at pH 8.5 (Fig. 4C). Because the phenotypic differences between the viruses with the wild-type KB9 and KB9-H66N envelope glycoproteins were evident at high pH, deprotonation of histidine 66 is apparently not sufficient for cold resistance.
FIG. 4.
Effect of pH on virus sensitivity to cold inactivation. (A) The recombinant HIV-1 viruses with wild-type KB9 and KB9-H66N envelope glycoproteins were incubated with Cf2Th-CD4/CXCR4 cells, and the infectivity was measured by luciferase assay. (B) The above recombinant viruses were incubated on ice at the indicated pHs for 48 h. Afterwards, viruses were returned to pH 7.5 and used to infect Cf2Th-CD4/CXCR4 cells. The infectivity was measured by luciferase assay. (C) The rate of decay of infectivity was measured for viruses with wild-type KB9 and KB9-H66N envelope glycoproteins at pH 5.0 and pH 8.5. Values are given as percentages of initial infectivity. Data are representative of results from independent experiments performed in triplicate.
Shedding of gp120 as a potential mechanism of cold inactivation.
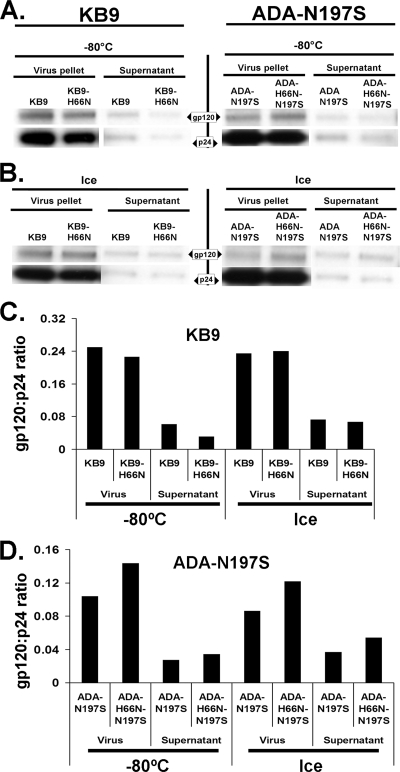
The noncovalent nature of the association between the gp120 and gp41 glycoproteins allows shedding of gp120 from the envelope glycoprotein complex. This shedding of gp120 occurs spontaneously at a low level, leading to functional inactivation of a subset of the envelope glycoprotein spikes on the virion (38). To address whether gp120 shedding contributes to cold inactivation, radiolabeled recombinant HIV-1 containing the wild-type KB9, KB9-H66N, ADA-N197S, and ADA-H66N-N197S envelope glycoproteins was prepared by transfecting 293T cells. Viruses in the supernatant were separated from shed gp120 by pelleting through a 20% sucrose cushion. Each virus preparation was divided into two portions, one kept at −80°C and the other placed on ice. After 60 h, virus was pelleted and pellets and supernatants were used for precipitation of viral proteins by an excess of a serum mixture from HIV-1-infected individuals. The gp120/p24 ratio reflects the amount of gp120 per virion and did not correlate with the infectivity pattern for the different viruses and treatments (Fig. 5). The low level of Gag p24 protein in the supernatant indicates that no significant dissolution of viral particles occurred during incubation on ice. Thus, the loss of HIV-1 infectivity on ice results mainly from processes other than gp120 shedding or disintegration of virion particles.
FIG. 5.
Effect of the H66N change on the shedding of gp120 in the cold. Radiolabeled HIV-1 bearing the ADA-N197S and ADA-H66N-N197S envelope glycoproteins (left) or KB9 and KB9-H66N envelope glycoproteins (right) was incubated on ice for 60 h or kept at −80°C. Viruses were pelleted and resuspended in radioimmunoprecipitation assay buffer. Viral pellets and supernatants were precipitated by a mixture of sera from HIV-1-infected individuals to determine the content of gp120 and p24 Gag proteins.
Changes in the HIV-1 envelope glycoprotein structure associated with incubation in the cold.
To determine whether changes in the conformation of the HIV-1 envelope glycoproteins accompanied cold inactivation, we examined envelope glycoproteins on cell surfaces and virions for the ability to bind a number of Env ligands.
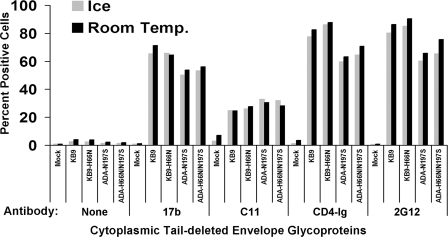
For the study of HIV-1 envelope glycoproteins on the cell surface, 293T cells were transfected with plasmids expressing the KB9, KB9-H66N, ADA-N197S, and ADA-H66N-N197S envelope glycoproteins with truncations of their gp41 cytoplasmic tails. These truncations allow more-efficient expression of these glycoproteins on the cell surface (25, 78). Pilot experiments demonstrated that truncating the gp41 cytoplasmic tail did not influence the sensitivity of the viral envelope glycoproteins to cold inactivation (see Fig. S4 in the supplemental material). Transfected cells were divided into two aliquots, one kept at room temperature and the other on ice. After 16 h at the respective temperature, the cells were stained with a panel of MAbs directed against HIV-1 gp120 and with CD4-Ig, a fusion of the N-terminal two CD4 domains and the Fc portion of human IgG (27). The binding of these gp120 ligands to the envelope glycoproteins on the cell surface was measured by incubation with a phycoerythrin-conjugated anti-human Ig antibody and FACS analysis (Fig. 6). As expected (22, 30, 63, 74), the gp120 ligands bound the HIV-1 envelope glycoproteins to different degrees; for example, the nonneutralizing C11 anti-gp120 antibody bound less efficiently than the 17b and 2G12 antibodies, which can neutralize the ADA-N197S envelope glycoproteins. However, no consistent differences between the recognition of the parental envelope glycoproteins and that of the H66N derivatives were observed.
FIG. 6.
Binding of gp120 ligands to cell surface HIV-1 envelope glycoproteins after incubation on ice. Mock-transfected 293T cells and 293T cells expressing cytoplasmic tail-deleted ADA-N197S, ADA-H66N-N197S, KB9, or KB9-H66N envelope glycoproteins were incubated on ice or at room temperature for 16 h. Binding of the indicated gp120 ligands was assessed by FACS. The percentages of antibody-positive cells are shown.
The functional inactivation of the HIV-1 envelope glycoproteins observed after prolonged cold incubation might involve a disruption of the formation of key intermediates induced by CD4 binding. CD4 binding allows gp120 to interact efficiently with the CCR5 or CXCR4 chemokine receptor and also results in the exposure of the HR1 groove on the gp41 transmembrane glycoprotein (24, 62, 67, 72). We tested whether cold incubation influences either of these processes and whether differences between the parental and H66 envelope glycoproteins exist.
To determine if cold inactivation alters the affinity of the envelope glycoproteins for the chemokine receptor, we took advantage of the observations that increased susceptibility to CCR5-directed inhibitors invariably accompanies decreases in the affinity of the gp120-CCR5 interaction (40, 57, 58). Partially cold-inactivated recombinant HIV-1 with cold-sensitive wild-type ADAfl envelope glycoproteins or cold-resistant YU2 envelope glycoproteins and their H66N counterparts were used to infect Cf2Th-CD4/CCR5 target cells in the presence of different concentrations of compound A, a small-molecule CCR5 ligand that blocks HIV-1 binding and infection (see Fig. S5 in the supplemental material) (21). Similarly, partially cold-inactivated recombinant HIV-1 with wild-type KB9 and KB9-H66N envelope glycoproteins was used to infect Cf2Th-CD4/CXCR4 cells in the presence of different concentrations of AMD3100, a CXCR4 ligand that blocks HIV-1 binding and infection (see Fig. S5 in the supplemental material). In all cases, there was no difference in sensitivity to chemokine receptor inhibitors after exposure to cold, indicating that incubation at 0°C does not necessarily alter the affinity of envelope glycoprotein binding to chemokine receptors.
To determine if cold incubation influences the spontaneous or CD4-induced exposure of the gp41 HR1 groove, we utilized C34-Ig to probe the HIV-1 envelope glycoproteins expressed transiently on the surfaces of 293T cells, as described above. C34-Ig consists of a peptide (C34) that corresponds to the gp41 HR2 region fused to the Fc portion of an IgG molecule. Mock-transfected 293T cells or 293T cells expressing wild-type KB9, KB9-H66N, ADA-N197S, and ADA-H66N-N197S envelope glycoproteins were kept at room temperature or on ice for 16 h. Then the cells were incubated with sCD4, C34-Ig, or a combination of both proteins at room temperature, which minimizes CD4-induced shedding but allows CD4-induced HR1 exposure (62). The bound C34-Ig or 2G12 antibody, which is used as a control to detect the gp120 envelope glycoprotein, was measured by FACS analysis using an anti-human Ig antibody. Under these conditions, gp120 shedding after sCD4 incubation was minimal, as indicated by the level of 2G12 staining (Fig. 7). The spontaneous level of HR1 exposure was greater for the wild-type KB9 and KB9-H66N envelope glycoproteins that had been incubated on ice than for those incubated at room temperature. The induction of C34-Ig binding by sCD4 was greater for the KB9 envelope glycoproteins than for the ADA-N197S envelope glycoproteins. However, the KB9 and KB9-H66N envelope glycoproteins exhibited similar degrees of induction of C34-Ig binding in the presence of sCD4. Thus, although some increased exposure of the gp41 HR1 region occurs following incubation on ice compared with room temperature incubation, this change is not significantly affected by the alteration of histidine 66 and therefore does not correlate with cold inactivation of infectivity.
FIG. 7.
Effect of cold incubation on the CD4-induced exposure of the gp41 HR1 region. Mock-transfected 293T cells and 293T cells expressing cytoplasmic-tail-deleted ADA-N197S, ADA-H66N-N197S, KB9, or KB9-H66N envelope glycoproteins were incubated on ice or at room temperature for 16 h. The binding of C34-Ig and 2G12, after incubation with the indicated ligands, was assessed by FACS.
Cold-induced exposure/formation of gp41 epitopes.
The above studies suggested that prolonged exposure to 0°C might alter the conformation of the gp41 ectodomain. We wished to examine this possibility in the context of the envelope glycoproteins on the virion surface. To do so, we adopted an assay in which antibodies recognizing envelope glycoprotein epitopes of interest are bound to a plate and used to capture virions. The recombinant, luciferase-expressing viruses used in this assay contain both HIV-1 envelope glycoproteins and VSV G. Antibodies specific for the HIV-1 envelope glycoproteins were bound to 96-well plates and used to capture virus particles; unbound particles were removed by thorough washing. Cf2Th canine thymocytes that do not express receptors for HIV-1 were then added to the wells, and infection of these cells (mediated by VSV G) was measured by luciferase assay. This provides a quantitative, albeit indirect, measure of the efficiency of recognition of the viral envelope glycoproteins by the antibody of interest (7, 48, 51, 55).
The envelope glycoproteins of three HIV-1 strains, the cold-sensitive ADAfl and KB9 and the cold-resistant YU2 viruses, were compared, along with their H66N counterparts. Viruses with no envelope glycoproteins and viruses with only VSV G were included as negative controls. Equivalent RT units of each virus were incubated on ice or at room temperature for 50 h or kept at −80°C as an untreated control.
Two major groups of epitopes in the HIV-1 gp41 ectodomain have been identified: cluster I and cluster II (76). To probe structural changes in the HIV-1 gp41 ectodomain, we used two antibodies: 2.2B, which recognizes a cluster II epitope, and 7B2, which recognizes a cluster I epitope (6). The gp120-directed 2G12 antibody was included as a positive control, allowing an assessment of any shedding of the exterior envelope glycoprotein. Indeed, some decreases in 2G12 binding, presumably reflecting spontaneous gp120 shedding, accompanied incubation of all viruses in the cold or at room temperature (Fig. 8). For the ADAfl and KB9 viruses, the H66N change appeared to decrease the amount of gp120 shedding on ice, but not during the room temperature incubation. The H66N change affected 2G12 binding to the YU2 envelope glycoproteins only minimally. These results suggest that gp120 shedding may contribute minimally to cold inactivation.
FIG. 8.
Exposure of HIV-1 envelope glycoprotein epitopes on virions. Viruses with VSV G and ADAfl, KB9, or YU2 envelope glycoproteins or their H66N counterparts were kept at −80°C (initial) or incubated on ice or at room temperature for 50 h. Control viruses with only VSV G (VSV) or lacking envelope glycoproteins [ENV(−)VSV(−)] were treated in parallel. Viruses were captured on wells coated with the indicated antibodies. After a washing, Cf2Th cells lacking HIV-1 receptors were added to the wells, and luciferase activity was assessed 48 h later. The results of a single experiment, performed in triplicate, are shown. The experiment was repeated twice with similar results.
Viruses with the cold-resistant YU2 envelope glycoproteins exhibited mild decreases in binding to 2.2B and 7B2 antibodies after incubation on ice or at room temperature. Viruses with the wild-type ADAfl and ADAfl-H66N envelope glycoproteins and with the wild-type KB9 and KB9-H66N envelope glycoproteins also showed slight decreases in binding of these antibodies after room temperature incubation. By contrast, 2.2B and 7B2 antibody binding to the viruses with wild-type ADAfl and KB9 envelope glycoproteins that had been incubated on ice increased significantly. Binding of the 2.2B antibody to the H66N derivatives of the ADAfl and KB9 envelope glycoproteins was less than that seen for the wild-type counterparts of these glycoproteins. Thus, the patterns of 2.2B epitope exposure roughly correlate with the functional inactivation of certain HIV-1 envelope glycoproteins in the cold.
Kinetics of exposure of cold-induced gp41 epitopes.
The virus capture assay was used to measure the binding of the 2G12, 2.2B, and 7B2 antibodies to the envelope glycoprotein variants described above at various times during incubation of the virus at 0°C. During the incubation period on ice, viruses shed some gp120, as evidenced by decreasing binding of the 2G12 antibody over time (Fig. 9). The temporal loss of 2G12 binding occurred slightly more rapidly for the viruses with the wild-type ADAfl, KB9, and YU2 envelope glycoproteins than for the viruses with the H66N variants. Thus, the H66N change allows slightly larger amounts of gp120 to be retained on virion envelope glycoprotein complexes for both cold-sensitive and cold-resistant viruses.
FIG. 9.
Timing of exposure of cold-induced epitopes on the HIV-1 envelope glycoproteins. Viruses with VSV G and ADAfl, KB9, or YU2 envelope glycoproteins or their H66N counterparts were kept at −80°C (initial) or incubated on ice for the indicated times. Viruses with only VSV G (VSV) or lacking envelope glycoproteins [ENV(−)VSV(−)] were studied in parallel as controls. Viruses were captured on antibody-coated wells and quantitated by adding Cf2Th target cells and measuring luciferase activity 48 h later.
The H66N variants of the ADAfl and wild-type KB9 envelope glycoproteins exhibited less 2.2B and 7B2 antibody binding immediately after the viruses were thawed from −80°C (Fig. 9). This was found to be an inherent feature of the H66N envelope glycoproteins and independent of freezing and thawing (see Fig. S6 and S7 in the supplemental material). With time, the binding of the 2.2B and 7B2 antibodies to the H66N envelope glycoproteins increased, until they achieved a level of binding similar to those of the ADAfl and wild-type KB9 envelope glycoproteins. The basal levels of 2.2B and 7B2 binding to the wild-type YU2 and YU2-H66N envelope glycoproteins were similar and exhibited slight increases during the incubation on ice. These results indicate that specific changes occur in the gp41 ectodomain of the HIV-1 envelope glycoproteins during functional inactivation at 0°C.
The CD4-bound conformation of the HIV-1 envelope glycoproteins is susceptible to cold inactivation.
The H66N change in HIV-1 gp120 has been shown to decrease the spontaneous transition of the unliganded envelope glycoproteins to the CD4-bound conformation (A. Kassa et al., unpublished data). Thus, selection of a temperature-stable virus resulted in an envelope glycoprotein with an altered propensity to sample the CD4-bound state. To investigate a potential relationship between the CD4-induced state of the HIV-1 envelope glycoproteins and temperature stability, we examined the cold susceptibilities of viruses bearing HIV-1 envelope glycoproteins with different propensities to assume the CD4-bound conformation. We compared the stabilities on ice of viruses with the HXBc2 and HXBc2-S375W envelope glycoproteins. The HXBc2-S375W mutant has a tryptophan side chain that fills the Phe 43 cavity, which predisposes gp120 to assume the CD4-bound conformation (75). We also used the CD4-mimetic drug NBD-556, which induces the CD4-bound conformation upon binding gp120 (42, 61). Viruses with the HXBc2, HXBc2-H66N, and HXBc2-S375W envelope glycoproteins were compared for sensitivity to cold inactivation in the absence or presence of NBD-556 (Fig. 10). In the absence of NBD-556, viruses with the HXBc2-S375W envelope glycoproteins exhibited greater sensitivity to cold than viruses with the wild-type HXBc2 envelope glycoproteins; in contrast, viruses with the HXBc2-H66N envelope glycoproteins were more resistant to cold inactivation (Fig. 10A). In the presence of increasing concentrations of NBD-556, viruses with wild-type HXBc2 envelope glycoproteins exhibited progressively greater inactivation on ice (Fig. 10B). In the presence of 2 μM NBD-556, viruses with the wild-type HXBc2 and HXBc2-S375W envelope glycoproteins exhibited similar rates of cold inactivation (Fig. 10C). NBD-556 did not influence the cold sensitivity of viruses with HXBc2-H66N or HXBc2-S375W envelope glycoproteins because these gp120 mutants do not efficiently bind the compound (42; A. Kassa et al., unpublished). Although NBD-556-treated viruses with the wild-type HXBc2 envelope glycoproteins exhibited mild reductions in infectivity following room temperature incubation, these viruses were much more sensitive to incubation on ice (Fig. 10D). Incubation of sCD4 with viruses bearing the wild-type HXBc2 envelope glycoproteins also resulted in increased inactivation in the cold (Fig. 10E). Viruses with the YU2 envelope glycoproteins, which are naturally resistant to cold inactivation, exhibited dramatic reductions in infectivity on ice following incubation with either NBD-556 or sCD4 (Fig. 10F). These results suggest that the CD4-bound state of the HIV-1 envelope glycoproteins is more sensitive to cold inactivation.
FIG. 10.
Susceptibility to cold inactivation of HIV-1 envelope glycoproteins in the CD4-bound conformation. (A) The infectivities of viruses with the HXBc2, HXBc2-H66N, and HXBc2-S375W envelope glycoproteins after incubation on ice for the indicated times were measured. The relative infectivity represents the percentage of the observed luciferase counts relative to those seen with freshly thawed virus. (B) Relative infectivities of viruses with the wild-type HXBc2 envelope glycoproteins following incubation on ice with the indicated concentrations of NBD-556. (C) Relative infectivities of viruses with the HXBc2, HXBc2-H66N, and HXBc2-S375W envelope glycoproteins after incubation on ice in the presence of 2 μM NBD-556. (D) Infectivities of viruses with the wild-type HXBc2 envelope glycoproteins following incubation on ice or at room temperature (r.t.) for 50 h in the presence of the indicated concentrations of NBD-556. (E) Viruses with the wild-type HXBc2 envelope glycoproteins were incubated on ice in the absence or presence of sCD4 (0.1 μg/ml), and the infectivities were measured. (F) Relative infectivities of viruses with the YU2 envelope glycoproteins following incubation on ice in the absence (control) or presence of 100 μM NBD-556 or 1 μg/ml sCD4. The means and standard deviations from three independent experiments performed in triplicate are shown.
DISCUSSION
By repeated passage of HIV-1 following intermittent exposure to increasingly higher temperatures, we obtained viruses that were able to withstand incubation at 42 to 56°C for longer periods of time than the parental virus (data not shown). Our preliminary observations suggest that multiple changes in the viral genome allow this heat resistance, consistent with previous suggestions that, at a minimum, the RT and envelope glycoproteins of HIV-1 are functionally inactivated at elevated temperatures (10, 17, 38, 64).
During the course of this work, we made the unexpected discovery that the infectivity of the parental virus decayed more rapidly at 0°C than at room temperature. The parental KB9 envelope glycoproteins and those of several other HIV-1 strains as well were found to be functionally inactivated by prolonged incubation at low temperatures. Viruses with some primary HIV-1 envelope glycoproteins did not appear to undergo cold inactivation. Although studies with a more extensive panel of HIV-1 envelope glycoproteins will be required to understand the biological significance of these differences, the results indicate that the envelope glycoproteins constitute the major cold-sensitive elements on at least some HIV-1 isolates. Notably, the H66N change in the gp120 envelope glycoprotein, which arose during the selection of heat-resistant viruses, rendered HIV-1 resistant to inactivation on ice. In some envelope glycoproteins, slight effects of the H66N change on sensitivity to heat inactivation were noted. However, because HIV-1 moieties other than the envelope glycoproteins are also inactivated at high temperatures (6, 38), it is more challenging to assess the effect of the H66N change on the heat sensitivity of the HIV-1 envelope glycoproteins.
Although most proteins denature after prolonged incubation at high temperatures, denaturation at temperatures near 0°C has been documented for a small, but growing, subset of proteins (56). The basis for cold denaturation of proteins is thought to be the formation of icelike water structures surrounding exposed hydrophobic amino acid residues that, in the native state, would be buried and contribute to stability. The favorable enthalpy change secondary to the formation of these water cages offsets the unfavorable entropy change, which makes less of a contribution to the free energy change of denaturation at lower temperatures (41, 56, 69). Of interest, cold denaturation has been documented mainly for oligomeric proteins and often involves weakening of intersubunit interactions (18, 31, 50, 54, 56). Notably, cold denaturation is often reversible; however, because of the high potential energy inherent in the unliganded HIV-1 envelope glycoprotein complex, any cold-induced change in the gp120-gp41 interaction may predispose to irreversible transitions to low-energy states. Indeed, despite long incubation at 20 to 37°C, cold-inactivated HIV-1 exhibited no evidence of restoration of function.
Although an asparagine residue was the only replacement for histidine 66 selected during the generation of the temperature-sensitive virus, substitution of amino acid residues with varied characteristics at position 66 also resulted in cold resistance. Histidine 66 is extremely well conserved among HIV-1 strains (Los Alamos HIV Sequence Database [http://www.hiv.lanl.gov/content/sequence/HIV/mainpage.html]). It is counterintuitive that HIV-1 strains retain histidine 66, which contributes to temperature-sensitive inactivation of virus infectivity. Although the consequences of temperature sensitivity for the survival of HIV-1 in its natural environment are unknown, there are no obvious advantages that would explain the observed high degree of conservation of histidine 66. A more satisfying rationale for this conservation arises from consideration of thermodynamic, mutagenic, and structural studies of the gp120 glycoprotein. The unliganded HIV-1 gp120 glycoprotein consists of an inner domain, an outer domain, and a bridging sheet and is thought to sample multiple conformations (36, 37, 49). The binding of CD4 and NBD-556 fixes the conformation of gp120, resulting in structuring of the inner domain and bridging sheet (49, 61). A recently solved X-ray crystal structure of the CD4-bound HIV-1 gp120 glycoprotein with intact N and C termini reveals that histidine 66 is located in a disulfide-bonded inner domain loop (layer 1) that is thought to undergo conformational transitions as gp120 binds CD4 (M. Pancera et al., unpublished data). Although the conformation of layer 1 in the unliganded envelope glycoproteins is currently unknown, in the CD4-bound state, histidine 66 contacts a number of aromatic and hydrophobic gp120 residues in the inner domain and bridging sheet. These contacts help to stabilize the CD4-bound state and thus contribute to CD4 binding. Indeed, gp120 mutants with alterations in histidine 66 have been shown to sample the CD4-bound conformation less than the wild-type HIV-1 gp120 (A. Kassa et al., unpublished). Some of the amino acid substitutions for histidine 66 decreased the ability of the mutant envelope glycoproteins to support virus infection, perhaps as a result of diminished capacity to bind CD4. The role of histidine 66 in facilitating the transition of gp120 from an unliganded state to the CD4-bound state provides a rationale for the high degree of conservation of this residue in HIV-1 strains.
Why did the selection of virus for temperature stability yield a variant envelope glycoprotein altered in its capacity to achieve the CD4-bound conformation? This outcome implies a relationship between HIV-1 envelope glycoprotein stability and the CD4-bound state. Recent work has demonstrated that, when induced on a virus that is distant from a target cell, the CD4-bound conformation is short-lived and decays into a nonfunctional state (H. Haim et al., submitted). Alteration of histidine 66 decreases the sampling of the CD4-bound state and thus allows the HIV-1 envelope glycoproteins to avoid this labile state. Conversely, promoting the transition of the HIV-1 envelope glycoproteins into the CD4-bound conformation by various means resulted in a dramatic increase in susceptibility to cold inactivation. These results support a model in which the CD4-bound state represents a major point of access for either successful virus entry or irreversible decay, depending on the availability and proximity of a suitable target cell.
Awareness of cold inactivation of HIV-1 may have practical implications for preservation of the natural phenotypes of viral samples. Moreover, studies that will be reported elsewhere indicate that changes in histidine 66 can exert major effects on HIV-1 sensitivity to some gp120-directed inhibitors, including neutralizing antibodies (A. Kassa et al., unpublished). The incorporation of HIV-1 gp120 glycoproteins that differ in residue 66 into further structural and mechanistic studies should provide important insights into the impact that the conformational lability of gp120 exerts on attempts to intervene in the HIV-1 entry process.
Supplementary Material
Acknowledgments
We thank Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation. We thank Peter Kwong at the National Institutes of Health Vaccine Research Center for helpful discussions.
This work was supported by NIH grants (AI24755, AI39420, AI40895, and GM56550), by a Center for HIV/AIDS Vaccine Immunology grant (AI67854), by a Center for AIDS Research grant (AI42848), by an unrestricted research grant from the Bristol-Myers Squibb Foundation, by a gift from the late William F. McCarty-Cooper, and by funds from the International AIDS Vaccine Initiative.
Footnotes
Published ahead of print on 11 February 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar-Cordova, E., J. Chinen, L. Donehower, D. E. Lewis, and J. W. Belmont. 1994. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum. Retrovir. 10295-301. [DOI] [PubMed] [Google Scholar]
- 3.Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 2281091-1094. [DOI] [PubMed] [Google Scholar]
- 4.Baltimore, D. 1970. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 2261209-1211. [DOI] [PubMed] [Google Scholar]
- 5.Bender, W., and N. Davidson. 1976. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell 7595-607. [DOI] [PubMed] [Google Scholar]
- 6.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavacini, L., and M. Posner. 2004. Native HIV type 1 virion surface structures: relationships between antibody binding and neutralization or lessons from the viral capture assay. AIDS Res. Hum. Retrovir. 20435-441. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti, S., M. Robert-Guroff, F. Wong-Staal, R. C. Gallo, and B. Moss. 1986. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature 320535-537. [DOI] [PubMed] [Google Scholar]
- 9.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89263-273. [DOI] [PubMed] [Google Scholar]
- 10.Chertova, E., J. W. Bess, Jr., B. J. Crise, I. R. Sowder, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 765315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 851135-1148. [DOI] [PubMed] [Google Scholar]
- 12.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312763-767. [DOI] [PubMed] [Google Scholar]
- 13.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381661-666. [DOI] [PubMed] [Google Scholar]
- 14.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 472-77. [DOI] [PubMed] [Google Scholar]
- 15.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381667-673. [DOI] [PubMed] [Google Scholar]
- 16.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70777-810. [DOI] [PubMed] [Google Scholar]
- 17.Einarsson, M., L. Perenius, J. S. McDougal, and S. Cort. 1989. Heat inactivation of human immunodeficiency virus in solutions of antithrombin III. Transfusion 29148-152. [DOI] [PubMed] [Google Scholar]
- 18.Fan, C. C., J. P. Lin, and G. W. Plaut. 1975. Effects of temperature on diphosphopyridine nucleotide-linked isocitrate dehydrogenase from bovine heart. Aspects of the kinetics, stability, and quarternary structure of the enzyme. J. Biol. Chem. 2502022-2027. [PubMed] [Google Scholar]
- 19.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272872-877. [DOI] [PubMed] [Google Scholar]
- 20.Fennie, C., and L. A. Lasky. 1989. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J. Virol. 63639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finke, P. E., B. Oates, S. G. Mills, M. MacCoss, L. Malkowitz, M. S. Springer, S. L. Gould, J. A. DeMartino, A. Carella, G. Carver, K. Holmes, R. Danzeisen, D. Hazuda, J. Kessler, J. Lineberger, M. Miller, W. A. Schleif, and E. A. Emini. 2001. Antagonists of the human CCR5 receptor as anti-HIV-1 agents. Part 4: synthesis and structure-activity relationships for 1-[N-(methyl)-N-(phenylsulfonyl)amino]-2-(phenyl)-4-(4-(N-(alkyl)-N-(benzy loxycarbonyl)amino)piperidin-1-yl)butanes. Bioorg. Med. Chem. Lett. 112475-2479. [DOI] [PubMed] [Google Scholar]
- 22.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 712779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu, Y. K., T. K. Hart, Z. L. Jonak, and P. J. Bugelski. 1993. Physicochemical dissociation of CD4-mediated syncytium formation and shedding of human immunodeficiency virus type 1 gp120. J. Virol. 673818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5276-279. [DOI] [PubMed] [Google Scholar]
- 25.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 663306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haim, H., I. Steiner, and A. Panet. 2005. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, R. J., K. L. Wagner, and M. W. Spellman. 1990. Structural characterization of a recombinant CD4-IgG hybrid molecule. Eur. J. Biochem. 194611-620. [DOI] [PubMed] [Google Scholar]
- 28.Hart, T. K., R. Kirsh, H. Ellens, R. W. Sweet, D. M. Lambert, S. R. Petteway, Jr., J. Leary, and P. J. Bugelski. 1991. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 882189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helseth, E., M. Kowalski, D. Gabuzda, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 642416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrera, C., C. Spenlehauer, M. S. Fung, D. R. Burton, S. Beddows, and J. P. Moore. 2003. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J. Virol. 771084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irias, J. J., M. R. Olmsted, and M. F. Utter. 1969. Pyruvate carboxylase. Reversible inactivation by cold. Biochemistry 85136-5148. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 714218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312767-768. [DOI] [PubMed] [Google Scholar]
- 34.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 753435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolchinsky, P., T. Mirzabekov, M. Farzan, E. Kiprilov, M. Cayabyab, L. J. Mooney, H. Choe, and J. Sodroski. 1999. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J. Virol. 738120-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420678-682. [DOI] [PubMed] [Google Scholar]
- 37.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189695-714. [DOI] [PubMed] [Google Scholar]
- 39.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobritz, M. A., A. J. Marozsan, R. M. Troyer, and E. J. Arts. 2007. Natural variation in the V3 crown of human immunodeficiency virus type 1 affects replicative fitness and entry inhibitor sensitivity. J. Virol. 818258-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez, C. F., R. K. Darst, and P. J. Rossky. 2008. Mechanistic elements of protein cold denaturation. J. Phys. Chem. B 1125961-5967. [DOI] [PubMed] [Google Scholar]
- 42.Madani, N., A. Schön, A. M. Princiotto, J. M. LaLonde, J. R. Courter, T. Soeta, D. Ng, L. Wang, E. T. Brower, S.-H. Xiang, Y. D. Kwon, C.-C. Huang, R. Wyatt, P. D. Kwong, E. Freire, A. B. Smith III, and J. G. Sodroski. 2008. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 161689-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 5355-67. [DOI] [PubMed] [Google Scholar]
- 44.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, J. P., and P. J. Klasse. 1992. Thermodynamic and kinetic analysis of sCD4 binding to HIV-1 virions and of gp120 dissociation. AIDS Res. Hum. Retrovir. 8443-450. [DOI] [PubMed] [Google Scholar]
- 46.Moore, J. P., J. A. McKeating, W. A. Norton, and Q. J. Sattentau. 1991. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J. Virol. 651133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 2501139-1142. [DOI] [PubMed] [Google Scholar]
- 48.Moore, P. L., E. T. Crooks, L. Porter, P. Zhu, C. S. Cayanan, H. Grise, P. Corcoran, M. B. Zwick, M. Franti, L. Morris, K. H. Roux, D. R. Burton, and J. M. Binley. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 802515-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myszka, D. G., R. W. Sweet, P. Hensley, M. Brigham-Burke, P. D. Kwong, W. A. Hendrickson, R. Wyatt, J. Sodroski, and M. L. Doyle. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 979026-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagradova, N. K., V. I. Muronetz, I. D. Grozdova, and T. O. Golovina. 1975. Cold inactivation of glyceraldehyde-phosphate dehydrogenase from rat skeletal muscle. Biochim. Biophys. Acta 37715-25. [DOI] [PubMed] [Google Scholar]
- 51.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zolla-Pazner. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 729384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien, W. A., S. H. Mao, Y. Cao, and J. P. Moore. 1994. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J. Virol. 685264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parolin, C., B. Taddeo, G. Palu, and J. Sodroski. 1996. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology 222415-422. [DOI] [PubMed] [Google Scholar]
- 54.Penefsky, H. S., and R. C. Warner. 1965. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J. Biol. Chem. 2404694-4702. [PubMed] [Google Scholar]
- 55.Poignard, P., M. Moulard, E. Golez, V. Vivona, M. Franti, S. Venturini, M. Wang, P. W. Parren, and D. R. Burton. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Privalov, P. L. 1990. Cold denaturation of proteins. Crit. Rev. Biochem. Mol. Biol. 25281-305. [DOI] [PubMed] [Google Scholar]
- 57.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 9916249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeves, J. D., J. L. Miamidian, M. J. Biscone, F. H. Lee, N. Ahmad, T. C. Pierson, and R. W. Doms. 2004. Impact of mutations in the coreceptor binding site on human immunodeficiency virus type 1 fusion, infection, and entry inhibitor sensitivity. J. Virol. 785476-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rho, H. M., B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112355-360. [DOI] [PubMed] [Google Scholar]
- 60.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 767293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schon, A., N. Madani, J. C. Klein, A. Hubicki, D. Ng, X. Yang, A. B. Smith III, J. Sodroski, and E. Freire. 2006. Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 4510973-10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si, Z., N. Madani, J. M. Cox, J. J. Chruma, J. C. Klein, A. Schon, N. Phan, L. Wang, A. C. Biorn, S. Cocklin, I. Chaiken, E. Freire, A. B. Smith III, and J. G. Sodroski. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc. Natl. Acad. Sci. USA 1015036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Si, Z., N. Phan, E. Kiprilov, and J. Sodroski. 2003. Effects of HIV type 1 envelope glycoprotein proteolytic processing on antigenicity. AIDS Res. Hum. Retrovir. 19217-226. [DOI] [PubMed] [Google Scholar]
- 64.Spire, B., D. Dormont, F. Barre-Sinoussi, L. Montagnier, and J. C. Chermann. 1985. Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet i188-189. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 694413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Temin, H. M., and S. Mizutani. 1970. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 2261211-1213. [DOI] [PubMed] [Google Scholar]
- 67.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384184-187. [DOI] [PubMed] [Google Scholar]
- 68.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 701100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai, C. J., J. V. Maizel, Jr., and R. Nussinov. 2002. The hydrophobic effect: a new insight from cold denaturation and a two-state water structure. Crit. Rev. Biochem. Mol. Biol. 3755-69. [DOI] [PubMed] [Google Scholar]
- 70.Veronese, F. D., A. L. DeVico, T. D. Copeland, S. Oroszlan, R. C. Gallo, and M. G. Sarngadharan. 1985. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science 2291402-1405. [DOI] [PubMed] [Google Scholar]
- 71.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387426-430. [DOI] [PubMed] [Google Scholar]
- 72.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384179-183. [DOI] [PubMed] [Google Scholar]
- 73.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 2801884-1888. [DOI] [PubMed] [Google Scholar]
- 74.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 674557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 769888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 654832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, X., S. Kurteva, X. Ren, S. Lee, and J. Sodroski. 2006. Subunit stoichiometry of human immunodeficiency virus type 1 envelope glycoprotein trimers during virus entry into host cells. J. Virol. 804388-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye, L., Z. Bu, A. Vzorov, D. Taylor, R. W. Compans, and C. Yang. 2004. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: role of the cytoplasmic domain. J. Virol. 7813409-13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 919564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.